Simple Summary
Many people are fascinated by beautiful flowers. Botanists have been intensively studying flowers to decipher their history. Various hypotheses have been advanced to account for the evolution of flowers. Unfortunately, most hypotheses are based on the understanding of modern flowers. Magnolia and related plants were once thought to bear the greatest resemblance to ancestral flowers. Naturally, botanists have been frequently surprised by new finds in the past century. A new flower named Lingyuananthus from the Early Cretaceous (about 125 million years ago) in China is the latest surprise for botanists. Distinct from all previously reported fossil flowers of similar ages, Lingyuananthus has an inferior ovary, syncarpy, and hypanthium. All three features were thought to be highly derived in angiosperms. Considering its Early Cretaceous age (the currently widely accepted earliest age of angiosperms and flowers), Lingyuananthus is a black swan that illustrates how astray botanists may have gone previously. Unable to account for the fossil flower diversity in the Early Cretaceous, botanical theories face a crisis. Among existing hypotheses, Zimmermann’s hypothesis, a long-time unfavored hypothesis, stands out like a black swan: it provides a better explanation for the origin and evolution of Lingyuananthus.
Abstract
Background: The origin and early evolution of angiosperms, by far the most important plant group for human beings, are questions demanding answers, mainly due to a lack of related fossils. The Yixian Formation (Lower Cretaceous) is famous for its fossils of early angiosperms, and several Early Cretaceous angiosperms with apocarpous gynoecia have been documented. However, a hypanthium and an inferior ovary are lacking in these fossil angiosperms. Methods: The specimen was collected from the outcrop of the Yixian Formation in Dawangzhangzi in the suburb of Lingyuan, Liaoning, China. The specimen was photographed using a Nikon D200 digital camera, and its details were photographed using a Nikon SMZ1500 stereomicroscope and a MAIA3 TESCAN SEM. Results: A fossil angiosperm, Lingyuananthus inexpectus gen. et sp. nov, is reported from the Lower Cretaceous of China. Differing from those documented previously, Lingyuananthus has a hypanthium, an inferior ovary, and ovules inside its ovary. Such a character assemblage indicates its angiospermous affinity, although not expected by any existing leading angiosperm evolutionary theory. Conclusions: New fossil material with a unique character assemblage falls beyond the expectation of the currently widely accepted theories of angiosperm evolution. Together with independently documented fossils of early angiosperms, Lingyuananthus suggests that at least some early angiosperms’ flowers can be derived in a way that has been ignored previously.
1. Introduction
The Early Cretaceous is currently the widely accepted earliest age for angiosperms, and the Yixian Formation, which is famous for its abundant fossil angiosperms [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16], belongs to the Lower Cretaceous (Barremian to Aptian, approximately 125 Ma) [17]. Theoretically, conduplicate carpels and their derivatives were thought to be ancestral among angiosperms [18]. This concept used to be widely accepted and frequently taught in classrooms. Although supported by some early angiosperms [2,3,4,5,8,10], this hypothesis is now facing increasing challenges from fossil evidence [1,7,11,14,19,20,21,22,23] and molecular studies [24,25,26,27,28,29]. Furthermore, a strong challenge is from the early Jurassic Nanjinganthus [21,22,30], which shows syncarpy and an inferior ovary, both unexpected for the assumed basalmost angiosperms [18]. Actually, Nanjinganthus is not peerless in challenging the traditional theories. Monetianthus Friis et al. 2009, a fossil representative of Nymphaeaceae [31], also has an inferior ovary and syncarpy. Other than Nanjinganthus and Monetianthus, fossil evidence of early angiosperms has raised similar challenges [1,6,9,11,12,14,15,20,32]. Here, I report a new angiosperm, Lingyuananthus inexpectus gen. et sp. nov, from the Yixian Formation (Lower Cretaceous, Barremian–Aptian) of Lingyuan, Liaoning, China. Distinct from early angiosperms previously reported from the formation, Lingyuananthus is a flower with an inferior ovary and multiple linear tepals arranged on the edge of the hypanthium. This not only adds to the great diversity of angiosperms in the formation but also joins Nanjinganthus and Monetianthus in demanding attention to a novel way to derive flowers in angiosperms.
