Simple Summary
C-type natriuretic peptide (CNP) is the third member of the natriuretic peptide family. Unlike atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), CNP was not previously regarded as an important cardiac modulator. However, recent studies have revealed the physiological and pathophysiological importance of CNP in the heart; in concert with its cognate natriuretic peptide receptor-B (NPR-B), CNP has come to be regarded as the major heart-protective natriuretic peptide in the failed heart. In this review, I introduce the history of research on CNP in the cardiac field.
Abstract
C-type natriuretic peptide (CNP) is the third member of the natriuretic peptide family. Unlike other members, i.e., atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), which are cardiac hormones secreted from the atrium and ventricle of the heart, respectively, CNP is regarded as an autocrine/paracrine regulator with broad expression in the body. Because of its low expression levels compared to ANP and BNP, early studies failed to show its existence and role in the heart. However, recent studies have revealed the physiological and pathophysiological importance of CNP in the heart; in concert with the distribution of its specific natriuretic peptide receptor-B (NPR-B), CNP has come to be regarded as the major heart-protective natriuretic peptide in the failed heart. NPR-B generates intracellular cyclic guanosine 3′,5′-monophosphate (cGMP) upon CNP binding, followed by various molecular effects including the activation of cGMP-dependent protein kinases, which generates diverse cytoprotective actions in cardiomyocytes, as well as in cardiac fibroblasts. CNP exerts negative inotropic and positive lusitropic responses in both normal and failing heart models. Furthermore, osteocrin, the intrinsic and specific ligand for the clearance receptor for natriuretic peptides, can augment the effects of CNP and may supply a novel therapeutic strategy for cardiac protection.
1. Introduction
C-type natriuretic peptide (CNP) was extracted from porcine brain in 1990 for the first time [1] and then cloned in pigs [2], as well as in rats [3] and humans [4]. CNP is the third member of the natriuretic peptide family, and, along with the other two natriuretic peptide family members, i.e., atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), it shares a similar ring structure of 17 amino acids with the essential residues needed to exert their biological actions through binding to their biological active receptors [1] (Figure 1A). CNP acts as an intrinsic and bioactive peptide ligand through the binding to its specific membrane guanylyl cyclase receptor [5], natriuretic peptide receptor-B (NPR-B) [6], whereas ANP and BNP are selective ligands for the other receptor membrane guanylyl cyclase, natriuretic peptide receptor-A (NPR-A) [7]; these NPRs exert their biological action through the generation of the second messenger, cyclic guanosine 3′,5′-monophosphate (cGMP) from GTP upon ligand binding [8]. Accordingly, they are also referred to as guanylyl cyclase-A (GC-A) for NPR-A and guanylyl cyclase-B (GC-B) for NPR-B (Figure 1B).
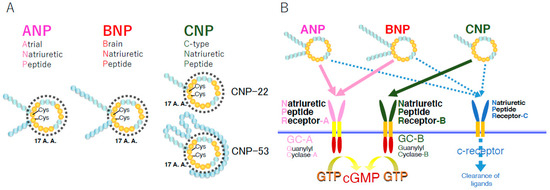
Figure 1.
Schematic representation of natriuretic peptides (A) and their receptors (B). A.A.: amino acids; Cys: cysteines making the disulfide bond in each ring structure.
Although ANP and BNP act as cardiac hormones secreted from the atrium and ventricle of the heart, respectively [9,10,11], CNP was shown to be expressed ubiquitously in the body [12]: CNP and its specific receptor, NPR-B [13], are expressed in the central nervous system including the brain [14], hypothalamus [15,16], and pituitary gland [14,17], in the circulatory system including the blood vessels [18] and heart, in the reproductive system [19,20], and in the skeletal system including the growth plate cartilage [21,22,23,24]. Together with the fact that CNP has considerably low blood concentrations in mammals [25,26,27], CNP is regarded as an autocrine/paracrine regulator, not an endocrine hormone [28].
For a few decades, the physiological and pathophysiological roles of ANP and BNP were intensively investigated, and these cardiac hormones were shown to be engaged in the protection of circulatory homeostasis, including the exertion of their cardioprotective effects. These discoveries have assisted in establishing the clinical implications of ANP and BNP as biomarkers that detect cardiac disease including heart failure and cardiac hypertrophy, and ANP and BNP themselves and molecules relevant to them have been implicated in the development of therapeutic agents for heart failure or related diseases [29,30].
On the other hand, along with the notion that CNP is a ubiquitously expressed autocrine/paracrine factor, research pursuing the physiological and pathophysiological roles of CNP in the body and their transition to clinical use has diverged into a wide variety of organs. The recent and most prominent studies were performed in the skeletal system [31,32]. Nevertheless, several groups have persisted with working to resolve the roles of CNP in the heart [33]. In this review, after a brief general description of CNP, I introduce studies of CNP in the heart.
2. General Features of CNP
2.1. Generation of CNP
The gene encoding CNP, NPPC, is located on the second chromosome, 2q37.1, in humans. CNP is first produced as the pre-pro-peptide of 126 amino acids, which is subsequently cleaved into proCNP with 103 amino acids by the endoprotease furin [34]. ProCNP is further cleaved by furin into biologically active and mature CNP with 53 amino acids (CNP-53) and the presumably bio-inactive N-terminal product amino-terminal proCNP with 50 amino acids (NTproCNP). A New Zealand group has been reporting on the significance of NTproCNP as a clinical biomarker for various physiological and pathophysiological conditions including issues concerning skeletal growth in humans and other experimental animal models [35]. In some cases, CNP-53 is further broken down into a variant with 22 amino acids (CNP-22), whose biological activity is thought to be equal to that of CNP-53, by an unknown enzyme [36] (Figure 1A).
As for the regulation of CNP production, cytokines (transforming growth factor β, tumor necrosis factor α, and interleukin 1β), bacterial endotoxin lipopolysaccharides [28], transcription factors (kruppel-like factor 2) [37], hypoxia [38], and shear stress [39] were reported to stimulate the production of CNP in blood vessels in in vivo and ex vivo experiments. Furthermore, molecules participating in Wnt signaling reportedly stimulated CNP production in the kidney [40], while transforming growth factor β stimulated CNP production in chondrocytes [41] and osteoblasts [21] in in vitro experiments. In the ovaries of mice, excess human chorionic gonadotropin treatment reportedly exhibited a marked decrease in CNP expression in granulosa cells of the preovulatory follicles [20].
2.2. Receptors for CNP and Their Downstream Signaling
As mentioned above, the biologically active receptor for CNP is NPR-B. NPR-B is a membranous receptor guanylyl cyclase that produces cGMP from GTP through selective ligand CNP binding. The downstream pathways of this CNP/NPR-B/cGMP signaling cascade include the pathways through cGMP-dependent protein kinases (abbreviated as cGKs or PKGs), cGMP-dependent phosphodiesterases (PDEs), and cGMP-gated ion channels, all of which cause a broad variety of physiological responses. Among these pathways, that through cGKs is regarded as the most important one; cGKs phosphorylate various downstream target proteins. One subtype of cGKs, cGKII, is reported to play an important role in skeletal growth as the downstream mediator of the CNP/NPR-B/cGMP pathway [42,43,44,45]. On the other hand, cGMP signaling is abrogated by cGMP hydrolysis via PDEs and cGMP export via multidrug resistance proteins.
Furthermore, there exists another receptor for CNP, named NPR-C, which was initially reported to be engaged in the clearance of ligands [46]. The catalytic effect of NPR-C is discussed in the next section. After the discovery of its clearance action, it was revealed that NPR-C contains Gi-binding domains in its intracellular C-terminal region, which induce the inhibition of adenylyl cyclase (through Gi α subunit) and the activation of phospholipase C-β (through Gi βγ subunits) [47,48,49,50].
2.3. Degradation of CNP
The plasma CNP concentration is considerably low [25,51]. This is because CNP is rapidly degraded in circulation or in the periphery where it acts as an autocrine/paracrine factor. The plasma half-life of CNP is reportedly 2.6 min [52]. The main catabolic pathways of CNP include its clearance receptor, NPR-C, referred to as the c-receptor, and neutrophil endopeptidase 24. 11 (NEP).
NPR-C is the third identified natriuretic peptide receptor, succeeding NPR-A and NPR-B. NPR-C has a similar affinity to all three natriuretic peptides; however, the binding affinity is as follows in both humans and rats: ANP > CNP > BNP [7]. Whereas NPR-A and NPR-B are biologically active receptor guanylyl cyclases with catalytic or enzymatic domains under the membranous portion, NPR-C does not have intracellular guanylyl cyclase domains and, thus, cannot produce cGMP as the second messenger. It internalizes the bound ligands and degrades them intracellularly [46]. The role of NPR-C in the metabolism of CNP is summarized elsewhere [53]. In addition, there exists an intrinsic and specific ligand for NPR-C named osteocrin or musclin. Osteocrin can regulate the effect of CNP by moderating the local CNP concentrations [54,55,56].
NEP is a zinc-dependent peptidase that is present in numerous tissues, including the lung, kidney, endothelial cells, and plasma. NEP degrades natriuretic peptide family members, and CNP is reportedly highly susceptible to degradation by NEP in vitro [57]. Later, NEP was shown to regulate CNP metabolism in in vivo infusion experiments [58], and its inhibition was exhibited to enhance CNP-related actions in several tissues [59,60,61].
