An Overview of the Importance and Value of Porcine Species in Sialic Acid Research
Abstract
:Simple Summary
Abstract
1. Introduction
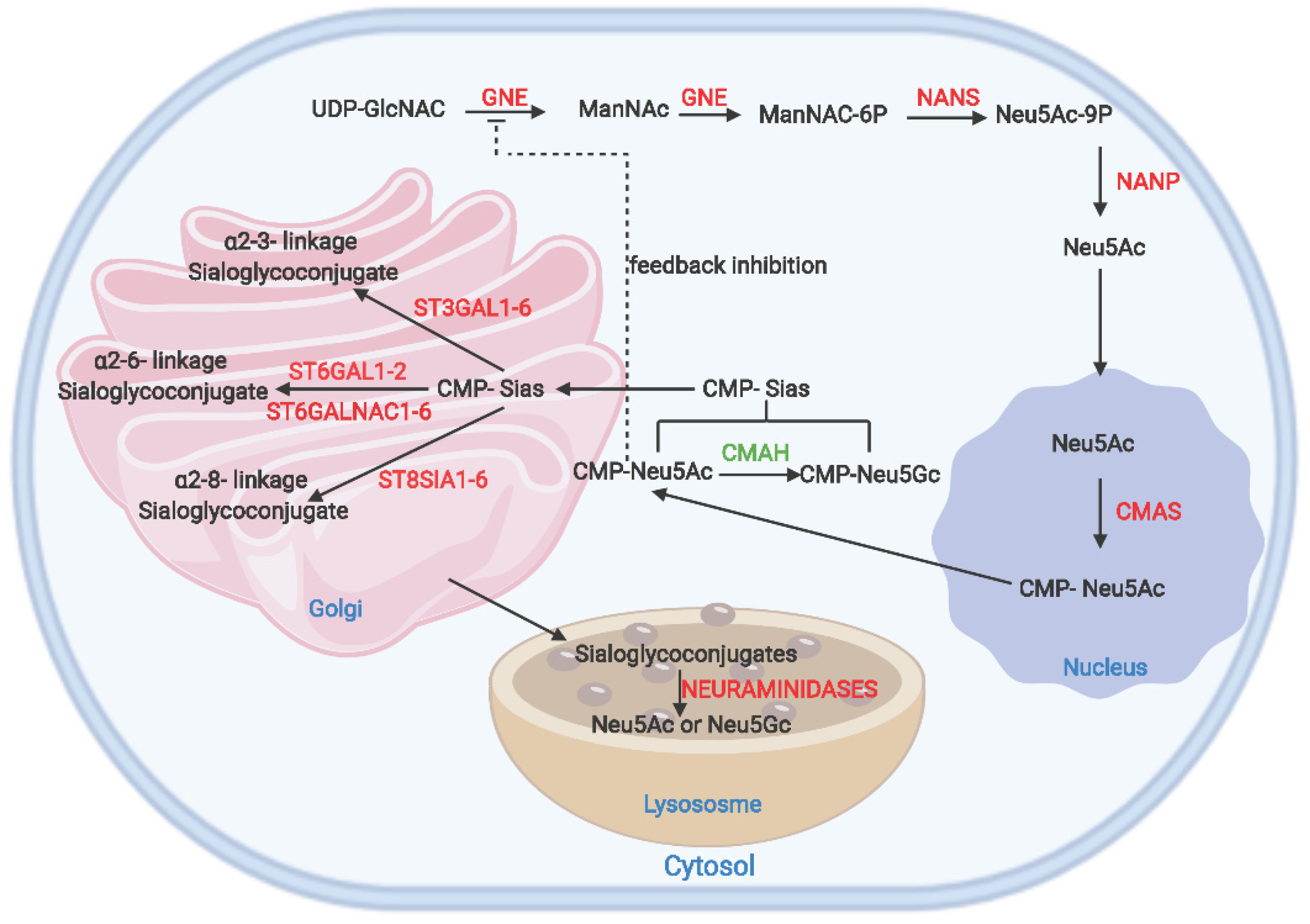
2. The Sialic Acid Metabolic Pathway
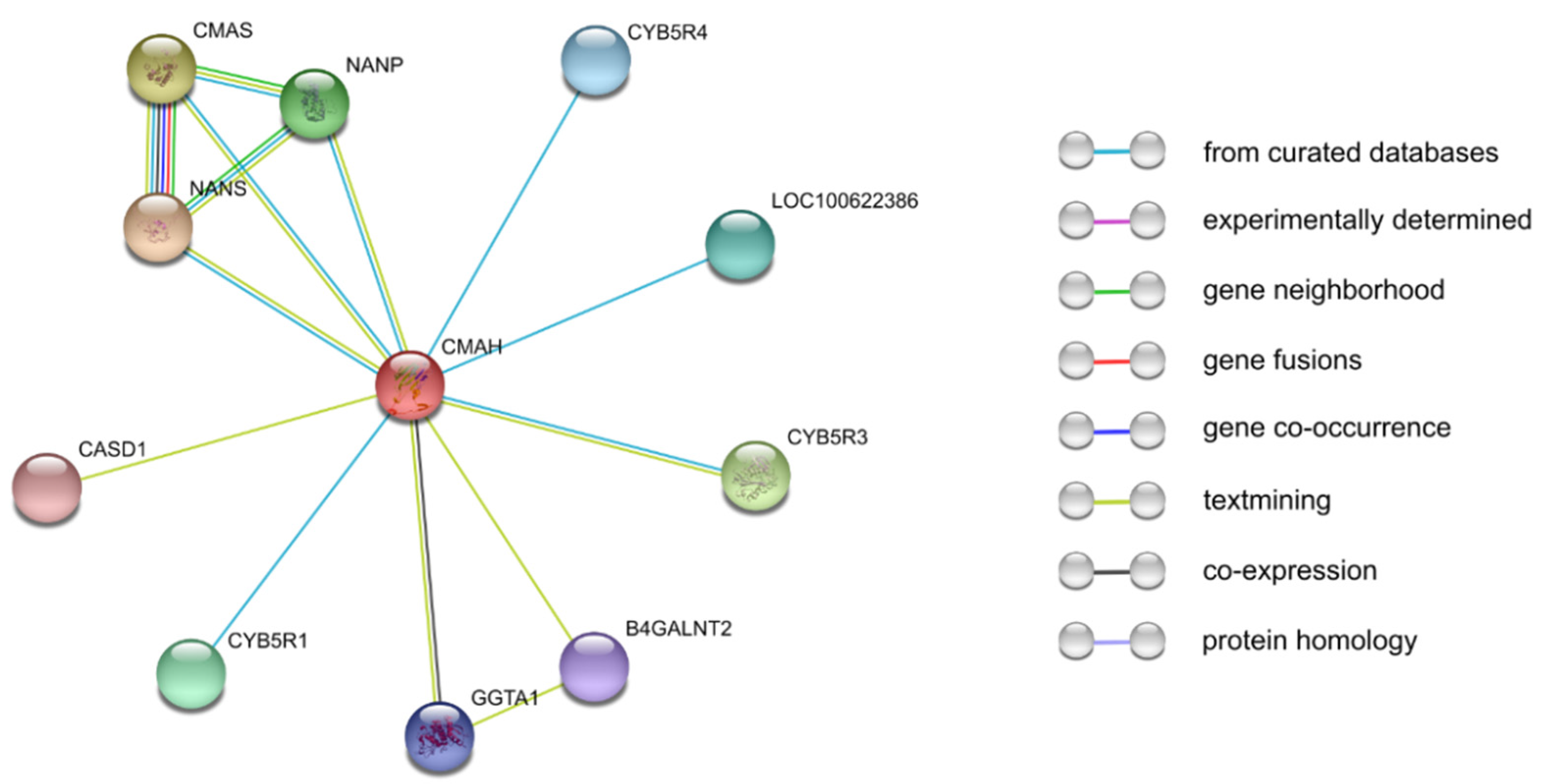
3. Porcine CMAH Structure
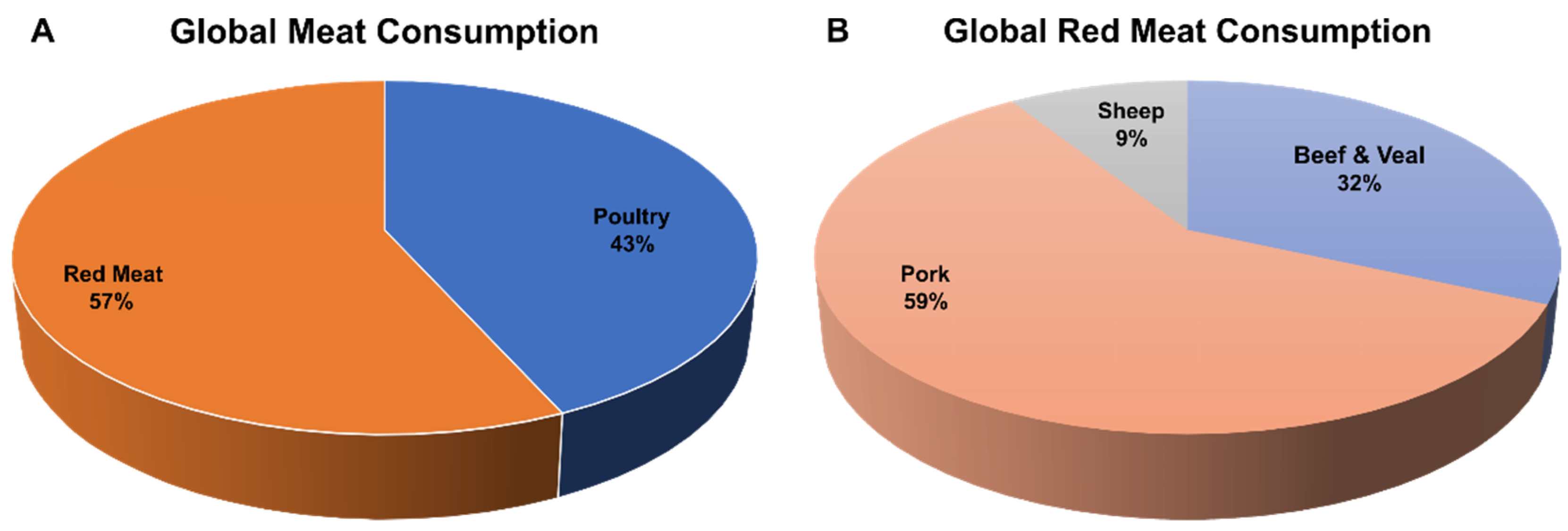
4. Pigs as a Supply for Food Protein
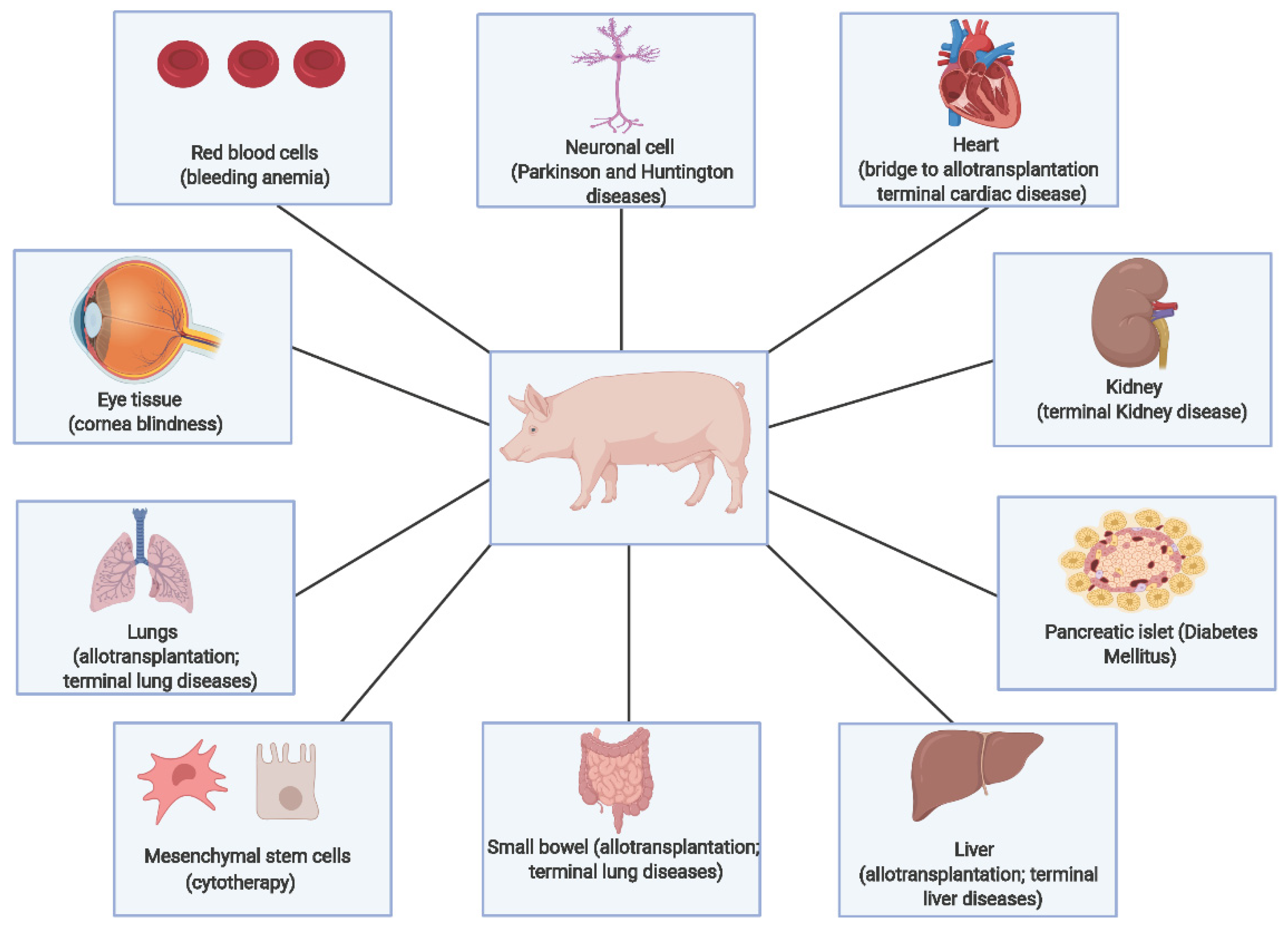
5. Pigs as a Model Organism
Significance for Xenotransplantation
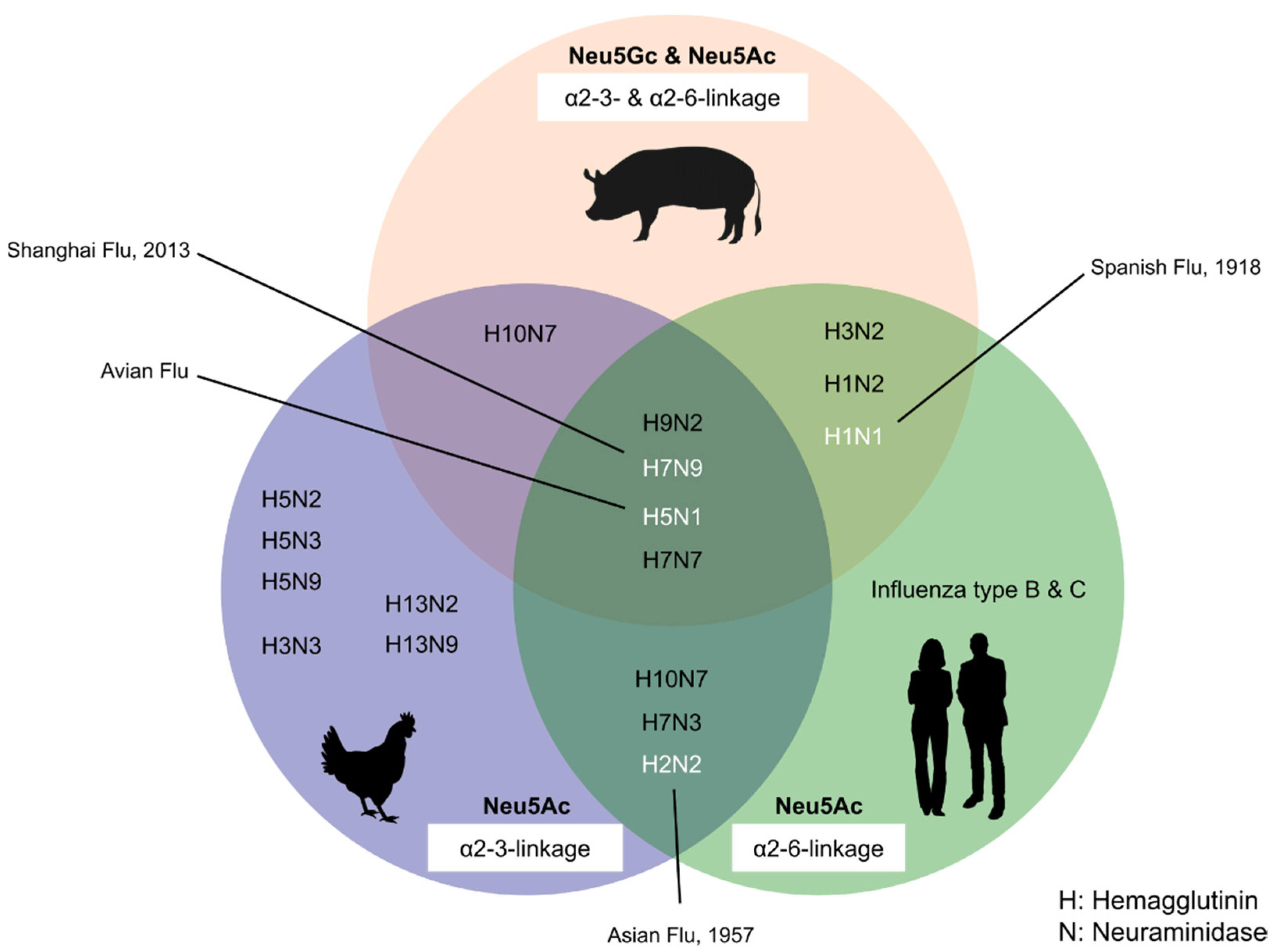
6. Porcine Sialic Acid in Infection, Cross-Species Transmission, and Emergence of Novel Viruses
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef]
- Varki, A.; Schauer, R. Sialic Acids. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor: Long Island, NY, USA, 2009. [Google Scholar]
- Varki, A. Colloquium paper: Uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. S2), 8939–8946. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, G.; Guan, F. Biological Functions and Analytical Strategies of Sialic Acids in Tumor. Cells 2020, 9, 273. [Google Scholar] [CrossRef]
- Matrosovich, M.; Herrler, G.; Klenk, H.D. Sialic Acid Receptors of Viruses. Top. Curr. Chem. 2015, 367, 1–28. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ito, T.; Suzuki, T.; Holland, R.E.; Chambers, T.M.; Kiso, M.; Ishida, H.; Kawaoka, Y. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 2000, 74, 11825–11831. [Google Scholar] [CrossRef]
- Magre, S.; Takeuchi, Y.; Bartosch, B. Xenotransplantation and pig endogenous retroviruses. Rev. Med. Virol. 2003, 13, 311–329. [Google Scholar] [CrossRef]
- Payne, S. Family Coronaviridae. In Viruses: From Understanding to Investigation; Payne, S., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 149–158. [Google Scholar]
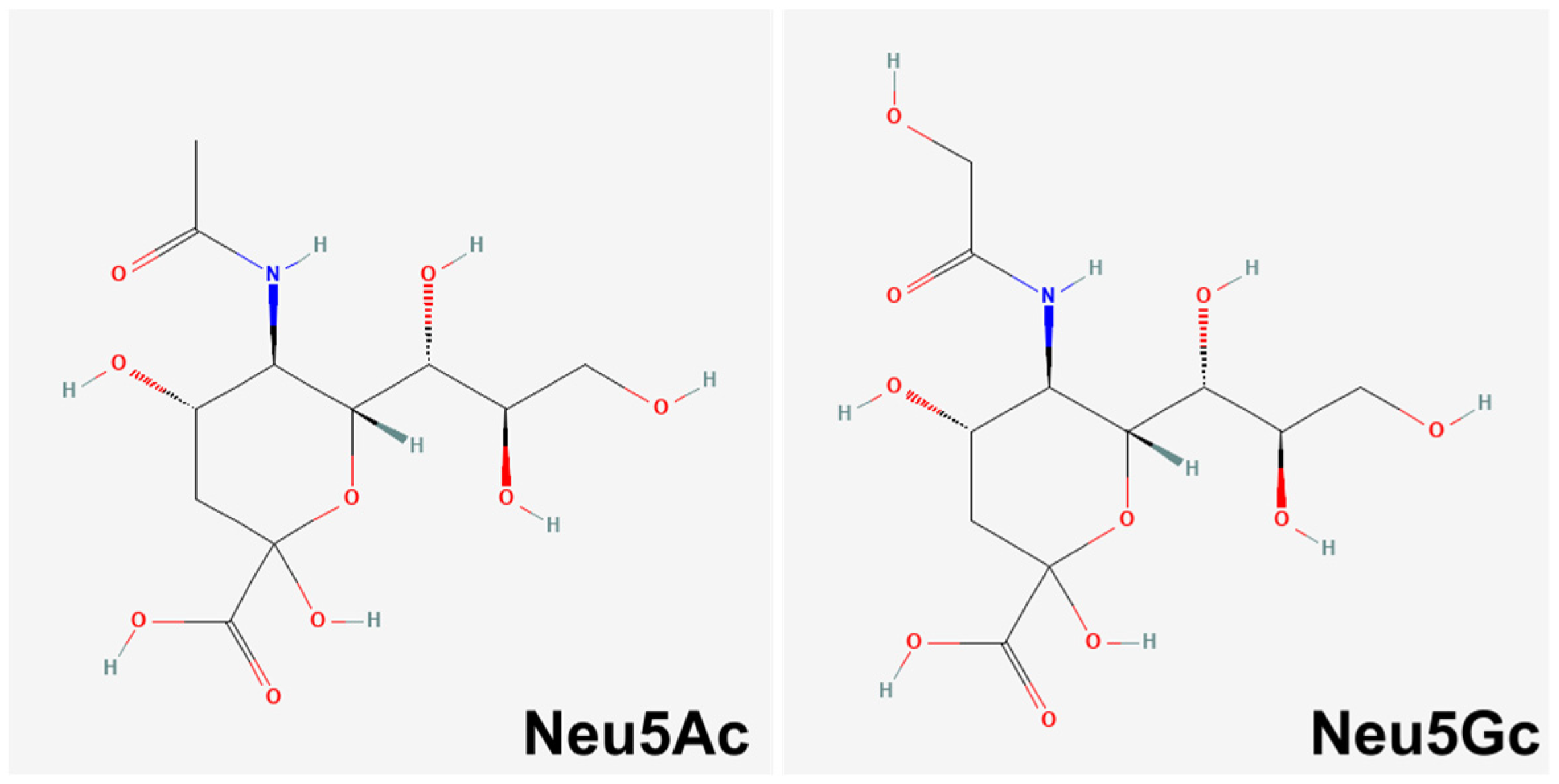
- Angata, T.; Varki, A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev. 2002, 102, 439–469. [Google Scholar] [CrossRef]
- Kooner, A.S.; Yu, H.; Chen, X. Synthesis of N-Glycolylneuraminic Acid (Neu5Gc) and Its Glycosides. Front. Immunol. 2019, 10, 2004. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Medrano, J.F. Primer on genes encoding enzymes in sialic acid metabolism in mammals. Biochimie 2011, 93, 1641–1646. [Google Scholar] [CrossRef]
- Samraj, A.N.; Laubli, H.; Varki, N.; Varki, A. Involvement of a non-human sialic Acid in human cancer. Front. Oncol. 2014, 4, 33. [Google Scholar] [CrossRef]