2. Materials and Methods
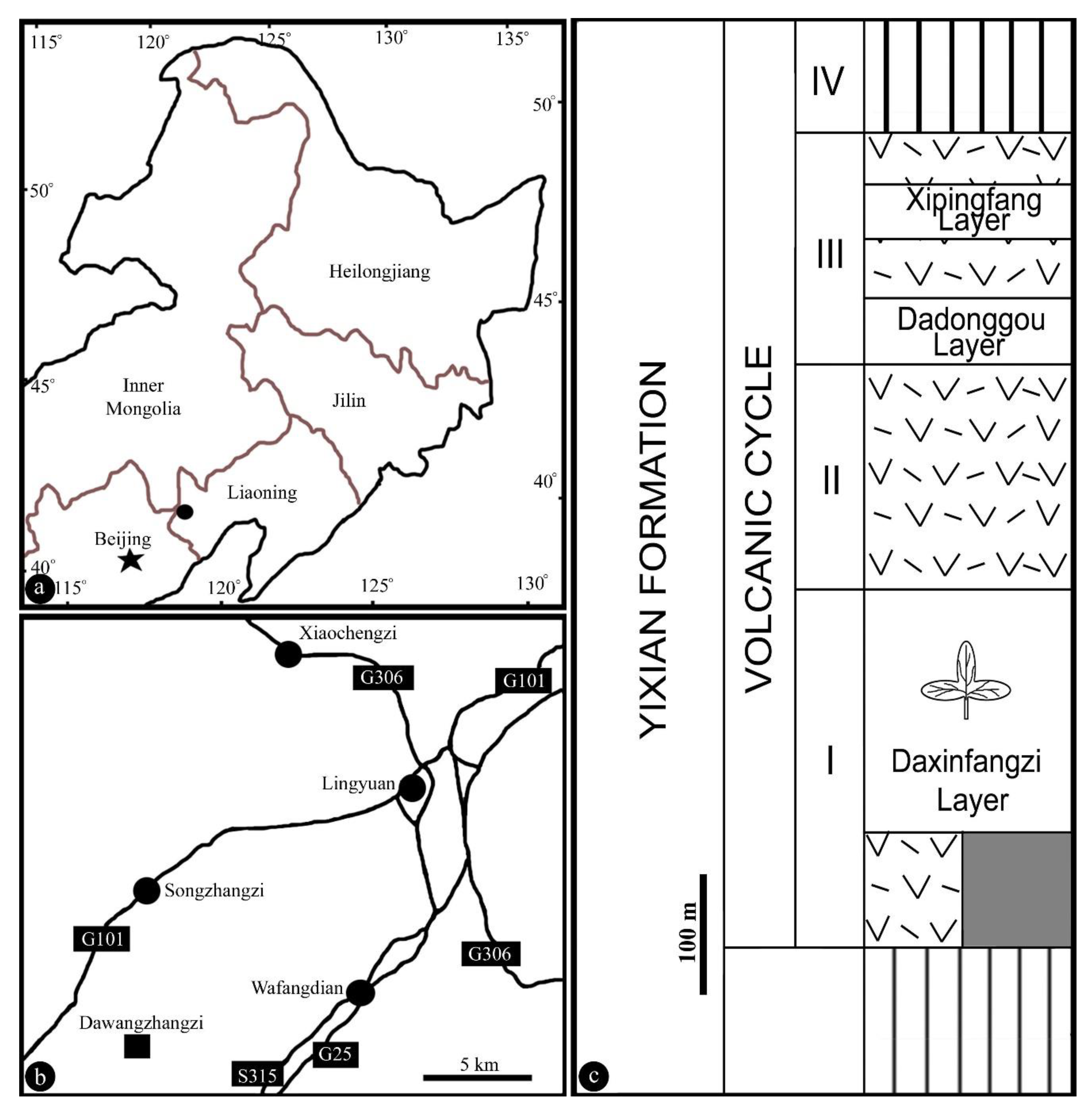
The specimen was collected from the Yixian Formation near Dawangzhangzi Village, Lingyuan, Liaoning, China (41°15′ N, 119°15′ E) (Figure 1a–c). The fossiliferous layer is a siltstone of the Daxinfangzi layer in the formation (Figure 1c). The specimen includes two facing parts preserved as a compression with some coalified residue (Figure 2a). The locality has yielded Archaefructus sinensis Sun et al. 2002 [3], Sinocarpus decussatus Leng and Friis 2003 [4,5], Nothodichocarpum lingyuanensis Han et al. 2017 [10], Neofructus lingyuanensis Liu and Wang 2018 [8], and Callianthus dilae Wang and Zheng 2009 [32] (Table 1). The specimen is preserved on gray siltstone slabs approximately 11 cm × 11 cm. The specimen was photographed using a Nikon D200 digital camera and a Nikon SMZ1500 stereomicroscope with a Nikon DS-Fi1 digital camera. SEM was performed using a MAIA3 TESCAN housed at the Nanjing Institute of Geology and Palaeontology, Nanjing, China. All figures were organized using Photoshop 7.0.

Figure 1.
Geographical position of the fossil locality of Lingyuananthus gen. nov. in western Liaoning, China. (a,b), Modified from Liu and Wang (2018); (c), modified from Wang et al. (2021). (a) Fossil locality (black dot) in northeastern China; (b) position of fossil locality (black square) in a suburb of the city Lingyuan, Liaoning; (c) stratigraphic column of the Yixian Formation yielding the specimen. The column includes various volcanic eruptions and intercalating siltstones. The specimen was uncovered from the Daxinfangzi Layer.

Table 1.
Comparison between Lingyuananthus inexpectus gen. et sp. nov. and other angiosperms from the Yixian Formation.
3. Results
Genus Lingyuananthus gen. nov
Diagnosis: Flower syncarpous, with an elongated pedicel, ovary, and tepals. Ovary cup-formed, flat-topped, surrounded by a hypanthium with asymmetrically arranged lateral appendages. Multiple tepals, linear, on hypanthium edge or surface. Multiple ovules/seeds within the ovary.
Species Lingyuananthus inexpectus gen. et sp. nov
Diagnosis: The same as the genus.
