Near-Infrared Spectroscopy Used to Assess Physiological Muscle Adaptations in Exercise Clinical Trials: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
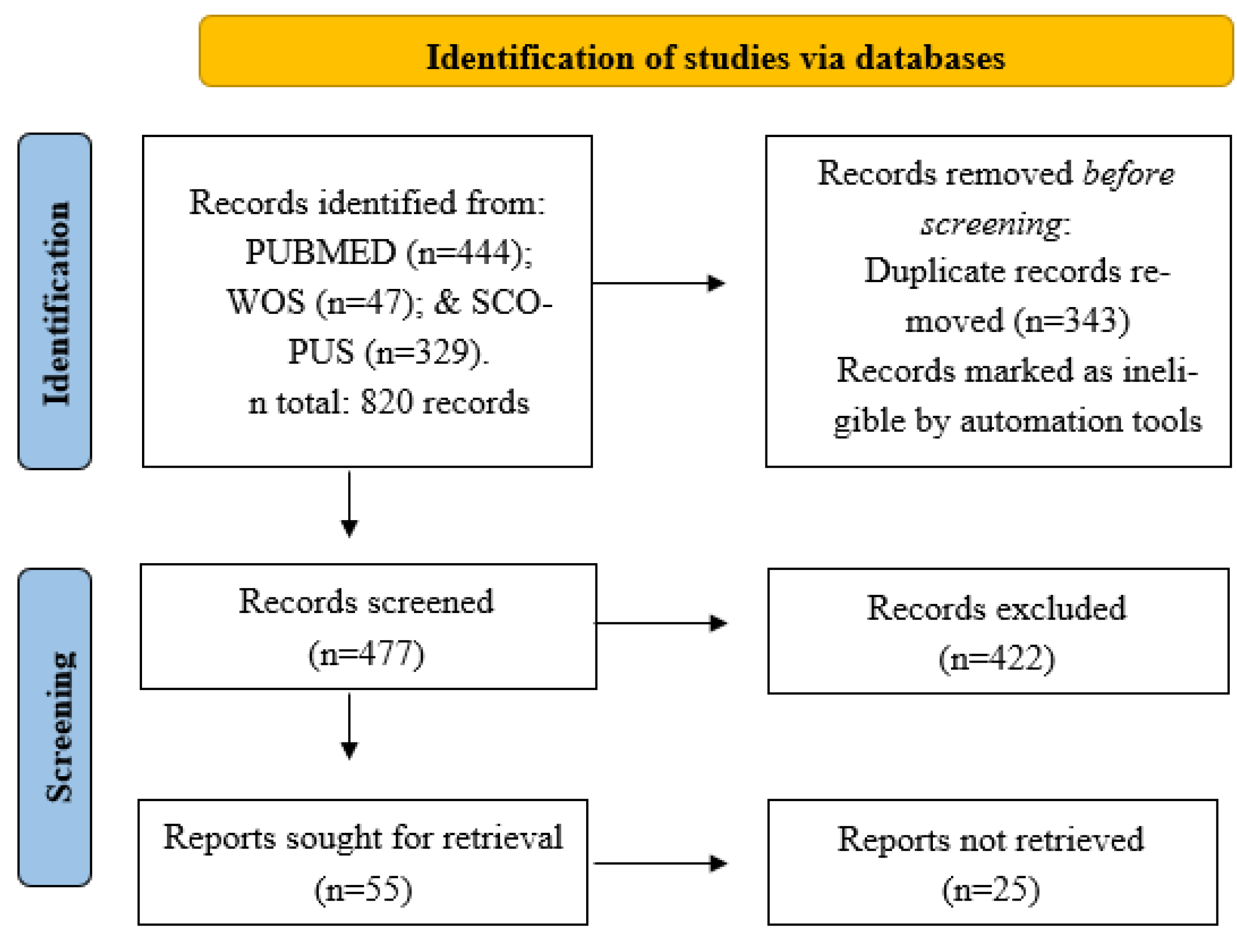
2.1. Scientific Literature Search
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
3. Results and Discussion
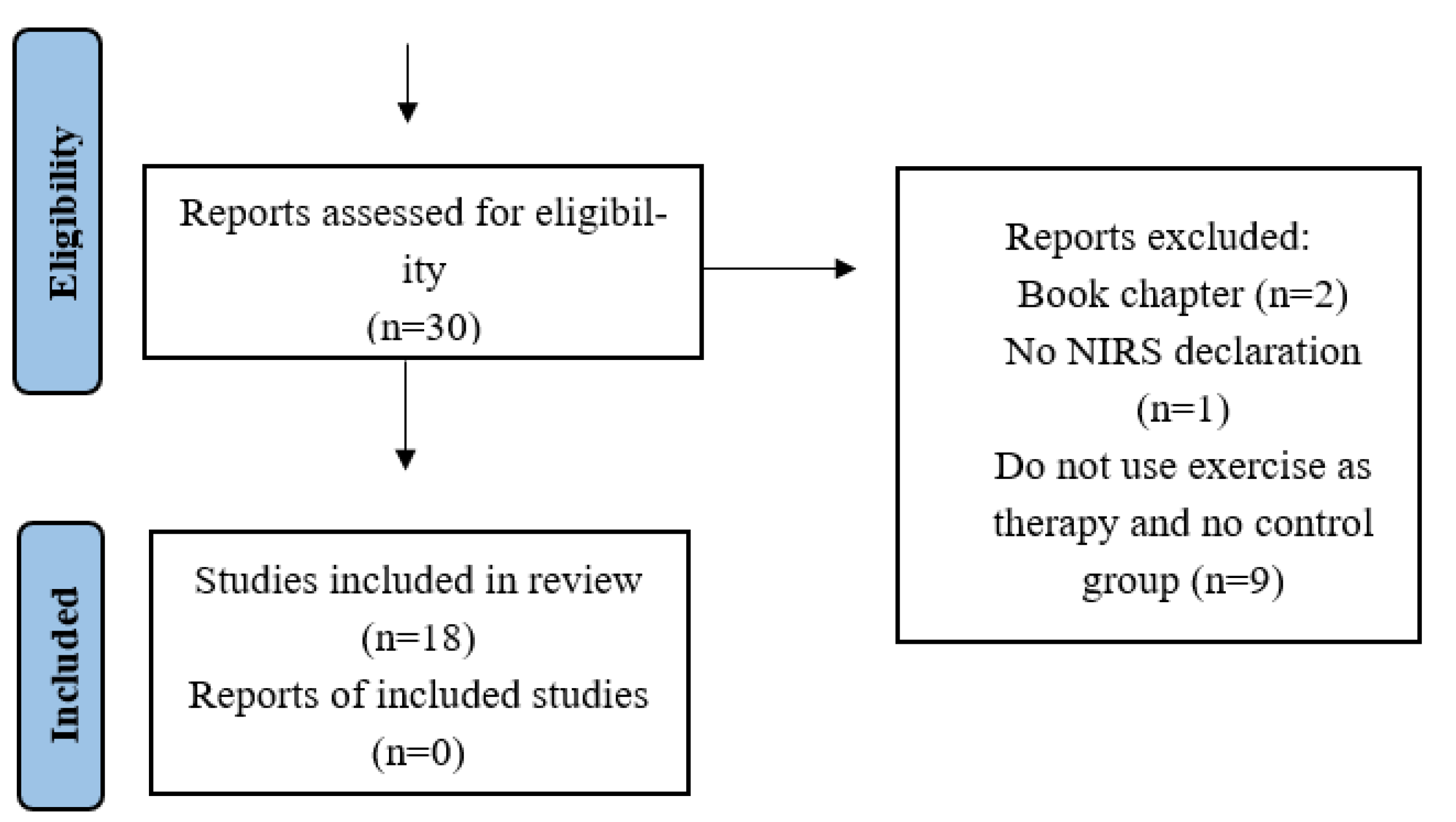
3.1. Description of the Selected Studies
3.2. Methodological Quality Assessment
3.3. Characteristics of the NIRS Devices in Exercise Clinical Trials
3.4. Adaptations in the Oxygenation, Blood Flow, and Muscle Metabolism in Exercise Clinical Trials
3.4.1. Peripheral Artery Disease
3.4.2. Metabolic Muscle Diseases
3.4.3. Chronic Kidney Disease
3.4.4. Type 2 Diabetes Mellitus
3.4.5. Heart Failure
3.4.6. Acute Myocardial Infarction
3.4.7. Orthopedic Disorders
3.4.8. Multiple Sclerosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Steps | Strategy | PubMed (444) | WoS (47) | Scopus (302) |
|---|---|---|---|---|
| 1 | Spectroscopy, Near-Infrared | 1101 | 473 | 1621 |
| 2 | NIRS | 417 | 150 | 558 |
| 3 | Exercise | 65,369 | 21,026 | 81,580 |
| 4 | Exercise therapy | 33,931 | 1299 | 14,021 |
| 5 | Physical exertion | 5735 | 58 | 1050 |
| 6 | Physical Fitness | 6696 | 543 | 3968 |
| 7 | Sports | 34,562 | 2502 | 9176 |
| 8 | Exercise Movement Techniques | 2820 | 24 | 321 |
| 9 | #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 74,620 | 24,939 | 87,414 |
| 10 | #1 AND #9 | 343 | 37 | 246 |
| 11 | #2 AND #9 | 101 | 10 | 83 |
References
- Weibel, E.R.; Taylor, C.R.; Hoppeler, H. The concept of symmorphosis: A testable hypothesis of structure-function relationship. Proc. Natl. Acad. Sci. USA 1991, 88, 10357–10361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamaoka, T.; McCully, K.K.; Niwayama, M.; Chance, B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: Recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4591–4604. [Google Scholar] [CrossRef] [Green Version]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef] [Green Version]
- Elcadi, G.H.; Forsman, M.; Crenshaw, A.G. The relationship between oxygenation and myoelectric activity in the forearm and shoulder muscles of males and females. Eur. J. Appl. Physiol. 2011, 111, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; McCully, K.K.; Quaresima, V.; Yamamoto, K.; Chance, B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J. Biomed. Opt. 2007, 12, 062105. [Google Scholar] [CrossRef] [PubMed]
- Özyener, F. Evaluation of intra-muscular oxygenation during exercise in humans. J. Sport. Sci. Med. 2002, 1, 15–19. [Google Scholar]
- Boushel, R.; Langberg, H.; Olesen, J.; Gonzales-Alonzo, J.; Bülow, J.; Kjær, M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand. J. Med. Sci. Sport. 2001, 11, 213–222. [Google Scholar] [CrossRef]
- Ramírez-García, S.; Carranza-Castro, P.H.; Gutiérrez-Salinas, J.; García-Ortiz, L.; Hernández-Rodríguez, S. Aplicación en medicina de la espectroscopia de infrarrojo cercano. Med. Interna. Mex. 2012, 28, 365–370. [Google Scholar]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, techniques, and limitations of near infrared spectroscopy. Can. J. Appl.Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [Green Version]