- Chou, H.H.; Takematsu, H.; Diaz, S.; Iber, J.; Nickerson, E.; Wright, K.L.; Muchmore, E.A.; Nelson, D.L.; Warren, S.T.; Varki, A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. USA 1998, 95, 11751–11756. [Google Scholar] [CrossRef]
- Samraj, A.N.; Pearce, O.M.; Laubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. USA 2015, 112, 542–547. [Google Scholar] [CrossRef]
- Dhar, C.; Sasmal, A.; Varki, A. From ”Serum Sickness” to “Xenosialitis”: Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc. Front. Immunol. 2019, 10, 807. [Google Scholar] [CrossRef]
- Gutierrez, K.; Dicks, N.; Glanzner, W.G.; Agellon, L.B.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 2015, 6, 293. [Google Scholar] [CrossRef]
- OECD. Agricultural Output—Meat Consumption. Available online: http://data.oecd.org/agroutput/meat-consumption.htm (accessed on 6 March 2022).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Hinderlich, S.; Weidemann, W.; Yardeni, T.; Horstkorte, R.; Huizing, M. UDP-GlcNAc 2-Epimerase/ManNAc Kinase (GNE): A Master Regulator of Sialic Acid Synthesis. Top. Curr. Chem. 2015, 366, 97–137. [Google Scholar] [CrossRef]
- Kurochkina, N.; Yardeni, T.; Huizing, M. Molecular modeling of the bifunctional enzyme UDP-GlcNAc 2-epimerase/ManNAc kinase and predictions of structural effects of mutations associated with HIBM and sialuria. Glycobiology 2010, 20, 322–337. [Google Scholar] [CrossRef]
- Traving, C.; Schauer, R. Structure, function and metabolism of sialic acids. Cell. Mol. Life Sci. 1998, 54, 1330–1349. [Google Scholar] [CrossRef]
- Huizing, M. Disease mechanisms associated with mutations of the GNE gene. Drug Discov. Today Dis. Mech. 2005, 2, 519–527. [Google Scholar] [CrossRef]
- Varki, A. Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. Yearb. Phys. Anthr. 2001, 44, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Hayakawa, T.; Diaz, S.; Krings, M.; Indriati, E.; Leakey, M.; Paabo, S.; Satta, Y.; Takahata, N.; Varki, A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11736–11741. [Google Scholar] [CrossRef]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.Q.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Chen, X.; Varki, A. Advances in the Biology and Chemistry of Sialic Acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef]