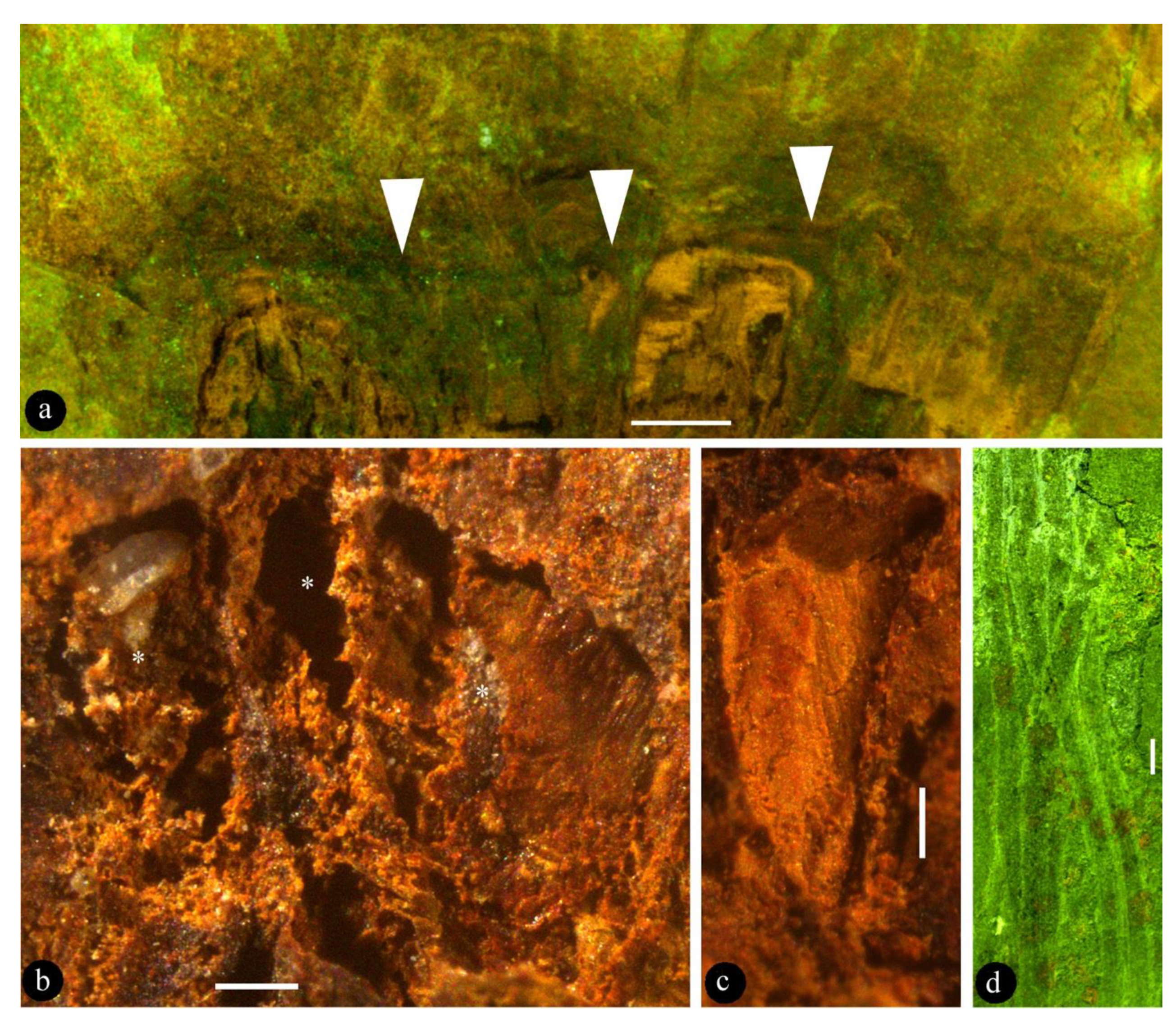
Description: The fossil is 6.4 cm long and 3.8 cm wide, including a pedicel, an ovary, and multiple tepals (Figure 2a). The pedicel is slightly curved, 13 mm long, and 1.5 mm wide (Figure 2a). The fossil is syncarpous, with an ovary that is cup-shaped, 13.5 mm long, widest at the top, and 10 mm wide (Figure 2b, Figure 3a, and Figure 4d). The outer surface of the hypanthium has multiple lateral appendages, which tend to be concentrated on one side of the hypanthium (Figure 2a,b and Figure 4d). The floral roof is generally flat, secluding the ovary, with a small process (Figure 2b and Figure 4d,e). The tepals are linear, 0.7 mm wide, and 41 mm long, and not differentiated in size and morphology (Figure 2a, Figure 3d, and Figure 5). At least 15 tepals are arranged along the hypanthium edge and outer surface (Figure 2a,b and Figure 5). Multiple (more than three) ovules of variable forms are in the ovary, 0.48–0.92 mm wide and 1.16–1.3 mm long (Figure 2b–e, Figure 3b,c, Figure 4a, and Figure 5). Round papillae are seen on the ovule surface, 16–23 μm in diameter (Figure 4b). Transmitting tissue is seen under the floral roof (Figure 4c). No trace of a seed coat is seen throughout the ovary.

Figure 2.
Lingyuananthus inexpectus gen. et sp. nov. and its details. (a) General view of the flower, showing pedicel (p), ovary (ov), and tepals (t). Scale bar = 1 cm. (b) Detailed view of the ovary, showing its obconical form, even top (triangles), ovary wall (=hypanthium) (ow), ovules (asterisks), and transmitting tissue (t). Refer to Figure 4c for details. Scale bar = 1 mm. (c) An ovule (asterisk) in situ in the ovary. Scale bar = 0.5 mm. (d) The ovule in Figure 2c, slightly dislocated from its original position, in the ovary. For papillae on the ovule, refer to Figure 4b. Scale bar = 0.5 mm. (e) Imprints left by three adjacent ovules (asterisks) of various forms in the ovary. Scale bar = 0.5 mm.

Figure 3.
Lingyuananthus inexpectus gen. et sp. nov. (a) Flat ovary top (= floral roof, triangles) secluding the ovary. Refer to Figure 4d. Scale bar = 1 mm. (b) Three adjacent ovules (asterisks) of various forms in the ovary. Scale bar = 0.2 mm. (c) Cuneate profiled cavity left by a fallen-off ovule. Scale bar = 0.2 mm. (d) Several linear-shaped tepals. Scale bar = 1 mm.

