Simple Summary
The aim of this work was to describe the trend of kidney tumors in a province of northern Italy through 25 years of registration. In the period examined, over 2300 patients with kidney cancer (mostly males of advanced age) and over 1200 deaths were registered, without differences between men and women but with significant age differences (12% among younger adults and 80% among the elderly). In men, we observed an increase in cases from 1996 to 2011, then the incidence decreased—probably in relation to the decline in cigarette smoking, which was also confirmed by the decline in lung cancers. Mortality decreased in both men and women, partly due to an earlier diagnosis of the disease and partly due to the availability of more advanced drugs that have made it possible to effectively treat the disease at a more advanced stage. In addition to the decrease in mortality from kidney cancer, we observed an increase in mortality from other causes, in particular from cardiovascular disease, which was also linked to the cardiotoxicity of some treatments. Therefore, along with early diagnosis and effective treatments, careful surveillance of cardiovascular episodes that may develop in these patients must be ensured.
Abstract
The aim of this study was to examine the incidence and mortality trends for tumors and cardiovascular disease (CVD) in a province of northern Italy. The study included kidney cancers recorded in the period 1996–2020, divided by sex, age, year of incidence and years from diagnosis. The standardized incidence rate was calculated using the European population, and the Annual Percent Change (APC) was reported. In total, 2331 patients with kidney cancers were identified, mainly males (1504 cases) aged 60–79 years (1240 cases). There were 1257 deaths; there were no differences according sex but there were differences according to age (12.1% among younger adults and 80.4% among 80+). The incidence rate increased in males between 1996 and 2011 (APC = 2.3), while the mortality rate decreased in both males (APC = −3.3%) and females (APC = −4.5%). Comparing the same periods, kidney cancer-specific mortality decreased from 81.8% to 43.7%, while in the same period there was an increasing trend for CVD mortality. Moreover, the risk of CVD mortality increased as we moved away from the diagnosis (from 6.2% to 27.5%, p < 0.01). The same trend was observed for other causes of death (from 12.6% to 32.1%, p < 0.01). Thus, a multidisciplinary approach seems necessary during the follow-up and treatments of patients with kidney cancer.
1. Introduction
Kidney cancer is the ninth most frequent malignancy in Italy (with over 13,500 new cases per year), with an incidence rate of 28.1 per 100,000 in males and 11.8 per 100,000 in females [1].
The incidence in Italy appears to be increasing in both males (+2.9% per year) and females (+2.2% per year) [1]. This has also been observed in other European countries [2,3], Canada [4] and the United States [5,6]. In particular, in some countries, the increase in cases up to the 1990s was followed by a decline in incidence, linked to lifestyle changes (reduced smoking and better management of hypertension and obesity) [5].
Several risk factors have been determined to be responsible for this increase [7]. Cigarette smoking is considered a certain risk factor for kidney cancer [8,9]. Compared with non-smokers, kidney cancer among smokers is 50% higher among males and 20% higher among females [10]. Excess body weight is also a risk factor in both the US and Europe (over 40% and 30% of cases, respectively, are associated with overweight and obesity) [11]. Hypertension is responsible for 20–40% of kidney cancers [12], while environmental exposures, especially in the workplace (for example, exposure to trichlorethylene) [13], are more controversial. Moreover, a certain genetic susceptibility is responsible for a small proportion of familial forms [14], and the occasional diagnosis of abdominal masses, so-called incidentaloma, could be responsible for another modest increase [4,15]. In fact, the increasing use of imaging technologies (e.g., ultrasonography, computed tomography, magnetic resonance imaging) has likely resulted in greater incidental detection of kidney cancer [16], especially of smaller tumors [17,18].
At the same time, mortality has decreased in many European countries [19], in the US [20] and in Canada [6]. Early diagnosis and therapy improvements,—especially in metastatic disease with the availability of targeted therapies, immunocheckpoint inhibitors and immune-based combinations—have certainly played a fundamental role in increasing the overall survival rates in these patients [21,22,23,24].
However, the decline in mortality is not homogeneous and is strongly correlated to factors such as age and sex (mortality increases only in women and only after 75 years) [2], race (mortality falls in whites but not in American Indian/Alaska Native) [25,26] and income (a high GDP is associated with better diagnoses, treatments and survival) [20,27,28].
The strong variability of incidence and mortality recorded in various countries reflects different behaviors for the reduction of risk factors and the use of more effective treatments. However, a decrease in cause-specific mortality from kidney cancer results in an increase in survival and drug-induced cardiotoxicity which can increase mortality from cardiovascular events. Cardiac toxicity, in fact, can range from asymptomatic subclinical abnormalities, such as electrocardiographic changes and left ventricular ejection fraction decline, to life-threatening events, like congestive heart failure and acute coronary syndromes [29].
The availability of population data referring to an entire territory (and not selected from hospital cases) could allow a descriptive analysis of the incidence of new cases and the trend in mortality due to specific causes over a long time period. The aim of this study was to describe incidence and mortality trends in patients with kidney cancer diagnosed from 1996 to 2020, and to provide details on cancer and CVD mortality.
2. Materials and Methods
2.1. Data Sources
Incidence data for kidney cancer for 1996–2020 were obtained from the Reggio Emilia Cancer Registry (RE-CR). The study was approved by the provincial Ethics Committee of Reggio Emilia, Protocol no. 2014/0019740, on 4 August 2014. The main information sources of the RE-CR were anatomic pathology reports, hospital discharge records, and mortality data, integrated with laboratory tests, diagnostic reports, and information from general practitioners. The RE-CR covered a population of 529,609 inhabitants and was characterized by good data quality: kidney tumors had 89% microscopic confirmations, while DCOs (Death Certificate Only) were less than 0.1%.
Kidney cancer cases were defined based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) [30] as topography C64.9. In total, 2331 cases of infiltrating malignant tumor (all morphologies) of the kidney incident in the Province of Reggio Emilia from 1996 to 2020 were included. The table shows the number of cases, the number of deaths and the percentages, while the age-standardized rates are shown in the figure with the trends from 1996 to 2020. In the same period, the number of total deaths from all causes in patients with kidney cancer was 1257. The causes of death were classified as overall mortality and cause-specific mortality. The latter was then divided into mortality from kidney cancer, mortality from other cancers, and mortality from CVD (heart disease, hypertension, cerebrovascular disease, atherosclerosis or aortic aneurysm/dissection; ICD-10 I00-I99).
Mortality data were selected based on classification C64 used in the International Classification of Disease, version 10 [31]. The objective of the analysis was to describe trends in incidence and mortality by age, sex and period and, for mortality, to separate cardiovascular causes of death from cancer mortality.
