Effects of Corilagin on Lipopolysaccharide-Induced Acute Lung Injury via Regulation of NADPH Oxidase 2 and ERK/NF-κB Signaling Pathways in a Mouse Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Experimental Procedures and Drug Treatment
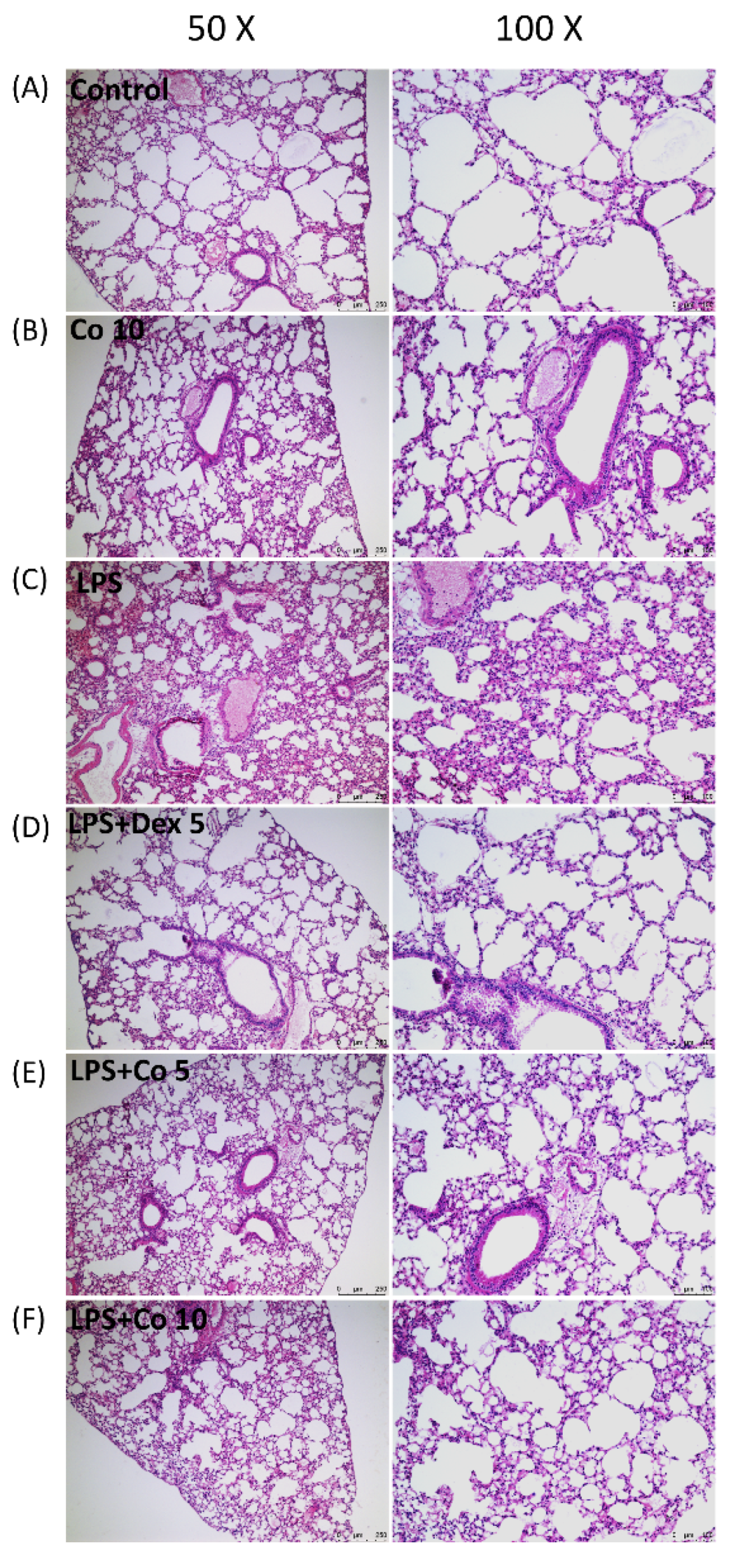
2.5. Histology Analysis of Lung Tissues
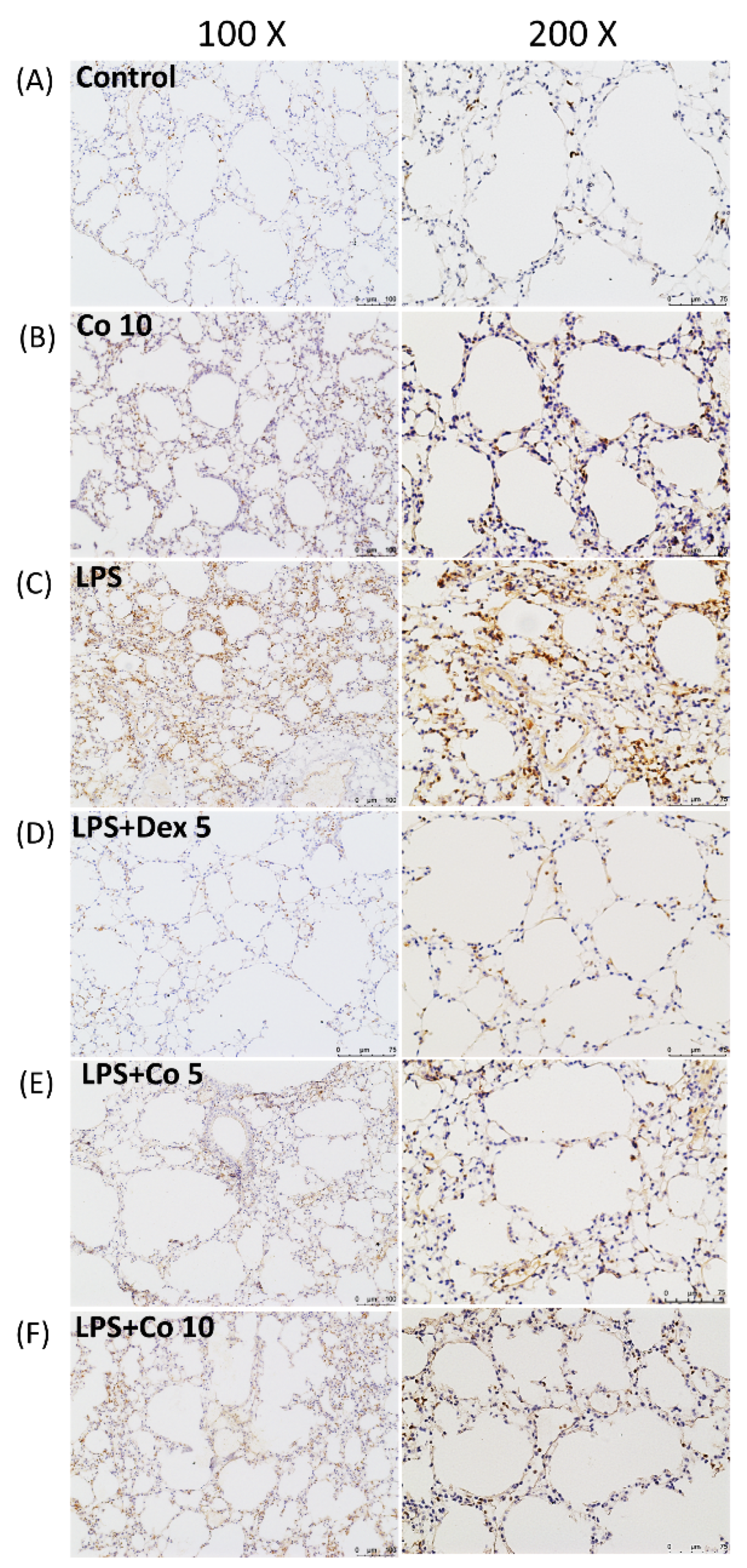
2.6. Immunohistochemistry on Lung Tissues
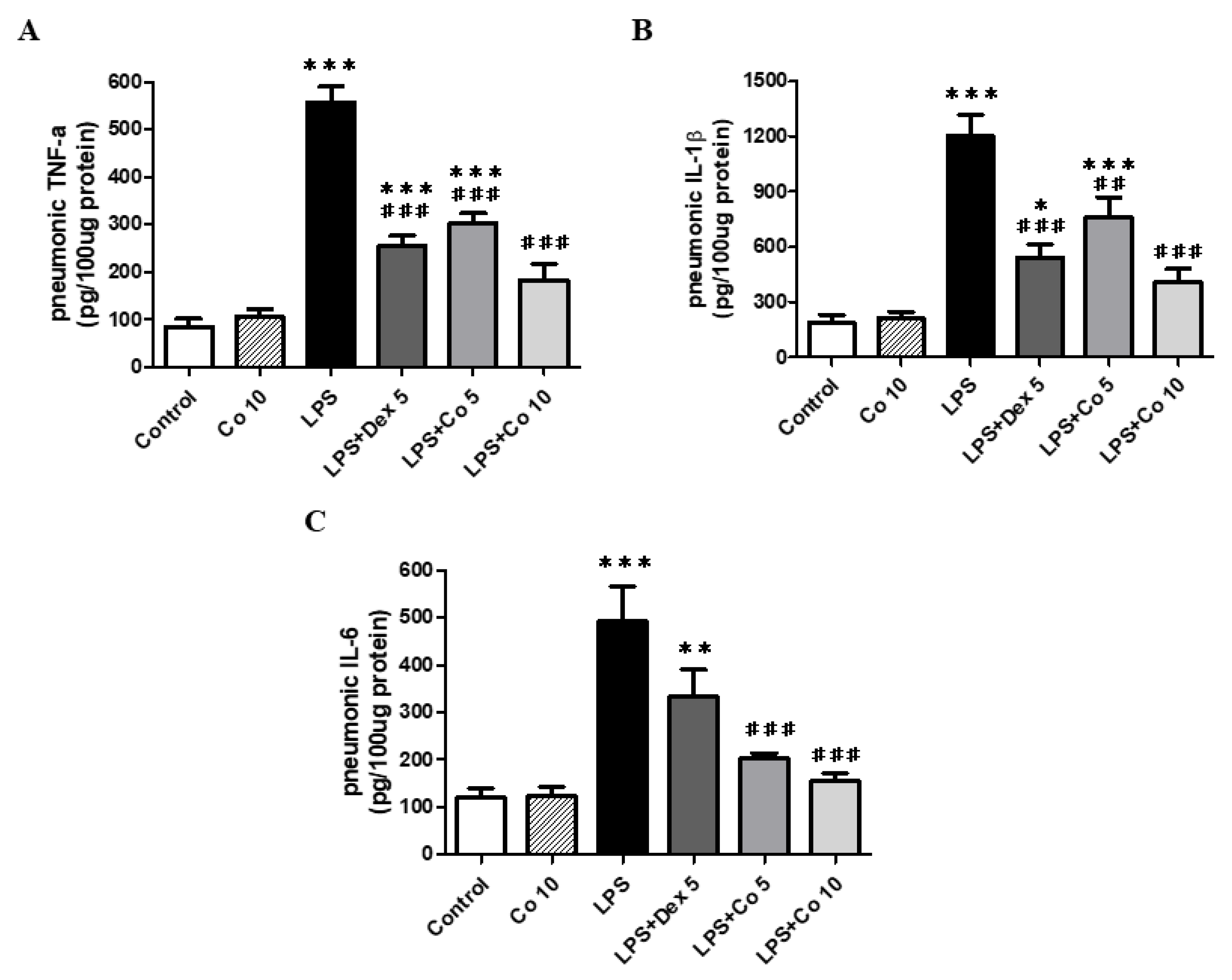
2.7. Measurement of Cytokine Levels in Lung Tissues
2.8. Measurement of Tissue Glutathione and Malondialdehyde Levels
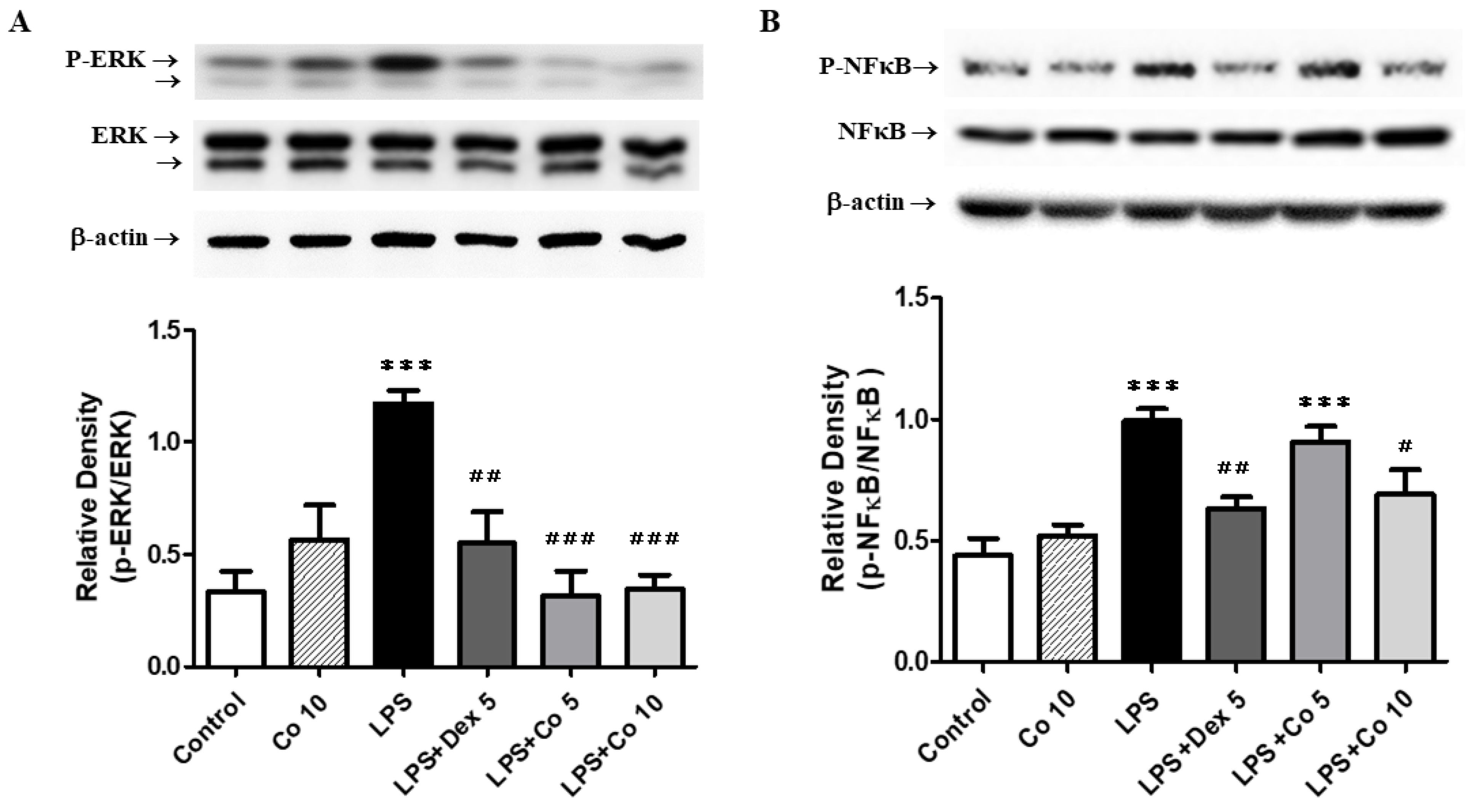
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of Corilagin on A549 Cell Viability
3.2. Effects of Corilagin on Histopathological Changes in LPS-Induced ALI
3.3. Effects of Corilagin on Neutrophil Infiltrations in LPS-Induced ALI
3.4. Effects of Corilagin on Inflammatory Cytokine Production in Lung Tissues
3.5. Effects of Corilagin on GSH and MDA Production in Lung Tissues
3.6. Effects of Corilagin on Lung NOX2 Expression in LPS-Induced ALI
3.7. Effects of Corilagin on ERK and NF-κB Expression in LPS-Induced ALI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, K.; King, L.S.; Aggarwal, N.R.; De Gorordo, A.; D’Alessio, F.R.; Kubo, K. Acute lung injury review. Intern. Med. 2009, 48, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Vincent, J.L. The changing pattern of acute respiratory distress syndrome over time: A comparison of two periods. Eur. Respir. J. 2012, 40, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, M.; Woods, C.R.; Mora, A.L.; Xu, J.; Brigham, K.L. Endotoxin-induced lung injury in mice: Structural, functional, and biochemical responses. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L333–L341. [Google Scholar] [CrossRef] [Green Version]