Figure 4.
SEM views of Lingyuananthus inexpectus gen. et sp. nov. (a) A portion of the interior of the ovary showing hypanthium (hy) and cavities (asterisks) left by several ovules. Scale bar = 1 mm. (b) Papillae on the dislocated ovule shown in Figure 2c,d. Scale bar = 50 μm. (c) Transmitting tissue within the ovary. Refer to Figure 2b. Scale bar = 0.1 mm. (d) SEM view of the ovary top (triangles). Note one of the lateral appendages (asterisk) to the left. Refer to Figure 3a. Scale bar = 1 mm. (e) Detailed view of the rectangular region in Figure 4d showing the process (triangles). Scale bar = 1 mm.
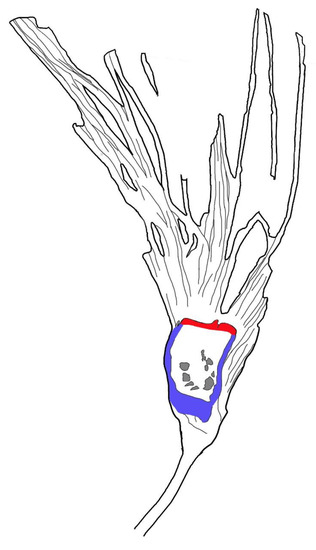
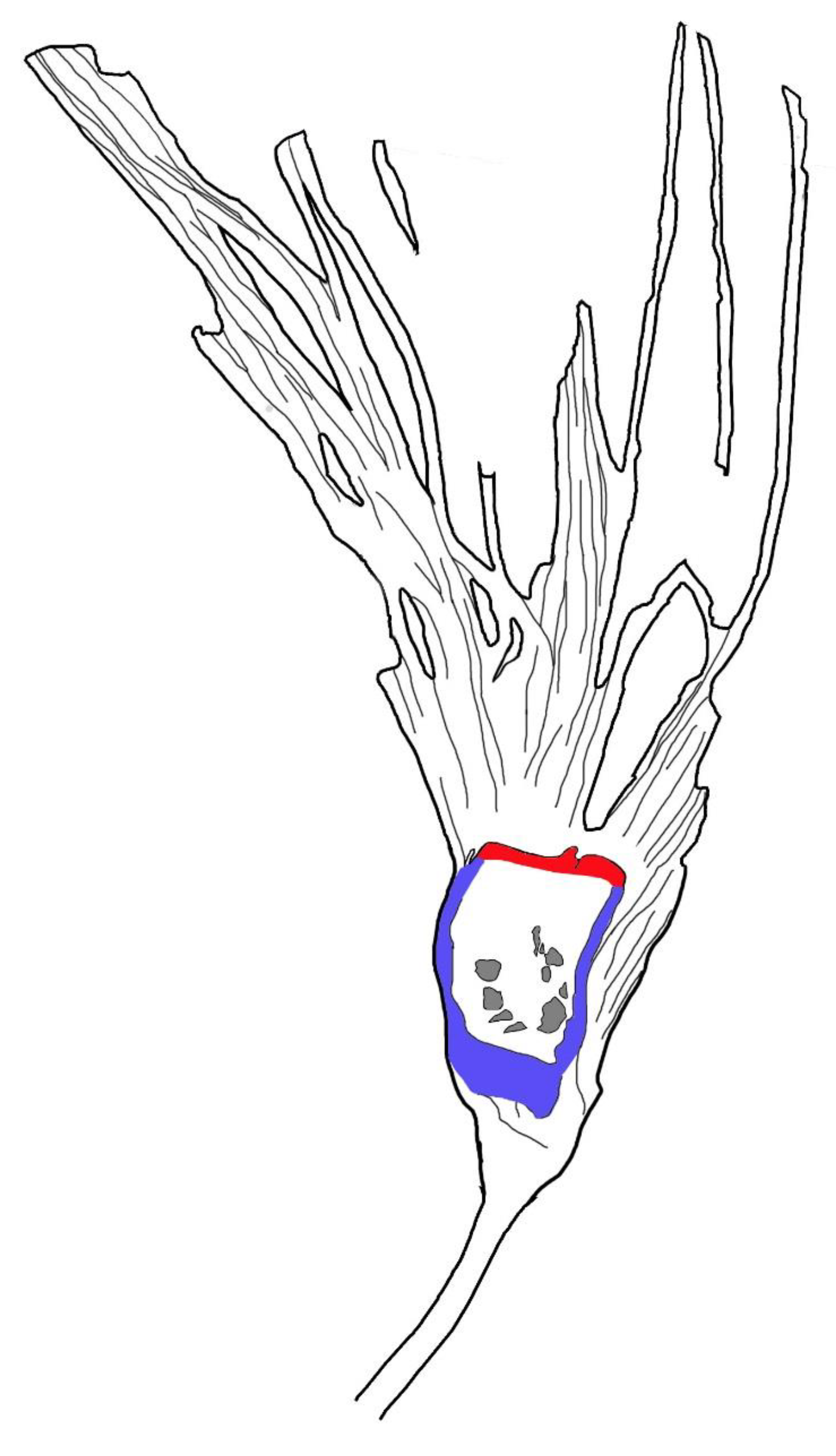
Figure 5.
Sketch of Lingyuananthus inexpectus gen. et sp. nov. Note the ovule cavities (gray) in the ovary, ovary wall (=hypanthium) (blue), and floral roof (red) secluding the ovary.
