Optical Fiber-Based Recording of Climbing Fiber Ca2+ Signals in Freely Behaving Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
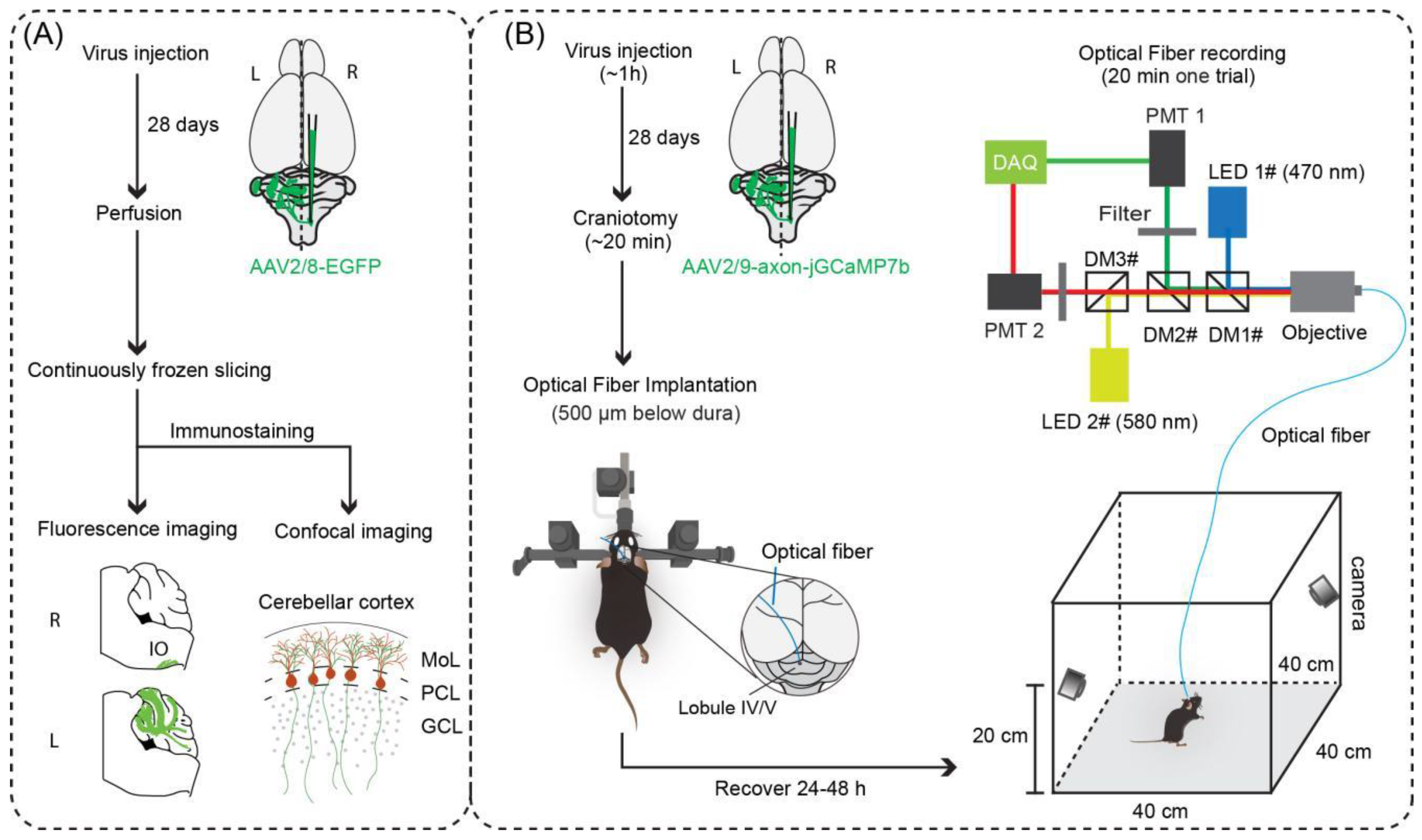
2.2. Surgery
2.3. Virus Injection
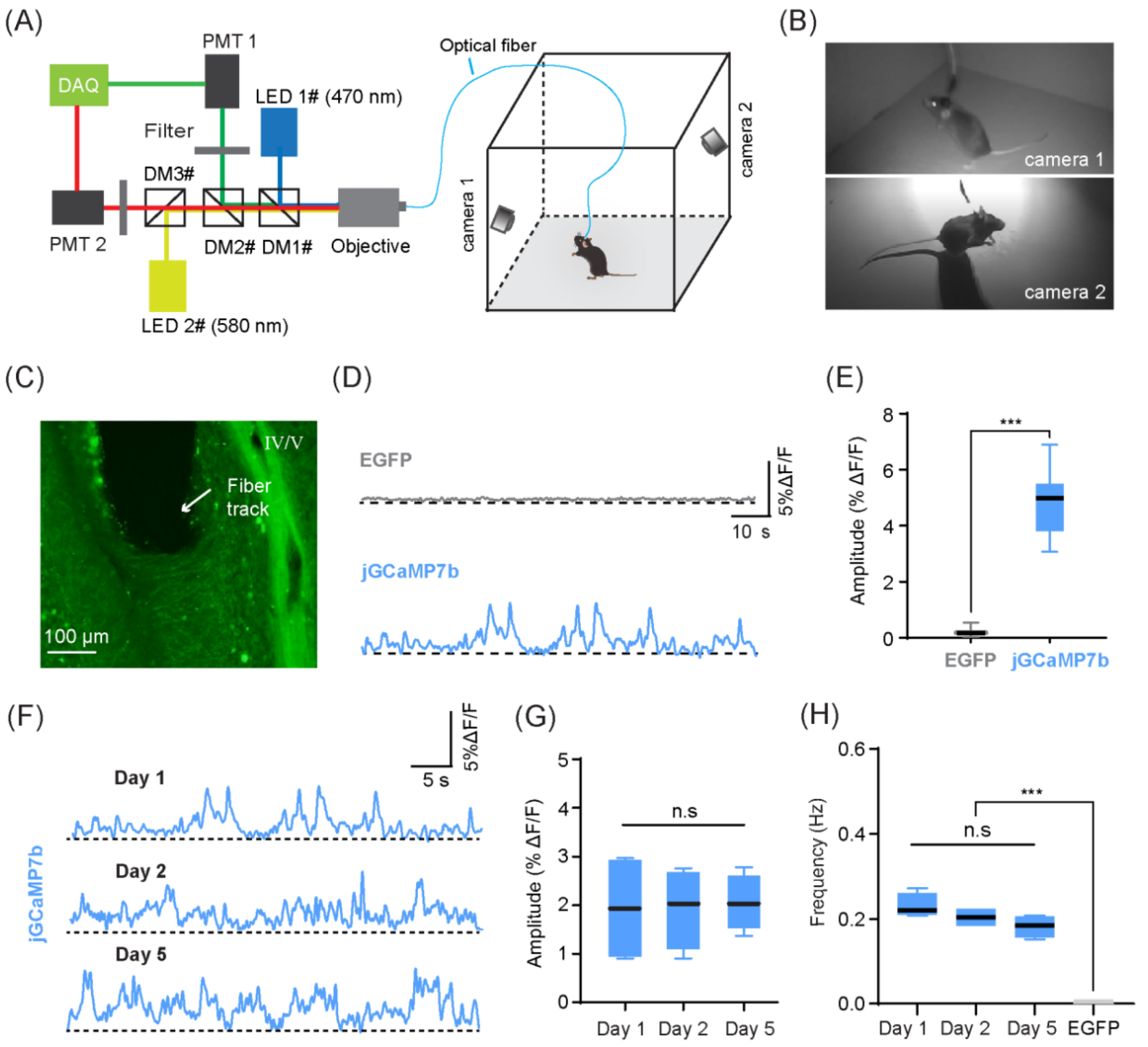
2.4. Optical Fiber Recording Set-Up
2.5. Optical Fiber Recordings in Freely Behaving Mice
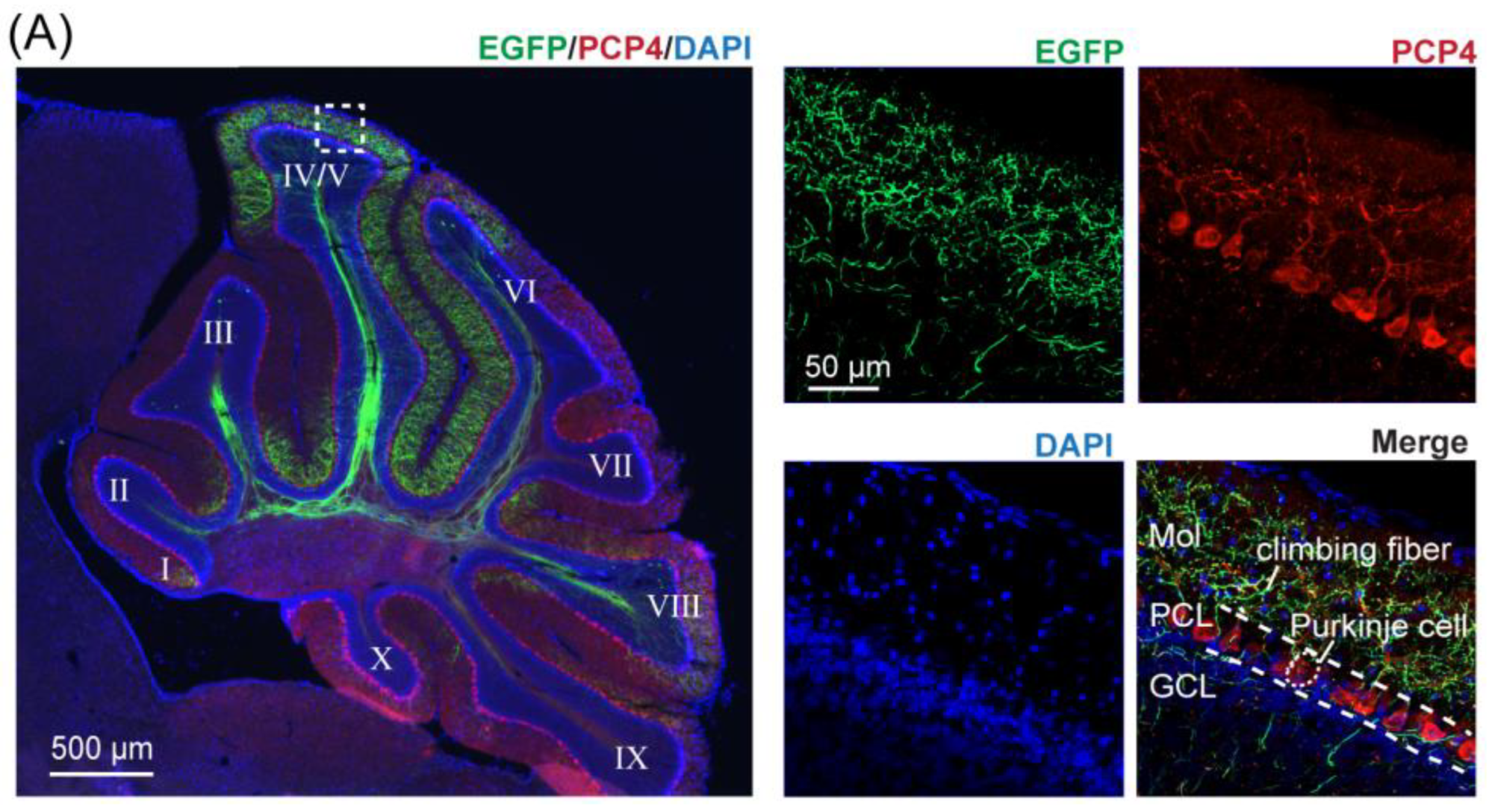
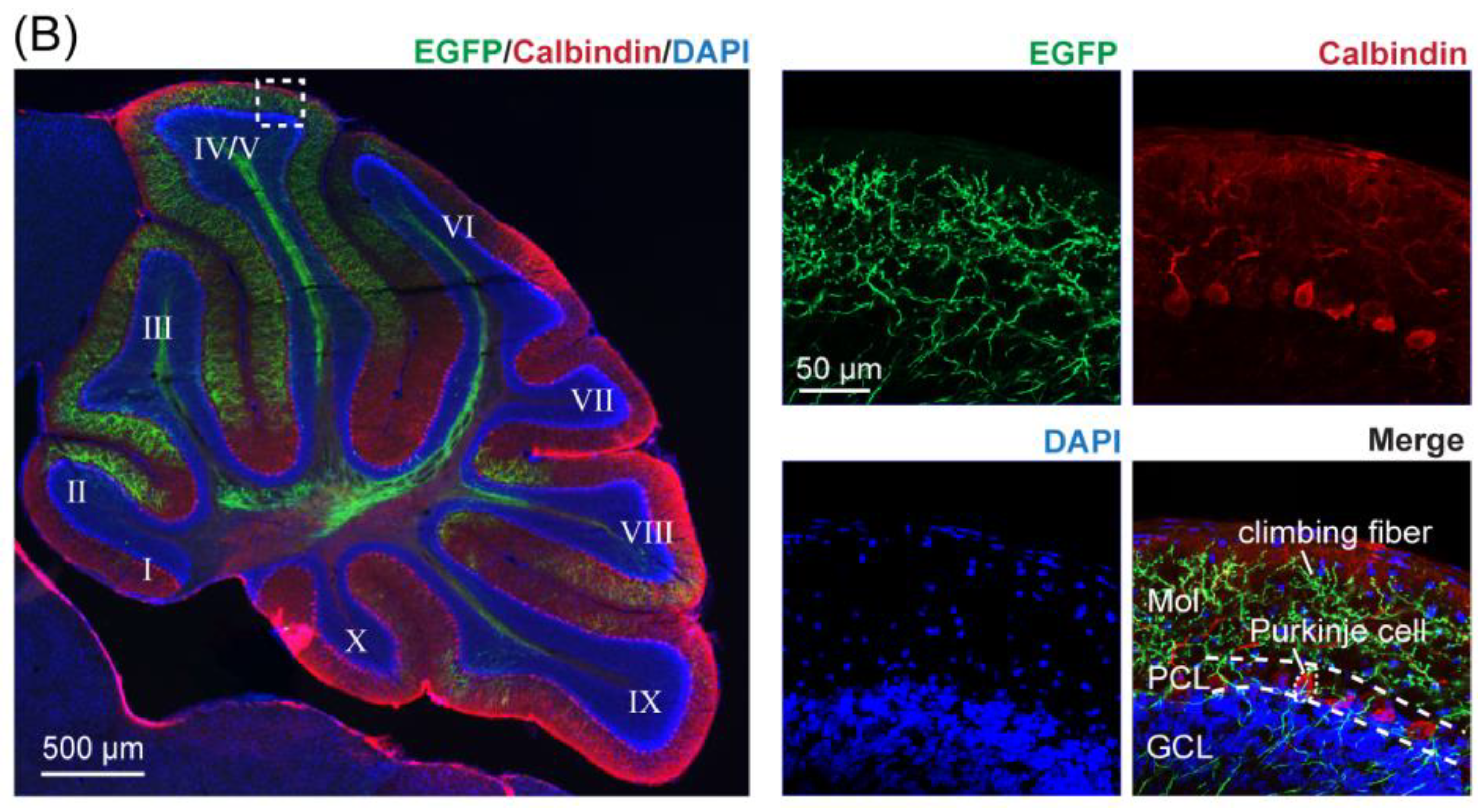
2.6. Histology and Fluorescence Imaging
3. Data Analysis
4. Results
4.1. Mapping CF Projections to Lobules of the Contralateral Cerebellar Cortex
4.2. Recording of CF Ca2+ Signals in Freely Behaving Mice
4.3. CF Ca2+ Signals in Lobule IV/V Were Synchronous with Exploratory Motor Behaviors
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| AAV2/8-hSyn-EGFP-WPRE-pA | OBiO | NA |
| AAV2/9-hSyn-mCherry-WPRE-pA | OBiO | NA |
| AAV2/9-hSyn-axon-GCaMP6s -WPRE-pA | Brain Case | NA |
| AAV2/9-hSyn-axon-jGCaMP7b -WPRE-pA | Taitool Bioscience Co., Ltd. | NA |
| Antibodies | ||
| Rabbit anti-PCP4 | Sigma | Cat#HPA005792, RRID:AB_1855086 |
| Chicken anti-GFP | Abcam | Cat#600-901-215S, RRID:AB_1537403 |
| Mouse anti-calbindin D-28K | Sigma | Cat#ABIN283971, RRID:AB_10783063 |
| Alexa Fluor 594 donkey anti-rabbit | Invitrogen | Cat#A-21207, RRID:AB_141637 |
| Alexa Fluor 568 donkey anti-mouse | Invitrogen | Cat#A10037, RRID:AB_2534013 |
| Alexa Fluor 488 donkey anti-chicken | Sigma | Cat#703-545-155, RRID:AB_2340375 |
References
- Sotelo, C. Viewing the cerebellum through the eyes of Ramon Y Cajal. Cerebellum 2008, 7, 517–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voogd, J.; Glickstein, M. The anatomy of the cerebellum. Trends Cogn. Sci. 1998, 2, 307–313. [Google Scholar] [CrossRef]
