A Novel Early Cretaceous Flower and Its Implications on Flower Derivation
Abstract
:Simple Summary
Abstract
1. Introduction
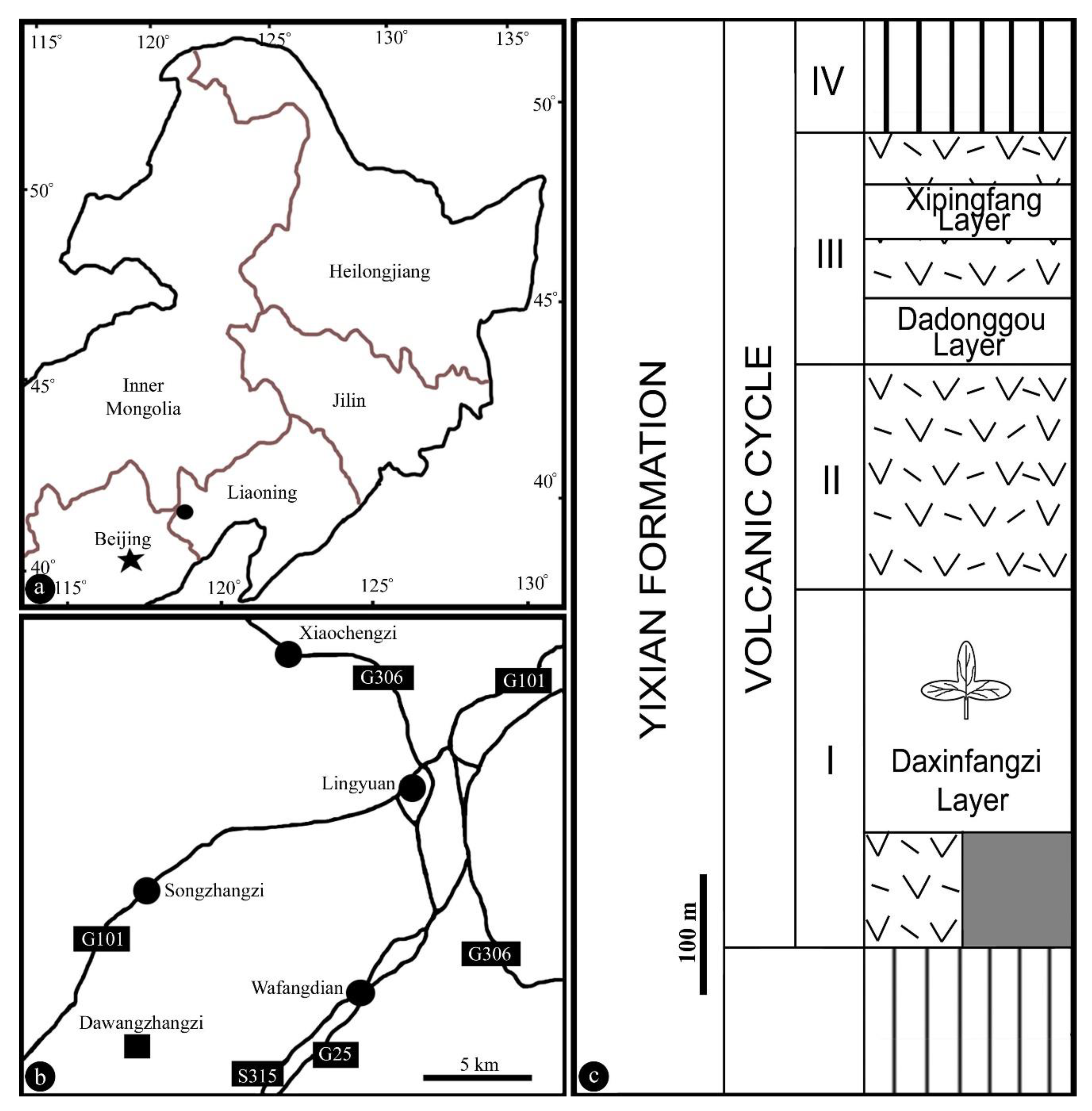
2. Materials and Methods
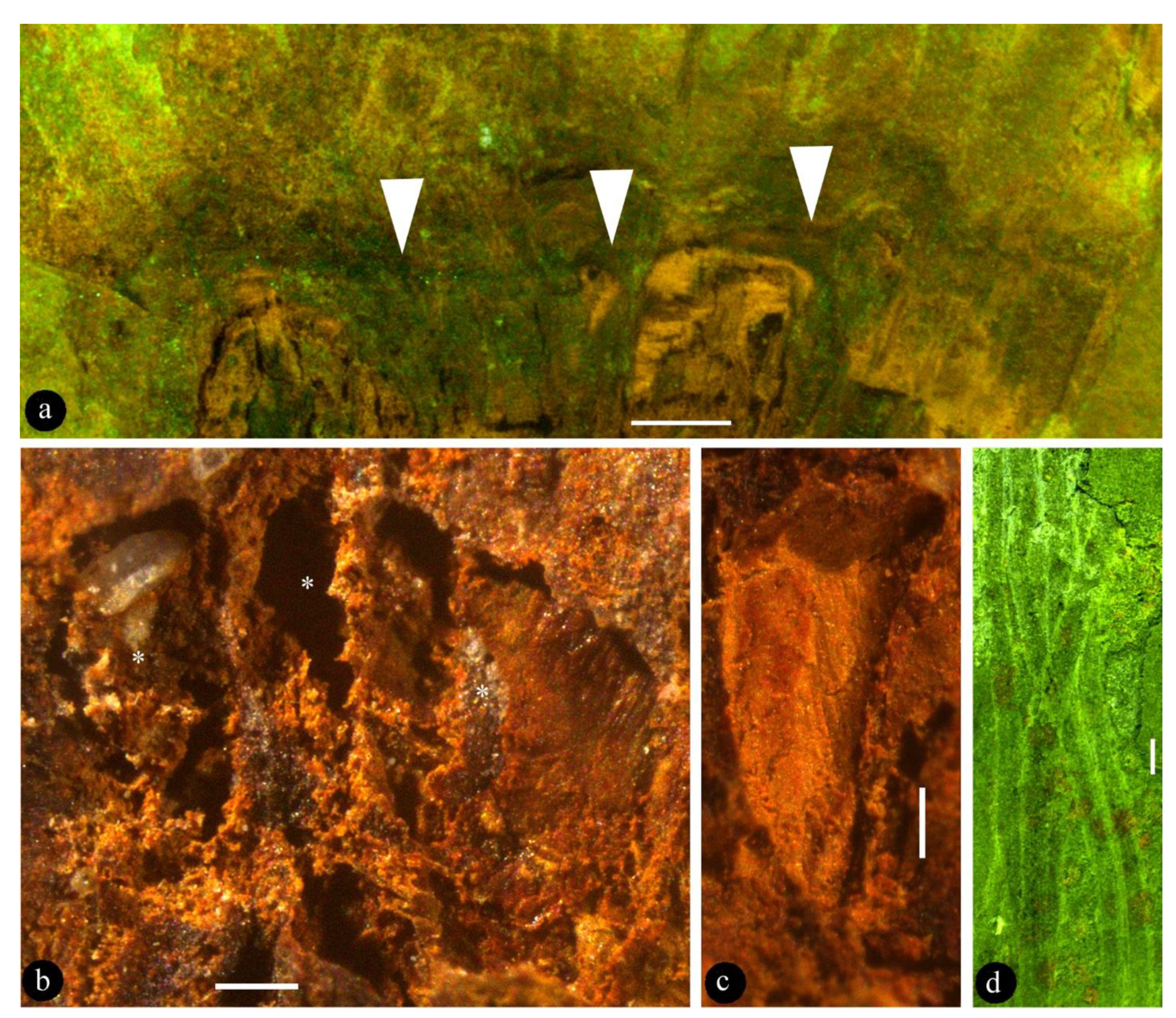
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, S. The oldest angiosperm—A tricarpous female reproductive fossil from western Liaoning Province, NE China. Sci. China D 1998, 41, 14–20. [Google Scholar] [CrossRef]
- Sun, G.; Dilcher, D.L.; Zheng, S.; Zhou, Z. In search of the first flower: A Jurassic angiosperm, Archaefructus, from Northeast China. Science 1998, 282, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ji, Q.; Dilcher, D.L.; Zheng, S.; Nixon, K.C.; Wang, X. Archaefructaceae, a new basal angiosperm family. Science 2002, 296, 899–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Q.; Friis, E.M. Sinocarpus decussatus gen. et sp. nov., a new angiosperm with basally syncarpous fruits from the Yixian Formation of Northeast China. Plant Syst. Evol. 2003, 241, 77–88. [Google Scholar] [CrossRef]
- Leng, Q.; Friis, E.M. Angiosperm leaves associated with Sinocarpus infructescences from the Yixian Formation (Mid-Early Cretaceous) of NE China. Plant Syst. Evol. 2006, 262, 173–187. [Google Scholar] [CrossRef]
- Wang, X. The Dawn Angiosperms, 2nd ed.; Springer: Cham, Switzerland, 2018; p. 407. [Google Scholar]
- Liu, Z.-J.; Chen, L.-J.; Wang, X. A whole-plant monocot from Lower Cretaceous. Palaeoworld 2021, 30, 169–175. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Wang, X. A novel angiosperm from the Early Cretaceous and its implications on carpel-deriving. Acta Geol. Sin. 2018, 92, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Fu, X.; Liu, Z.-J.; Wang, X. A new angiosperm genus from the Lower Cretaceous Yixian Formation, Western Liaoning, China. Acta Geol. Sin. 2013, 87, 916–925. [Google Scholar]
- Han, G.; Liu, Z.; Wang, X. A Dichocarpum-like angiosperm from the Early Cretaceous of China. Acta Geol. Sin. 2017, 90, 1–8. [Google Scholar] [CrossRef]
- Han, G.; Wang, X. A new infructescence of angiosperms from the Early Cretaceous of China. Acta Geol. Sin. 2020, 94, 1711–1713. [Google Scholar] [CrossRef]
- Ji, Q.; Li, H.; Bowe, M.; Liu, Y.; Taylor, D.W. Early Cretaceous Archaefructus eoflora sp. nov. with bisexual flowers from Beipiao, Western Liaoning, China. Acta Geol. Sin. 2004, 78, 883–896. [Google Scholar]