2.4. Distribution of CNP
Whereas ANP and BNP are known as cardiac hormones which are produced in the atrium and ventricle of the heart, respectively, CNP is expressed ubiquitously throughout the body and is produced in various tissues. Together with the fact that the plasma concentrations of CNP are relatively low as mentioned above, CNP is thought to be an autocrine/paracrine factor. Suga et al. revealed that CNP is expressed in endothelial cells for the first time [18]. Since then, various studies have exhibited that endothelial cells express and secret CNP, predisposing that the endothelium is an important tissue in which CNP plays a pivotal role [62,63,64]. Generally, CNP is thought to have vasodilatory and antimitogenic actions there. Furthermore, Komatsu et al. first reported that CNP exists in the brain, including the pituitary gland [14], and many studies followed with the discovery of the production and the expression of CNP in the central nervous system [65,66,67,68,69,70,71], indicating important roles of CNP there.
As the most prominent phenotype of systemic CNP knockout mice was impaired skeletal growth [23], the physiological role of CNP in skeletal tissues, especially that in the growth plate cartilage, was presumed to be the most important among those in all tissues in the mammalian body. The notion that the CNP/NPR-B pathway is crucial for skeletal growth was confirmed in rat experimental models [72,73] and further in humans through the observation of several pathophysiological phenotypes in cases with genetic mutations in the genes coding for relevant molecules included in this pathway [74,75,76]. Using this prominent growth-promoting effect of the activation of the CNP/NPR-B pathway on skeletal tissues, a CNP analogue was developed to improve the impaired skeletal growth observed in patients with achondroplasia, one of the most common forms of skeletal dysplasia [31,32].
Another obvious phenotype of systemic CNP knockout mice is infertility. Concerning this point, the CNP/NPR-B system in the reproductive system was investigated, and its critical role in female fertility was elucidated using mutant mice with impaired CNP or NPR-B function [19,20].
3. Physiological Roles of CNP in the Heart
3.1. Distribution of CNP and NPR-B in the Heart
Although widely expressed throughout the body as mentioned above, CNP is also found in the heart [77]. However, the expression levels are much lower than those of ANP or BNP [78,79]. Researchers could not detect CNP in the heart of rats in the earliest studies using radioimmunoassay [12,14,80]. Later, in various species including cartilaginous fish [81], Squalus acanthias [82], and Triakis scyllia [83], CNP itself and its gene expression were detected in rat heart [84]. CNP and its receptor NPR-B were also detected in goat cardiomyocytes [85]. Likewise, researchers were unable to detect CNP in human hearts in early studies [86], but the augmentation of CNP production in the failing heart made it easy to evaluate the existence of CNP in the heart [87]; as discussed in a later section, in the failing heart, the expression of CNP and the plasma concentrations of CNP are increased [27,88,89,90,91].
The hearts of vertebrates are roughly composed of two types of cells: cardiomyocytes and their interstitial fibroblasts. As for cardiomyocytes, Wei et al. confirmed the presence of CNP within the cardiomyocytes by immunohistochemistry and radioimmunoassay in human subjects for the first time [77]. Soon after, the expression of NPR-B was detected in cardiomyocytes isolated from rat ventricle, but the cGMP genesis by CNP in the experimental preparation was reportedly low [92]. Later, CNP and NPR-B were detected in rat cardiomyocytes both in vitro and ex vivo [78].
On the other hand, CNP was shown to be synthesized in and secreted from cardiac fibroblasts in in vitro experiments using rat cultured ventricular cells. In an immunohistochemical study, NPR-B was detected at much greater levels in cardiac fibroblasts than in cardiomyocytes in frozen sections of rat ventricle. This was further confirmed by an immunoblot study using protein extracts of distinct cardiac cell types. Taken together, NPR-B in adult rat ventricle was reported to be predominantly confined to the nonmyocyte population [93]. In humans, NPR-B activity is increased in nonmyocytes in failing ventricles, possibly as a result of increased fibrosis, and human ventricular cardiomyocytes were reported to express much lower levels or possibly no NPR-B [94].
3.2. Downstream Signaling of CNP in the Cardiac Cells
As for the downstream signaling of CNP in the cardiac cells, the signaling pathway through cGKs is regarded as one of the main pathways of the CNP/NPR-B/cGMP signaling cascade, as reviewed elsewhere [95]. Briefly, cGKs activated by cGMP inhibit calcium (Ca) signaling and suppress the calcineurin nuclear factor of activated T cells (NFAT) pathway in cardiac myocytes [96]. A nonselective non-voltage-gated cation channel, L-type Ca channel [97], and the transient potential canonical 6 (TRPC6) [98] are phosphorylated by cGKs and are, thus, involved in attenuating Ca entry, while also inhibiting Ca/calmodulin-activated kinase II (CaMKII). CNP greatly increased the phosphorylation of phospholamban (PLN) a and increased that of troponin I (TnI) to some extent in a failing heart model [99]. Several studies have revealed the central roles of the regulators of G-protein signaling (RGSs) (Figure 2).
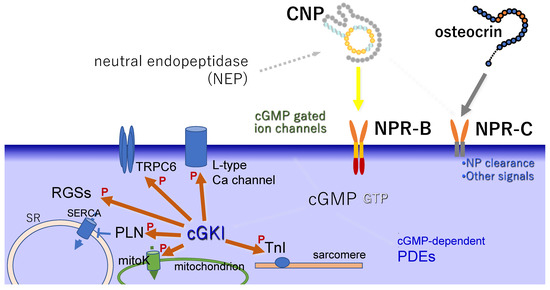
Figure 2.
Schema of intracellular signaling molecules downstream of CNP/NPR-B. ‘P’ indicates the action of phosphorylation. SR: sarcoplasmic reticulum, SERCA: SR Ca-ATPase, PLN: phospholamban, RGS: regulator of G-protein signaling, TRPC6: transient potential canonical 6, TnI: troponin I, cGKI: cyclic GMP-dependent protein kinase I, mitoK: mitochondrial ATP-sensitive K channels and Ca-activated K channels of the BK type, PDE: phosphodiesterase.
Among PDEs, PDE3 is dominant in cardiomyocytes [93]. In particular, the CNP-mediated increase in cGMP is generally regulated by PDE2; however, through inhibition experiments, PED3 was shown to be more functionally important than PDE2 [99]. On the other hand, CNP sensitizes cAMP-mediated signaling in the non-failing heart via the NPR-B-mediated increase in cGMP, which inhibits the cAMP-PDE activity of PDE3 [100].
Natriuretic peptides including CNP reportedly modulate the current of ATP-sensitive potassium (K) channel in cardiomyocytes from the ventricle [101], which may be relevant to the fact that they can increase intracellular cGMP levels through NPR-A or NPR-B.
3.3. Physiological Effects of CNP on the Heart
In a physiological situation, CNP expression levels in cardiomyocytes are much smaller than those of ANP and BNP [78], and, as was the case with the exhibition of its existence, early studies failed to prove any effects of CNP on the heart [92]. Although a gene-targeting approach is fascinating to reveal the physiological roles of a gene product, mice depleted with CNP in cardiomyocytes or fibroblasts showed no obvious changes in the contractility, structure, or fibrosis of the heart, supporting the previous idea that CNP plays a minimal role in the heart in healthy conditions [102]. Nevertheless, NPR-B activity was shown to represent a significant portion of the natriuretic peptide-dependent guanylyl cyclase activity in the normal heart [103]. Yoshizumi et al. showed that CNP stimulates Na-dependent Ca efflux from freshly isolated adult rat cardiomyocytes [104]. Using ex vivo preparations of rat papillary muscle, CNP was shown to exert a positive lusitropic effect, in that the putative mechanism involved a cGMP-dependent enhancement of the rate of relaxation with a slowly developing negative inotropic effect [105]. CNP caused a significant reduction in the amplitude of contraction of cultured neonatal rat beating cardiomyocytes [106]. In contrast to endothelin-1, CNP reduced the contractility of these cells and further induced apoptosis via the accumulation of cGMP [107,108,109]. By using the hypertrophic rabbit heart model, negative inotropic effects of CNP were shown to be attenuated in hypertrophied ventricular myocytes because of reduced cyclic GMP production [110]. A recent study using ventricular myocytes isolated from transgenic mice expressing the highly sensitive cytosolic cGMP biosensor exhibited that NPR-B is evenly distributed across the ventricular muscle membrane and produces far-reaching, diffusible cGMP signals, whereas NPR-A is exclusively found in T-tubules where it creates a microdomain with restricted cGMP diffusion locally confined by PDE2 [111]. A Norway group also used targeted cGMP biosensors in rats and showed that CNP increases cGMP production near TnI, as well as sarcoplasmic reticulum Ca ATPase (SERCA), indicating that CNP can promote lusitropic and negative inotropic actions [112]. As for the molecular signaling pathway of the contractile effects of CNP in the heart, cGKI was demonstrated to be a downstream target, and cGMP/cGKI-stimulated phosphorylation of Ser16-phosphorylated PLN and subsequent activation of SERCA pump appear to mediate the positive lusitropic responses to CNP [113,114].