2.2. Statistical Analyses
Descriptive analyses of patient characteristics with kidney cancer were performed by number of deaths for all causes, for CVD, for kidney cancer and for other cancers. To determine the differences between these groups, we performed a one-way ANOVA test. The proportions of the causes of death were calculated by sex, age, calendar period of cancer diagnosis, and years since cancer diagnosis. We reported the trend of the proportion of CVD and malignant cancer survivors by calendar period of death.
Population estimates, which were used to derive rates, were represented by the general population of Province of Reggio Emilia recorded on January 1st of each year. Incidence rates and incidence-based mortality rates were adjusted to the 2013 European standard population and calculated per 100,000 person-years. Analyses were performed using STATA 16.1 software. In this study, we reported 95% confidence intervals (CI) and we defined a p-value < 0.05 as statistically significant.
Trends over time were analyzed by calculating the annual percent change (APC) in age-standardized rates using Joinpoint Regression.
3. Results
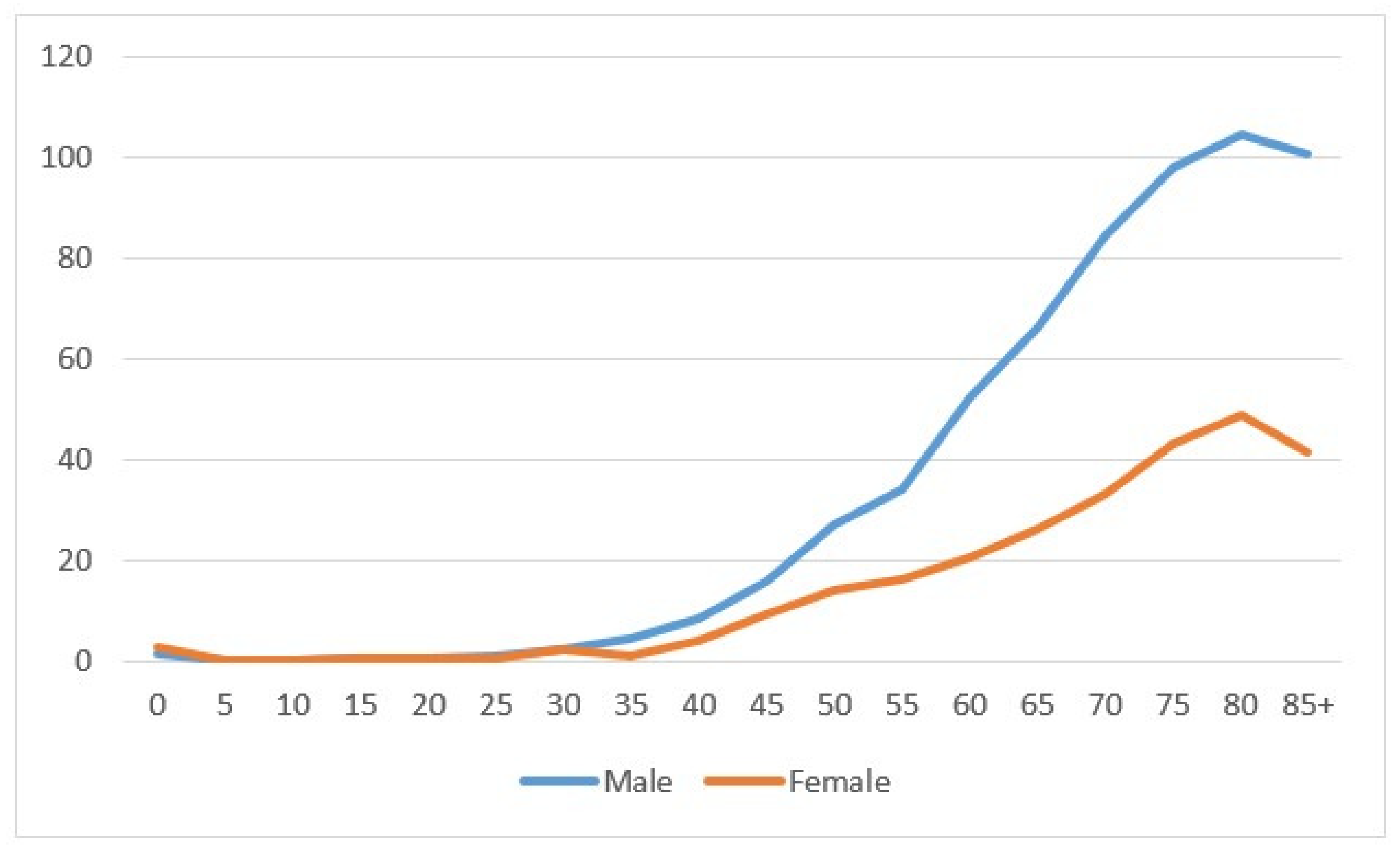
In the period 1996–2020, 2331 kidney cancers were diagnosed—1504 in males and 827 in females. The age-specific rate showed a net increase related to age, more marked in males than in females (Figure 1). Most cancers were diagnosed at the age of 60–79 (1240), followed by the age of 40–59 (535 cases) and then 80+ (490 cases); tumors diagnosed under the age of 40 were rare (66 cases). The number of cases between 1996–2000 was 349, and this increased to 559 in years 2011–2015, then showed a small decline of 512 in the most recent years 2016–2020. (Table 1).
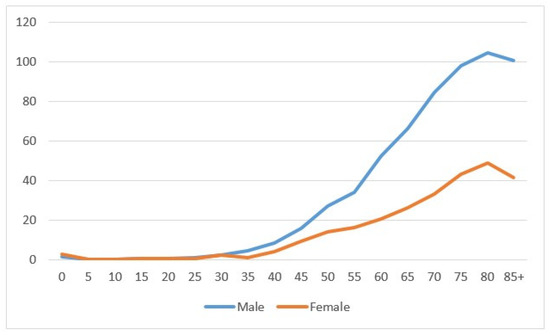
Figure 1.
Age-specific incidence rate by sex in the Province of Reggio Emilia in the period 1996–2020.

Table 1.
Cancer patients and overall and cause-specific mortality in the Province of Reggio Emilia in the period 1996–2020.
The figure shows the incidence specific rates for five-year classes for males (blue line) and females (orange line).