- Owen-Reece, H.; Smith, M.; Elwell, C.E.; Goldstone, J.C. Near infrared spectroscopy. Br. J. Anaesth. 1999, 82, 418–426. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery Res. 2016, 16, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanni, A.A.; McCully, K.K. Interpretation of Near-Infrared Spectroscopy (NIRS) Signals in Skeletal Muscle. J. Func. Morphol. Kinesiol. 2019, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatterer, H.; Menz, V.; Salazar-Martinez, E.; Sumbalova, Z.; Garcia-Souza, L.F.; Velika, B.; Gnaiger, E.; Burtscher, M. Exercise performance, muscle oxygen extraction and blood cell mitochondrial respiration after repeated-sprint and sprint interval training in hypoxia: A pilot study. J. Sport. Sci. Med. 2018, 17, 339–347. [Google Scholar]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta. 2018, 1026, 8–36. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.N.; George, M.A.; Proctor, D.N.; Murias, J.M. Differences in vascular function between trained and untrained limbs assessed by near-infrared spectroscopy. Eur. J. Appl. Physiol. 2018, 118, 2241–2248. [Google Scholar] [CrossRef]
- Hamasaki, A.; Arima, S.; Hirakoba, K. Changes in pulmonary oxygen uptake and muscle deoxygenation kinetics during cycling exercise in older women performing walking training for 12 weeks. Eur. J. Appl. Physiol. 2018, 118, 2179–2188. [Google Scholar] [CrossRef]
- Perrey, S.; Ferrari, M. Muscle Oximetry in Sports Science: A Systematic Review. Sport Med. 2018, 48, 597–616. [Google Scholar] [CrossRef]
- Ding, H.; Wang, G.; Lei, W.; Wang, R.; Huang, L.; Xia, Q.; Wu, J. Non-invasive quantitative assessment of oxidative metabolism in quadriceps muscles by near infrared spectroscopy. Br. J. Sports. Med. 2001, 35, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Neary, J.P. Application of near infrared spectroscopy to exercise sports science. Can. J. Appl. Physiol. 2004, 29, 488–503. [Google Scholar] [CrossRef]
- Davis, S.L.; Fadel, P.J.; Cui, J.; Thomas, G.D.; Crandall, C.G. Skin blood flow influences near-infrared spectroscopy-derived measurements of tissue oxygenation during heat stress. J. Appl. Physiol. 2006, 100, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.F.; Koga, S.; Barstow, T.J. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J. Appl. Physiol. 2007, 103, 1999–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, D.M.; Bolinger, L.; Li, H.; Kendrick, K.; Chance, B.; Wilson, J.R. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994, 77, 2740–2747. [Google Scholar] [CrossRef] [PubMed]
- Seiyama, A.; Hazeki, O.; Tamura, M. Noninvasive quantitative analysis of blood oxygenation in rat skeletal muscle. J. Biochem. 1988, 103, 419–424. [Google Scholar] [CrossRef]
- Jones, B.; Dat, M.; Cooper, C.E. Underwater near-infrared spectroscopy measurements of muscle oxygenation: Laboratory validation and preliminary observations in swimmers and triathletes. J. Biomed. Opt. 2014, 19, 127002. [Google Scholar] [CrossRef] [Green Version]