Summing up the above concept of the effect of CNP on heart contraction, NPR-B stimulation by CNP increases cGMP, and its downstream signaling cascade eventually causes a positive lusitropic and negative inotropic action in the myocardium. These effects are not mimicked by NPR-A stimulation by BNP, despite a similar cGMP increase.
CNP was reported to decrease fibroblast proliferation and extracellular matrix production in a NPR-B-mediated cGMP-dependent manner, i.e., CNP produced by cardiac fibroblasts is proposed to play a role in inhibiting cardiac fibrosis as an autocrine/paracrine factor [115].
4. Effects of CNP on Heart Failure
As mentioned, ANP and BNP are cardiac hormones secreted from the atrium and ventricle of the heart, respectively, and their clinical roles in patients with heart failure are well established; both of them are markers of the severity of heart failure and are further used as drugs for heart failure in clinical settings. Unlike ANP and BNP, CNP is thought to be a ubiquitous autocrine/paracrine regulator, and its expression levels in the heart are much lower than those of ANP and BNP; thus, so the role of CNP in heart failure did not initially attract much attention. Nevertheless, the gene expression of CNP and the plasma levels of NTproCNP were reported to be increased in case of heart failure, as along with ANP and BNP [27,88,89,90,91]. CNP production was increased in the hearts of patients with chronic heart failure, and this increase was correlated with the severity of heart failure [89,116]. Furthermore, the severity of the disease correlated with plasma NTproCNP levels, predicting all-cause mortality and hospitalization in patients suffering from heart failure with preserved ejection fraction (HFpEF) [117]. As CNP is an autocrine/paracrine regulator, in order to clarify whether increased levels of plasma proCNP are caused by increased production of CNP in the heart, CNP levels in the coronary sinus were compared with those in the aortic root in subjects with heart failure [116]. As a result, CNP levels in the coronary sinus were significantly increased compared with those in systemic circulation, indicating that CNP production is augmented in the failing heart. Dickey et al. reported that CNP generated twice as much cGMP as ANP in the mouse model of pressure-overloaded heart failure. They supposed that, in this condition, NPR-A activity decreased whereas NPR-B activity was not changed, indicating that NPR-B accounts for the majority of the natriuretic peptide-dependent activity in the failed heart [103].
As is the case with non-failing hearts, CNP is reported to show negative inotropic and positive lusitropic responses in rat failing heart models [118]. Concerning the role of PDEs that mediate the effects of CNP on failing hearts, the Oslo University group reported that the increase in global cGMP by CNP was mainly regulated by PDE2, not PDE3, in left-ventricular muscle strips and ventricular cardiomyocytes in the failing hearts of Wistar rats, but the functional consequences were different from the changes in cGMP, i.e., PDE3 inhibition induced the CNP-mediated negative inotropic response and lusitropic response, whereas PDE2 inhibition desensitized the CNP-induced negative inotropic response, but not lusitropic response. The increase in cGMP necessarily coincides with the functional responses and, generally, the functional responses induced by CNP are intermediately regulated by PDEs [99,100]. Furthermore, CNP sensitizes cAMP-mediated signaling via NPR-B-mediated increase of cGMP, which inhibits the cAMP-PDE activity of PDE3 in failing hearts [100].
The same group reported the involvement of SERCA activity as one of the effectors of the downstream molecules of CNP/NPR-B signaling, which mediates the negative inotropic and positive lusitropic effects of CNP in the failing heart. CNP-induced PLN and TnI phosphorylation by cGK in concert mediate both negative inotropic and positive lusitropic effects in failing hearts [119].
In addition, during the early phases of pressure overload, NPR-B/cGMP/cGKI signaling activated by CNP in cardiomyocytes protects from myocyte stiffening caused via titin [120].
5. Roles of CNP on Cardiac Hypertrophy
Similar to the case of heart failure, ANP and BNP are also biomarkers for cardiac hypertrophy. At the early stage of research on CNP, the role of CNP in cardiac hypertrophy was investigated as one of the cGMP generators along with other natriuretic peptides. The first intensive study on the specific effect of CNP on cardiac hypertrophy was performed by Tokudome et al. [121]. They investigated the effects of CNP on cultured cardiac myocyte hypertrophy and the interaction between CNP and endothelin-1 (ET-1), which is a representative stimulator of cardiac hypertrophy. Resultantly, CNP attenuated basal and ET-1-induced hypertrophy-related gene expression and inhibited ET-1-induced cardiomyocyte hypertrophy via a cGMP-dependent mechanism. The same group further reported that CNP affected antihypertrophic action in rat myocardial infarction (MI) models [122]. In addition, CNP reportedly attenuated angiotensin II-induced cardiac hypertrophy, fibrosis, and contractile dysfunction, which were accompanied by reduced cardiac superoxide production, in in vivo experiments using mice models [123]. On the contrary, the effects of CNP on cardiac contractility, guanylyl cyclase activity, and phosphorylation of cGMP-dependent protein were dampened in myocytes from the hypertrophied heart of mice induced by aortic banding [124].
As for other model animals, NPR-B dominant-negative transgenic rats displayed progressive, blood pressure-independent cardiac hypertrophy, and the hypertrophic phenotype was further enhanced in chronic volume overload-induced congestive heart failure, suggesting the preventing effect of CNP/NPR-B on cardiac hypertrophy [125].
6. Roles of CNP on MI
The vasculature is the major tissue where CNP abundantly exists and on which CNP potently works. As for the physiological and pathophysiological roles of CNP in the vasculature, the vascular natriuretic peptide system is proposed, in that endothelium-derived CNP affects vascular smooth muscle cells expressing its cognate receptor, NPR-B, and regulates vascular tone, remodeling, and regeneration [63,126]. On the other hand, endothelium-derived CNP is reported to maintain vascular homeostasis through NPR-C [127], and, in the case of MI, the CNP/NPR-C signaling governs coronary blood flow and protects against ischemia/reperfusion injury (I/R) complicated by MI [128].
In vivo administration of CNP was shown to attenuate cardiac remodeling after MI through its antifibrotic and antihypertrophic action [122]. In a mouse model with targeted overexpression of CNP in cardiomyocytes, overexpressed CNP did not affect I/R-induced infarct size but prevented cardiac hypertrophy induced by MI [129]. On the other hand, in a swine model of induced MI with preserved left-ventricular ejection fraction, CNP expression was locally increased in the infarct-remodeled myocardium in the presence of a dense capillary network, and a high concentration of CNP was required in the vasculogenic response there together with VEGF-A [130].
During MI and I/R, cGMP triggers cytoprotective responses and improves cardiomyocyte survival; cGMP production leads to the activation of cGKI, which in turn phosphorylates many substrates and eventually facilitates the opening of mitochondrial ATP-sensitive K channels and Ca-activated K channels of the BK type [131,132]. PDEs and SERCA2, which were mentioned above as downstream molecules in the CNP/NPR-B signaling cascade in the failing heart, are thought to be effective in MI states because the in vitro experimental model of heart failure included ventricular cardiomyocytes from Wistar rats with heart failure after MI [99,119].
In clinical settings, although circulating signal peptides of CNP could not identify patients with MI or those with unstable angina, it was significantly lower in patients with a history of previous MI and could identify those at risk of death or reinfarction within 1 year [133]. Similarly, plasma NTproCNP is reported to be an independent predictor of mortality and cardiac readmission in individuals with unstable angina [134]. A recent study performed in Denmark hospitals showed that increased proCNP levels at admission are an independent risk of all-cause mortality in female patients with ST-elevated MI [135].
7. Effects of CNP on Heart Rate and Electrical Conduction in the Sinoatrial Node (SAN)
Chronotropic effects of CNP, i.e., effects of CNP on the heart conduction system, have also been reported. After indicating that CNP exerts a significant and prolonged positive chronotropic effect both in vivo and in vitro using a dog model [136], Beaulieu et al. showed CNP modifies cardiac ionic currents to produce positive chronotropic effects by stimulation of NPR-B located in the SAN region [137]. Subsequently, natriuretic peptides (including CNP) and their cognate receptors were shown to modulate ion channel function in the SAN [138,139,140]. Recently, to investigate the physiological roles of NPR-B signaling in regulating heart rate and SAN function, NPR-B-deficient mice were used, and it was revealed that NPR-B plays an essential physiological role in maintaining normal heart rate and SAN function by modulating ion channel function in SAN myocytes via a cGMP/PDE3/cAMP signaling mechanism [141].
8. Conclusions and Further Discussion
Despite fewer dynamic changes compared to ANP or BNP, CNP plays a distinct role in cardiac physiology and pathophysiology. Cardiac cell-specific manipulation of CNP elucidated its autocrine/paracrine mechanism of action on the heart; a recent study reported that CNP originating from cardiomyocytes, endothelial cells, and cardiac fibroblasts is essential in maintaining the cardiac structure, function, and coronary vasoreactivity [102]. In the physiological state, cardiomyocyte- and fibroblast-specific knockout mice showed no alteration compared to control mice regarding cardiac contractility, structure, or fibrosis, supporting the concept that CNP plays a minimal role in a healthy state. On the other hand, when artificial heart failure was induced by pressure overload after aortic banding, mice with specific depletion of CNP in cardiomyocytes and in fibroblasts both had decreased ejection fraction, increased ventricular dilation, and increased collagen deposition; in particular, in cardiomyocyte-specific knockout mice, cardiac hypertrophy was observed. Similar effects were observed in clinical settings; therefore, the development of drugs related to the cardioprotective effect of CNP-NPR-B signaling is expected. As an analogue for CNP was recently approved as a drug for impaired skeletal growth in achondroplasia, relevant remedies targeting cardiovascular disorders may be developed in the future. In addition, the development of a method for measuring CNP or NTproCNP is lacking compared to other natriuretic peptides. Authentic or standard measuring procedures for CNP or NTproCNP should be explored.