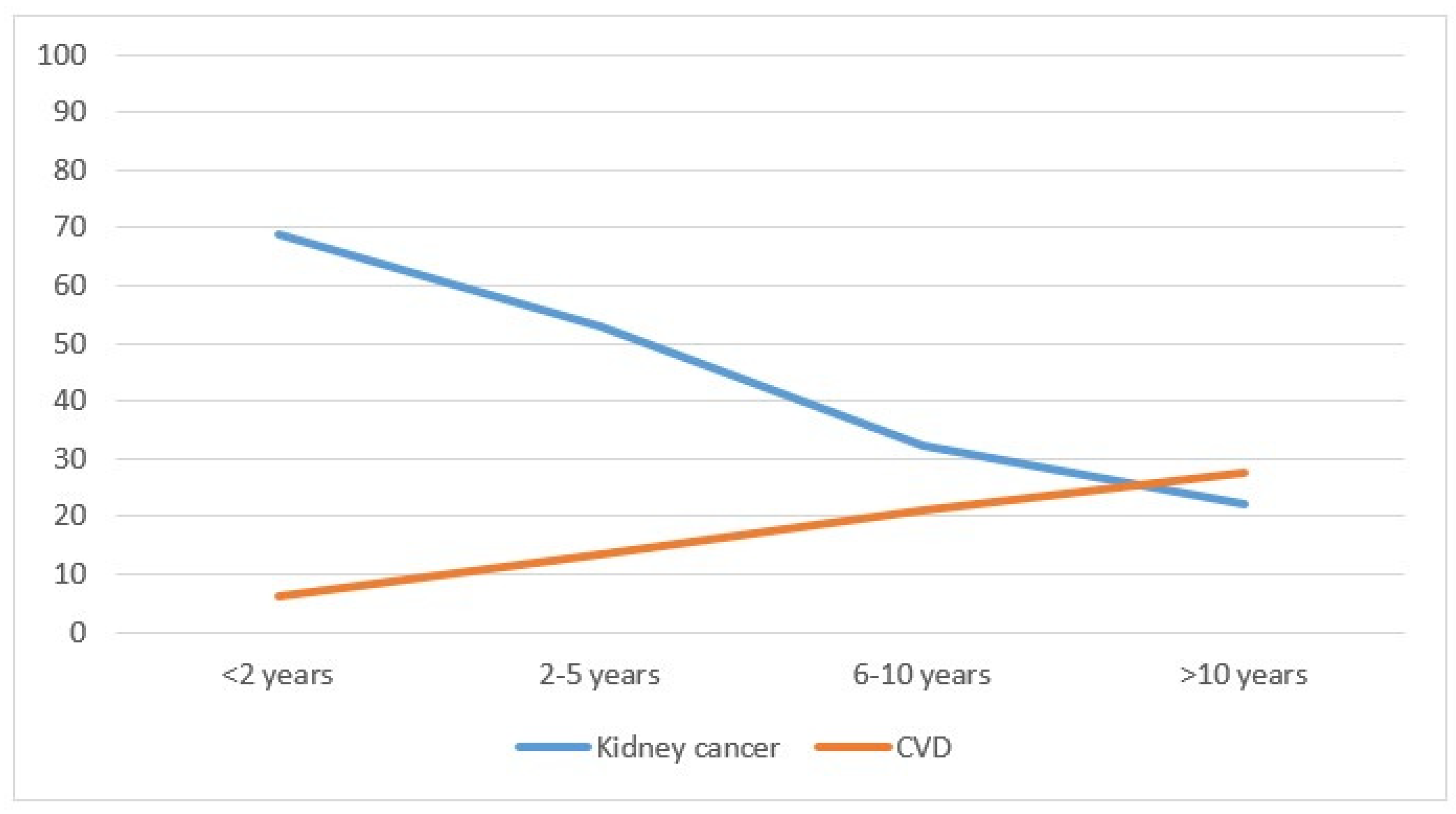
Considering mortality, kidney cancer deaths represented the majority of cases (688 cases, equal to 54.7%), followed by 231 deaths (18.4%) from other causes, including 8 from renal failure, 183 (14.6%) from other cancers and 155 (12.3%) from CVD. (Table 1). Mortality did not differ by sex, whereas the differences were significant by age: younger adults had a significantly higher mortality from kidney cancer (p < 0.01), from cardiovascular disease (p < 0.01) and from other causes (p < 0.01), while there was no significant difference for other types of cancer (p = 0.75). Cancers diagnosed after 2010 had higher mortality from kidney cancer compared with previous years (p < 0.01) and reduced mortality from CVD (p < 0.01). Kidney cancer mortality decreased with the distance from the diagnosis (from 68.9% in the first two years to 22.1% after 10 years; p < 0.01), while the opposite occurred for CVD mortality (from 6.2% to 27.5%; p < 0.01) (Table 1 and Figure 2). Considering the year of death, in 1996–2000, the percentage of patients who died from kidney cancer was 81.8%; this dropped to 43.7% in the years 2016–2020 (p < 0.01), while CVD mortality rose from 6.4% to 12.3%. The increase was not significant (p = 0.07).
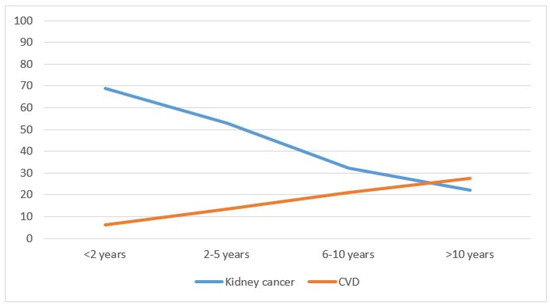
Figure 2.
Percentage of deaths from kidney cancer and cardiovascular disease by year of cancer diagnosis in the Province of Reggio Emilia in the period 1996–2020.