- Alisson-Silva, F.; Liu, J.Z.; Diaz, S.L.; Deng, L.Q.; Gareau, M.G.; Marchelletta, R.; Chen, X.; Nizet, V.; Varki, N.; Barrett, K.E.; et al. Human evolutionary loss of epithelial Neu5Gc expression and species-specific susceptibility to cholera. PLoS Pathog. 2018, 14, e1007133. [Google Scholar] [CrossRef]
- Malykh, Y.N.; Schauer, R.; Shaw, L. N-Glycolylneuraminic acid in human tumours. Biochimie 2001, 83, 623–634. [Google Scholar] [CrossRef]
- Tangvoranuntakul, P.; Gagneux, P.; Diaz, S.; Bardor, M.; Varki, N.; Varki, A.; Muchmore, E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. USA 2003, 100, 12045–12050. [Google Scholar] [CrossRef]
- Song, K.H.; Kang, Y.J.; Jin, U.H.; Park, Y.I.; Kim, S.M.; Seong, H.H.; Hwang, S.; Yang, B.S.; Im, G.S.; Min, K.S.; et al. Cloning and functional characterization of pig CMP-N-acetylneuraminic acid hydroxylase for the synthesis of N-glycolylneuraminic acid as the xenoantigenic determinant in pig-human xenotransplantation. Biochem. J. 2010, 427, 179–188. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Kozutsumi, Y.; Kawano, T.; Yamakawa, T.; Suzuki, A. Participation of Cytochrome-B5 in Cmp-N-Acetylneuraminic Acid Hydroxylation in Mouse-Liver Cytosol. J. Biochem. 1990, 108, 704–706. [Google Scholar] [CrossRef]
- Kawano, T.; Koyama, S.; Takematsu, H.; Kozutsumi, Y.; Kawasaki, H.; Kawashima, S.; Kawasaki, T.; Suzuki, A. Molecular-Cloning of Cytidine Monophospho-N-Acetylneuraminic Acid Hydroxylase—Regulation of Species-Specific and Tissue-Specific Expression of N-Glycolylneuraminic Acid. J. Biol. Chem. 1995, 270, 16458–16463. [Google Scholar] [CrossRef]
- Ji, S.; Wang, F.; Chen, Y.; Yang, C.; Zhang, P.; Zhang, X.; Troy, F.A., 2nd; Wang, B. Developmental changes in the level of free and conjugated sialic acids, Neu5Ac, Neu5Gc and KDN in different organs of pig: A LC-MS/MS quantitative analyses. Glycoconj. J. 2017, 34, 21–30. [Google Scholar] [CrossRef]
- Lepers, A.; Shaw, L.; Schneckenburger, P.; Cacan, R.; Verbert, A.; Schauer, R. A study on the regulation of N-glycoloylneuraminic acid biosynthesis and utilization in rat and mouse liver. Eur. J. Biochem. 1990, 193, 715–723. [Google Scholar] [CrossRef]