As for the receptor, NPR-B is regarded as biologically active because of its massive cGMP generation upon ligand CNP binding. However, NPR-C has come to attract attention as it can augment regional CNP content. Therefore, osteocrin, the intrinsic and specific ligand for NPR-C, may play a major role in cardiac physiology and pathophysiology (Figure 2). Using a wildtype or osteocrin knockout mouse model, Miyazaki et al. showed the possibility that osteocrin suppresses the worsening of chronic heart failure after MI by inhibiting the clearance of the natriuretic peptide family including CNP [55]. Szaroszyk et al. performed RNA sequencing on wasting murine skeletal muscles in the condition of heart failure and found a reduced osteocrin expression. Furthermore, by generating mice with skeletal muscle-targeted depletion or overexpression of osteocrin, they demonstrated that, under the pressure overload condition, the progression of cardiac dysfunction and myocardial fibrosis is adversely correlated with skeletal muscle osteocrin levels. As for the mechanism, osteocrin enhanced the abundance of CNP, which promoted cardiomyocyte contractility via protein kinase A and further inhibited fibroblast activity via cGK signaling. They also found that osteocrin expression was reduced in the skeletal muscle of patients with heart failure, suggesting the therapeutic potency of the augmentation of osteocrin for cardiac cachexia [142]. The activation of NPR-C by its cognate ligands osteocrin or its analogues may represent a novel therapeutic approach to various cardiac disorders.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI, Grant Number JP 20K08877.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Sudoh, T.; Minamino, N.; Kangawa, K.; Matsuo, H. C-type natriuretic peptide (CNP): A new member of natriuretic peptide family identified in porcine brain. Biochem. Biophys. Res. Commun. 1990, 168, 863–870. [Google Scholar] [CrossRef]
- Tawaragi, Y.; Fuchimura, K.; Nakazato, H.; Tanaka, S.; Minamino, N.; Kangawa, K.; Matsuo, H. Gene and precursor structure of porcine C-type natriuretic peptide. Biochem. Biophys. Res. Commun. 1990, 172, 627–632. [Google Scholar] [CrossRef]
- Kojima, M.; Minamino, N.; Kangawa, K.; Matsuo, H. Cloning and sequence analysis of a cDNA encoding a precursor for rat C-type natriuretic peptide (CNP). FEBS Lett. 1990, 276, 209–213. [Google Scholar] [CrossRef]
- Tawaragi, Y.; Fuchimura, K.; Tanaka, S.; Minamino, N.; Kangawa, K.; Matsuo, H. Gene and precursor structures of human C-type natriuretic peptide. Biochem. Biophys. Res. Commun. 1991, 175, 645–651. [Google Scholar] [CrossRef]
- Furuya, M.; Tawaragi, Y.; Minamitake, Y.; Kitajima, Y.; Fuchimura, K.; Tanaka, S.; Minamino, N.; Kangawa, K.; Matsuo, H. Structural requirements of C-type natriuretic peptide for elevation of cyclic GMP in cultured vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1992, 183, 964–969. [Google Scholar] [CrossRef]
- Koller, K.J.; Lowe, D.G.; Bennett, G.L.; Minamino, N.; Kangawa, K.; Matsuo, H.; Goeddel, D.V. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 1991, 252, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Suga, S.; Nakao, K.; Hosoda, K.; Mukoyama, M.; Ogawa, Y.; Shirakami, G.; Arai, H.; Saito, Y.; Kambayashi, Y.; Inouye, K. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 1992, 130, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Takehisa, M.; Minamitake, Y.; Kitajima, Y.; Hayashi, Y.; Ohnuma, N.; Ishihara, T.; Minamino, N.; Kangawa, K.; Matsuo, H. Novel natriuretic peptide, CNP, potently stimulates cyclic GMP production in rat cultured vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1990, 170, 201–208. [Google Scholar] [CrossRef]
- Saito, Y.; Nakao, K.; Itoh, H.; Yamada, T.; Mukoyama, M.; Arai, H.; Hosoda, K.; Shirakami, G.; Suga, S.; Minamino, N. Brain natriuretic peptide is a novel cardiac hormone. Biochem. Biophys. Res. Commun. 1989, 158, 360–368. [Google Scholar] [CrossRef]
- Mukoyama, M.; Nakao, K.; Hosoda, K.; Suga, S.; Saito, Y.; Ogawa, Y.; Shirakami, G.; Jougasaki, M.; Obata, K.; Yasue, H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Investig. 1991, 87, 1402–1412. [Google Scholar] [CrossRef]
- Ogawa, Y.; Nakao, K.; Mukoyama, M.; Hosoda, K.; Shirakami, G.; Arai, H.; Saito, Y.; Suga, S.; Jougasaki, M.; Imura, H. Natriuretic peptides as cardiac hormones in normotensive and spontaneously hypertensive rats. The ventricle is a major site of synthesis and secretion of brain natriuretic peptide. Circ. Res. 1991, 69, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Minamino, N.; Aburaya, M.; Kangawa, K.; Matsukura, S.; Matsuo, H. Distribution and characterization of immunoreactive porcine C-type natriuretic peptide. Biochem. Biophys. Res. Commun. 1991, 175, 759–767. [Google Scholar] [CrossRef]
- Konrad, E.M.; Thibault, G.; Schiffrin, E.L. Autoradiographic visualization of the natriuretic peptide receptor-B in rat tissues. Regul. Pept. 1992, 39, 177–189. [Google Scholar] [CrossRef]
- Komatsu, Y.; Nakao, K.; Suga, S.; Ogawa, Y.; Mukoyama, M.; Arai, H.; Shirakami, G.; Hosoda, K.; Nakagawa, O.; Hama, N. C-type natriuretic peptide (CNP) in rats and humans. Endocrinology 1991, 129, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Langub, M.C.; Watson, R.E. Localization of C-type natriuretic peptide mRNA in rat hypothalamus. Endocrinology 1993, 133, 1903–1906. [Google Scholar] [CrossRef][Green Version]
- Langub, M.C.; Dolgas, C.M.; Watson, R.E.; Herman, J.P. The C-type natriuretic peptide receptor is the predominant natriuretic peptide receptor mRNA expressed in rat hypothalamus. J. Neuroendocrinol. 1995, 7, 305–309. [Google Scholar] [CrossRef]
- Yandle, T.G.; Fisher, S.; Charles, C.; Espiner, E.A.; Richards, A.M. The ovine hypothalamus and pituitary have markedly different distribution of C-type natriuretic peptide forms. Peptides 1993, 14, 713–716. [Google Scholar] [CrossRef]
- Suga, S.; Nakao, K.; Itoh, H.; Komatsu, Y.; Ogawa, Y.; Hama, N.; Imura, H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J. Clin. Investig. 1992, 90, 1145–1149. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef]
- Tsuji, T.; Kiyosu, C.; Akiyama, K.; Kunieda, T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol. Reprod. Dev. 2012, 79, 795–802. [Google Scholar] [CrossRef]
- Suda, M.; Tanaka, K.; Fukushima, M.; Natsui, K.; Yasoda, A.; Komatsu, Y.; Ogawa, Y.; Itoh, H.; Nakao, K. C-type natriuretic peptide as an autocrine/paracrine regulator of osteoblast. Evidence for possible presence of bone natriuretic peptide system. Biochem. Biophys. Res. Commun. 1996, 223, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yasoda, A.; Ogawa, Y.; Suda, M.; Tamura, N.; Mori, K.; Sakuma, Y.; Chusho, H.; Shiota, K.; Tanaka, K.; Nakao, K. Natriuretic peptide regulation of endochondral ossification. Evidence for possible roles of the C-type natriuretic peptide/guanylyl cyclase-B pathway. J. Biol. Chem. 1998, 273, 11695–11700. [Google Scholar] [CrossRef] [PubMed]
- Chusho, H.; Tamura, N.; Ogawa, Y.; Yasoda, A.; Suda, M.; Miyazawa, T.; Nakamura, K.; Nakao, K.; Kurihara, T.; Komatsu, Y.; et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Doolittle, L.K.; Hammer, R.E.; Shelton, J.M.; Richardson, J.A.; Garbers, D.L. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc. Natl. Acad. Sci. USA 2004, 101, 17300–17305. [Google Scholar] [CrossRef]
- Stingo, A.J.; Clavell, A.L.; Heublein, D.M.; Wei, C.M.; Pittelkow, M.R.; Burnett, J.C. Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am. J. Physiol. 1992, 263, H1318–H1321. [Google Scholar] [CrossRef]
