Morphological and Molecular Characterization, and Demonstration of a Definitive Host, for Sarcocystis masoni from an Alpaca (Vicugna pacos) in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Examination for Sarcocysts in Alpaca
2.2. Experimental Infection of Potential Definitive Hosts
2.3. Molecular Characterization
3. Results
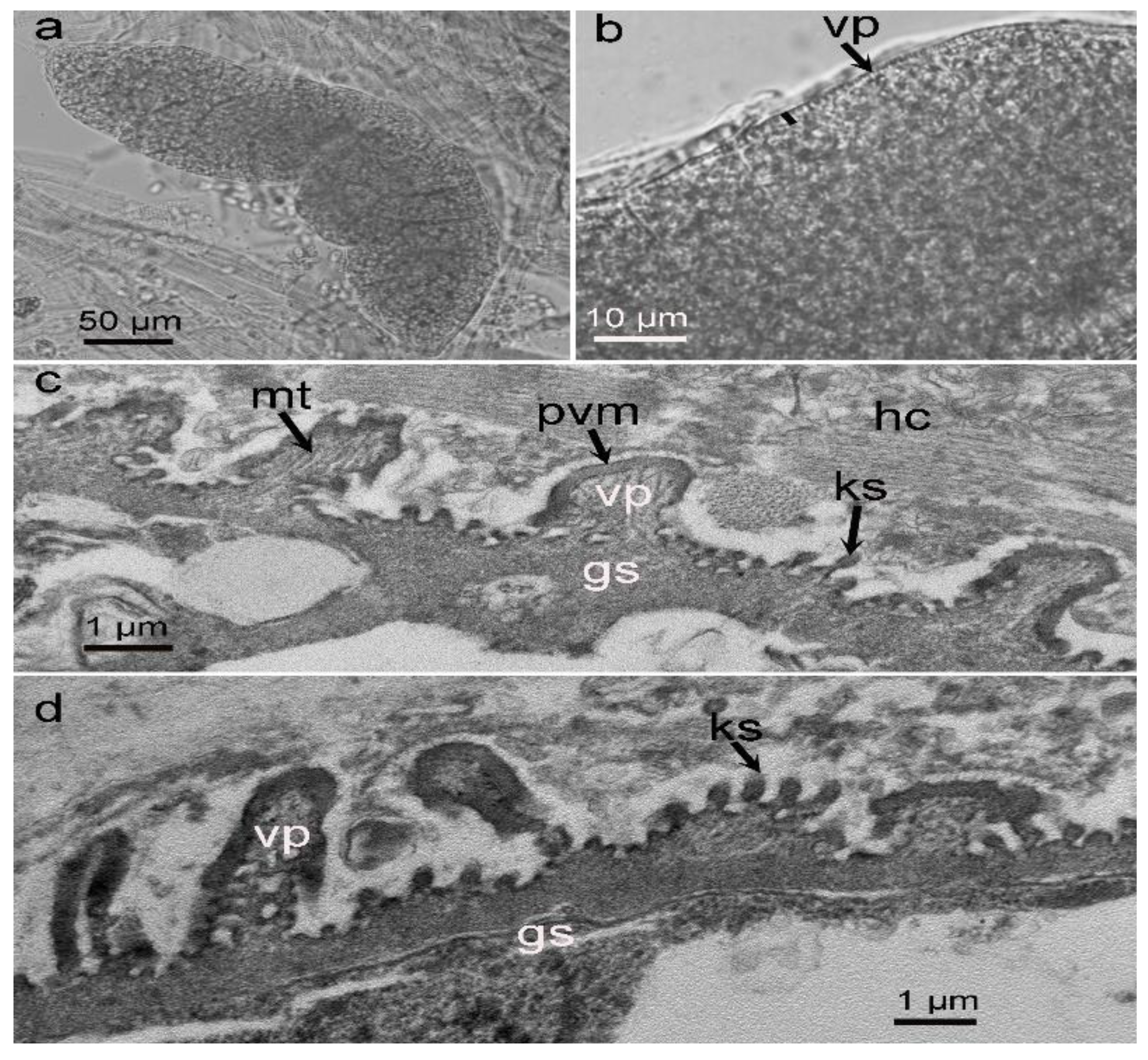
3.1. Morphological Observation of Sarcocysts in the Alpaca
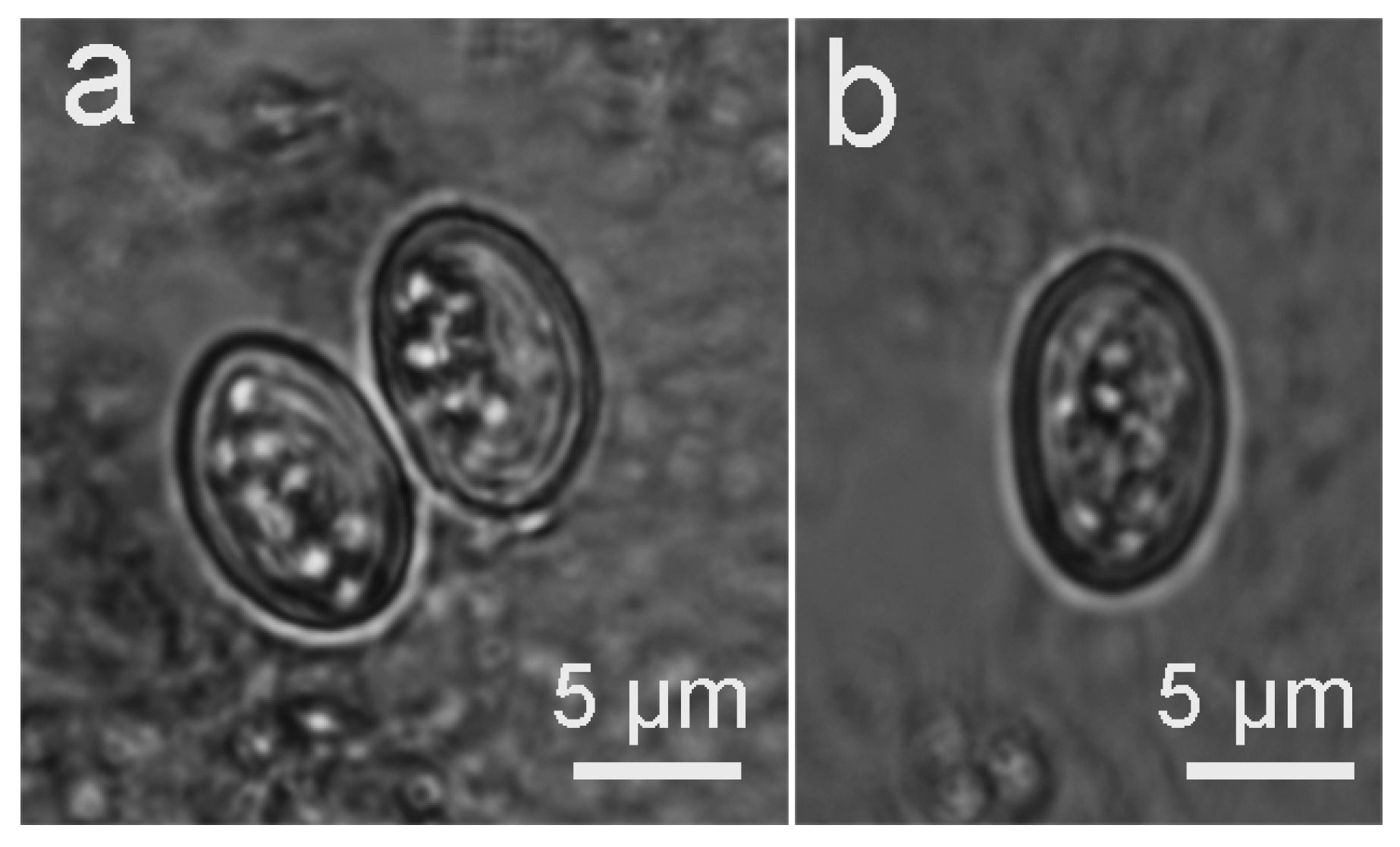
3.2. Infection of the Definitive Hosts
3.3. Molecular Analysis
3.4. PCR-RFLP Based on Mitochondrial cox1 Obtained from S. masoni Sarcocysts and Oocysts
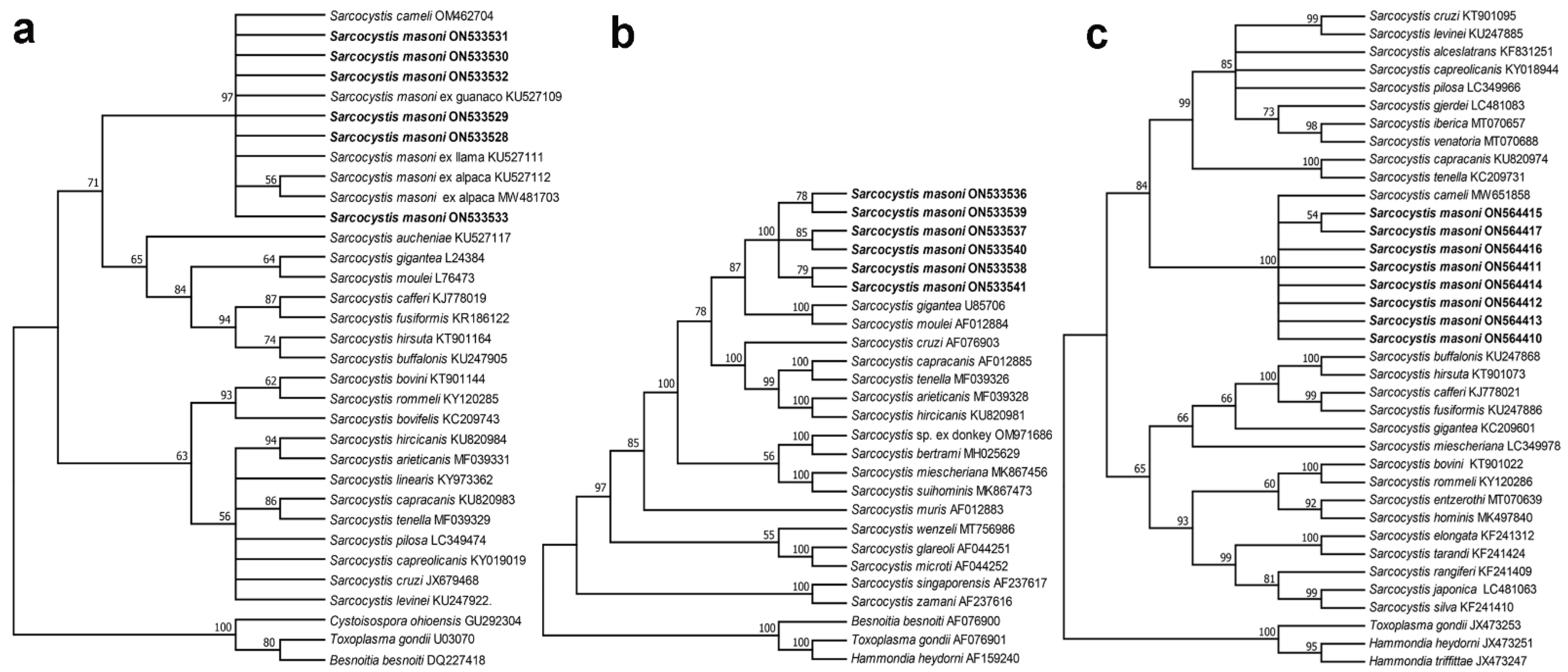
3.5. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 257–267. [Google Scholar]
- Reyna, J. Alpaca breeding in Peru and perspectives for the future. N. Z. Alpacas. 2005, 18, 21. [Google Scholar]
- She, Q.; Zhang, H.; Lou, P.; Ji, X.; Zhao, Z. Current situation and development countermeasures of alpaca Breeding. Agric. Sci. Technol. 2011, 12, 733–736. [Google Scholar]
- La Perle, K.M.; Silveria, F.; Anderson, D.E.; Blomme, E.A. Dalmeny disease in an alpaca (Lama pacos): Sarcocystosis, eosinophilic myositis and abortion. J. Comp. Pathol. 1999, 121, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Chávez, V.; Leyva, V.; Panez, L.; Ticona, S.; García, V.; Pezo, C. Sarcocystiosis and productive efficiency of the alpaca. Rev. Investig. Vet. del Perú. 2008, 19, 160–167. [Google Scholar]
- Moré, G.; Regensburger, C.; Gos, M.L.; Pardini, L.; Verma, S.K.; Ctibor, J.; Serrano-Martínez, M.E.; Dubey, J.P.; Venturini, M.C. Sarcocystis masoni, n. sp. (Apicomplexa: Sarcocystidae), and redescription of Sarcocystis aucheniae from llama (Lama glama), guanaco (Lama guanicoe) and alpaca (Vicugna pacos). Parasitology 2016, 143, 617–626. [Google Scholar] [CrossRef]