- Jeyaseelan, S.; Chu, H.W.; Young, S.K.; Worthen, G.S. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect. Immun. 2004, 72, 7247–7256. [Google Scholar] [CrossRef] [Green Version]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. CRC Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Yu, S.M.; Kim, S.J. The thymoquinone-induced production of reactive oxygen species promotes dedifferentiation through the ERK pathway and inflammation through the p38 and PI3K pathways in rabbit articular chondrocytes. Int. J. Mol. Med. 2015, 35, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Schuh, K.; Pahl, A. Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury. Biochem. Pharmacol. 2009, 77, 1827–1834. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Yin, C.; Shou, S.; Wang, J.; Yu, L.; Li, X.; Chai, Y. Ulinastatin Protects Against LPS-Induced Acute Lung Injury by Attenuating TLR4/NF-kappaB Pathway Activation and Reducing Inflammatory Mediators. Shock 2018, 50, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, L.; Lu, S.; Li, K.; Su, Z.; Wang, Y.; Fan, X.; Li, X.; Zhao, G. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-kappaB and PI3K/Akt/mTOR pathways. Mol. Immunol. 2018, 94, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Li, T.J.; Zhao, L.L.; Qiu, J.; Zhang, H.Y.; Bai, G.X.; Chen, L. Interleukin-17 antagonist attenuates lung inflammation through inhibition of the ERK1/2 and NF-kappaB pathway in LPS-induced acute lung injury. Mol. Med. Rep. 2017, 16, 2225–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Tasaka, S.; Amaya, F.; Hashimoto, S.; Ishizaka, A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid. Redox Signal. 