Remarks: The disadvantage of the present study is its single specimen, undermining the validity of the conclusion. However, this does not reduce the significance of the fossil, as it follows the routine practiced in paleobotany. For example, both Archaefructus [2] and Monetianthus [33], published in Science and Nature, respectively, are based on only one specimen.
Due to its compression preservation, it is hard to judge the symmetry of the flower. However, the asymmetrical arrangement of lateral appendages on the outer surface of the ovary wall (hypanthium) seems to suggest that the flower of Lingyuananthus more likely has bilateral symmetry.
No trace of stamens or pollen grains is seen in the specimen, even when examined using SEM. This leads to the conclusion that Lingyuananthus is an incomplete flower, namely, a pistillate flower.
The “tepals” of Lingyuananthus are uniform in morphology: linear, with smooth margins, attached to the hypanthium edge or outer surface. They constitute the “perianth” in Lingyuananthus. Their uniform morphology suggests that they should not be termed “sepals” or “petals” (differentiated perianth elements) and are better termed as “tepals” (undifferentiated perianth elements). The preservation of the present specimen does not allow the author to tell the exact arrangement of the tepals (whorled or not, number of whorls) in Lingyuananthus.
The number of tepals is characterized as “multiple” since there are more than 3 (approximately 15) tepals preserved in the specimen. The exact number of tepals is to be determined by future studies of more specimens. The venation in the tepals is most likely parallel, but this cannot be ascertained with the current specimen.
Theoretically, ovules within a single ovary should be of uniform shape, but the outlines of ovules in Lingyuananthus vary. This variation may be due to different orientations, developmental stages, and preservation of the ovules.
Since Lingyuananthus is an obvious reproductive organ, the dimension and lack of pollen traces in the fossil leaves only one alternative, that it is a female reproductive organ. The inner bodies are millimetric in size, so they are interpreted as ovules because a seed coat, expected for seeds, is not seen in the fossil. These ovules are embedded within a closed structure, suggesting angio-ovuly in Lingyuananthus. This leads me to interpret the fossil as a flower rather than a fruit.
Horizon: The Yixian Formation, Barremian–Aptian, Lower Cretaceous.
Holotype: PB328297.
Etymology: Lingyuan- for the fossil locality, Lingyuan City; -anthus for flower; inexpectus for unexpected morphology of the flower.
Depository: The Herbarium, Nanjing Institute of Geology and Palaeontology, Nanjing, China.
4. Discussion
Angiosperms are the most diversified plant group on Earth. There are more than 300,000 species of angiosperms, accounting for more than 90% of the species diversity of land plants [34]. Ecologically and historically, angiosperms are important elements in the background for the origin and evolution of human beings. Considering the importance of angiosperms, it is rather natural that the origin and evolution of angiosperms have been constant foci of botanical studies for a long time. Literally, angiosperms are distinguished from their gymnosperm peers from their defining feature, an enclosed seed. However, all organisms tend to protect their offspring in one way or another [35]; e.g., some gymnosperms (e.g., conifers) also protect their seeds by secluding them after pollination [36]. Therefore, distinguishing angiosperms and gymnosperms using angiospermy alone is not completely accurate. A more critical survey indicates that a constant difference between angiosperms and gymnosperms occurs at the time of pollination; namely, at the time of pollination, the ovules of gymnosperms are exposed to exterior space and accessible to pollen grains, while those of angiosperms are secluded and not directly accessible to pollen grains. Although Herendeen et al. [37] tried to challenge this criterion by enumerating “unique angiosperm features” as “diagnostic features of the angiosperm crown group”, they refuted themselves by listing five angiosperms published by themselves that did not honor their own criterion [37,38]. This refutation was repeated implicitly in 2019 by Friis et al. [39,40]. Therefore, I prefer to be consistent and adopt a stricter criterion for angiosperms: angio-ovuly, namely, ovule enclosed before pollination [6,22,36].
It is well-known that the only foliar structures (bracts) in cones are uniformly borne on the cone axis rather than on the periphery of the cones. In contrast, the tepals in Lingyuanthus are arranged on the upper rim and outer surface of the cup-shaped structure (ovary/hypanthium). Lingyuanthus has no trace of a cone axis, which would be indispensable and required for a conifer cone. Therefore, as conifer cones are distinct from Lingyuananthus in morphology, it is reasonable to interpret Lingyuananthus as a flower.
Traditionally, Magnoliales were thought to be the most ancestral of angiosperms. Logically, apocarpy, conduplicate carpels, and follicles (features of Magnoliales) were taken as proxies of ancestrality in angiosperms [18]. In contrast to Magnoliales, flowers with an inferior ovary and hypanthium were thought to be derived or advanced in angiosperms, since these features occur only in the assumedly more derived taxa [18]. Although supported by Archaefructus [2,3], Sinocarpus [4,5], and Neofructus [8], with free carpels, this thinking is now challenged by Lingyuananthus, which is from the same formation and distinguishes itself from previously reported contemporary early angiosperms by its non-apocarpy, inferior ovary, and hypanthium. The co-occurrence of Archaefructus [2,3], Sinocarpus [4,5], Callianthus [14,32], Nothodichocarpum [10], Sinoherba [13], Neofructus [8], and now Lingyuananthus underscores the great diversity of flowers and angiosperms in the Yixian Formation.
The occurrence of transmitting tissue (Figure 2b and Figure 4c) within the ovary of Lingyuananthus is noteworthy and intriguing. The function of transmitting tissue in living plants is guiding pollen tubes to the micropyles of ovules for successful pollination. Such function and tissue are unnecessary in gymnosperms, in which pollen grains have direct access to the micropyles. The occurrence of transmitting tissue in Lingyuananthus further reinforces its angiospermous affinity.