- Malykh, Y.N.; King, T.P.; Logan, E.; Kelly, D.; Schauer, R.; Shaw, L. Regulation of N-glycolylneuraminic acid biosynthesis in developing pig small intestine. Biochem. J. 2003, 370, 601–607. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Estrada, J.L.; Martens, G.; Li, P.; Adams, A.; Newell, K.A.; Ford, M.L.; Butler, J.R.; Sidner, R.; Tector, M.; Tector, J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 2015, 22, 194–202. [Google Scholar] [CrossRef]
- Baumann, A.M.T.; Bakkers, M.J.G.; Buettner, F.F.R.; Hartmann, M.; Grove, M.; Langereis, M.A.; de Groot, R.J.; Muhlenhoff, M. 9-O-Acetylation of sialic acids is catalysed by CASD1 via a covalent acetyl-enzyme intermediate. Nat. Commun. 2015, 6, 7673. [Google Scholar] [CrossRef]
- Jones, G.F. Genetic Aspects of Domestication, Common Breeds and Their Origin. In The Genetics of the Pig; Rothschild, M.F., Ruvinsky, A., Eds.; CAB International: Wallingford, UK, 1998. [Google Scholar]
- Amills, M.; Clop, A.; Ramírez, O.; Pérez-Enciso, M. Origin and Genetic Diversity of Pig Breeds. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Bosse, M.; Megens, H.J.; Frantz, L.A.; Madsen, O.; Larson, G.; Paudel, Y.; Duijvesteijn, N.; Harlizius, B.; Hagemeijer, Y.; Crooijmans, R.P.; et al. Genomic analysis reveals selection for Asian genes in European pigs following human-mediated introgression. Nat. Commun. 2014, 5, 4392. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Romero, E.; Garnier, J.; Aguilera, E. How changes in diet and trade patterns have shaped the N cycle at the national scale: Spain (1961–2009). Reg. Environ. Change 2014, 14, 785–797. [Google Scholar] [CrossRef]
- Bai, Z.H.; Ma, W.Q.; Ma, L.; Velthof, G.L.; Wei, Z.B.; Havlik, P.; Oenema, O.; Lee, M.R.F.; Zhang, F.S. China’s livestock transition: Driving forces, impacts, and consequences. Sci. Adv. 2018, 4, eaar8534. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Imran, A.; Hussain, M.B. Nutritional Composition of Meat. In Meat Science and Nutrition; Arshad, M.S., Ed.; IntechOpen: London, UK, 2018; pp. 61–77. [Google Scholar] [CrossRef]
- Williams, P. Nutritional composition of red meat. Nutr. Diet 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Sinha, R.; Chow, W.H.; Kulldorff, M.; Denobile, J.; Butler, J.; Garcia-Closas, M.; Weil, R.; Hoover, R.N.; Rothman, N. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res. 1999, 59, 4320–4324. [Google Scholar]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Red meat consumption and mortality: Results from 2 prospective cohort studies. Arch. Intern. Med. 2012, 172, 555–563. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef]