- Llinas, R.; Sasaki, K. The Functional Organization of the Olivo-Cerebellar System as Examined by Multiple Purkinje Cell Recordings. Eur. J. Neurosci. 1989, 1, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Llinas, R.; Walton, K.; Hillman, D.E.; Sotelo, C. Inferior olive: Its role in motor learing. Science 1975, 190, 1230–1231. [Google Scholar] [CrossRef]
- Nguyen-Vu, T.D.; Kimpo, R.R.; Rinaldi, J.M.; Kohli, A.; Zeng, H.; Deisseroth, K.; Raymond, J.L. Cerebellar Purkinje cell activity drives motor learning. Nat. Neurosci. 2013, 16, 1734–1736. [Google Scholar] [CrossRef] [Green Version]
- Lang, E.J.; Apps, R.; Bengtsson, F.; Cerminara, N.L.; De Zeeuw, C.I.; Ebner, T.J.; Heck, D.H.; Jaeger, D.; Jorntell, H.; Kawato, M.; et al. The Roles of the Olivocerebellar Pathway in Motor Learning and Motor Control. A Consensus Paper. Cerebellum 2017, 16, 230–252. [Google Scholar] [CrossRef] [Green Version]
- Marr, D. A theory of cerebellar cortex. J. Physiol. 1969, 202, 437–470. [Google Scholar] [CrossRef]
- De Schutter, E.; Maex, R. The cerebellum: Cortical processing and theory. Curr. Opin. Neurobiol. 1996, 6, 759–764. [Google Scholar] [CrossRef]
- Hansel, C.; Linden, D.J. Long-term depression of the cerebellar climbing fiber--Purkinje neuron synapse. Neuron 2000, 26, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Mathy, A.; Ho, S.S.; Davie, J.T.; Duguid, I.C.; Clark, B.A.; Hausser, M. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron 2009, 62, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Najafi, F.; Medina, J.F. Beyond “all-or-nothing” climbing fibers: Graded representation of teaching signals in Purkinje cells. Front. Neural Circuits 2013, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, Y.; De Schutter, E. Climbing Fibers Provide Graded Error Signals in Cerebellar Learning. Front. Syst. Neurosci. 2019, 13, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benardo, L.S.; Foster, R.E. Oscillatory behavior in inferior olive neurons mechanism, modulation, cell aggregates. Brain Res. Bull. 1986, 17, 773–784. [Google Scholar] [CrossRef]