The above implications on angiosperm evolution are compatible with and reinforced by Jurassic angiosperm fossils. A feature of Nanjinganthus that surprised many, as seen in the title of the paper, is its “noncarpellate epigynous flower” [21]. Comparing Lingyuananthus and Nanjinganthus, it is obvious that both taxa share an inferior ovary and a hypanthium, although these features are not expected theoretically for early angiosperms [18]. It is natural for a botanist to make a difficult choice between theory and evidence, especially when they contradict each other. A wise strategy when facing such a dilemma is doing nothing and watching for further progress before taking steps. The good news liberating scientists from such a dilemma is that such awaited fossil evidence actually has been documented: independently studied and well-documented flowers, including Monetianthus [31], Divisestylus Hermsen et al. 2003, Antiquacupula Sims et al. 1998, Archaefagacea Takahashi et al. 2008, Calathiocarpus Knobloch and Mai 1986, Caryanthus Friis 1983, Manningia Friis 1983, Normanthus Schönenberger et al. 2001, Esgueiria Friis et al. 1992, Tylerianthus Gandolfo 1998, and Scandianthus Friis and Skarby 1982 [23] from the Cretaceous, are all examples of flowers with an inferior ovary and syncarpy. All of these fossils unanimously suggest either that not all early angiosperms are apocarpous with a superior ovary, that there is another lineage of early angiosperms (namely, that angiosperms are most likely polyphyletic), that angiosperms originated much earlier, or all three. All of these implications contradict the current understanding of angiosperm evolution.
Traditionally, apocarpy, conduplicate carpels, and marginal placentation were thought to be ancestral in angiosperms. This image of ancestral angiosperms appeared to be supported by some fossil evidence, e.g., Archaeanthus Dilcher and Crane 1984 [41], Archaefructus [2,3], and Sinocarpus [4,5], from the Cretaceous (Table 1). However, later more careful studies indicate that Archaeanthus [41] and Archaefructus [2,3] were previously wrongly interpreted, and their support for the above image of ancestral angiosperms is spurious [42]. Numerous early angiosperms from the Yixian Formation, including Chaoyangia Duan [1,6], Callianthus Wang and Zheng [14,32], Baicarpus Han et al. 2013 [9], Eofructus Han and Wang 2020 [11], Neofructus Liu and Wang [8], and Liaoningfructus Wang and Han 2011 [16] (Table 1), cast further doubt over this image. Interestingly, reviewing the history of the Magnolia-ancestral theory indicates that the theory was actually founded on a famous dictum of a celebrity, Goethe [43,44,45], rather than solid scientific evidence. Apparently, this theory, of great influence in the past century, needs to be updated with the progress in paleobotany and gene function studies in living plants [26,27,28,29].
Based on a wide spectrum of carpel variations in a single tree or a single flower of Michelia Linn., Zhang et al. proposed that a conduplicate carpel typical of Magnoliales is derived from a leaf and its axillary ovule-bearing branch [46]. This generalization was coherent with the study of Magnolia Linn. and Illicium, and was adopted by Wang [6] as one of the ways to derive carpels. However, the flowers of Lingyuananthus, plus Nanjinganthus from the Early Jurassic [21,22] and Monetianthus, as well as other fossil angiosperms from the Early Cretaceous [23,31], constitute hard-to-ignore exceptions to this generalization of Magnoliales. One feasible way to bypass this challenge is to derive these gynoecia in another way through floral axis invagination (also see below).
A retrospective of botany and paleobotany indicates that Zimmermann had long applied the telome theory for the evolution of early land plants, which was successful for ferns, less so for gymnosperms, and almost of no influence for angiosperms. Its failure in angiosperms can be partially attributed to the then dominance of the Magnolia-ancestral theory. Now, since this roadblock has been removed by the rise of molecular systematics, it is necessary for us to reevaluate Zimmermann’s hypothesis. In his Figure 302 on page 558, Zimmermann depicted the derivation of flowers with inferior ovaries through floral axis invagination. This depiction appeared unconvincing when Magnolia was placed at the basalmost position in angiosperms. However, it becomes more promising now with new paleobotanical progress in mind. One of the characteristics of Nanjinganthus is its inferior ovary and scale-like structures on the outer surface of the ovary; such observations can be accounted for by floral axis invagination, just as Zimmermann suggested. In addition, Monetianthus [31], another flower independently studied, has scars of fallen-off lateral appendages on the outer surface of the ovary. Although this fossil has been well documented, the authors did not offer any explanation on the origin of its gynoecium. Parallel to Nanjinganthus and Monetianthus, the appendages on the hypanthium surface of Lingyuananthus reinforce such a possibility in plant evolution, although the arrangement of the lateral appendages in Lingyuananthus appears asymmetrical (Figure 2a and Figure 5). Wang [6], after a systematic survey of all reproductive organs in land plants, proposed several ways to derive gynoecia in angiosperms, including floral axis invagination. Now, Lingyuananthus, with its unique gynoecium morphology meeting the expectation of Wang [6], suggests that Zimmermann’s hypothesis appears to be a more promising hypothesis accounting for the derivation of this kind of gynoecium.