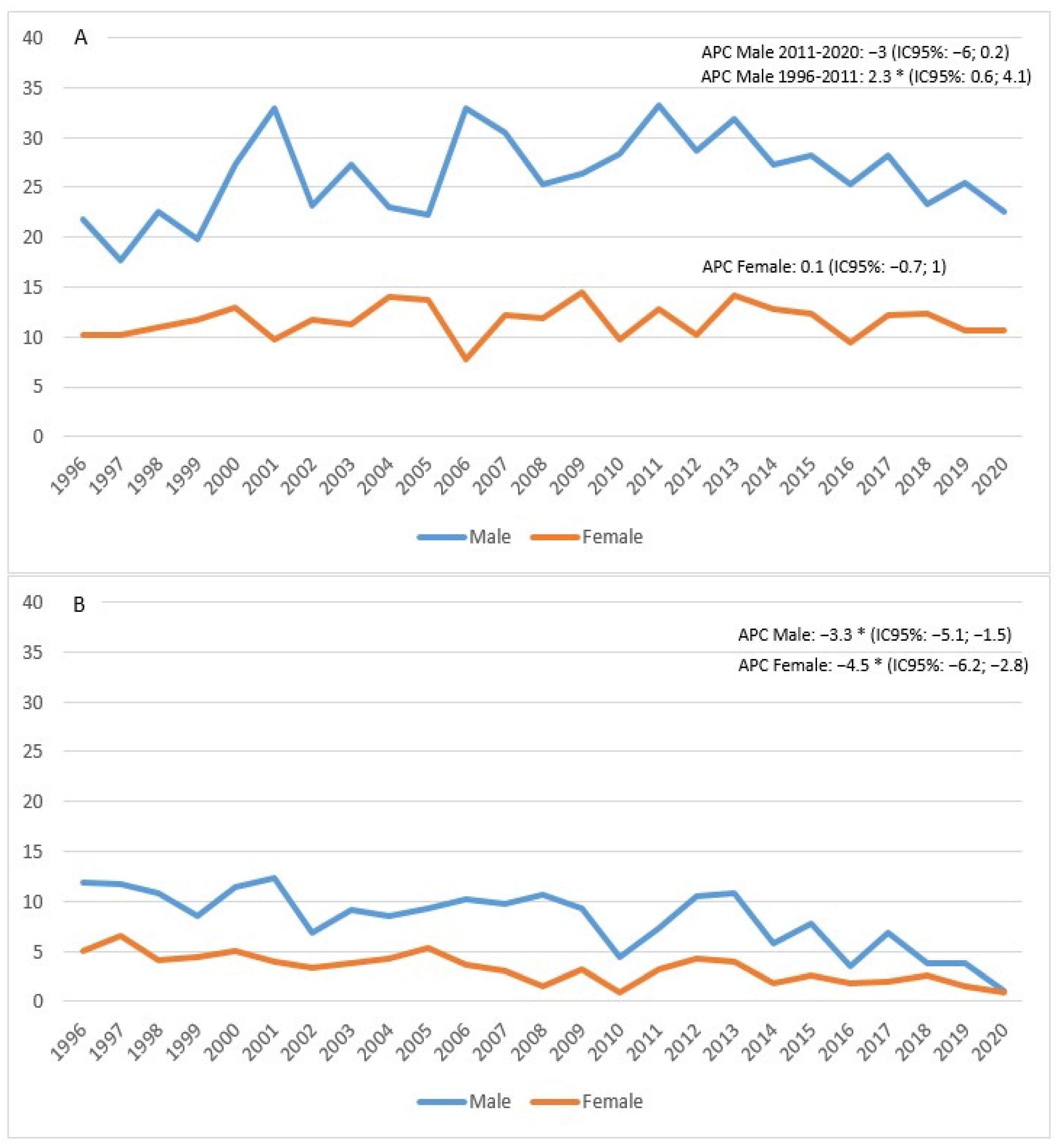
The standardized incidence rate showed an increase in males between 1996 and 2011 (APC = 2.3; 95% CI 0.6; 4.1), followed by a decline in subsequent years, although not a significant one (APC = −3; 95% CI −6; 0.2). In females, on the other hand, the trend appeared stable (APC = 0.1; 95% CI −0.7; 1) (Figure 3A). Mortality appeared to be in sharp decline in both males (APC = −3.3%; 95% CI −5.1; −1.5) and females (APC = −4.5%; 95% CI −6.2; −2.8) (Figure 3B).
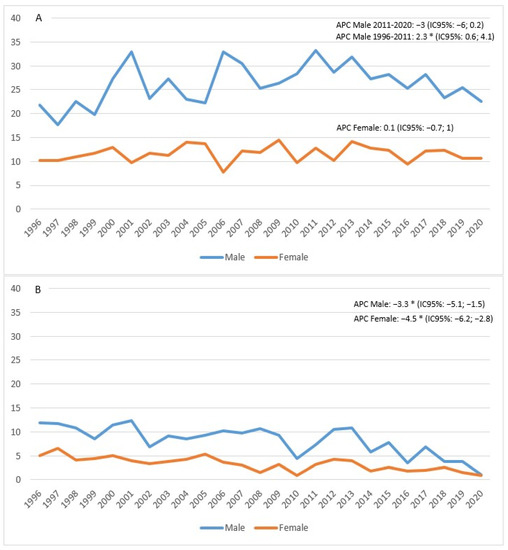
Figure 3.
Age-standardized incidence (A) and mortality (B) rates per 100,000 p-y in the Province of Reggio Emilia in the period 1996–2020.
The figure shows the APC (Annual Percentage Change) for males (blue line) and females (orange line). The asterisk indicates statistical significance * p < 0.05.
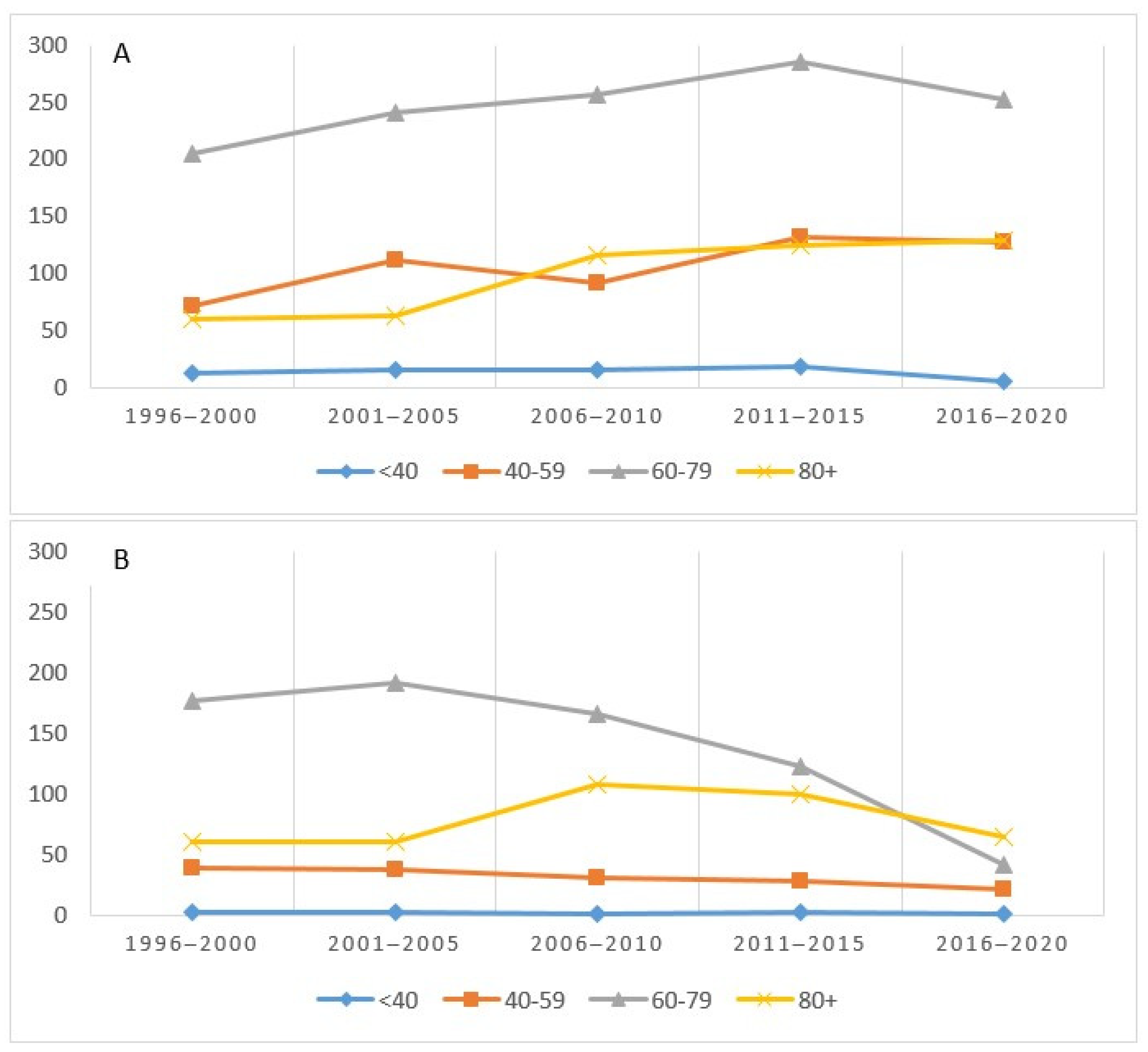
Figure 4 shows the incidence (A) and deaths (B) for the four age groups considered. The incidence, highest in the 60–79 age group, had a sharp increase until 2015 and then decreased. The trends in the 40–59 and 80+ groups were almost overlapping (Figure 4A). As regards mortality, the 60–79 age group recorded a higher absolute value in the first five years and then decreased, starting from 2006–2010, while an inverse trend was observed in those over 80 (Figure 4B).