- Liu, X.; Ma, L.; Liu, B.; Liu, Z.-J.; Wang, X. A novel angiosperm including various parts from the Early Cretaceous sheds new light on flower evolution. Hist. Biol. 2021, 33, 2706–2714. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, S. The earliest normal flower from Liaoning Province, China. J. Integr. Plant Biol. 2009, 51, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, X.-T. Reconsiderations on two characters of early angiosperm Archaefructus. Palaeoworld 2012, 21, 193–201. [Google Scholar] [CrossRef]
- Wang, X.; Han, G. The earliest ascidiate carpel and its implications for angiosperm evolution. Acta Geol. Sin. 2011, 85, 998–1002. [Google Scholar] [CrossRef]
- Dilcher, D.L.; Sun, G.; Ji, Q.; Li, H. An early infructescence Hyrcantha decussata (comb. nov.) from the Yixian Formation in northeastern China. Proc. Natl. Acad. Sci. USA 2007, 104, 9370–9374. [Google Scholar] [CrossRef] [Green Version]
- Cronquist, A. The Evolution and Classification of Flowering Plants, 2nd ed.; New York Botanical Garden: Bronx, NY, USA, 1988; p. 555. [Google Scholar]
- Liu, Z.-J.; Wang, X. A perfect flower from the Jurassic of China. Hist. Biol. 2016, 28, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Liu, Z.-J.; Liu, X.; Mao, L.; Jacques, F.M.B.; Wang, X. A whole plant herbaceous angiosperm from the Middle Jurassic of China. Acta Geol. Sin. 2016, 90, 19–29. [Google Scholar]
- Fu, Q.; Diez, J.B.; Pole, M.; Garcia-Avila, M.; Liu, Z.-J.; Chu, H.; Hou, Y.; Yin, P.; Zhang, G.-Q.; Du, K.; et al. An unexpected noncarpellate epigynous flower from the Jurassic of China. eLife 2018, 7, e38827. [Google Scholar] [CrossRef]
- Fu, Q.; Diez, J.B.; Pole, M.; García-Ávila, M.; Wang, X. Nanjinganthus is an angiosperm, isn’t it? China Geol. 2020, 3, 359–361. [Google Scholar] [CrossRef]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. The Early Flowers and Angiosperm Evolution; Cambridge University Press: Cambridge, UK, 2011; p. 596. [Google Scholar]
- APG. APG IV: An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.-L.; Lee, J.; Bernasconi-Quadroni, F.; Soltis, D.E.; Soltis, P.S.; Zanis, M.; Zimmer, E.A.; Chen, Z.; Savolainen, V.; Chase, M.W. The Earliest Angiosperms: Evidence from Mitochondrial, Plastid and Nuclear Genomes. Nature 1999, 402, 404–407. Available online: http://www.nature.com/nature/journal/v402/n6760/suppinfo/402404a0_S1.html (accessed on 8 July 2009). [CrossRef] [PubMed]