- Hama, N.; Itoh, H.; Shirakami, G.; Suga, S.; Komatsu, Y.; Yoshimasa, T.; Tanaka, I.; Mori, K.; Nakao, K. Detection of C-type natriuretic peptide in human circulation and marked increase of plasma CNP level in septic shock patients. Biochem. Biophys. Res. Commun. 1994, 198, 1177–1182. [Google Scholar] [CrossRef]
- Prickett, T.C.; Yandle, T.G.; Nicholls, M.G.; Espiner, E.A.; Richards, A.M. Identification of amino-terminal pro-C-type natriuretic peptide in human plasma. Biochem. Biophys. Res. Commun. 2001, 286, 513–517. [Google Scholar] [CrossRef]
- Suga, S.; Itoh, H.; Komatsu, Y.; Ogawa, Y.; Hama, N.; Yoshimasa, T.; Nakao, K. Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells--evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology 1993, 133, 3038–3041. [Google Scholar] [CrossRef]
- Kuwahara, K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol. Ther. 2021, 227, 107863. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Savarirayan, R.; Irving, M.; Bacino, C.A.; Bostwick, B.; Charrow, J.; Cormier-Daire, V.; Le Quan Sang, K.H.; Dickson, P.; Harmatz, P.; Phillips, J.; et al. C-Type Natriuretic Peptide Analogue Therapy in Children with Achondroplasia. N. Engl. J. Med. 2019, 381, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Savarirayan, R.; Tofts, L.; Irving, M.; Wilcox, W.; Bacino, C.A.; Hoover-Fong, J.; Ullot Font, R.; Harmatz, P.; Rutsch, F.; Bober, M.B.; et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: A randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet 2020, 396, 684–692. [Google Scholar] [CrossRef]
- Lumsden, N.G.; Khambata, R.S.; Hobbs, A.J. C-type natriuretic peptide (CNP): Cardiovascular roles and potential as a therapeutic target. Curr. Pharm. Des. 2010, 16, 4080–4088. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, F.; Pan, J.; Morser, J.; Wu, Q. Furin-mediated processing of Pro-C-type natriuretic peptide. J. Biol. Chem. 2003, 278, 25847–25852. [Google Scholar] [CrossRef]
- Prickett, T.C.; Espiner, E.A. Circulating products of C-type natriuretic peptide and links with organ function in health and disease. Peptides 2020, 132, 170363. [Google Scholar] [CrossRef]
- Minamino, N.; Kangawa, K.; Matsuo, H. N-terminally extended form of C-type natriuretic peptide (CNP-53) identified in porcine brain. Biochem. Biophys. Res. Commun. 1990, 170, 973–979. [Google Scholar] [CrossRef]
- Parmar, K.M.; Larman, H.B.; Dai, G.; Zhang, Y.; Wang, E.T.; Moorthy, S.N.; Kratz, J.R.; Lin, Z.; Jain, M.K.; Gimbrone, M.A.; et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Investig. 2006, 116, 49–58. [Google Scholar] [CrossRef]
- Klinger, J.R.; Siddiq, F.M.; Swift, R.A.; Jackson, C.; Pietras, L.; Warburton, R.R.; Alia, C.; Hill, N.S. C-type natriuretic peptide expression and pulmonary vasodilation in hypoxia-adapted rats. Am. J. Physiol. 1998, 275, L645–L652. [Google Scholar] [CrossRef]
- Chun, T.H.; Itoh, H.; Ogawa, Y.; Tamura, N.; Takaya, K.; Igaki, T.; Yamashita, J.; Doi, K.; Inoue, M.; Masatsugu, K.; et al. Shear stress augments expression of C-type natriuretic peptide and adrenomedullin. Hypertension 1997, 29, 1296–1302. [Google Scholar] [CrossRef]
- Surendran, K.; Simon, T.C. CNP gene expression is activated by Wnt signaling and correlates with Wnt4 expression during renal injury. Am. J. Physiol. Ren. Physiol. 2003, 284, F653–F662. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sakaguchi, H.; Itakura, M.; Yoshimoto, T.; Furuya, M.; Tanaka, S.; Hirose, S. Autocrine regulation of rat chondrocyte proliferation by natriuretic peptide C and its receptor, natriuretic peptide receptor-B. J. Biol. Chem. 1994, 269, 10729–10733. [Google Scholar] [CrossRef]
- Pfeifer, A.; Aszódi, A.; Seidler, U.; Ruth, P.; Hofmann, F.; Fässler, R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 1996, 274, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Ogawa, Y.; Chusho, H.; Yasoda, A.; Tamura, N.; Komatsu, Y.; Pfeifer, A.; Hofmann, F.; Nakao, K. Cyclic GMP-dependent protein kinase II plays a critical role in C-type natriuretic peptide-mediated endochondral ossification. Endocrinology 2002, 143, 3604–3610. [Google Scholar] [CrossRef][Green Version]
- Chikuda, H.; Kugimiya, F.; Hoshi, K.; Ikeda, T.; Ogasawara, T.; Shimoaka, T.; Kawano, H.; Kamekura, S.; Tsuchida, A.; Yokoi, N.; et al. Cyclic GMP-dependent protein kinase II is a molecular switch from proliferation to hypertrophic differentiation of chondrocytes. Genes Dev. 2004, 18, 2418–2429. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Kugimiya, F.; Chikuda, H.; Kamekura, S.; Ikeda, T.; Kawamura, N.; Saito, T.; Shinoda, Y.; Higashikawa, A.; Yano, F.; et al. Phosphorylation of GSK-3beta by cGMP-dependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes. J. Clin. Investig. 2008, 118, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Nussenzveig, D.R.; Lewicki, J.A.; Maack, T. Cellular mechanisms of the clearance function of type C receptors of atrial natriuretic factor. J. Biol. Chem. 1990, 265, 20952–20958. [Google Scholar] [CrossRef]
- Anand-Srivastava, M.B.; Sairam, M.R.; Cantin, M. Ring-deleted analogs of atrial natriuretic factor inhibit adenylate cyclase/cAMP system. Possible coupling of clearance atrial natriuretic factor receptors to adenylate cyclase/cAMP signal transduction system. J. Biol. Chem. 1990, 265, 8566–8572. [Google Scholar] [CrossRef]
- Murthy, K.S.; Makhlouf, G.M. Identification of the G protein-activating domain of the natriuretic peptide clearance receptor (NPR-C). J. Biol. Chem. 1999, 274, 17587–17592. [Google Scholar] [CrossRef]
- Murthy, K.S.; Teng, B.Q.; Zhou, H.; Jin, J.G.; Grider, J.R.; Makhlouf, G.M. Gi−1/Gi−2-dependent signaling by single-transmembrane natriuretic peptide clearance receptor. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G974–G980. [Google Scholar] [CrossRef]
- Pagano, M.; Anand-Srivastava, M.B. Cytoplasmic domain of natriuretic peptide receptor C constitutes Gi activator sequences that inhibit adenylyl cyclase activity. J. Biol. Chem. 2001, 276, 22064–22070. [Google Scholar] [CrossRef]
- Sangaralingham, S.J.; McKie, P.M.; Ichiki, T.; Scott, C.G.; Heublein, D.M.; Chen, H.H.; Bailey, K.R.; Redfield, M.M.; Rodeheffer, R.J.; Burnett, J.C. Circulating C-type natriuretic peptide and its relationship to cardiovascular disease in the general population. Hypertension 2015, 65, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.J.; Richards, A.M.; Espiner, E.A.; Nicholls, M.G.; Yandle, T.G. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 1994, 78, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.R.; Heublein, D.M.; Aarhus, L.L.; Lewicki, J.A.; Burnett, J.C. Role of natriuretic peptide clearance receptor in in vivo control of C-type natriuretic peptide. Am. J. Physiol. 1995, 269, H326–H331. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Yasoda, A.; Mori, K.P.; Watanabe-Takano, H.; Nagai-Okatani, C.; Yamashita, Y.; Hirota, K.; Ueda, Y.; Yamauchi, I.; Kondo, E.; et al. Circulating osteocrin stimulates bone growth by limiting C-type natriuretic peptide clearance. J. Clin. Investig. 2017, 127, 4136–4147. [Google Scholar] [CrossRef]
- Miyazaki, T.; Otani, K.; Chiba, A.; Nishimura, H.; Tokudome, T.; Takano-Watanabe, H.; Matsuo, A.; Ishikawa, H.; Shimamoto, K.; Fukui, H.; et al. A New Secretory Peptide of Natriuretic Peptide Family, Osteocrin, Suppresses the Progression of Congestive Heart Failure After Myocardial Infarction. Circ. Res. 2018, 122, 742–751. [Google Scholar] [CrossRef]
- Watanabe-Takano, H.; Ochi, H.; Chiba, A.; Matsuo, A.; Kanai, Y.; Fukuhara, S.; Ito, N.; Sako, K.; Miyazaki, T.; Tainaka, K.; et al. Mechanical load regulates bone growth via periosteal Osteocrin. Cell Rep. 2021, 36, 109380. [Google Scholar] [CrossRef]
- Kenny, A.J.; Bourne, A.; Ingram, J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem. J. 1993, 291 Pt 1, 83–88. [Google Scholar] [CrossRef]