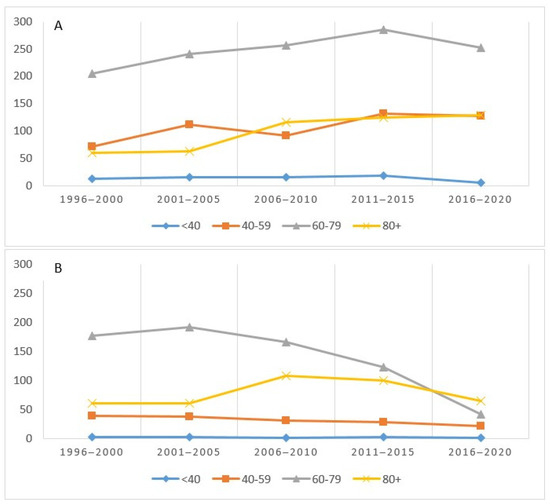
Figure 4.
Number of cases (incidence (A) and mortality (B)) by age group and period in the Province of Reggio Emilia.
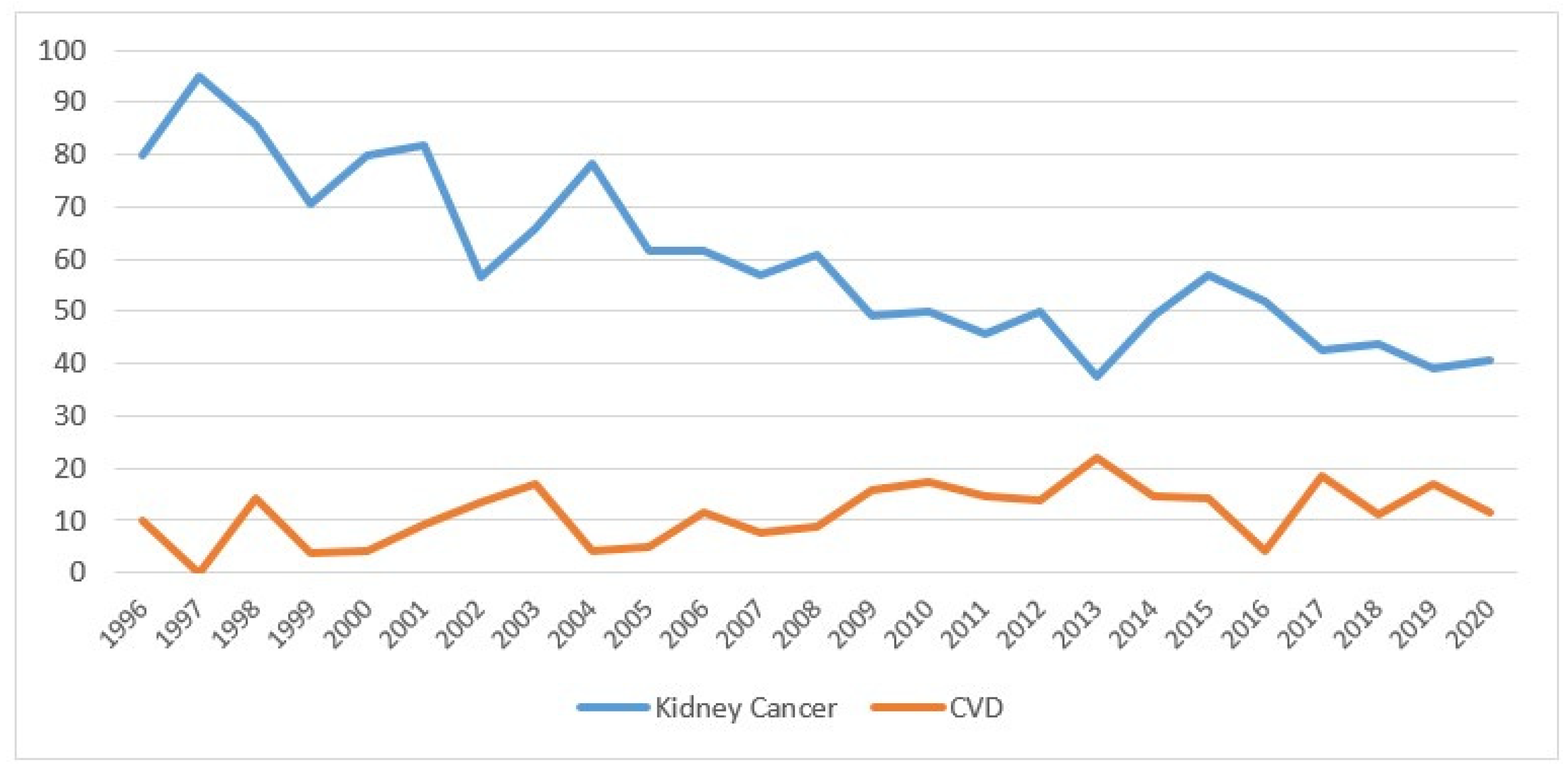
Overall, the percentage of mortality from kidney cancer decreased over the years, going from 80% in 1996 to 41% in 2020, while mortality from CVD increased slightly, starting from 2009 at 16% (Figure 5).