2008, 10, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Pache, J.C.; Barazzone-Argiroffo, C. NOX enzymes: Potential target for the treatment of acute lung injury. Cell Mol. Life Sci. 2012, 69, 2373–2385. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wei, H.; Tien, Y.T.; Frei, B. Genetic ablation of phagocytic NADPH oxidase in mice limits TNFalpha-induced inflammation in the lungs but not other tissues. Free Radic. Biol. Med. 2011, 50, 1517–1525. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Frey, R.S.; Rahman, A.; Malik, A.B. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J. Biol. Chem. 2002, 277, 3404–3411. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Cheng, D.; Tao, J.Y.; Zhang, S.L.; Pang, R.; Guo, Y.J.; Ye, P.; Dong, J.H.; Zhao, L. Anti-inflammatory and anti-oxidative effects of corilagin in a rat model of acute cholestasis. BMC Gastroenterol. 2013, 13, 79. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Zhang, G.; Li, Y.; Xu, J.; Yuan, J.; Zhang, B.; Hu, T.; Song, G. Corilagin inhibits breast cancer growth via reactive oxygen species-dependent apoptosis and autophagy. J. Cell. Mol. Med. 2018, 22, 3795–3807. [Google Scholar] [CrossRef] [Green Version]
- Gambari, R.; Borgatti, M.; Lampronti, I.; Fabbri, E.; Brognara, E.; Bianchi, N.; Piccagli, L.; Yuen, M.C.; Kan, C.W.; Hau, D.K.; et al. Corilagin is a potent inhibitor of NF-kappaB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int. Immunopharmacol. 2012, 13, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Inoue, Y.; Nakama, S.; Ichiba, T.; Aniya, Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine 2007, 14, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Muresan, X.M.; Cervellati, F.; Sticozzi, C.; Belmonte, G.; Chui, C.H.; Lampronti, I.; Borgatti, M.; Gambari, R.; Valacchi, G. The loss of cellular junctions in epithelial lung cells induced by cigarette smoke is attenuated by corilagin. Oxid. Med. Cell. Longev. 