- Cerminara, N.L.; Lang, E.J.; Sillitoe, R.V.; Apps, R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat. Rev. Neurosci. 2015, 16, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, G.; Häusser, M. Initiation and spread of sodium action potentials in cerebellar purkinje cells. Neuron 1994, 13, 703–712. [Google Scholar] [CrossRef]
- Llinás, R.; Sugimori, M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J. Physiol. 1980, 305, 197–213. [Google Scholar] [CrossRef]
- Lefler, Y.; Amsalem, O.; Vrieler, N.; Segev, I.; Yarom, Y. Using subthreshold events to characterize the functional architecture of the electrically coupled inferior olive network. Elife 2020, 9, e43560. [Google Scholar] [CrossRef]
- Roth, R.H.; Ding, J.B. From Neurons to Cognition: Technologies for Precise Recording of Neural Activity Underlying Behavior. BME Front. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Thach, W.T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J. Neurophysiol. 1968, 31, 785–797. [Google Scholar] [CrossRef]
- Armstrong, D.M.; Edgley, S.A. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion. J. Physiol. 1984, 352, 403–424. [Google Scholar] [CrossRef]
- Guo, D.; Gurkan Ozer, A.; Uusisaari, M.Y. In vivo Calcium Imaging in Mouse Inferior Olive. J. Vis. Exp. 2021, 172, e62222. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, H.; Fukaya, M.; Watanabe, M.; Linden, D.J. Axonal motility and its modulation by activity are branch-type specific in the intact adult cerebellum. Neuron 2007, 56, 472–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffield, M.A.; Rowan, M.J.M.; Amat, S.B.; Hirai, H.; Christie, J.M. Inhibition gates supralinear Ca(2+) signaling in Purkinje cell dendrites during practiced movements. Elife 2018, 7, e36246. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kovalchuk, Y.; Adelsberger, H.; Henning, H.A.; Sausbier, M.; Wietzorrek, G.; Ruth, P.; Yarom, Y.; Konnerth, A. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proc. Natl. Acad. Sci. USA 2010, 107, 12323–12328. [Google Scholar] [CrossRef] [Green Version]
- Ozden, I.; Dombeck, D.A.; Hoogland, T.M.; Tank, D.W.; Wang, S.S. Widespread state-dependent shifts in cerebellar activity in locomoting mice. PLoS ONE 2012, 7, e42650. [Google Scholar] [CrossRef]
- Tsubota, T.; Ohashi, Y.; Tamura, K.; Sato, A.; Miyashita, Y. Optogenetic manipulation of cerebellar Purkinje cell activity in vivo. PLoS ONE 2011, 6, e22400. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Chadney, O.; Yiu, T.L.; Baumler, E.; Faraggiana, L.; Beau, M.; Hausser, M. Purkinje Cell Activity Determines the Timing of Sensory-Evoked Motor Initiation. Cell Rep. 2020, 33, 108537. [Google Scholar] [CrossRef] [PubMed]
- Balmer, T.S.; Trussell, L.O. Selective targeting of unipolar brush cell subtypes by cerebellar mossy fibers. Elife 2019, 8, e44964. [Google Scholar] [CrossRef]
- Lutcke, H.; Murayama, M.; Hahn, T.; Margolis, D.J.; Astori, S.; Zum Alten Borgloh, S.M.; Gobel, W.; Yang, Y.; Tang, W.; Kugler, S.; et al. Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice. Front. Neural Circuits 2010, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Schonewille, M.; Luo, C.; Ruigrok, T.J.; Voogd, J.; Schmolesky, M.T.; Rutteman, M.; Hoebeek, F.E.; De Jeu, M.T.; De Zeeuw, C.I. Zonal organization of the mouse flocculus: Physiology, input, and output. J. Comp. Neurol. 2006, 497, 670–682. [Google Scholar] [CrossRef]