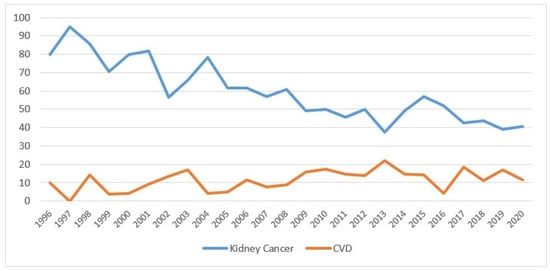
Figure 5.
Percentage of deaths for kidney cancer and cardiovascular disease by year of death (the percentages are calculated on the total of deaths from all causes) in the Province of Reggio Emilia in the period 1996–2020.
The figure shows the percentage values of mortality from kidney cancer (blue line) and from cardiovascular disease (orange line), after kidney cancer diagnosis.
4. Discussion
The aim of our study was to examine trends in incidence and mortality for tumors and for CVD in renal cell carcinoma (RCC) patients in the Province of Reggio Emilia using data updated to 2020.
The male to female ratio was about 2 to 1, lower than that in the literature [2,5], where the former study reported a ratio of 5 to 1 and the latter 3 to 1. This derived from the fact that smoking has decreased in males in recent years, which has also been confirmed by the decrease in cases of lung cancer (Figure S1). It has been confirmed that kidney cancer is a neoplasm that affects mainly the elderly, with an increase in incidence after 40 years of age and a peak after 80 years in both males and females [32].
The incidence in males increased until 2011 (Figure 3A) and then decreased in the following period. In females, the trend appeared stable. In particular, we observed a reduction in the incidence rate that dropped since 2014, but only in males, perhaps in relation to a decrease in risk factors (smoking, in particular).
Mortality, on the other hand, decreased in both sexes (Figure 3B). In 2020, the year that coincided with the COVID-19 pandemic and consequent lockdown in Italy, there was a decline in diagnoses, more evident in males. Compared to the rest of Italy, the incidence in males in our study was lower, while in females, the figure was comparable. In Italy, the incidence trend increased, but only in the 0–49 age group, as regards males (+2.5%), and in the 50–69 age group for females (+2%) [1]. In the Province of Reggio Emilia, the trend by age was higher in the 60–79 group until 2015, after which it decreased. This trend was also confirmed by a study carried out in Denmark [2].
Incidence has increased since 2000 in males under 70 years of age [2]; Chow’s study [5] also showed an increase in Europe and North America from the 1970s to the ‘90s, which then stabilized or dropped slightly, probably due to the reduction of risk factors (smoking, obesity, hypertension). De et al. [4] also confirmed an increase in incidence from 1986 to 2007, from 13.4 to 17.9 per 100,000 in males, especially in the <65 age group, and from 7.7 to 10.3 in females. There was an increase in incidence in the United States in the period 1975–2009 [6], where the standardized rate rose from 6 to 17 per 100,000 with an APC of +2.8%. An increase was also recorded for stage I, from 4 to 12 per 100,000 with an APC value of +4.5% per year, probably due to early diagnosis. Evidence suggests that incidentally discovered renal cell carcinomas continue to constitute a major segment of all newly diagnosed renal cancers. These tumors have favorable prognoses, as they are smaller and of lower stage. On the other hand, a considerable amount of RCCs are still discovered late, after metastasis has occurred. The question was, therefore, whether early detection and hence, a screening program, would be appropriate in this setting. The available evidence did not allow for a recommendation of screening for RCC, but emphasized the importance of diagnostic biopsy of small renal tumors [33]. Even in our territory, despite the absence of a dedicated diagnostic pathway, in recent years there has been an increase in ultrasonography in subjects who request it spontaneously.
An increase in the early stages of renal cell carcinoma is associated with less invasive surgery: compared with radical nephrectomy, partial nephrectomy is associated with decreased mortality and a lower rate of postoperative decline in kidney function. Hypertension and cardiovascular events are also less frequent in conservative treatments than in demolitive ones [34]. The risk of kidney failure and worsening survival after nephrectomy could be linked, as kidney failure is itself a risk factor for cardiovascular disease and mortality [35]. Even in our series, renal failure appeared in 70% of patients over 50 and in 80% of patients over 70 undergoing nephrectomy.
The study by Li et al. [26] also confirmed an increase in the incidence in the United States among Native Americans, though much less than that observed among whites. Spain also had an increase in incidence in the years 1989–1998, mainly due to an increase in early diagnoses [3]. GLOBOCAN data [36] updated to 2012 [20,27,28] also confirmed an increase in the incidence in the United States and in northern and eastern Europe.
In northern Italy, the standardized mortality rates were 11.8 and 4.3 per 100,000 for males and females, respectively, in the years 2010–2015 [1]. In the literature, the rate has remained almost constant [2,6] or has decreased slightly, in particular in Canada, where it dropped by 0.4% per year in males and 0.8% in females in the period 1986–2009 [4]. It has decreased in the United States, but only for whites, suggesting the socioeconomic disparities present there [3,26]. Indeed, some studies have shown the extent to which GDP (Gross Domestic Product) has a negative impact on developing countries. The study by Sung et al. [20] reported a drop in mortality in the United States as opposed to an increase in the African countries. Znaor et al. [28] also reported a decline in developed countries. Azawi et al. [2] showed a decline in mortality in the 60–79 age group over the period 2001–2005. Young people with kidney cancer died from the disease, while the elderly mostly died from CVD, as confirmed by the Mangone study [37]. Kidney cancer mortality, which appeared high in the first two years after diagnosis (Figure 2), tended to decrease with the passing of the years (from 68.9% to 22.1%). The opposite appeared to hold for cardiovascular mortality, which increased gradually moving away from the date of diagnosis (from 6% to 27.5%) (Table 1). Looking at calendar years, kidney cancer patients diagnosed in 1996–2000 had nearly 82% kidney cancer mortality, which nearly halved in 2016–2020. This reduction in mortality could be linked to diagnostic anticipation and, above all, to the introduction of new drugs, especially in metastatic forms. However, the lengthening of life and the addition of cardiotoxic drugs could have contributed to the increase in CVD mortality, which rose from 6% to 12% over the years (the small numbers could explain the statistical nonsignificance). The decrease of mortality in kidney cancer by almost half in the 25 years considered could be related to better (and sometimes earlier) surgery and certainly to the introduction of effective therapies in advanced disease [38,39,40,41,42,43,44,45]. During the last decade, the treatment of advanced or metastatic kidney cancer was revolutionized with the advent of antiangiogenic drugs and tyrosine-kinase inhibitors. Several agents targeting the vascular endothelial growth factor (VEGF) pathway or the mammalian target of rapamycin pathway have been progressively approved for first-line or later-line use in the treatment of patients with advanced RCC and have become the new standard of care. As a result, the survival of patients with advanced RCC has significantly improved.