- Perota, A.; Galli, C. N-Glycolylneuraminic Acid (Neu5Gc) Null Large Animals by Targeting the CMP-Neu5Gc Hydroxylase (CMAH). Front. Immunol. 2019, 10, 2396. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Panepinto, L.M. Miniature Swine Breeds used Worldwide in Research. In Advances in Swine in Biomedical Research; Tumbleson, M.E., Schook, L.B., Eds.; Springer: Boston, MA, USA, 1996; pp. 681–691. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J.; Frazier, K.S. Swine as Models in Biomedical Research and Toxicology Testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef]
- Nielsen, V.H.; Bendixen, C.; Arnbjerg, J.; Sorensen, C.M.; Jensen, H.E.; Shukri, N.M.; Thomsen, B. Abnormal growth plate function in pigs carrying a dominant mutation in type X collagen. Mamm. Genome 2000, 11, 1087–1092. [Google Scholar] [CrossRef]
- Amin, Z.; Woo, R.; Danford, D.A.; Froemming, S.E.; Reddy, V.M.; Lof, J.; Overman, D. Robotically assisted perventricular closure of perimembranous ventricular septal defects: Preliminary results in Yucatan pigs. J. Thorac. Cardiovasc. Surg. 2006, 131, 427–432. [Google Scholar] [CrossRef]
- Köhn, F. History and Development of Miniature, Micro and Minipigs. In The Minipig in Biomedical Research, 1st ed.; McAnulty, P.A., Dayan, A.D., Ganderup, N.-C., Hastings, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 3–16. [Google Scholar] [CrossRef]
- Bollen, P.; Ellegaard, L. The Gottingen minipig in pharmacology and toxicology. Pharm. Toxicol. 1997, 80 (Suppl. S2), 3–4. [Google Scholar] [CrossRef]
- Mahl, J.A.; Vogel, B.E.; Court, M.; Kolopp, M.; Roman, D.; Nogues, V. The minipig in dermatotoxicology: Methods and challenges. Exp. Toxicol. Pathol. 2006, 57, 341–345. [Google Scholar] [CrossRef]
- Shrader, S.M.; Greentree, W.F. Gottingen Minipigs in Ocular Research. Toxicol. Pathol. 2018, 46, 403–407. [Google Scholar] [CrossRef]
- Chapman, L.E.; Folks, T.M.; Salomon, D.R.; Patterson, A.P.; Eggerman, T.E.; Noguchi, P.D. Xenotransplantation and xenogeneic infections. N. Engl. J. Med. 1995, 333, 1498–1501. [Google Scholar] [CrossRef]
- EDQM. The European Day for Organ Donation and Transplantation [Fact Sheet]. Available online: https://www.edqm.eu/sites/default/files/factsheet_organ_tissue_cell_donation_eodd_2018.pdf (accessed on 6 March 2022).