- Roe, J.L.; Nemhauser, J.L.; Zambryski, P.C. TOUSLED participates in apical tissue formation during gynoecium development in Arabidopsis. Plant Cell 1997, 9, 335–353. [Google Scholar] [PubMed] [Green Version]
- Rounsley, S.D.; Ditta, G.S.; Yanofsky, M.F. Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 1995, 7, 1259–1269. [Google Scholar]
- Skinner, D.J.; Hill, T.A.; Gasser, C.S. Regulation of ovule development. Plant Cell 2004, 16, S32–S45. [Google Scholar] [CrossRef]
- Mathews, S.; Kramer, E.M. The evolution of reproductive structures in seed plants: A re-examination based on insights from developmental genetics. New Phytol. 2012, 194, 910–923. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.W.; Li, H. Paleobotany: Did flowering plants exist in the Jurassic period? eLife 2018, 7, e43421. [Google Scholar] [CrossRef]
- Friis, E.M.; Pedersen, K.R.; von Balthazar, M.; Grimm, G.W.; Crane, P.R. Monetianthus mirus gen. et sp. nov., a nymphaealean flower from the Early Cretaceous of Portugal. Int. J. Plant Sci. 2009, 170, 1086–1101. [Google Scholar] [CrossRef]
- Wang, X.; Shih, C.; Liu, Z.-J.; Lin, L.; Singh, K.J. Reconstructing the Callianthus plant–An early aquatic angiosperm from the Lower Cretaceous of China. Cretac. Res. 2021, 128, 104983. [Google Scholar] [CrossRef]
- Friis, E.M.; Pedersen, K.R.; Crane, P.R. Fossil evidence of water lilies (Nymphaeales) in the Early Cretaceous. Nature 2001, 410, 357–360. [Google Scholar] [CrossRef]
- Crepet, W.L.; Niklas, K.J. Darwin’s second “abominable mystery”: Why are there so many angiosperm species? Am. J. Bot. 2009, 96, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Liu, J.; Wang, X. Offspring development conditioning (ODC): A universal evolutionary trend in sexual reproduction of organisms. J. Northwest Univ. 2021, 51, 163–172. [Google Scholar] [CrossRef]
- Tomlinson, P.B.; Takaso, T. Seed cone structure in conifers in relation to development and pollination: A biological approach. Can. J. Bot. 2002, 80, 1250–1273. [Google Scholar] [CrossRef]
- Herendeen, P.S.; Friis, E.M.; Pedersen, K.R.; Crane, P.R. Palaeobotanical redux: Revisiting the age of the angiosperms. Nat. Plants 2017, 3, 17015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X. Criterion is a touchstone in study of early angiosperms. Open J. Plant Sci. 2021, 6, 91–93. [Google Scholar] [CrossRef]