- Saeed, M.A.; Rashid, M.H.; Vaughan, J.; Jabbar, A. Sarcocystosis in South American camelids: The state of play revisited. Parasit. Vectors 2018, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Brumpt, E. Précis de Parasitologie, 2nd ed.; Masson et Cie: Paris, France, 1913. [Google Scholar]
- Leguía, G. The epidemiology and economic impact of llama parasites. Parasitol. Today 1991, 7, 54–56. [Google Scholar] [CrossRef]
- Lucas, J.R. Sarcocystis spp. in Peru. Peruv. J. Parasitol. 2012, 20, e64e73. [Google Scholar]
- Gorman, T.R.; Alcaíno, H.A.; Muñoz, H.; Cunazza, C. Sarcocystis sp. in guanaco (Lama guanicoe) and effect of temperature on its viability. Vet. Parasitol. 1984, 15, 95–101. [Google Scholar] [CrossRef]
- Schnieder, T.; Kaup, F.J.; Drommer, W.; Thiel, W.; Rommel, M. Fine structure and development of Sarcocystis aucheniae in llamas. Z. Parasitenkd. 1984, 70, 451–458. [Google Scholar] [CrossRef]
- Gjerde, B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013, 43, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Sarcocystis species in red deer revisited: With a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. Sp. based on mitochondrial cox1 sequences. Parasitology 2014, 141, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Huang, S.; Wen, T.; Esch, G.W.; Liang, Y.; Li, H.L. Sarcocystis spp. in domestic sheep in Kunming City, China: Prevalence, morphology, and molecular characteristics. Parasite 2017, 24, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barta, J.R.; Martin, D.S.; Liberator, P.A.; Dashkevicz, M.; Anderson, J.W.; Feighner, S.D.; Elbrecht, A.; Perkins-Barrow, A.; Jenkins, M.C.; Danforth, H.D.; et al. Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 1997, 83, 262–271. [Google Scholar] [CrossRef]