The above proposed floral axis invagination not only gives rise to this special kind of gynoecium but also introduces a special structure idiosyncratic of angiosperms, a hypanthium. This structure used to be thought to be highly derived in angiosperms, as it is remote from any structure in gymnosperms and basalmost angiosperms (Magnolia or Amborella, in traditional or current theory), and its presence in Lingyuananthus, in the Early Cretaceous Yixian Formation, suggests that it is not as derived as previously thought. One of the key functions of a hypanthium is to provide strong protection for the ovules/seeds within the ovary. This protection is one of many implementations of the universal trend underlying the evolution of reproduction in plants, Offspring Development Conditioning (ODC), as advanced recently by Fu et al. [35]. The derivation of conduplicate carpels, which were formerly thought to be ancestral in angiosperms, in Michelia (Magnoliaceae) is another good example of implementing an ODC strategy. Although appearing ubiquitous in sexually reproducing organisms, apparently, how widely applicable ODC is among other organisms is still an open question. I expect more studies to test the ODC hypothesis, using evidence of either fossil or living plants.
5. Conclusions
Lingyuananthus is a novel flower uncovered from the Yixian Formation (the Lower Cretaceous), adding to the already great diversity of angiosperms in the Yixian Formation (Lower Cretaceous). Integrated with previous reports of fossil angiosperms and recent progress made on living angiosperms, Lingyuananthus, with its inferior ovary, prompts botanists to reevaluate the existing hypotheses on flower derivation.
Funding
This research was supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB26000000) and the National Natural Science Foundation of China (42288201, 41688103, 91514302).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The specimen is deposited in the Herbarium, Nanjing Institute of Geology and Palaeontology, Nanjing, China (http://www.nigpas.cas.cn/).
Acknowledgments
I thank Jingjing Tang for help with photography using Nikon D800, Jingyi Yang and Yan Fang for help with photography using MAIA3 TESCAN SEM, and Mike Pole at Queensland Herbarium, Brisbane Botanical Gardens, for help on the English in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duan, S. The oldest angiosperm—A tricarpous female reproductive fossil from western Liaoning Province, NE China. Sci. China D 1998, 41, 14–20. [Google Scholar] [CrossRef]
- Sun, G.; Dilcher, D.L.; Zheng, S.; Zhou, Z. In search of the first flower: A Jurassic angiosperm, Archaefructus, from Northeast China. Science 1998, 282, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ji, Q.; Dilcher, D.L.; Zheng, S.; Nixon, K.C.; Wang, X. Archaefructaceae, a new basal angiosperm family. Science 2002, 296, 899–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Q.; Friis, E.M. Sinocarpus decussatus gen. et sp. nov., a new angiosperm with basally syncarpous fruits from the Yixian Formation of Northeast China. Plant Syst. Evol. 2003, 241, 77–88. [Google Scholar] [CrossRef]
- Leng, Q.; Friis, E.M. Angiosperm leaves associated with Sinocarpus infructescences from the Yixian Formation (Mid-Early Cretaceous) of NE China. Plant Syst. Evol. 2006, 262, 173–187. [Google Scholar] [CrossRef]
- Wang, X. The Dawn Angiosperms, 2nd ed.; Springer: Cham, Switzerland, 2018; p. 407. [Google Scholar]
- Liu, Z.-J.; Chen, L.-J.; Wang, X. A whole-plant monocot from Lower Cretaceous. Palaeoworld 2021, 30, 169–175. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Wang, X. A novel angiosperm from the Early Cretaceous and its implications on carpel-deriving. Acta Geol. Sin. 2018, 92, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Fu, X.; Liu, Z.-J.; Wang, X. A new angiosperm genus from the Lower Cretaceous Yixian Formation, Western Liaoning, China. Acta Geol. Sin. 2013, 87, 916–925. [Google Scholar]
- Han, G.; Liu, Z.; Wang, X. A Dichocarpum-like angiosperm from the Early Cretaceous of China. Acta Geol. Sin. 2017, 90, 1–8. [Google Scholar] [CrossRef]
- Han, G.; Wang, X. A new infructescence of angiosperms from the Early Cretaceous of China. Acta Geol. Sin. 2020, 94, 1711–1713. [Google Scholar] [CrossRef]
- Ji, Q.; Li, H.; Bowe, M.; Liu, Y.; Taylor, D.W. Early Cretaceous Archaefructus eoflora sp. nov. with bisexual flowers from Beipiao, Western Liaoning, China. Acta Geol. Sin. 2004, 78, 883–896. [Google Scholar]
- Liu, X.; Ma, L.; Liu, B.; Liu, Z.-J.; Wang, X. A novel angiosperm including various parts from the Early Cretaceous sheds new light on flower evolution. Hist. Biol. 2021, 33, 2706–2714. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, S. The earliest normal flower from Liaoning Province, China. J. Integr. Plant Biol. 2009, 51, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, X.-T. Reconsiderations on two characters of early angiosperm Archaefructus. Palaeoworld 2012, 21, 193–201. [Google Scholar] [CrossRef]
- Wang, X.; Han, G. The earliest ascidiate carpel and its implications for angiosperm evolution. Acta Geol. Sin. 2011, 85, 998–1002. [Google Scholar] [CrossRef]
- Dilcher, D.L.; Sun, G.; Ji, Q.; Li, H. An early infructescence Hyrcantha decussata (comb. nov.) from the Yixian Formation in northeastern China. Proc. Natl. Acad. Sci. USA 2007, 104, 9370–9374. [Google Scholar] [CrossRef] [Green Version]