- Wang, X. Groundless research published on the International Journal of Plant Sciences. Voice Publ. 2020, 6, 167–169. [Google Scholar] [CrossRef]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. Hedyosmum-like fossils in the Early Cretaceous diversification of angiosperms. Int. J. Plant Sci. 2019, 180, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Dilcher, D.L.; Crane, P.R. Archaeanthus: An early angiosperm from the Cenomanian of the Western Interior of North America. Ann. Mo. Bot. Gard. 1984, 71, 351–383. [Google Scholar] [CrossRef]
- Wang, X. New observation on seed/ovule position in the fruit of Archaeanthus and its systematic implications. China Geol. 2021, 4, 752–755. [Google Scholar] [CrossRef]
- Arber, A. Introduction to Goethe’s botany. Chron. Bot. 1946, 10, 63–87. [Google Scholar]
- Arber, E.A.N.; Parkin, J. On the origin of angiosperms. J. Linn. Soc. Lond. Bot. 1907, 38, 29–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, Z.-J.; Liu, W.; Liao, W.; Zhang, X.; Liu, Z.; Hu, G.; Guo, X.; Wang, Y. Stepping out of the shadow of Goethe: For a more scientific plant systematics. Chin. Bull. Bot. 2020, 55, 505–512. [Google Scholar]
- Zhang, X.; Liu, W.; Wang, X. How the ovules get enclosed in magnoliaceous carpels. PLoS ONE 2017, 12, e0174955. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Archae-fructus sinensis | Sinocarpus decussatus | Nothodicho-carpum lingyuanensis | Neofructus lingyuanensis | Callianthus dilae | Baicarpus | Sinoherba ningchengen-sis | Lingyuananthus inexpectus gen. et sp. nov. | |
|---|---|---|---|---|---|---|---|---|
| Sexuality | Bisexual | Female | Bisexual | Female | Bisexual | Female | Female | Female |
| Ovule enclosed | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Carpellate | Yes | Yes | Yes | Yes | ? | Yes | Yes | No |
| Carpel arrangement | Free | Semi-free | Free | Free | ? | Free | ? | No |
| Carpel number | Multiple | 4 | 2 | Multiple | 2 | 3+ | ? | ? |
| Reference | Sun et al., 1998, 2002 | Leng and Friis, 2003 | Han et al., 2017 | Liu and Wang, 2018 | Wang et al., 2021 | Han et al., 2013 | Liu et al., 2021 | present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X. A Novel Early Cretaceous Flower and Its Implications on Flower Derivation. Biology 2022, 11, 1036. https://doi.org/10.3390/biology11071036
Wang X. A Novel Early Cretaceous Flower and Its Implications on Flower Derivation. Biology. 2022; 11(7):1036. https://doi.org/10.3390/biology11071036
Chicago/Turabian StyleWang, Xin. 2022. "A Novel Early Cretaceous Flower and Its Implications on Flower Derivation" Biology 11, no. 7: 1036. https://doi.org/10.3390/biology11071036