- Kilic, I.D.; Caiazzo, G.; Fabris, E.; Serdoz, R.; Abou-Sherif, S.; Madden, S.; Moreno, P.R.; Goldstein, J.; Di Mario, C. Near-infrared spectroscopy-intravascular ultrasound: Scientific basis and clinical applications. Eur. Heart J. Cardiovasc. Imag. 2015, 16, 1299–1306. [Google Scholar] [CrossRef]
- Negi, S.I.; Didier, R.; Ota, H.; Magalhaes, M.A.; Popma, C.J.; Kollmer, M.R.; Spad, M.A.; Torguson, R.; Suddath, W.; Satler, L.F.; et al. Role of near-infrared spectroscopy in intravascular coronary imaging. Cardiovasc. Revascularization Med. 2015, 16, 299–305. [Google Scholar] [CrossRef]
- Hampton, D.A.; Schreiber, M.A. Near infrared spectroscopy: Clinical and research uses. Transfusion 2013, 53, 52–58. [Google Scholar] [CrossRef]
- Sakudo, A. Near-infrared spectroscopy for medical applications: Current status and future perspectives. Clin. Chim. Acta 2016, 455, 181–188. [Google Scholar] [CrossRef]
- Manfredini, F.; Malagoni, A.M.; Mandini, S.; Felisatti, M.; Mascoli, F.; Basaglia, N.; Manfredini, R.; Mikhailidis, D.P.; Zamboni, P. Near-infrared spectroscopy assessment following exercise training in patients with intermittent claudication and in untrained healthy participants. Vasc. Endovascular. Surg. 2012, 46, 315–324. [Google Scholar] [CrossRef]
- Manfredini, F.; Lamberti, N.; Malagoni, A.M.; Felisatti, M.; Zuccalà, A.; Torino, C.; Tripepi, G.; Catizone, L.; Mallamaci, F.; Zoccali, C.; et al. The role of deconditioning in the end-stage renal disease myopathy: Physical exercise improves altered resting muscle oxygen consumption. Am. J. Nephrol. 2015, 41, 329–336. [Google Scholar] [CrossRef]
- Chen, M.L.; Lin, B.S.; Su, C.W.; Lin, Y.B.; Chen, M.Y.; Shen, J.H.; Chang, C.C. The application of wireless near infrared spectroscopy on detecting peripheral circulation in patients with diabetes foot ulcer when doing Buerger’s exercise. Lasers. Surg. Med. 2017, 49, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Delagarde, H.; Ouadraougo, N.; Grall, S.; Macchi, L.; Roy, P.M.; Abraham, P.; Prunier, F. Remote ischaemic preconditioning in intermittent claudication. Arch. Cardiovasc. Dis. 2015, 108, 472–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layec, G.; Hart, C.R.; Trinity, J.D.; Kwon, O.S.; Rossman, M.J.; Broxterman, R.M.; Le Fur, Y.; Jeong, E.K.; Richardson, R.S. Oxygen delivery and the restoration of the muscle energetic balance following exercise: Implications for delayed muscle recovery in patients with COPD. Am. J. Physiol. Endocrinol Metab. 2017, 313, E94–E104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julian, V.; Thivel, D.; Pereira, B.; Costes, F.; Richard, R.; Duclos, M. Improving peripheral and central vascular adjustments during exercise through a training program in adolescents with obesity. Obes. Facts 2016, 9, 321–331. [Google Scholar] [CrossRef]
- Müller-Mottet, S.; Hildenbrand, F.F.; Keusch, S.; Hasler, E.; Maggiorini, M.; Speich, R.; Bloch, K.E.; Ulrich, S. Effects of exercise and vasodilators on cerebral tissue oxygenation in pulmonary hypertension. Lung 2015, 193, 113–120. [Google Scholar] [CrossRef]
- Archiza, B.; Andaku, D.K.; Caruso, F.C.R.; Bonjorno, J.C., Jr.; Oliveira, C.R.D.; Ricci, P.A.; Amaral, A.C.D.; Mattiello, S.M.; Libardi, C.A.; Phillips, S.A.; et al. Effects of inspiratory muscle training in professional women football players: A randomized sham-controlled trial. J. Sports Sci. 2018, 36, 771–780. [Google Scholar] [CrossRef]