- Aristizabal, A.M.; Caicedo, L.A.; Martinez, J.M.; Moreno, M.; Echeverri, G.J. Clinical xenotransplantation, a closer reality: Literature review. Cir. Esp. 2017, 95, 62–72. [Google Scholar] [CrossRef]
- Tector, A.J.; Mosser, M.; Tector, M.; Bach, J.M. The Possible Role of Anti-Neu5Gc as an Obstacle in Xenotransplantation. Front. Immunol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Galili, U. Natural anti-carbohydrate antibodies contributing to evolutionary survival of primates in viral epidemics? Glycobiology 2016, 26, 1140–1150. [Google Scholar] [CrossRef]
- Phelps, C.J.; Koike, C.; Vaught, T.D.; Boone, J.; Wells, K.D.; Chen, S.H.; Ball, S.; Specht, S.M.; Polejaeva, I.A.; Monahan, J.A.; et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003, 299, 411–414. [Google Scholar] [CrossRef]
- Galili, U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol. Cell Biol. 2005, 83, 674–686. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Woods, J.R.; Hardin, J.W.; Campana, G.L.; Ortiz, M.A.; Jaeger, B.R.; Reichart, B.; Bonnin, J.M.; Currin, A.; Cosgrove, S.; et al. Early prediction of cardiac allograft vasculopathy and heart transplant failure. Am. J. Transpl. 2011, 11, 528–535. [Google Scholar] [CrossRef]
- Miwa, Y.; Kobayashi, T.; Nagasaka, T.; Liu, D.; Yu, M.; Yokoyama, I.; Suzuki, A.; Nakao, A. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation 2004, 11, 247–253. [Google Scholar] [CrossRef]
- Ezzelarab, M.; Ayares, D.; Cooper, D.K. Carbohydrates in xenotransplantation. Immunol. Cell Biol. 2005, 83, 396–404. [Google Scholar] [CrossRef]
- Chen, G.; Qian, H.; Starz, T.; Sun, H.T.; Garcia, B.; Wang, X.M.; Wise, Y.; Liu, Y.Q.; Xiang, Y.; Copeman, L.; et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat. Med. 2005, 11, 1295–1298. [Google Scholar] [CrossRef]
- Byrne, G.W.; Du, Z.; Stalboerger, P.; Kogelberg, H.; McGregor, C.G. Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation 2014, 21, 543–554. [Google Scholar] [CrossRef]
- Fischer, K.; Rieblinger, B.; Hein, R.; Sfriso, R.; Zuber, J.; Fischer, A.; Klinger, B.; Liang, W.; Flisikowski, K.; Kurome, M.; et al. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation 2020, 27, e12560. [Google Scholar] [CrossRef]
- Scobie, L.; Padler-Karavani, V.; Le Bas-Bernardet, S.; Crossan, C.; Blaha, J.; Matouskova, M.; Hector, R.D.; Cozzi, E.; Vanhove, B.; Charreau, B.; et al. Long-Term IgG Response to Porcine Neu5Gc Antigens without Transmission of PERV in Burn Patients Treated with Porcine Skin Xenografts. J. Immunol. 2013, 191, 2907–2915. [Google Scholar] [CrossRef]
- Couvrat-Desvergnes, G.; Salama, A.; Le Berre, L.; Evanno, G.; Viklicky, O.; Hruba, P.; Vesely, P.; Guerif, P.; Dejoie, T.; Rousse, J.; et al. Rabbit antithymocyte globulin-induced serum sickness disease and human kidney graft survival. J. Clin. Investig. 2015, 125, 4655–4665. [Google Scholar] [CrossRef]
- Hauschild, J.; Petersen, B.; Santiago, Y.; Queisser, A.L.; Carnwath, J.W.; Lucas-Hahn, A.; Zhang, L.; Meng, X.D.; Gregory, P.D.; Schwinzer, R.; et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc. Natl. Acad. Sci. USA 2011, 108, 12013–12017. [Google Scholar] [CrossRef]
- Carlson, D.F.; Tan, W.F.; Lillico, S.G.; Stverakova, D.; Proudfoot, C.; Christian, M.; Voytas, D.F.; Long, C.R.; Whitelaw, C.B.A.; Fahrenkrug, S.C. Efficient TALEN-mediated gene knockout in livestock. Proc. Natl. Acad. Sci. USA 2012, 109, 17382–17387. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.L.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.B.; Jiang, W.Y.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Burlak, C.; Bern, M.; Brito, A.E.; Isailovic, D.; Wang, Z.Y.; Estrada, J.L.; Li, P.; Tector, A.J. N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens. Xenotransplantation 2013, 20, 277–291. [Google Scholar] [CrossRef]
- Burlak, C.; Paris, L.L.; Lutz, A.J.; Sidner, R.A.; Estrada, J.; Li, P.; Tector, M.; Tector, A.J. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am. J. Transpl. 2014, 14, 1895–1900. [Google Scholar] [CrossRef]
- Miyagawa, S.; Matsunari, H.; Watanabe, M.; Nakano, K.; Umeyama, K.; Sakai, R.; Takayanagi, S.; Takeishi, T.; Fukuda, T.; Yashima, S.; et al. Generation of alpha1,3-galactosyltransferase and cytidine monophospho-N-acetylneuraminic acid hydroxylase gene double-knockout pigs. J. Reprod. Dev. 2015, 61, 449–457. [Google Scholar] [CrossRef]
- Martens, G.R.; Reyes, L.M.; Li, P.; Butler, J.R.; Ladowski, J.M.; Estrada, J.L.; Sidner, R.A.; Eckhoff, D.E.; Tector, M.; Tector, A.J. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells from GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs. Transplantation 2017, 101, e86–e92. [Google Scholar] [CrossRef]
- Wang, R.G.; Ruan, M.; Zhang, R.J.; Chen, L.; Li, X.X.; Fang, B.; Li, C.; Ren, X.Y.; Liu, J.Y.; Xiong, Q.; et al. Antigenicity of tissues and organs from GGTA1/CMAH/beta4GalNT2 triple gene knockout pigs. J. Biomed. Res. 2018, 33, 235–243. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Burlak, C.; Estrada, J.L.; Li, P.; Tector, M.F.; Tector, A.J. Erythrocytes from GGTA1/CMAH knockout pigs: Implications for xenotransfusion and testing in non-human primates. Xenotransplantation 2014, 21, 376–384. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, M.R.; Bui, H.T.; Kwon, D.N.; Kang, M.H.; Oh, M.; Han, J.W.; Cho, S.G.; Park, C.; Shim, H.; et al. alpha1,3-galactosyltransferase deficiency in germ-free miniature pigs increases N-glycolylneuraminic acids as the xenoantigenic determinant in pig-human xenotransplantation. Cell. Reprogram. 2012, 14, 353–363. [Google Scholar] [CrossRef]