- Brandt, R.R.; Mattingly, M.T.; Clavell, A.L.; Barclay, P.L.; Burnett, J.C. Neutral endopeptidase regulates C-type natriuretic peptide metabolism but does not potentiate its bioactivity in vivo. Hypertension 1997, 30, 184–190. [Google Scholar] [CrossRef]
- Ohbayashi, H.; Yamaki, K.; Suzuki, R.; Kume, H.; Takagi, K. Neutral endopeptidase 3.4.24.11 inhibition potentiates the inhibitory effects of type-C natriuretic peptide on leukotriene D4-induced airway changes. Clin. Exp. Pharmacol. Physiol. 1998, 25, 986–991. [Google Scholar] [CrossRef]
- Márton, Z.; Pataricza, J.; Krassói, I.; Varró, A.; Papp, J.G. NEP inhibitors enhance C-type natriuretic peptide-induced relaxation in porcine isolated coronary artery. Vasc. Pharmacol. 2005, 43, 207–212. [Google Scholar] [CrossRef]
- Hu, P.; Xia, X.; Xuan, Q.; Huang, B.Y.; Liu, S.Y.; Zhang, D.D.; Jiang, G.M.; Xu, Y.; Qin, Y.H. Neutral endopeptidase and natriuretic peptide receptors participate in the regulation of C-type natriuretic peptide expression in renal interstitial fibrosis. J. Recept. Signal Transduct. 2017, 37, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Itoh, H.; Komatsu, Y.; Igaki, T.; Chun, T.H.; Takaya, K.; Yamashita, J.; Inoue, M.; Yoshimasa, T.; Nakao, K. Vascular endothelial growth factor suppresses C-type natriuretic peptide secretion. Hypertension 1996, 27, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Itoh, H.; Suga, S.; Ogawa, Y.; Hama, N.; Kishimoto, I.; Nakagawa, O.; Igaki, T.; Doi, K.; Yoshimasa, T.; et al. Regulation of endothelial production of C-type natriuretic peptide in coculture with vascular smooth muscle cells. Role of the vascular natriuretic peptide system in vascular growth inhibition. Circ. Res. 1996, 78, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.H.; Itoh, H.; Saito, T.; Yamahara, K.; Doi, K.; Mori, Y.; Ogawa, Y.; Yamashita, J.; Tanaka, T.; Inoue, M.; et al. Oxidative stress augments secretion of endothelium-derived relaxing peptides, C-type natriuretic peptide and adrenomedullin. J. Hypertens. 2000, 18, 575–580. [Google Scholar] [CrossRef]
- Yeung, V.T.; Mak, A.S.; Chui, Y.L.; Ho, S.K.; Lai, K.N.; Nicholls, M.G.; Cockram, C.S. Identification of C-type natriuretic peptide gene transcripts in glial cells. Neuroreport 1996, 7, 1709–1712. [Google Scholar] [CrossRef]
- Middendorff, R.; Maronde, E.; Paust, H.J.; Muller, D.; Davidoff, M.; Olcese, J. Expression of C-type natriuretic peptide in the bovine pineal gland. J. Neurochem. 1996, 67, 517–524. [Google Scholar] [CrossRef]
- Middendorff, R.; Paust, H.J.; Davidoff, M.S.; Olcese, J. Synthesis of C-type natriuretic peptide (CNP) by immortalized LHRH cells. J. Neuroendocrinol. 1997, 9, 177–182. [Google Scholar] [CrossRef]
- Yamamoto, S.; Morimoto, I.; Yanagihara, N.; Kangawa, K.; Inenaga, K.; Eto, S.; Yamashita, H. C-type natriuretic peptide suppresses arginine-vasopressin secretion from dissociated magnocellular neurons in newborn rat supraoptic nucleus. Neurosci. Lett. 1997, 229, 97–100. [Google Scholar] [CrossRef]
- Decker, J.M.; Wójtowicz, A.M.; Ul Haq, R.; Braunewell, K.H.; Heinemann, U.; Behrens, C.J. C-type natriuretic peptide decreases hippocampal network oscillations in adult rats in vitro. Neuroscience 2009, 164, 1764–1775. [Google Scholar] [CrossRef]
- Decker, J.M.; Wójtowicz, A.M.; Bartsch, J.C.; Liotta, A.; Braunewell, K.H.; Heinemann, U.; Behrens, C.J. C-type natriuretic peptide modulates bidirectional plasticity in hippocampal area CA1 in vitro. Neuroscience 2010, 169, 8–22. [Google Scholar] [CrossRef]
- Yamada-Goto, N.; Katsuura, G.; Ebihara, K.; Inuzuka, M.; Ochi, Y.; Yamashita, Y.; Kusakabe, T.; Yasoda, A.; Satoh-Asahara, N.; Ariyasu, H.; et al. Intracerebroventricular administration of C-type natriuretic peptide suppresses food intake via activation of the melanocortin system in mice. Diabetes 2013, 62, 1500–1504. [Google Scholar] [CrossRef]
- Fujii, T.; Hirota, K.; Yasoda, A.; Takizawa, A.; Morozumi, N.; Nakamura, R.; Yotsumoto, T.; Kondo, E.; Yamashita, Y.; Sakane, Y.; et al. Rats deficient C-type natriuretic peptide suffer from impaired skeletal growth without early death. PLoS ONE 2018, 13, e0194812. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Furuya, M.; Morozumi, N.; Yoshikiyo, K.; Yotsumoto, T.; Jindo, T.; Nakamura, R.; Murakami, K.; Ueda, Y.; Hanada, T.; et al. Exogenous C-type natriuretic peptide restores normal growth and prevents early growth plate closure in its deficient rats. PLoS ONE 2018, 13, e0204172. [Google Scholar] [CrossRef] [PubMed]
- Bartels, C.F.; Bukulmez, H.; Padayatti, P.; Rhee, D.K.; van Ravenswaaij-Arts, C.; Pauli, R.M.; Mundlos, S.; Chitayat, D.; Shih, L.Y.; Al-Gazali, L.I.; et al. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am. J. Hum. Genet. 2004, 75, 27–34. [Google Scholar] [CrossRef]
- Miura, K.; Namba, N.; Fujiwara, M.; Ohata, Y.; Ishida, H.; Kitaoka, T.; Kubota, T.; Hirai, H.; Higuchi, C.; Tsumaki, N.; et al. An overgrowth disorder associated with excessive production of cGMP due to a gain-of-function mutation of the natriuretic peptide receptor 2 gene. PLoS ONE 2012, 7, e42180. [Google Scholar] [CrossRef]
- Hisado-Oliva, A.; Ruzafa-Martin, A.; Sentchordi, L.; Funari, M.F.A.; Bezanilla-López, C.; Alonso-Bernáldez, M.; Barraza-García, J.; Rodriguez-Zabala, M.; Lerario, A.M.; Benito-Sanz, S.; et al. Mutations in C-natriuretic peptide (NPPC): A novel cause of autosomal dominant short stature. Genet. Med. 2018, 20, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.M.; Heublein, D.M.; Perrella, M.A.; Lerman, A.; Rodeheffer, R.J.; McGregor, C.G.; Edwards, W.D.; Schaff, H.V.; Burnett, J.C. Natriuretic peptide system in human heart failure. Circulation 1993, 88, 1004–1009. [Google Scholar] [CrossRef]
- Del Ry, S.; Cabiati, M.; Vozzi, F.; Battolla, B.; Caselli, C.; Forini, F.; Segnani, C.; Prescimone, T.; Giannessi, D.; Mattii, L. Expression of C-type natriuretic peptide and its receptor NPR-B in cardiomyocytes. Peptides 2011, 32, 1713–1718. [Google Scholar] [CrossRef]
- Del Ry, S. C-type natriuretic peptide: A new cardiac mediator. Peptides 2013, 40, 93–98. [Google Scholar] [CrossRef]
- Minamino, N.; Aburaya, M.; Kojima, M.; Miyamoto, K.; Kangawa, K.; Matsuo, H. Distribution of C-type natriuretic peptide and its messenger RNA in rat central nervous system and peripheral tissue. Biochem. Biophys. Res. Commun. 1993, 197, 326–335. [Google Scholar] [CrossRef]
- Suzuki, R.; Takahashi, A.; Hazon, N.; Takei, Y. Isolation of high-molecular-weight C-type natriuretic peptide from the heart of a cartilaginous fish (European dogfish, Scyliorhinus canicula). FEBS Lett. 1991, 282, 321–325. [Google Scholar] [CrossRef]
- Schofield, J.P.; Jones, D.S.; Forrest, J.N. Identification of C-type natriuretic peptide in heart of spiny dogfish shark (Squalus acanthias). Am. J. Physiol. 1991, 261, F734–F739. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Takahashi, A.; Takei, Y. Different molecular forms of C-type natriuretic peptide isolated from the brain and heart of an elasmobranch, Triakis scyllia. J. Endocrinol. 1992, 135, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A.M.; Gerbes, A.L.; Nemer, M.; Schulz, R. Detection of C-type natriuretic peptide (CNP) transcript in the rat heart and immune organs. Endocrinology 1993, 132, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Öztop, M.; Cinar, K.; Turk, S. Immunolocalization of natriuretic peptides and their receptors in goat (Capra hircus) heart. Biotech. Histochem. 2018, 93, 389–404. [Google Scholar] [CrossRef]