- Fischer, S.; Odening, K. Characterization of bovine Sarcocystis species by analysis of their 18S ribosomal DNA sequences. J. Parasitol. 1998, 84, 50–54. [Google Scholar] [CrossRef]
- Fenger, C.K.; Granstrom, D.E.; Langemeier, J.L.; Stamper, S.; Donahue, J.M.; Patterson, J.S.; Gajadhar, A.A.; Marteniuk, J.V.; Xiaomin, Z.; Dubey, J.P. Identification of opossums (Didelphis virginiana) as the putative definitive host of Sarcocystis neurona. J. Parasitol. 1995, 81, 916–919. [Google Scholar] [CrossRef]
- Mugridge, N.B.; Morrison, D.A.; Johnson, A.M.; Luton, K.; Dubey, J.P.; Votýpka, J.; Tenter, A.M. Phylogenetic relationships of the genus Frenkelia: A review of its history and new knowledge gained from comparison of large subunit ribosomal ribonucleic acid gene sequences. Int. J. Parasitol. 1999, 29, 957–972. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, T.T.; Liu, Q.; Esch, G.W.; Chen, J.Q.; Huang, S.; Wen, T. Prevalence, morphology, and molecular characteristics of Sarcocystis spp. in domestic goats (Capra hircus) from Kunming, China. Parasitol. Res. 2016, 115, 3973–3981. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jiang, N.; Xin, S.; Zhu, N.; Yang, L.; Huang, W.; Hu, J.; Zhu, X.; Yang, Y. First report of Sarcocystis masoni in a captive alpaca (Vicugna pacos) from China. Front. Vet. Sci. 2021, 8, 759252. [Google Scholar] [CrossRef]