- Soares, R.N.; Colosio, A.L.; Murias, J.M.; Pogliaghi, S. Noninvasive and in vivo assessment of upper and lower limb skeletal muscle oxidative metabolism activity and microvascular responses to glucose ingestion in humans. Appl. Physiol. Nutr. Metab. 2019, 44, 10, 1105–1111. [Google Scholar] [CrossRef]
- Soares, R.N.; Reimer, R.A.; Doyle-Baker, P.K.; Murias, J.M. Metabolic inflexibility in individuals with obesity assessed by near-infrared spectroscopy. Diab. Vasc. Dis. Res. 2017, 14, 502–509. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, n71. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Beckitt, T.A.; Day, J.; Morgan, M.; Lamont, P.M. Calf muscle oxygen saturation and the effects of supervised exercise training for intermittent claudication. J. Vasc. Surg. 2012, 56, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Baker, W.B.; Li, Z.; Schenkel, S.S.; Chandra, M.; Busch, D.R.; Englund, E.K.; Schmitz, K.H.; Yodh, A.G.; Floyd, T.F.; Mohler, E.R., III. Effects of exercise training on calf muscle oxygen extraction and blood flow in patients with peripheral artery disease. J. Appl. Physiol. 2017, 123, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.G.; McBurney, C.; Butler, J.; Jelinek, C.; O’Connell, S.; Fritschi, C.; Reda, D. The Effects of Walking or Walking-with-Poles Training on Tissue Oxygenation in Patients with Peripheral Arterial Disease. Int. J. Vasc. Med. 2012, 2012, 985025. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.C.; Wang, C.H.; Lin, P.S.; Hsu, C.C.; Cherng, W.J.; Huang, S.C.; Liu, M.H.; Chiang, C.L.; Wang, J.S. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int. J. Cardiol. 2013, 167, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: A randomized controlled trial. J. Am. Heart Assoc. 2014, 3, e001107. [Google Scholar] [CrossRef] [Green Version]
- Gildea, N.; McDermott, A.; Rocha, J.; O’Shea, D.; Green, S.; Egaña, M. Time-course of Vo2 kinetics responses during moderate-intensity exercise subsequent to HIIT versus moderate-intensity continuous training in type 2 diabetes. J. Appl. Physiol. 2021, 130, 1646–1659. [Google Scholar] [CrossRef]
- Guimarães, G.V.; Ribeiro, F.; Castro, R.E.; Roque, J.M.; Machado, A.D.T.; Antunes-Correa, L.M.; Ferreira, S.A.; Bocchi, E.A. Effects of the exercise training on skeletal muscle oxygen consumption in heart failure patients with reduced ejection fraction. Int. J. Cardiol. 2021, 343, 73–79. [Google Scholar] [CrossRef]
- Kuge, N.; Suzuki, T.; Isoyama, S. Does handgrip exercise training increase forearm ischemic vasodilator responses in patients receiving hemodialysis? Tohoku J. Exp. Med. 2005, 207, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Manfredini, F.; Straudi, S.; Lamberti, N.; Patergnani, S.; Tisato, V.; Secchiero, P.; Bernardi, F.; Ziliotto, N.; Marchetti, G.; Basaglia, N.; et al. Rehabilitation Improves Mitochondrial Energetics in Progressive Multiple Sclerosis: The Significant Role of Robot-Assisted Gait Training and of the Personalized Intensity. Diagnostics 2020, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Mezzani, A.; Grassi, B.; Jones, A.M.; Giordano, A.; Corrà, U.; Porcelli, S.; Della Bella, S.; Taddeo, A.; Giannuzzi, P. Speeding of pulmonary VO2 on-kinetics by light-to-moderate- intensity aerobic exercise training in chronic heart failure: Clinical and pathophysiological correlates. Int. J. Cardiol. 