2015, 2015, 631758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Guo, Q.Y.; Zhang, X.J.; Li, X.; Li, W.T.; Ma, X.T.; Ma, L.J. Corilagin attenuates aerosol bleomycin-induced experimental lung injury. Int. J. Mol. Sci. 2014, 15, 9762–9779. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Fan, L.; Li, Y.; Zou, Z.; Scott, M.J.; Xiao, G.; Li, S.; Billiar, T.R.; Wilson, M.A.; Shi, X.; et al. Neutrophils counteract autophagy-mediated anti-inflammatory mechanisms in alveolar macrophage: Role in posthemorrhagic shock acute lung inflammation. J. Immunol. 2014, 193, 4623–4633. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Johnston, L.K.; Rims, C.R.; Gill, S.E.; McGuire, J.K.; Manicone, A.M. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012, 47, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.T.; Yang, C.M. Inflammatory signalings involved in airway and pulmonary diseases. Mediators Inflamm. 2013, 2013, 791231. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Yeo, C.D.; Lee, H.Y.; Rhee, C.K.; Kim, I.K.; Lee, D.G.; Lee, S.H.; Kim, J.W. Inhibition of neutrophil elastase contributes to attenuation of lipopolysaccharide-induced acute lung injury during neutropenia recovery in mice. J. Anesth. 2017, 31, 397–404. [Google Scholar] [CrossRef]
- Kang, O.H.; Choi, J.G.; Lee, J.H.; Kwon, D.Y. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells. Molecules 2010, 15, 385–398. [Google Scholar] [CrossRef] [Green Version]