Starting from 2015, the treatment of advanced RCC underwent a second revolution with the advent of immune checkpoint inhibitors, especially agents targeting the programmed cell death-1 receptor, as well as with the advent of new generation tyrosine-kinase receptor inhibitors. More recently, the outcomes of patients affected by this once-orphan disease were further improved by the use of immune-based combinations [46].
Targeted agents have significantly improved outcomes in metastatic renal cell carcinoma and have changed long-term expectation in these patients. Although we did not have direct data on individual patient treatments, we knew that during treatments, cardiovascular adverse events have been observed, mainly in the elderly and in patients with significant comorbidities. Sunitinib has been associated with congestive heart failure (CHF) and left ventricular ejection fraction (LVEF) decline in some patients, while acute coronary syndrome has been reported with sorafenib [29]. In addition, inhibition of the VEGF receptor might be responsible for the occurrence of hypertension which may cause or exacerbate other cardiovascular diseases [47]. Regarding immune checkpoint inhibitor and novel combinations, the risk of long-term cardiotoxicity is still unknown, since most of these therapies have only been approved in the last few years. Thus, sufficient long-term safety data were not yet available.
The occurrence of cardiovascular adverse events linked to anticancer therapies could partly explain the increase in mortality related to CVD observed in the same period.
A final consideration concerned the impact that COVID-19 had on new cancer diagnoses. In 2020, the Province of Reggio Emilia was deeply affected by COVID-19. However, the urinary tract showed an increased odds ratio for hospitalization but not for death [48], while the 3-month lockdown resulted in a decrease of 35% in cancer diagnoses and the number of new kidney cancer diagnoses dropped from 117 in 2019 to 107 in 2020 [49].
One of the strengths of this study was that it involved population data referring to very recent years. A significant limitation of the study was the lack of information on the tumor stage and on treatment, which certainly had a strong impact on the prognosis of this type of tumor. However, this limit was inherent in the data collected by the Population Cancer Registers which, by definition, collect few variables but refer to an entire population and not just to a hospital case history. Additionally, the availability of data updated to 2020, which represented a limit for clinical studies, is uncommon for the population CRs, which generally have a delay of 3–4 years between incidence and publication. A more specific study, updated with data from 2021, could include other variables, such as hypertension, diabetes, comorbidities, etc., that could better explain the outcome of these patients.
5. Conclusions
In 25 years of registration, the kidney cancer incidence trends appeared to be decreasing in men, probably linked to the reduction of cigarette smoking. These trends appeared stable in women. Overall mortality fell in both sexes; however, ten years after diagnosis of renal cancer, mortality from cardiovascular events exceeded that of cause-specific kidney cancer.
Overall, it was determined that it will be important to monitor patients after tumor diagnosis and to provide for a multidisciplinary approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11071048/s1, Figure S1: Reggio Emilia Province: incidence and mortality trend for lung cancer by sex, years 2019–2020.
Author Contributions
Conceptualization, L.M. and C.P.; methodology, F.M.; validation, L.M., C.P., L.T. and C.M.; formal analysis, F.M.; investigation, L.M.; writing—original draft preparation, L.M., L.T. and C.M.; writing—review and editing, I.B.; visualization, C.P., L.T. and C.M; supervision, A.N. and S.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the Italian Ministry of Health-Ricerca Corrente Annual Program 2023.
Institutional Review Board Statement
This population-based cohort study uses data from the Reggio Emilia Cancer Registry, approved by the Provincial Ethics Committee of Reggio Emilia (ref. no. 2014/0019740 of 4 August 2014). The Ethics Committee authorized, even in the absence of consent, the processing of personal data, including those suitable for revealing the state of health of patients who are deceased or untraceable for the execution of the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical and privacy issues; requests for data must be approved by the Ethics Committee after the presentation of a study protocol.
Conflicts of Interest
The authors declare no conflict of interest.
References
- AIOM-AIRTUM-SIAPEC-IAP. I Numeri del Cancro in Italia; Intermedia Editore: Brescia, Italy, 2020. [Google Scholar]
- Azawi, N.H.; Joergensen, S.M.; Jensen, N.V.; Clark, P.E.; Lund, L. Academy of Geriatric Cancer Research (AgeCare). Trends in kidney cancer among the elderly in Denmark, 1980–2012. Acta Oncol. 2016, 55 (Suppl. 1), 79–84. [Google Scholar] [CrossRef]
- López-Abente, G.; Aragonés, N.; Pérez-Gómez, B.; Ramis, R.; Vidal, E.; García-Pérez, J.; Fernández-Navarro, P.; Pollán, M. Kidney cancer mortality in Spain: Geographic patterns and possible hypotheses. BMC Cancer 2008, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Otterstatter, M.C.; Semenciw, R.; Ellison, L.F.; Marrett, L.D.; Dryer, D. Trends in incidence, mortality, and survival for kidney cancer in Canada, 1986–2007. Cancer Causes Control. 2014, 25, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Ravi, P.; Abdollah, F.; Abd-El-Barr, A.E.; Becker, A.; Popa, I.; Briganti, A.; Karakiewicz, P.I.; Trinh, Q.D.; Jewett, M.A.; et al. Contemporary incidence and mortality rates of kidney cancer in the United States. Can. Urol. Assoc. J. 2014, 8, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, L.; Tarone, R.E.; Lund, L.; Mclaughlin, J.K. Epidemiologic characteristics and risk factors for renal cell cancer. Clin. Epidemiol. 2009, 1, 33–43. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenesis Risks to Humans: Tobacco Smoke and Involuntary Smoking; International Agency for Research on Cancer: Lyon, France, 2004. [Google Scholar]
- US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General; Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2004.