- Voogd, J.; Ruigrok, T.J. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis, the congruence of projection zones and the zebrin pattern. J. Neurocytol. 2004, 33, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Hawkes, R. Cerebellar cortical organization: A one-map hypothesis. Nat. Rev. Neurosci. 2009, 10, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Holmes, T.C.; Luo, M.H.; Beier, K.T.; Horwitz, G.D.; Zhao, F.; Zeng, W.; Hui, M.; Semler, B.L.; Sandri-Goldin, R.M. Viral Vectors for Neural Circuit Mapping and Recent Advances in Trans-synaptic Anterograde Tracers. Neuron 2020, 107, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.E.; Tsien, R.Y. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat. Protoc. 2006, 1, 1057–1065. [Google Scholar] [CrossRef]
- Romano, V.; Zhai, P.; van der Horst, A.; Mazza, R.; Jacobs, T.; Bauer, S.; Wang, X.; White, J.J.; De Zeeuw, C.I. Olivocerebellar control of movement symmetry. Curr. Biol. 2022, 32, 654–670.e4. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Guo, Q.; Luo, M. Long-term Fiber Photometry for Neuroscience Studies. Neurosci. Bull. 2019, 35, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Zhao, Z.; Zhang, J.; Wu, B.; Zhu, Y.; Chang, E.F.; Wu, J.; Duffau, H.; Berger, M.S. Functional maps of direct electrical stimulation-induced speech arrest and anomia: A multicentre retrospective study. Brain 2021, 144, 2541–2553. [Google Scholar] [CrossRef]
- Rosina, A.; Provini, L. Somatotopy of Climbing Fiber Branching to the Cerebellar Cortex in Cat. Brain Res. 1983, 289, 45–63. [Google Scholar] [CrossRef]
- Sugihara, I.; Quy, P.N. Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. J. Comp. Neurol. 2007, 500, 1076–1092. [Google Scholar] [CrossRef]
- Sarna, J.R.; Marzban, H.; Watanabe, M.; Hawkes, R. Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. J. Comp. Neurol. 2006, 496, 303–313. [Google Scholar] [CrossRef]