- Takahashi, T.; Allen, P.D.; Izumo, S. Expression of A-, B-, and C-type natriuretic peptide genes in failing and developing human ventricles. Correlation with expression of the Ca(2+)-ATPase gene. Circ. Res. 1992, 71, 9–17. [Google Scholar] [CrossRef]
- Kalra, P.R.; Clague, J.R.; Bolger, A.P.; Anker, S.D.; Poole-Wilson, P.A.; Struthers, A.D.; Coats, A.J. Myocardial production of C-type natriuretic peptide in chronic heart failure. Circulation 2003, 107, 571–573. [Google Scholar] [CrossRef]
- Wright, S.P.; Prickett, T.C.; Doughty, R.N.; Frampton, C.; Gamble, G.D.; Yandle, T.G.; Sharpe, N.; Richards, M. Amino-terminal pro-C-type natriuretic peptide in heart failure. Hypertension 2004, 43, 94–100. [Google Scholar] [CrossRef][Green Version]
- Del Ry, S.; Passino, C.; Maltinti, M.; Emdin, M.; Giannessi, D. C-type natriuretic peptide plasma levels increase in patients with chronic heart failure as a function of clinical severity. Eur. J. Heart Fail. 2005, 7, 1145–1148. [Google Scholar] [CrossRef]
- Del Ry, S.; Cabiati, M.; Lionetti, V.; Emdin, M.; Recchia, F.A.; Giannessi, D. Expression of C-type natriuretic peptide and of its receptor NPR-B in normal and failing heart. Peptides 2008, 29, 2208–2215. [Google Scholar] [CrossRef]
- Palmer, S.C.; Prickett, T.C.; Espiner, E.A.; Yandle, T.G.; Richards, A.M. Regional release and clearance of C-type natriuretic peptides in the human circulation and relation to cardiac function. Hypertension 2009, 54, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hänze, J.; Heese, F.; Sodmann, R.; Lang, R.E. Gene expression of natriuretic peptide receptors in myocardial cells. Circ. Res. 1995, 77, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.D.; Upshaw-Earley, J.; Bell, E.L.; Palfrey, H.C. Natriuretic peptide receptor-B in adult rat ventricle is predominantly confined to the nonmyocyte population. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2117–H2123. [Google Scholar] [CrossRef] [PubMed]
- Dickey, D.M.; Dries, D.L.; Margulies, K.B.; Potter, L.R. Guanylyl cyclase (GC)-A and GC-B activities in ventricles and cardiomyocytes from failed and non-failed human hearts: GC-A is inactive in the failed cardiomyocyte. J. Mol. Cell. Cardiol. 2012, 52, 727–732. [Google Scholar] [CrossRef]
- Nakamura, T.; Tsujita, K. Current trends and future perspectives for heart failure treatment leveraging cGMP modifiers and the practical effector PKG. J. Cardiol. 2021, 78, 261–268. [Google Scholar] [CrossRef]
- Dorn, G.W.; Force, T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Investig. 2005, 115, 527–537. [Google Scholar] [CrossRef]
- Fiedler, B.; Lohmann, S.M.; Smolenski, A.; Linnemuller, S.; Pieske, B.; Schroder, F.; Molkentin, J.D.; Drexler, H.; Wollert, K.C. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 11363–11368. [Google Scholar] [CrossRef]
- Kuwahara, K.; Wang, Y.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Hill, J.A.; Olson, E.N. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Investig. 2006, 116, 3114–3126. [Google Scholar] [CrossRef]
- Moltzau, L.R.; Meier, S.; Aronsen, J.M.; Afzal, F.; Sjaastad, I.; Skomedal, T.; Osnes, J.B.; Levy, F.O.; Qvigstad, E. Differential regulation of C-type natriuretic peptide-induced cGMP and functional responses by PDE2 and PDE3 in failing myocardium. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 407–417. [Google Scholar] [CrossRef]
- Meier, S.; Andressen, K.W.; Aronsen, J.M.; Sjaastad, I.; Hougen, K.; Skomedal, T.; Osnes, J.B.; Qvigstad, E.; Levy, F.O.; Moltzau, L.R. PDE3 inhibition by C-type natriuretic peptide-induced cGMP enhances cAMP-mediated signaling in both non-failing and failing hearts. Eur. J. Pharmacol. 2017, 812, 174–183. [Google Scholar] [CrossRef]
- Burley, D.S.; Cox, C.D.; Zhang, J.; Wann, K.T.; Baxter, G.F. Natriuretic peptides modulate ATP-sensitive K+ channels in rat ventricular cardiomyocytes. Basic Res. Cardiol. 2014, 109, 402. [Google Scholar] [CrossRef] [PubMed]
- Moyes, A.J.; Chu, S.M.; Aubdool, A.A.; Dukinfield, M.S.; Margulies, K.B.; Bedi, K.C.; Hodivala-Dilke, K.; Baliga, R.S.; Hobbs, A.J. C-type natriuretic peptide co-ordinates cardiac structure and function. Eur. Heart J. 2020, 41, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Dickey, D.M.; Flora, D.R.; Bryan, P.M.; Xu, X.; Chen, Y.; Potter, L.R. Differential regulation of membrane guanylyl cyclases in congestive heart failure: Natriuretic peptide receptor (NPR)-B, Not NPR-A, is the predominant natriuretic peptide receptor in the failing heart. Endocrinology 2007, 148, 3518–3522. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Houchi, H.; Tsuchiya, K.; Minakuchi, K.; Horike, K.; Kitagawa, T.; Katoh, I.; Tamaki, T. Atrial natriuretic peptide stimulates Na+-dependent Ca2+ efflux from freshly isolated adult rat cardiomyocytes. FEBS Lett. 1997, 419, 255–258. [Google Scholar] [CrossRef]
- Brusq, J.M.; Mayoux, E.; Guigui, L.; Kirilovsky, J. Effects of C-type natriuretic peptide on rat cardiac contractility. Br. J. Pharmacol. 1999, 128, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Nir, A.; Zhang, D.F.; Fixler, R.; Burnett, J.C.; Eilam, Y.; Hasin, Y. C-type natriuretic peptide has a negative inotropic effect on cardiac myocytes. Eur. J. Pharmacol. 2001, 412, 195–201. [Google Scholar] [CrossRef]
- Fixler, R.; Hasin, Y.; Eilam, Y.; Zhang, D.F.; Nir, A. Opposing effects of endothelin-1 on C-type natriuretic peptide actions in rat cardiomyocytes. Eur. J. Pharmacol. 2001, 423, 95–98. [Google Scholar] [CrossRef]
- Han, B.; Fixler, R.; Beeri, R.; Wang, Y.; Bachrach, U.; Hasin, Y. The opposing effects of endothelin-1 and C-type natriuretic peptide on apoptosis of neonatal rat cardiac myocytes. Eur. J. Pharmacol. 2003, 474, 15–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Moalem, J.; Tse, J.; Scholz, P.M.; Weiss, H.R. Effects of natriuretic peptides on ventricular myocyte contraction and role of cyclic GMP signaling. Eur. J. Pharmacol. 2005, 510, 209–215. [Google Scholar] [CrossRef]
- Moalem, J.; Davidov, T.; Zhang, Q.; Grover, G.J.; Weiss, H.R.; Scholz, P.M. Negative inotropic effects of C-type natriuretic peptide are attenuated in hypertrophied ventricular myocytes associated with reduced cyclic GMP production. J. Surg. Res. 2006, 135, 38–44. [Google Scholar] [CrossRef]
- Subramanian, H.; Froese, A.; Jönsson, P.; Schmidt, H.; Gorelik, J.; Nikolaev, V.O. Distinct submembrane localisation compartmentalises cardiac NPR1 and NPR2 signalling to cGMP. Nat. Commun. 2018, 9, 2446. [Google Scholar] [CrossRef] [PubMed]
- Manfra, O.; Calamera, G.; Froese, A.; Arunthavarajah, D.; Surdo, N.C.; Meier, S.; Melleby, A.O.; Aasrum, M.; Aronsen, J.M.; Nikolaev, V.O.; et al. CNP regulates cardiac contractility and increases cGMP near both SERCA and TnI—Difference from BNP visualized by targeted cGMP biosensors. Cardiovasc. Res. 2022, 118, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Yurukova, S.; Kilic, A.; Begrow, F.; Fiedler, B.; Gambaryan, S.; Walter, U.; Lohmann, S.M.; Kuhn, M. Increased effects of C-type natriuretic peptide on contractility and calcium regulation in murine hearts overexpressing cyclic GMP-dependent protein kinase I. Br. J. Pharmacol. 2003, 140, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Scholz, P.M.; Pilzak, A.; Su, J.; Weiss, H.R. Role of phospholamban in cyclic GMP mediated signaling in cardiac myocytes. Cell Physiol. Biochem. 2007, 20, 157–166. [Google Scholar] [CrossRef]
- Horio, T.; Tokudome, T.; Maki, T.; Yoshihara, F.; Suga, S.; Nishikimi, T.; Kojima, M.; Kawano, Y.; Kangawa, K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology 2003, 144, 2279–2284. [Google Scholar] [CrossRef]
- Del Ry, S.; Maltinti, M.; Piacenti, M.; Passino, C.; Emdin, M.; Giannessi, D. Cardiac production of C-type natriuretic peptide in heart failure. J. Cardiovasc. Med. 2006, 7, 397–399. [Google Scholar] [CrossRef]