- Cronquist, A. The Evolution and Classification of Flowering Plants, 2nd ed.; New York Botanical Garden: Bronx, NY, USA, 1988; p. 555. [Google Scholar]
- Liu, Z.-J.; Wang, X. A perfect flower from the Jurassic of China. Hist. Biol. 2016, 28, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Liu, Z.-J.; Liu, X.; Mao, L.; Jacques, F.M.B.; Wang, X. A whole plant herbaceous angiosperm from the Middle Jurassic of China. Acta Geol. Sin. 2016, 90, 19–29. [Google Scholar]
- Fu, Q.; Diez, J.B.; Pole, M.; Garcia-Avila, M.; Liu, Z.-J.; Chu, H.; Hou, Y.; Yin, P.; Zhang, G.-Q.; Du, K.; et al. An unexpected noncarpellate epigynous flower from the Jurassic of China. eLife 2018, 7, e38827. [Google Scholar] [CrossRef]
- Fu, Q.; Diez, J.B.; Pole, M.; García-Ávila, M.; Wang, X. Nanjinganthus is an angiosperm, isn’t it? China Geol. 2020, 3, 359–361. [Google Scholar] [CrossRef]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. The Early Flowers and Angiosperm Evolution; Cambridge University Press: Cambridge, UK, 2011; p. 596. [Google Scholar]
- APG. APG IV: An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.-L.; Lee, J.; Bernasconi-Quadroni, F.; Soltis, D.E.; Soltis, P.S.; Zanis, M.; Zimmer, E.A.; Chen, Z.; Savolainen, V.; Chase, M.W. The Earliest Angiosperms: Evidence from Mitochondrial, Plastid and Nuclear Genomes. Nature 1999, 402, 404–407. Available online: http://www.nature.com/nature/journal/v402/n6760/suppinfo/402404a0_S1.html (accessed on 8 July 2009). [CrossRef] [PubMed]
- Roe, J.L.; Nemhauser, J.L.; Zambryski, P.C. TOUSLED participates in apical tissue formation during gynoecium development in Arabidopsis. Plant Cell 1997, 9, 335–353. [Google Scholar] [PubMed] [Green Version]
- Rounsley, S.D.; Ditta, G.S.; Yanofsky, M.F. Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 1995, 7, 1259–1269. [Google Scholar]
- Skinner, D.J.; Hill, T.A.; Gasser, C.S. Regulation of ovule development. Plant Cell 2004, 16, S32–S45. [Google Scholar] [CrossRef]
- Mathews, S.; Kramer, E.M. The evolution of reproductive structures in seed plants: A re-examination based on insights from developmental genetics. New Phytol. 2012, 194, 910–923. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.W.; Li, H. Paleobotany: Did flowering plants exist in the Jurassic period? eLife 2018, 7, e43421. [Google Scholar] [CrossRef]
- Friis, E.M.; Pedersen, K.R.; von Balthazar, M.; Grimm, G.W.; Crane, P.R. Monetianthus mirus gen. et sp. nov., a nymphaealean flower from the Early Cretaceous of Portugal. Int. J. Plant Sci. 2009, 170, 1086–1101. [Google Scholar] [CrossRef]
- Wang, X.; Shih, C.; Liu, Z.-J.; Lin, L.; Singh, K.J. Reconstructing the Callianthus plant–An early aquatic angiosperm from the Lower Cretaceous of China. Cretac. Res. 2021, 128, 104983. [Google Scholar] [CrossRef]
- Friis, E.M.; Pedersen, K.R.; Crane, P.R. Fossil evidence of water lilies (Nymphaeales) in the Early Cretaceous. Nature 2001, 410, 357–360. [Google Scholar] [CrossRef]
- Crepet, W.L.; Niklas, K.J. Darwin’s second “abominable mystery”: Why are there so many angiosperm species? Am. J. Bot. 2009, 96, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Liu, J.; Wang, X. Offspring development conditioning (ODC): A universal evolutionary trend in sexual reproduction of organisms. J. Northwest Univ. 2021, 51, 163–172. [Google Scholar] [CrossRef]
- Tomlinson, P.B.; Takaso, T. Seed cone structure in conifers in relation to development and pollination: A biological approach. Can. J. Bot. 2002, 80, 1250–1273. [Google Scholar] [CrossRef]
- Herendeen, P.S.; Friis, E.M.; Pedersen, K.R.; Crane, P.R. Palaeobotanical redux: Revisiting the age of the angiosperms. Nat. Plants 2017, 3, 17015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X. Criterion is a touchstone in study of early angiosperms. Open J. Plant Sci. 2021, 6, 91–93. [Google Scholar] [CrossRef]
- Wang, X. Groundless research published on the International Journal of Plant Sciences. Voice Publ. 2020, 6, 167–169. [Google Scholar] [CrossRef]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. Hedyosmum-like fossils in the Early Cretaceous diversification of angiosperms. Int. J. Plant Sci. 2019, 180, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Dilcher, D.L.; Crane, P.R. Archaeanthus: An early angiosperm from the Cenomanian of the Western Interior of North America. Ann. Mo. Bot. Gard. 1984, 71, 351–383. [Google Scholar] [CrossRef]
- Wang, X. New observation on seed/ovule position in the fruit of Archaeanthus and its systematic implications. China Geol. 2021, 4, 752–755. [Google Scholar] [CrossRef]
- Arber, A. Introduction to Goethe’s botany. Chron. Bot. 1946, 10, 63–87. [Google Scholar]
- Arber, E.A.N.; Parkin, J. On the origin of angiosperms. J. Linn. Soc. Lond. Bot. 1907, 38, 29–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, Z.-J.; Liu, W.; Liao, W.; Zhang, X.; Liu, Z.; Hu, G.; Guo, X.; Wang, Y. Stepping out of the shadow of Goethe: For a more scientific plant systematics. Chin. Bull. Bot. 2020, 55, 505–512. [Google Scholar]
- Zhang, X.; Liu, W.; Wang, X. How the ovules get enclosed in magnoliaceous carpels. PLoS ONE 2017, 12, e0174955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).