- Reeber, S.L.; Otis, T.S.; Sillitoe, R.V. New roles for the cerebellum in health and disease. Front. Syst. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, E.J.; Sugihara, I.; Welsh, J.P.; Llinás, R. Patterns of spontaneous purkinje cell complex spike activity in the awake rat. J. Neurosci. 1999, 19, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.P.; Lang, E.J.; Suglhara, I.; Llinás, R. Dynamic organization of motor control within the olivocerebellar system. Nature 1995, 374, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Mukamel, E.A.; Nimmerjahn, A.; Schnitzer, M.J. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 2009, 63, 747–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonewille, M.; Khosrovani, S.; Winkelman, B.H.; Hoebeek, F.E.; De Jeu, M.T.; Larsen, I.M.; Van der Burg, J.; Schmolesky, M.T.; Frens, M.A.; De Zeeuw, C.I. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat. Neurosci. 2006, 9, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Savall, J.; Hernandez, O.; Mel, G.; Inan, H.; Rumyantsev, O.; Lecoq, J.; Kim, T.H.; Li, J.Z.; Ramakrishnan, C.; et al. A neural circuit state change underlying skilled movements. Cell 2021, 184, 3731–3747.e21. [Google Scholar] [CrossRef]
- O’Dell, D.E.; Schreurs, B.G.; Smith-Bell, C.; Wang, D. Disruption of rat deep cerebellar perineuronal net alters eyeblink conditioning and neuronal electrophysiology. Neurobiol. Learn Mem. 2021, 177, 107358. [Google Scholar] [CrossRef]
- Shambes, G.M.; Gibson, J.M.; Welker, W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav. Evol. 1978, 15, 94–140. [Google Scholar] [CrossRef]
- Guell, X.; Schmahmann, J.D.; Gabrieli, J.; Ghosh, S.S. Functional gradients of the cerebellum. Elife 2018, 7, e36652. [Google Scholar] [CrossRef]