2013, 167, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.P.; Ribeiro-Samora, G.A.; Britto, R.R.; Pereira, D.A.G. Effects of modified aerobic training on muscle metabolism in individuals with peripheral arterial disease: A randomized clinical trial. Sci. Rep. 2019, 9, 15966. [Google Scholar] [CrossRef] [PubMed]
- Olivier, N.; Weissland, T.; Legrand, R.; Berthoin, S.; Rogez, J.; Thevenon, A.; Prieur, F. The effect of a one-leg cycling aerobic training program during the rehabilitation period in soccer players with anterior cruciate ligament reconstruction. Clin. J. Sport Med. 2010, 20, 28–33. [Google Scholar] [CrossRef]
- Porcelli, S.; Marzorati, M.; Morandi, L.; Grassi, B. Home-based aerobic exercise training improves skeletal muscle oxidative metabolism in patients with metabolic myopathies. J. Appl. Physiol. 2016, 121, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Søgaard, K.; Blangsted, A.K.; Nielsen, P.K.; Hansen, L.; Andersen, L.L.; Vedsted, P.; Sjøgaard, G. Changed activation, oxygenation, and pain response of chronically painful muscles to repetitive work after training interventions: A randomized controlled trial. Eur. J. Appl. Physiol. 2012, 112, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Takagi, S.; Murase, N.; Kime, R.; Niwayama, M.; Osada, T.; Katsumura, T. Aerobic training enhances muscle deoxygenation in early post-myocardial infarction. Eur. J. Appl. Physiol. 2016, 116, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Tew, G.; Nawaz, S.; Zwierska, I.; Saxton, J.M. Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin. Sci. 2009, 117, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutron, I.; Guittet, L.; Estellat, C.; Moher, D.; Hróbjartsson, A.; Ravaud, P. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Med. 2007, 4, e61. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Miranda-Fuentes, C.; Chirosa-Ríos, L.J.; Guisado-Requena, I.M.; Delgado-Floody, P.; Jerez-Mayorga, D. Changes in Muscle Oxygen Saturation Measured Using Wireless Near-Infrared Spectroscopy in Resistance Training: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 4293. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, F.K.; Seiberl, W. Comparison of Different Approaches Estimating Skeletal Muscle Oxygen Consumption Using Continuous-Wave Near-Infrared Spectroscopy at a Submaximal Contraction Level—A Comparative Study. Appl. Sci. 2022, 12, 2272. [Google Scholar] [CrossRef]
- Iannetta, D.; Qahtani, A.; Mattioni Maturana, F.; Murias, J.M. The near-infrared spectroscopy-derived deoxygenated haemoglobin breaking-point is a repeatable measure that demarcates exercise intensity domains. J. Sci. Med. Sport 2017, 20, 873–877. [Google Scholar] [CrossRef]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Phil. Trans. R. Soc. A 2011, 369, 4577–4590. [Google Scholar] [CrossRef]