- Rooney, A.L.; Limon, G.; Vides, H.; Cortez, A.; Guitian, J. Sarcocystis spp. in llamas (Lama glama) in Southern Bolivia: A cross sectional study of the prevalence, risk factors and loss in income caused by carcass downgrades. Prev. Vet. Med. 2014, 116, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, C.; Gos, M.L.; Ctibor, J.; Moré, G. Morphological and molecular characteristics of Sarcocystis aucheniae isolated from meat of guanaco (Lama guanicoe). J. Food Qual. Hazards Control 2015, 2, 118–121. [Google Scholar]
- Lucas, J.R.; Barrios-Arpi, M.; Rodríguez, J.; Balcázarnakamatsu, S.; Zarria, J.; Namiyama, G.; Taniwaki, N.; Gonzales-Viera, O. Ultrastructural description of Sarcocystis sp. in cardiac muscle of naturally infected alpacas (Vicugna pacos). Iran. J. Parasitol. 2019, 14, 174–179. [Google Scholar] [PubMed]
- Abdel-Ghaffar, F.; Mehlhorn, H.; Bashtar, A.R.; Al-Rasheid, K.; Sakran, T.; El-Fayoumi, H. Life cycle of Sarcocystis camelicanis infecting the camel (Camelus dromedarius) and the dog (Canis familiaris), light and electron microscopic study. Parasitol. Res. 2009, 106, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Hilali, M.; Van Wilpe, E.; Calero-Bernal, R.; Verma, S.K.; Abbas, I.E. A review of sarcocystosis in camels and redescription of Sarcocystis cameli and Sarcocystis ippeni sarcocysts from the one-humped camel (Camelus dromedarius). Parasitology 2015, 142, 1481–1492. [Google Scholar] [CrossRef] [Green Version]

| DNA Region | Primer Name | Primer Sequence (5′–3′) | References |
|---|---|---|---|
| 18S rDNA | ERIB1 a | ACCTGGTTGATCCTGCCAG | [16] |
| S2 b | CTGATCGTCTTCGAGCCCCTA | [17] | |
| S3 a | TTGTTAAAGACGAACTACTGCG | [17] | |
| B b | GATCCTTCTGCAGGTTCACCTAC | [18] | |