- Proudfoot, A.; Bayliffe, A.; O’Kane, C.M.; Wright, T.; Serone, A.; Bareille, P.J.; Brown, V.; Hamid, U.I.; Chen, Y.; Wilson, R.; et al. Novel anti-tumour necrosis factor receptor-1 (TNFR1) domain antibody prevents pulmonary inflammation in experimental acute lung injury. Thorax 2018, 73, 723–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jantan, I.; Ilangkovan, M.; Yuandani; Mohamad, H.F. Correlation between the major components of Phyllanthus amarus and Phyllanthus urinaria and their inhibitory effects on phagocytic activity of human neutrophils. BMC Complement. Altern. Med. 2014, 14, 429. [Google Scholar] [CrossRef] [Green Version]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Hong, L.; Tian, Y.; Yin, C.; Zhu, C.; Feng, H. Corilagin alleviates acetaminophen-induced hepatotoxicity via enhancing the AMPK/GSK3beta-Nrf2 signaling pathway. Cell Commun. Signal. 2019, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Kadiiska, M.B.; Ghio, A.J.; Corbett, J.; Fann, Y.C.; Holland, S.M.; Thurman, R.G.; Mason, R.P. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: A model for ARDS. FASEB J. 2002, 16, 1713–1720. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C.; Chatterjee, S.; Feinstein, S.I. A Peptide Inhibitor of NADPH Oxidase (NOX2) Activation Markedly Decreases Mouse Lung Injury and Mortality Following Administration of Lipopolysaccharide (LPS). Int. J. Mol. Sci. 2019, 20, 2395. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.R.; Kim, Y.H.; Hwang, J.H.; Gang, G.T.; Kim, K.S.; Lee, I.K.; Yun, B.S.; Lee, C.H. Davallialactone protects against acetaminophen overdose-induced liver injuries in mice. Food Chem. Toxicol. 2013, 58, 14–21. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular basis of NF-kappaB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Guo, S.; Yang, C.; Yang, J.; Chen, Y.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-kappaB pathway. Int. Immunopharmacol. 2018, 64, 140–150. [Google Scholar] [CrossRef]