- Malagoni, A.M.; Felisatti, M.; Mandini, S.; Mascoli, F.; Manfredini, R.; Basaglia, N.; Zamboni, P.; Manfredini, F. Resting muscle oxygen consumption by near-infrared spectroscopy in peripheral arterial disease: A parameter to be considered in a clinical setting? Angiology 2010, 61, 530–536. [Google Scholar] [CrossRef]
- Andreozzi, G.M.; Leone, A.; Laudani, R.; Deinite, G.; Martini, R. Acute impairment of the endothelial function by maximal treadmill exercise in patients with intermittent claudication, and its improvement after supervised physical training. Int. Angiol. 2007, 26, 12–17. [Google Scholar]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M.; Teague, A.M.; Casanegra, A.I. Monitored daily ambulatory activity, inflammation, and oxidative stress in patients with claudication. Angiology 2013, 65, 491–496. [Google Scholar] [CrossRef]
- Diesel, W.; Knight, B.K.; Noakes, T.D.; Swanepoel, C.R.; van Zyl Smit, R.; Kaschula, R.O.; Sinclair-Smith, C.C. Morphologic features of the myopathy associated with chronic renal failure. Am. J. Kidney Dis. 1993, 22, 677–684. [Google Scholar] [CrossRef]
- Green, H.J.; Helyar, R.; Ball-Burnett, M.; Kowalchuk, N.; Symon, S.; Farrance, B. Metabolic adaptations to training precede changes in muscle mitochondrial capacity. J. Appl. Physiol. 1992, 72, 484–491. [Google Scholar] [CrossRef]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; MacDonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef]
- Wilund, K.R.; Viana, J.L.; Perez, L.M. A Critical Review of Exercise Training in Hemodialysis Patients: Personalized Activity Prescriptions Are Needed. Exerc. Sport Sci. Rev. 2020, 48, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Tagougui, S.; Leclair, E.; Fontaine, P.; Matran, R.; Marais, G.; Aucouturier, J.; Descatoire, A.; Vambergue, A.; Oussaidene, K.; Baquet, G.; et al. Muscle oxygen supply impairment during exercise in poorly controlled type 1 diabetes. Med. Sci. Sports Exerc. 2015, 47, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyman, E.; Daussin, F.; Wieczorek, V.; Caiazzo, R.; Matran, R.; Berthon, P.; Aucouturier, J.; Berthoin, S.; Descatoire, A.; Leclair, E.; et al. Muscle Oxygen Supply and Use in Type 1 Diabetes, From Ambient Air to the Mitochondrial Respiratory Chain: Is There a Limiting Step? Diabetes Care 2020, 43, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zores, F.; Iliou, M.C.; Gellen, B.; Kubas, S.; Berthelot, E.; Guillo, P.; Bauer, F.; Lamblin, N.; Bosser, G.; Damy, T.; et al. Physical activity for patients with heart failure: Position paper from the heart failure (GICC) and cardiac rehabilitation (GERS-P) Working Groups of the French Society of Cardiology. Arch. Cardiovasc. Dis. 2019, 112, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Cattadori, G.; Segurini, C.; Picozzi, A.; Padeletti, L.; Anzà, C. Exercise and heart failure: An update. ESC Heart Fail. 2018, 5, 222–232. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Lech, G.; Nioka, S.; Intes, X.; Chance, B. Noninvasive, low-noise, fast imaging of blood volume and deoxygenation changes in muscle using light-emitting diode continuous-wave imager. Rev. Sci. Instrum. 2002, 73, 3065–3074. [Google Scholar] [CrossRef]