| 28S rDNA | KL1 a | TACCCGCTGAACTTAAGC | [19] |
| KL3 b | CCACCAAGATCTGCACTAG | [19] | |
| KL6a a | GGATTGGCTCTGAGGG | [19] | |
| KL2 b | ACTTAGAGGCGTTCAGTC | [19] | |
| KL4 a | AGCAGGACGGTGGTCATG | [19] | |
| KL5b b | CTCAAGCTCAACAGGGTC | [19] | |
| ITS | ITSF a | GGAATGGAAAGTTTTGTGA | This study |
| ITSR b | TTTCTTCTCCTCCGCTTA | This study | |
| cox1 | SF1 a | ATGGCGTACAACAATCATAAAGAA | [13] |
| SR9 b | ATATCCATACCRCCATTGCCCAT | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Sun, J.; Hu, J.; Song, J.; Deng, S.; Zhu, N.; Yang, Y.; Tao, J. Morphological and Molecular Characterization, and Demonstration of a Definitive Host, for Sarcocystis masoni from an Alpaca (Vicugna pacos) in China. Biology 2022, 11, 1016. https://doi.org/10.3390/biology11071016
Wu Z, Sun J, Hu J, Song J, Deng S, Zhu N, Yang Y, Tao J. Morphological and Molecular Characterization, and Demonstration of a Definitive Host, for Sarcocystis masoni from an Alpaca (Vicugna pacos) in China. Biology. 2022; 11(7):1016. https://doi.org/10.3390/biology11071016
Chicago/Turabian StyleWu, Zhipeng, Jun Sun, Junjie Hu, Jingling Song, Shuangsheng Deng, Niuping Zhu, Yurong Yang, and Jianping Tao. 2022. "Morphological and Molecular Characterization, and Demonstration of a Definitive Host, for Sarcocystis masoni from an Alpaca (Vicugna pacos) in China" Biology 11, no. 7: 1016. https://doi.org/10.3390/biology11071016
APA StyleWu, Z., Sun, J., Hu, J., Song, J., Deng, S., Zhu, N., Yang, Y., & Tao, J. (2022). Morphological and Molecular Characterization, and Demonstration of a Definitive Host, for Sarcocystis masoni from an Alpaca (Vicugna pacos) in China. Biology, 11(7), 1016. https://doi.org/10.3390/biology11071016