- Hunt, J.D.; van der Hel, O.L.; McMillan, G.P.; Boffetta, P.; Brennan, P. Renal cell carcinoma in relation to cigarette smoking: Meta-analysis of 24 studies. Int. J. Cancer 2005, 114, 101–108. [Google Scholar] [CrossRef]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef]
- Mittal, B.V.; Singh, A.K. Hypertension in the developing world: Challenges and opportunities. Am. J. Kidney Dis. 2010, 55, 590–598. [Google Scholar] [CrossRef]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals; International Agency for Research on Cancer: Lyon, France, 1995. [Google Scholar]
- Kaelin, W.G. Von Hippel-Lindau disease. Annu. Rev. Pathol. 2007, 2, 145–173. [Google Scholar] [CrossRef]
- Janzen, N.K.; Kim, H.L.; Figlin, R.A.; Belldegrun, A.S. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol. Clin. N. Am. 2003, 30, 843–852. [Google Scholar] [CrossRef]
- Patard, J.J. Incidental renal tumours. Curr. Opin. Urol. 2009, 19, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Devesa, S.S. Contemporary epidemiology of renal cell cancer. Cancer J. 2008, 14, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.S.; Aron, M.; Gervais, D.A.; Jewett, M.A. Clinical practice. Small renal mass. N. Engl. J. Med. 2010, 362, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Mangone, L.; Bossard, N.; Marcos-Gragera, R.; Pezzarossi, A.; Roncaglia, F.; Giorgi Rossi, P.; GRELL EUROCARE-5 Working Group. Trends in net survival from kidney cancer in six European Latin countries: Results from the SUDCAN population-based study. Eur. J. Cancer Prev. 2017, 26, S121–S127. [Google Scholar] [CrossRef]
- Sung, W.W.; Wang, S.C.; Hsieh, T.Y.; Ho, C.J.; Huang, C.Y.; Kao, Y.L.; Chen, W.J.; Chen, S.L. Favorable mortality-to-incidence ratios of kidney Cancer are associated with advanced health care systems. BMC Cancer 2018, 18, 792. [Google Scholar] [CrossRef]
- Li, P.; Wong, Y.N.; Armstrong, K.; Haas, N.; Subedi, P.; Davis-Cerone, M.; Doshi, J.A. Survival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy eras. Cancer Med. 2016, 5, 169–181. [Google Scholar] [CrossRef]
- Shao, N.; Wan, F.; Abudurexiti, M.; Wang, J.; Zhu, Y.; Ye, D. Causes of Death and Conditional Survival of Renal Cell Carcinoma. Front. Oncol. 2019, 9, 591. [Google Scholar] [CrossRef]
- Kang, M.; Park, J.Y.; Jeong, C.W.; Hwang, E.C.; Song, C.; Hong, S.H.; Kwak, C.; Chung, J.; Sung, H.H.; Jeon, H.G.; et al. Changeable Conditional Survival Rates and Associated Prognosticators in Patients with Metastatic Renal Cell Carcinoma Receiving First Line Targeted Therapy. J. Urol. 2018, 200, 989–995. [Google Scholar] [CrossRef]
- Soerensen, A.V.; Donskov, F.; Hermann, G.G.; Jensen, N.V.; Petersen, A.; Spliid, H.; Sandin, R.; Fode, K.; Geertsen, P.F. Improved overall survival after implementation of targeted therapy for patients with metastatic renal cell carcinoma: Results from the Danish Renal Cancer Group (DARENCA) study-2. Eur. J. Cancer 2014, 50, 553–562. [Google Scholar] [CrossRef]
- Wallen, E.M.; Pruthi, R.S.; Joyce, G.F.; Wise, M. Urologic Diseases in America Project. Kidney cancer. J. Urol. 2007, 177, 2006. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Weir, H.K.; Jim, M.A.; King, S.M.; Wilson, R.; Master, V.A. Kidney cancer incidence and mortality among American Indians and Alaska Natives in the United States, 1990–2009. Am. J. Public Health 2014, 104 (Suppl. 3), S396–S403. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Goggins, W.B.; Yip, B.H.K.; Fung, F.D.H.; Leung, C.; Fang, Y.; Wong, S.Y.S.; Ng, C.F. Incidence and mortality of kidney cancer: Temporal patterns and global trends in 39 countries. Sci. Rep. 2017, 7, 15698. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Orphanos, G.S.; Ioannidis, G.N.; Ardavanis, A.G. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009, 48, 964–970. [Google Scholar] [CrossRef]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.; Whelan, S. International Classification of Disease for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th ed.; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rabjerg, M.; Mikkelsen, M.N.; Walter, S.; Marcussen, N. Incidental renal neoplasms: Is there a need for routine screening? A Danish single-center epidemiological study. APMIS 2014, 122, 708–714. [Google Scholar] [CrossRef]
- Capitanio, U.; Larcher, A.; Cianflone, F.; Trevisani, F.; Nini, A.; Mottrie, A.; Mari, A.; Campi, R.; Tellini, R.; Briganti, A.; et al. Hypertension and Cardiovascular Morbidity Following Surgery for Kidney Cancer. Eur. Urol. Oncol. 2020, 3, 209–215. [Google Scholar] [CrossRef]
- Li, L.; Lau, W.L.; Rhee, C.M.; Harley, K.; Kovesdy, C.P.; Sim, J.J.; Jacobsen, S.; Chang, A.; Landman, J.; Kalantar-Zadeh, K. Risk of chronic kidney disease after cancer nephrectomy. Nat. Rev. Nephrol. 2014, 10, 135–145. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Mangone, L.; Mancuso, P.; Tarantini, L.; Larocca, M.; Bisceglia, I.; Damato, A.; Giorgi Rossi, P.; Navazio, A.; Pinto, C. A Population-Based Study of Cardiovascular Disease Mortality in Italian Cancer Patients. Cancers 2021, 13, 5903. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Clin. Oncol. 2009, 7, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.E.; Szczylik, C.; Porta, C.; Bracarda, S.; Bjarnason, G.A.; Oudard, S.; Hariharan, S.; Lee, S.H.; Haanen, J.; Castellano, D.; et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: An expanded-access trial. Lancet Oncol. 2009, 10, 757–763. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. METEOR investigators. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 917–927. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Porta, C.; Rizzo, M. Recent advances in the frontline treatment of metastatic renal cell carcinoma. J. Cancer Metastasis Treat. 2021, 7, 49. [Google Scholar] [CrossRef]
- Neagoe, P.E.; Lemieux, C.; Sirois, M.G. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J. Biol. Chem. 2005, 280, 9904–9912. [Google Scholar] [CrossRef] [PubMed]
- Mangone, L.; Gioia, F.; Mancuso, P.; Bisceglia, I.; Ottone, M.; Vicentini, M.; Pinto, C.; Giorgi Rossi, P. Cumulative COVID-19 incidence, mortality and prognosis in cancer survivors: A population-based study in Reggio Emilia, Northern Italy. Int. J. Cancer 2021, 149, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Mangone, L.; Giorgi Rossi, P.; Bisceglia, I.; Grilli, R.; Pinto, C. The impact of COVID-19 on new cancer diagnoses. Ann. Oncol. Res. 2021, 1, 36–42. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).