- Lutz, A.J.; Li, P.; Estrada, J.L.; Sidner, R.A.; Chihara, R.K.; Downey, S.M.; Burlak, C.; Wang, Z.Y.; Reyes, L.M.; Ivary, B.; et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013, 20, 27–35. [Google Scholar] [CrossRef]
- Li, P.; Estrada, J.L.; Burlak, C.; Montgomery, J.; Butler, J.R.; Santos, R.M.; Wang, Z.Y.; Paris, L.L.; Blankenship, R.L.; Downey, S.M.; et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation 2015, 22, 20–31. [Google Scholar] [CrossRef]
- Ekser, B.; Ezzelarab, M.; Hara, H.; van der Windt, D.J.; Wijkstrom, M.; Bottino, R.; Trucco, M.; Cooper, D.K. Clinical xenotransplantation: The next medical revolution? Lancet 2012, 379, 672–683. [Google Scholar] [CrossRef]
- IUMS. Virology. Part II: The Viruses—The Negative Sense Single Stranded RNA Viruses. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 651–782. [Google Scholar] [CrossRef]
- Payne, S. Family Orthomyxoviridae. In Viruses: From Understanding to Investigation; Payne, S., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 197–208. [Google Scholar]
- Xu, G.; Suzuki, T.; Maejima, Y.; Mizoguchi, T.; Tsuchiya, M.; Kiso, M.; Hasegawa, A.; Suzuki, Y. Sialidase of swine influenza A viruses: Variation of the recognition specificities for sialyl linkages and for the molecular species of sialic acid with the year of isolation. Glycoconj. J. 1995, 12, 156–161. [Google Scholar] [CrossRef]
- Laver, G.; Garman, E. Pandemic influenza: Its origin and control. Microbes Infect. 2002, 4, 1309–1316. [Google Scholar] [CrossRef]
- Schauer, R. Sialic Acids as Antigenic Determinants of Complex Carbohydrates. In The Molecular Immunology of Complex Carbohydrates; Wu, A.M., Adams, L.G., Eds.; Springer: Boston, MA, USA, 1988; pp. 47–72. [Google Scholar] [CrossRef]
- Kelm, S.; Schauer, R. Sialic acids in molecular and cellular interactions. Int. Rev. Cytol. 1997, 175, 137–240. [Google Scholar] [CrossRef] [PubMed]
- Schwegmann-Wessels, C.; Herrler, G. Sialic acids as receptor determinants for coronaviruses. Glycoconj. J. 2006, 23, 51–58. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef]
- Schauer, R.; Kelm, S.; Reuter, G.; Roggentin, P.; Shaw, L. Biochemsitry and Role of Sialic Acids. In Biology of the Sialic Acids, 1st ed.; Rosenberg, A., Ed.; Springer: Boston, MA, USA, 1995; pp. 7–68. [Google Scholar] [CrossRef]
- Ito, T.; Couceiro, J.N.; Kelm, S.; Baum, L.G.; Krauss, S.; Castrucci, M.R.; Donatelli, I.; Kida, H.; Paulson, J.C.; Webster, R.G.; et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 1998, 72, 7367–7373. [Google Scholar] [CrossRef]
- Nelli, R.K.; Kuchipudi, S.V.; White, G.A.; Perez, B.B.; Dunham, S.P.; Chang, K.C. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet. Res. 2010, 6, 4. [Google Scholar] [CrossRef]
- Webster, R.G. Antigenic Variation in Influenza Viruses. In Origin and Evolution of Viruses; Domingo, E., Webster, R., Holland, J., Eds.; Academic Press: London, UK, 1999; pp. 377–390. [Google Scholar] [CrossRef]
- Treanor, J. Influenza vaccine—Outmaneuvering antigenic shift and drift. N. Engl. J. Med. 2004, 350, 218–220. [Google Scholar] [CrossRef]
- Brown, I. The Role of Pigs in Interspecies Transmission. In Avian Influenza; Klenk, H.-D., Matrosovich, M.N., Stech, J., Eds.; Karger: Basel, Switzerland, 2008; pp. 88–100. [Google Scholar] [CrossRef]
- Rajao, D.S.; Gauger, P.C.; Anderson, T.K.; Lewis, N.S.; Abente, E.J.; Killian, M.L.; Perez, D.R.; Sutton, T.C.; Zhang, J.; Vincent, A.L. Novel Reassortant Human-Like H3N2 and H3N1 Influenza A Viruses Detected in Pigs Are Virulent and Antigenically Distinct from Swine Viruses Endemic to the United States. J. Virol. 2015, 89, 11213–11222. [Google Scholar] [CrossRef]
- Scholtissek, C. Pigs as ‘Mixing Vessels’ for the Creation of New Pandemic Influenza A Viruses. Med. Princ. Pract. 1990, 2, 65–71. [Google Scholar] [CrossRef]
- Ma, W.; Kahn, R.E.; Richt, J.A. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. J. Mol. Genet. Med. 2008, 3, 158–166. [Google Scholar] [CrossRef]
- Hinshaw, V.S.; Bean, W.J.; Webster, R.G.; Rehg, J.E.; Fiorelli, P.; Early, G.; Geraci, J.R.; St Aubin, D.J. Are seals frequently infected with avian influenza viruses? J. Virol. 1984, 51, 863–865. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Kawaoka, Y.; Gorman, O.; Ito, T.; Saito, T.; Webster, R.G. Characterization of a new avian-like influenza A virus from horses in China. Virology 1992, 188, 245–255. [Google Scholar] [CrossRef]
- Pensaert, M.; Ottis, K.; Vandeputte, J.; Kaplan, M.M.; Bachmann, P.A. Evidence for the Natural Transmission of Influenza-a Virus from Wild Ducks to Swine and Its Potential Importance for Man. Bull. World Health Organ. 1981, 59, 75–78. [Google Scholar]
- Olsen, C.W. The emergence of novel swine influenza viruses in North America. Virus Res. 2002, 85, 199–210. [Google Scholar] [CrossRef]
- Karasin, A.I.; Schutten, M.M.; Cooper, L.A.; Smith, C.B.; Subbarao, K.; Anderson, G.A.; Carman, S.; Olsen, C.W. Genetic characterization of H3N2 influenza viruses isolated from pigs in North America, 1977–1999: Evidence for wholly human and reassortant virus genotypes. Virus Res. 2000, 68, 71–85. [Google Scholar] [CrossRef]
- Karasin, A.I.; Landgraf, J.; Swenson, S.; Erickson, G.; Goyal, S.; Woodruff, M.; Scherba, G.; Anderson, G.; Olsen, C.W. Genetic characterization of H1N2 influenza A viruses isolated from pigs throughout the United States. J. Clin. Microbiol. 2002, 40, 1073–1079. [Google Scholar] [CrossRef]