- Apps, R.; Hawkes, R.; Aoki, S.; Bengtsson, F.; Brown, A.M.; Chen, G.; Ebner, T.J.; Isope, P.; Jorntell, H.; Lackey, E.P.; et al. Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper [corrected]. Cerebellum 2018, 17, 654–682. [Google Scholar] [CrossRef] [Green Version]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-García, J.M.; da Guarda, S.N.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Mariën, P.; et al. Consensus paper: Roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Chao, O.Y.; Zhang, H.; Pathak, S.S.; Huston, J.P.; Yang, Y.M. Functional Convergence of Motor and Social Processes in Lobule IV/V of the Mouse Cerebellum. Cerebellum 2021, 20, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Syme, L.A. Influence of age and sex on the behavior of rats deprived of the Rearing Response. Dev. Psychobiol. 1974, 8, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Manes, M.; Garcia-Gomes, M.S.A.; Sandini, T.M.; Zaccarelli-Magalhaes, J.; Florio, J.C.; Alexandre-Ribeiro, S.R.; Wadt, D.; Bernardi, M.M.; Massironi, S.M.G.; Mori, C.M.C. Behavioral and neurochemical characterization of the mlh mutant mice lacking otoconia. Behav. Brain Res. 2019, 359, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Osti, F.R.; de Souza, C.R.; Teixeira, L.A. Improvement of Balance Stability in Older Individuals by On-Water Training. J. Aging Phys. Act. 2018, 26, 222–226. [Google Scholar] [CrossRef]
- Thompson, S.M.; Berkowitz, L.E.; Clark, B.J. Behavioral and Neural Subsystems of Rodent Exploration. Learn. Motiv. 2018, 61, 3–15. [Google Scholar] [CrossRef]
- Takeuchi, E.; Ito-Ishida, A.; Yuzaki, M.; Yanagihara, D. Improvement of cerebellar ataxic gait by injecting Cbln1 into the cerebellum of cbln1-null mice. Sci. Rep. 2018, 8, 6184. [Google Scholar] [CrossRef] [Green Version]
- Gerlai, R.; Millen, K.J.; Herrup, K.; Fabien, K.; Joyner, A.L. Impaired Motor Learning Performance in cerebellar En-2 mutant mice. Behav. Neurosci. 1996, 110, 126–133. [Google Scholar] [CrossRef]