- Harikrishnan, H.; Jantan, I.; Haque, M.A.; Kumolosasi, E. Anti-inflammatory effects of Phyllanthus amarus Schum. & Thonn. through inhibition of NF-kappaB, MAPK, and PI3K-Akt signaling pathways in LPS-induced human macrophages. BMC Complement. Altern. Med. 2018, 18, 224. [Google Scholar] [CrossRef]
- Jacob, J.; Lakshmanapermalsamy, P.; Illuri, R.; Bhosle, D.; Sangli, G.K.; Mundkinajeddu, D. In vitro Evaluation of Antioxidant Potential of Isolated Compounds and Various Extracts of Peel of Punica granatum L. Pharmacogn. Res. 2018, 10, 44–48. [Google Scholar] [CrossRef]
- Soyocak, A.; Kurt, H.; Cosan, D.T.; Saydam, F.; Calis, I.U.; Kolac, U.K.; Koroglu, Z.O.; Degirmenci, I.; Mutlu, F.S.; Gunes, H.V. Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum. Exp. Toxicol. 2019, 38, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.-C.; Liao, C.-C.; Lee, H.-C.; Chou, A.-H.; Yu, H.-P. Effects of Corilagin on Lipopolysaccharide-Induced Acute Lung Injury via Regulation of NADPH Oxidase 2 and ERK/NF-κB Signaling Pathways in a Mouse Model. Biology 2022, 11, 1058. https://doi.org/10.3390/biology11071058
Liu F-C, Liao C-C, Lee H-C, Chou A-H, Yu H-P. Effects of Corilagin on Lipopolysaccharide-Induced Acute Lung Injury via Regulation of NADPH Oxidase 2 and ERK/NF-κB Signaling Pathways in a Mouse Model. Biology. 2022; 11(7):1058. https://doi.org/10.3390/biology11071058
Chicago/Turabian StyleLiu, Fu-Chao, Chia-Chih Liao, Hung-Chen Lee, An-Hsun Chou, and Huang-Ping Yu. 2022. "Effects of Corilagin on Lipopolysaccharide-Induced Acute Lung Injury via Regulation of NADPH Oxidase 2 and ERK/NF-κB Signaling Pathways in a Mouse Model" Biology 11, no. 7: 1058. https://doi.org/10.3390/biology11071058