- Castrucci, M.R.; Donatelli, I.; Sidoli, L.; Barigazzi, G.; Kawaoka, Y.; Webster, R.G. Genetic Reassortment between Avian and Human Influenza-a Viruses in Italian Pigs. Virology 1993, 193, 503–506. [Google Scholar] [CrossRef]
- Ninomiya, A.; Takada, A.; Okazaki, K.; Shortridge, K.F.; Kida, H. Seroepidemiological evidence of avian H4, H5, and H9 influenza A virus transmission to pigs in southeastern China. Vet. Microbiol. 2002, 88, 107–114. [Google Scholar] [CrossRef]
- Pereda, A.; Rimondi, A.; Cappuccio, J.; Sanguinetti, R.; Angel, M.; Ye, J.; Sutton, T.; Dibarbora, M.; Olivera, V.; Craig, M.I.; et al. Evidence of reassortment of pandemic H1N1 influenza virus in swine in Argentina: Are we facing the expansion of potential epicenters of influenza emergence? Influenza Other Respir. Viruses 2011, 5, 409–412. [Google Scholar] [CrossRef]
- Sun, Y.F.; Wang, X.H.; Li, X.L.; Zhang, L.; Li, H.H.; Lu, C.; Yang, C.L.; Feng, J.; Han, W.; Ren, W.K.; et al. Novel triple-reassortant H1N1 swine influenza viruses in pigs in Tianjin, Northern China. Vet. Microbiol. 2016, 183, 85–91. [Google Scholar] [CrossRef]
- He, P.; Wang, G.; Mo, Y.; Yu, Q.; Xiao, X.; Yang, W.; Zhao, W.; Guo, X.; Chen, Q.; He, J.; et al. Novel triple-reassortant influenza viruses in pigs, Guangxi, China. Emerg. Microbes Infect. 2018, 7, 85. [Google Scholar] [CrossRef]
- Brown, I.H. The pig as an intermediate host for influenza A viruses between birds and humans. Int. Congr. Ser. 2001, 1219, 173–178. [Google Scholar] [CrossRef]
- Cui, T.; Theuns, S.; Xie, J.; Van den Broeck, W.; Nauwynck, H.J. Role of Porcine Aminopeptidase N and Sialic Acids in Porcine Coronavirus Infections in Primary Porcine Enterocytes. Viruses 2020, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.X.; Zu, S.P.; Zhang, Y.F.; Zhao, F.J.; Jin, X.H.; Hu, H. Porcine Deltacoronavirus Utilizes Sialic Acid as an Attachment Receptor and Trypsin Can Influence the Binding Activity. Viruses 2021, 13, 2442. [Google Scholar] [CrossRef]
- Nova, N. Cross-Species Transmission of Coronaviruses in Humans and Domestic Mammals, What Are the Ecological Mechanisms Driving Transmission, Spillover, and Disease Emergence? Front. Public Health 2021, 9, 717941. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Huang, Y.; Lau, S.K.; Yuen, K.Y. Coronavirus genomics and bioinformatics analysis. Viruses 2010, 2, 1804–1820. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Huang, Y.; Yuen, K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 2009, 234, 1117–1127. [Google Scholar] [CrossRef]
- Opriessnig, T.; Huang, Y.W. Coronavirus disease 2019 (COVID-19) outbreak: Could pigs be vectors for human infections? Xenotransplantation 2020, 27, e12591. [Google Scholar] [CrossRef]
- Chen, W.J.; Yan, M.H.; Yang, L.; Ding, B.L.; He, B.; Wang, Y.Z.; Liu, X.L.; Liu, C.H.; Zhu, H.; You, B.; et al. SARS-associated coronavirus transmitted from human to pig. Emerg. Infect. Dis 2005, 11, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Z.J.; You, Z.; Wu, D.Y.; Liu, S.J.; Zhang, W.L.; Fan, K.R.; Luo, R.; Qiu, Y.; Ge, X.Y. Epidemiology and evolution of novel deltacoronaviruses in birds in central China. Transbound. Emerg. Dis. 2021, 69, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef]
- Boley, P.A.; Alhamo, M.A.; Lossie, G.; Yadav, K.K.; Vasquez-Lee, M.; Saif, L.J.; Kenney, S.P. Porcine Deltacoronavirus Infection and Transmission in Poultry, United States. Emerg. Infect. Dis. 2020, 26, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Hu, H.; Saif, L.J. Calves are susceptible to infection with the newly emerged porcine deltacoronavirus, but not with the swine enteric alphacoronavirus, porcine epidemic diarrhea virus. Arch. Virol. 2017, 162, 2357–2362. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, H.; Li, B.; Ding, Q.; Wang, Y.; Gao, W.; Guo, D.; Wei, Z.; Hu, H. Susceptibility of Chickens to Porcine Deltacoronavirus Infection. Viruses 2019, 11, 573. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Liang, Q.Z.; Shi, F.S.; Ji, C.M.; Yang, X.L.; Yang, Y.L.; Qin, P.; Chen, R.; Huang, Y.W. Roles of Two Major Domains of the Porcine Deltacoronavirus S1 Subunit in Receptor Binding and Neutralization. J. Virol. 2021, 95, e0111821. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Tagliamonte, M.S.; White, S.K.; Elbadry, M.A.; Alam, M.M.; Stephenson, C.J.; Bonny, T.S.; Loeb, J.C.; Telisma, T.; Chavannes, S.; et al. Independent infections of porcine deltacoronavirus among Haitian children. Nature 2021, 600, 133–137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogun, O.J.; Thaller, G.; Becker, D. An Overview of the Importance and Value of Porcine Species in Sialic Acid Research. Biology 2022, 11, 903. https://doi.org/10.3390/biology11060903
Ogun OJ, Thaller G, Becker D. An Overview of the Importance and Value of Porcine Species in Sialic Acid Research. Biology. 2022; 11(6):903. https://doi.org/10.3390/biology11060903
Chicago/Turabian StyleOgun, Oluwamayowa Joshua, Georg Thaller, and Doreen Becker. 2022. "An Overview of the Importance and Value of Porcine Species in Sialic Acid Research" Biology 11, no. 6: 903. https://doi.org/10.3390/biology11060903
APA StyleOgun, O. J., Thaller, G., & Becker, D. (2022). An Overview of the Importance and Value of Porcine Species in Sialic Acid Research. Biology, 11(6), 903. https://doi.org/10.3390/biology11060903