- Hong, J.; Yoon, D.; Nam, Y.; Seo, D.; Kim, J.H.; Kim, M.S.; Lee, T.Y.; Kim, K.S.; Ko, P.W.; Lee, H.W.; et al. Lipopolysaccharide administration for a mouse model of cerebellar ataxia with neuroinflammation. Sci. Rep. 2020, 10, 13337. [Google Scholar] [CrossRef]
- Starowicz-Filip, A.; Chrobak, A.A.; Moskala, M.; Krzyzewski, R.M.; Kwinta, B.; Kwiatkowski, S.; Milczarek, O.; Rajtar-Zembaty, A.; Przewoznik, D. The role of the cerebellum in the regulation of language functions. Psychiatr. Pol. 2017, 51, 661–671. [Google Scholar] [CrossRef]
- De Smet, H.J.; Paquier, P.; Verhoeven, J.; Marien, P. The cerebellum: Its role in language and related cognitive and affective functions. Brain Lang. 2013, 127, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Scelfo, B.; Strata, P. The cerebellum: Synaptic changes and fear conditioning. Neuroscientist 2005, 11, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Ernst, T.M.; Brol, A.E.; Gratz, M.; Ritter, C.; Bingel, U.; Schlamann, M.; Maderwald, S.; Quick, H.H.; Merz, C.J.; Timmann, D. The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. Elife 2019, 8, e46831. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Xue, R.; Wang, Y.; Li, M.; Jia, H.; Pakan, J.M.P.; Li, L.; Chen, X.; Li, X. Optical Fiber-Based Recording of Climbing Fiber Ca2+ Signals in Freely Behaving Mice. Biology 2022, 11, 907. https://doi.org/10.3390/biology11060907
Tang J, Xue R, Wang Y, Li M, Jia H, Pakan JMP, Li L, Chen X, Li X. Optical Fiber-Based Recording of Climbing Fiber Ca2+ Signals in Freely Behaving Mice. Biology. 2022; 11(6):907. https://doi.org/10.3390/biology11060907
Chicago/Turabian StyleTang, Jiechang, Rou Xue, Yan Wang, Min Li, Hongbo Jia, Janelle M. P. Pakan, Longhui Li, Xiaowei Chen, and Xingyi Li. 2022. "Optical Fiber-Based Recording of Climbing Fiber Ca2+ Signals in Freely Behaving Mice" Biology 11, no. 6: 907. https://doi.org/10.3390/biology11060907