- Bhambhani, Y.N. Muscle oxygenation trends during dynamic exercise measured by near infrared spectroscopy. Can. J. Appl. Physiol. 2004, 29, 504–523. [Google Scholar] [CrossRef]
- Barcelos, I.P.; Troxell, R.M.; Graves, J.S. Mitochondrial Dysfunction and Multiple Sclerosis. Biology 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Willingham, T.B.; Backus, D.; McCully, K.K. Muscle Dysfunction and Walking Impairment in Women with Multiple Sclerosis. Int. J. MS Care 2019, 21, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Ryan, T.E.; Erickson, M.L.; Brizendine, J.T.; Young, H.J.; McCully, K.K. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: Correcting for blood volume changes. J. Appl. Physiol. 2012, 113, 175–183. [Google Scholar] [CrossRef]
- Heinonen, I.; Koga, S.; Kalliokoski, K.K.; Musch, T.I.; Poole, D.C. Heterogeneity of muscle blood flow and metabolism: Influence of exercise, aging, and disease states. Exerc. Sport Sci. Rev. 2015, 43, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Mac Gabhann, F.; Popel, A.S. Effects of fiber type and size on the heterogeneity of oxygen distribution in exercising skeletal muscle. PLoS ONE 2012, 7, e44375. [Google Scholar] [CrossRef] [PubMed]
| Studies | PEDro Quality Criteria | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Results | Rating | Quality | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Baker et al., 2017 [44] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 | Medium |
| Beckitt et al., 2012 [43] | Yes | No | No | Yes | No | No | No | Yes | Yes | No | Yes | 4 | Medium |
| Collins et al., 2012 [45] | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes | Yes | 6 | Medium |
| Fu et al., 2013 [46] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 | Medium |
| Gardner et al., 2014 [47] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | Medium |
| Gildea et al., 2021 [48] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | Medium |
| Guimarães et al., 2021 [49] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 | Medium |
| Kuge et al., 2005 [50] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 | Medium |
| Manfredini et al., 2012 [30] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 | Medium |
| Manfredini et al., 2015 [31] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 | Medium |
| Manfredini et al., 2020 [51] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 | Medium |
| Mezzani et al., 2013 [52] | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 7 | Medium |
| Monteiro et al., 2019 [53] | Yes | Yes | No | Yes | No | No | Yes | No | Yes | Yes | Yes | 6 | Medium |
| Olivier et al., 2010 [54] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 | Medium |
| Porcelli et al., 2016 [55] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 | Medium |
| Søgaard et al., 2012 [56] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | No | Yes | 5 | Medium |
| Takagi et al., 2016 [57] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 | Medium |
| Tew et al., 2009 [58] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | Medium |