- Lok, D.J.; Klip, I.T.; Voors, A.A.; Lok, S.I.; Bruggink-André de la Porte, P.W.; Hillege, H.L.; Jaarsma, T.; van Veldhuisen, D.J.; van der Meer, P. Prognostic value of N-terminal pro C-type natriuretic peptide in heart failure patients with preserved and reduced ejection fraction. Eur. J. Heart Fail. 2014, 16, 958–966. [Google Scholar] [CrossRef]
- Qvigstad, E.; Moltzau, L.R.; Aronsen, J.M.; Nguyen, C.H.; Hougen, K.; Sjaastad, I.; Levy, F.O.; Skomedal, T.; Osnes, J.B. Natriuretic peptides increase beta1-adrenoceptor signalling in failing hearts through phosphodiesterase 3 inhibition. Cardiovasc. Res. 2010, 85, 763–772. [Google Scholar] [CrossRef]
- Moltzau, L.R.; Aronsen, J.M.; Meier, S.; Nguyen, C.H.; Hougen, K.; Ørstavik, Ø.; Sjaastad, I.; Christensen, G.; Skomedal, T.; Osnes, J.B.; et al. SERCA2 activity is involved in the CNP-mediated functional responses in failing rat myocardium. Br. J. Pharmacol. 2013, 170, 366–379. [Google Scholar] [CrossRef]
- Michel, K.; Herwig, M.; Werner, F.; Špiranec Spes, K.; Abeßer, M.; Schuh, K.; Dabral, S.; Mügge, A.; Baba, H.A.; Skryabin, B.V.; et al. C-type natriuretic peptide moderates titin-based cardiomyocyte stiffness. JCI Insight 2020, 5, e139910. [Google Scholar] [CrossRef]
- Tokudome, T.; Horio, T.; Soeki, T.; Mori, K.; Kishimoto, I.; Suga, S.; Yoshihara, F.; Kawano, Y.; Kohno, M.; Kangawa, K. Inhibitory effect of C-type natriuretic peptide (CNP) on cultured cardiac myocyte hypertrophy: Interference between CNP and endothelin-1 signaling pathways. Endocrinology 2004, 145, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Soeki, T.; Kishimoto, I.; Okumura, H.; Tokudome, T.; Horio, T.; Mori, K.; Kangawa, K. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Araki, S.; Usuku, H.; Rokutanda, T.; Hanatani, S.; Ogawa, H. Chronic C-Type Natriuretic Peptide Infusion Attenuates Angiotensin II-Induced Myocardial Superoxide Production and Cardiac Remodeling. Int. J. Vasc. Med. 2012, 2012, 246058. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, Q.; Moalem, J.; Tse, J.; Scholz, P.M.; Weiss, H.R. Functional effects of C-type natriuretic peptide and nitric oxide are attenuated in hypertrophic myocytes from pressure-overloaded mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1367–H1373. [Google Scholar] [CrossRef][Green Version]
- Langenickel, T.H.; Buttgereit, J.; Pagel-Langenickel, I.; Lindner, M.; Monti, J.; Beuerlein, K.; Al-Saadi, N.; Plehm, R.; Popova, E.; Tank, J.; et al. Cardiac hypertrophy in transgenic rats expressing a dominant-negative mutant of the natriuretic peptide receptor B. Proc. Natl. Acad. Sci. USA 2006, 103, 4735–4740. [Google Scholar] [CrossRef]
- Nakao, K.; Kuwahara, K.; Nishikimi, T.; Nakagawa, Y.; Kinoshita, H.; Minami, T.; Kuwabara, Y.; Yamada, C.; Yamada, Y.; Tokudome, T.; et al. Endothelium-Derived C-Type Natriuretic Peptide Contributes to Blood Pressure Regulation by Maintaining Endothelial Integrity. Hypertension 2017, 69, 286–296. [Google Scholar] [CrossRef]
- Moyes, A.J.; Khambata, R.S.; Villar, I.; Bubb, K.J.; Baliga, R.S.; Lumsden, N.G.; Xiao, F.; Gane, P.J.; Rebstock, A.S.; Worthington, R.J.; et al. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J. Clin. Investig. 2014, 124, 4039–4051. [Google Scholar] [CrossRef]
- Hobbs, A.; Foster, P.; Prescott, C.; Scotland, R.; Ahluwalia, A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: Novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation 2004, 110, 1231–1235. [Google Scholar] [CrossRef]
- Wang, Y.; de Waard, M.C.; Sterner-Kock, A.; Stepan, H.; Schultheiss, H.P.; Duncker, D.J.; Walther, T. Cardiomyocyte-restricted over-expression of C-type natriuretic peptide prevents cardiac hypertrophy induced by myocardial infarction in mice. Eur. J. Heart Fail. 2007, 9, 548–557. [Google Scholar] [CrossRef]
- Del Ry, S.; Cabiati, M.; Martino, A.; Cavallini, C.; Caselli, C.; Aquaro, G.D.; Battolla, B.; Prescimone, T.; Giannessi, D.; Mattii, L.; et al. High concentration of C-type natriuretic peptide promotes VEGF-dependent vasculogenesis in the remodeled region of infarcted swine heart with preserved left ventricular ejection fraction. Int. J. Cardiol. 2013, 168, 2426–2434. [Google Scholar] [CrossRef]
- Frankenreiter, S.; Bednarczyk, P.; Kniess, A.; Bork, N.I.; Straubinger, J.; Koprowski, P.; Wrzosek, A.; Mohr, E.; Logan, A.; Murphy, M.P.; et al. cGMP-Elevating Compounds and Ischemic Conditioning Provide Cardioprotection Against Ischemia and Reperfusion Injury via Cardiomyocyte-Specific BK Channels. Circulation 2017, 136, 2337–2355. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, R.; Cruz Santos, M.; Kuret, A.; Ruth, P. cGMP and mitochondrial K+ channels—Compartmentalized but closely connected in cardioprotection. Br. J. Pharmacol. 2021, 179, 2344–2360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Than, M.; Aldous, S.; Troughton, R.; Richards, M.; Pemberton, C.J. CNP Signal Peptide in Patients with Cardiovascular Disease. Front. Cardiovasc. Med. 2015, 2, 28. [Google Scholar] [CrossRef][Green Version]
- Prickett, T.C.; Doughty, R.N.; Troughton, R.W.; Frampton, C.M.; Whalley, G.A.; Ellis, C.J.; Espiner, E.A.; Richards, A.M. C-Type Natriuretic Peptides in Coronary Disease. Clin. Chem. 2017, 63, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.D.; Frydland, M.; Helgestad, O.K.L.; Holmvang, L.; Møller, J.E.; Johansson, P.I.; Ostrowski, S.R.; Prickett, T.; Hassager, C.; Goetze, J.P. Sex-specific mortality prediction by pro-C-type natriuretic peptide measurement in a prospective cohort of patients with ST-elevation myocardial infarction. BMJ. Open 2021, 11, e048312. [Google Scholar] [CrossRef]
- Beaulieu, P.; Cardinal, R.; De Léan, A.; Lambert, C. Direct chronotropic effects of atrial and C-type natriuretic peptides in anaesthetized dogs. Br. J. Pharmacol. 1996, 118, 1790–1796. [Google Scholar] [CrossRef]
- Beaulieu, P.; Cardinal, R.; Pagé, P.; Francoeur, F.; Tremblay, J.; Lambert, C. Positive chronotropic and inotropic effects of C-type natriuretic peptide in dogs. Am. J. Physiol. 1997, 273, H1933–H1940. [Google Scholar] [CrossRef]
- Rose, R.A.; Lomax, A.E.; Kondo, C.S.; Anand-Srivastava, M.B.; Giles, W.R. Effects of C-type natriuretic peptide on ionic currents in mouse sinoatrial node: A role for the NPR-C receptor. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1970–H1977. [Google Scholar] [CrossRef][Green Version]
- Springer, J.; Azer, J.; Hua, R.; Robbins, C.; Adamczyk, A.; McBoyle, S.; Bissell, M.B.; Rose, R.A. The natriuretic peptides BNP and CNP increase heart rate and electrical conduction by stimulating ionic currents in the sinoatrial node and atrial myocardium following activation of guanylyl cyclase-linked natriuretic peptide receptors. J. Mol. Cell. Cardiol. 2012, 52, 1122–1134. [Google Scholar] [CrossRef]
- Azer, J.; Hua, R.; Vella, K.; Rose, R.A. Natriuretic peptides regulate heart rate and sinoatrial node function by activating multiple natriuretic peptide receptors. J. Mol. Cell. Cardiol. 2012, 53, 715–724. [Google Scholar] [CrossRef]
- Dorey, T.W.; Mackasey, M.; Jansen, H.J.; McRae, M.D.; Bohne, L.J.; Liu, Y.; Belke, D.D.; Atkinson, L.; Rose, R.A. Natriuretic peptide receptor B maintains heart rate and sinoatrial node function via cyclic GMP-mediated signaling. Cardiovasc. Res. 2021, cvab245. [Google Scholar] [CrossRef] [PubMed]
- Szaroszyk, M.; Kattih, B.; Martin-Garrido, A.; Trogisch, F.A.; Dittrich, G.M.; Grund, A.; Abouissa, A.; Derlin, K.; Meier, M.; Holler, T.; et al. Skeletal muscle derived MusClin. protects the heart during pathological overload. Nat. Commun. 2022, 13, 149. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).