| NIRS Device (Trademark, Model) | Technique | Measurements (Units) | Wavelength (nm) | Research Articles (Reference) |
|---|---|---|---|---|
| Artinis Medical Systems | ||||
| Portamon | CW, multi-distance | TSI (%), ∆HHb, ∆O2Hb, ∆tHb. (μmol) | 750, 760, 841, 850 | [46,53,55] |
| Oxymon Mk-III | CW, multi-distance | TSI (%), ∆HHb, ∆O2Hb, ∆tHb (μmol) | 765, 770, 850, 905 | [30,31,51] |
| Astem Co | ||||
| NIR srs Hb11 | CW, multi-distance | StO2 (%), ∆HHb, ∆O2Hb, ∆tHb (μmol) | 770, 830 | [57] |
| Hamamatsu Photonics K.K. | ||||
| NIRO-300 | CW, multi-distance | TOI (%), ∆HHb, ∆O2Hb, ∆tHb (μmol) | 776, 826, 845, 905 | [43,56,58] |
| NIRO-200 | CW, multi-distance | TOI (%), ∆HHb, ∆O2Hb, ∆tHb (μmol) | 735, 810, 850 | [48] |
| Hutchinson Technology Inc. | ||||
| Inspectra Spectrometer 325 | CW, multi-distance | StO2 (%) | 680, 720, 760, 800 | [45,47] |
| NIM Inc. | ||||
| CW, multi-distance | StO2 (%), ∆HHb, ∆O2Hb, ∆tHb (arbitrary units) | 730, 850 | [54] | |
| OMEGA | ||||
| BOM-L1TR | CW, multi-distance | StO2 (%), ∆HHb, ∆O2Hb, ∆tHb (μmol) | 730, 810, 830 | [50] |
| OMRON | ||||
| HEO-100 | CW, single-distance | ∆HHb, ∆O2Hb, ∆tHb (%) | 760, 840 | [52] |
| Thorlabs | ||||
| DCS FD-NIRS | FD, multi-distance | StO2 (%), aHHb, aO2Hb, atHb (μmol) | 685, 785, 830 | [44] |
| SS Inc., Champaign, IL | ||||
| Oxiplex TS | FD, multi-distance | SO2m (%), aHHb, aO2Hb, atHb (μmol) | 692, 834 | [49] |
| Author | Oxygenation Objective | Participants | Training Protocols | Intervention Length | Sampling Area | Results after Training Protocol |
|---|---|---|---|---|---|---|
| Baker et al., 2017 [44] | AET on microvascular blood flow and muscle oxygen extraction in PAD | 64 pt. with PAD. AET (n = 29): 66 (59, 69) y. CON (n = 35): 67 (60, 76) y | AET: 60 min of treadmill walking intervals at 2 mph with increasing gradient (2%/2 min) until claudication. CON: non-exercise | 3 ses/wk/ 3 mo | Gastrocnemius | AET: Higher blood flow and oxygen desaturation during maximal exercise test. CON: without changes |
| Beckitt et al., 2012 [43] | Exercise training (ET) versus angioplasty (AG) on oxygen muscle saturation in stable claudication patients | 56 pt. with stable claudication PAD. ET (n = 42): 66 ± 6.1 y. AG (n = 14): 68 ± 5.8 y | ET: 10′ warm-up, 5 station circuit, 8′ each station. AG: angioplasty without exercise | 2 ses/wk/ 3 mo | Lateral gastrocnemius | ET and AG: Higher reoxygenation during recovery after an ischemia occlusion AG: A lower hemoglobin desaturation during submaximal exercise test |
| Collins et al., 2012 [45] | Oxygen muscle saturation in PAD after a TWP versus a WPP exercise program in PAD | 85 pt. with PAD. TWP (n = 40): 66.8 ± 8.5 y. WPP (n = 45): 71.7 ± 9.2 y | TWP and WPP: 9 wk. for 6 min at 25–44% VO2peak (LIn), 18 min at 45–59% VO2peak (MIn), and 6 min at 60–84% VO2peak (HI) according to maximal pain tolerance. Following ~3 wk, pt. walked for 5.5 min at LI, ~25 min at MI, and ~25 min at HI (WPP: with poles) | 3 ses/wk/ 3 mo | Medial gastrocnemius | TWP: Higher muscle saturation during submaximal intensity in a treadmill exercise test. WWP: without changes |
| Fu et al., 2013 [46] | MCT and AIT on central and peripheral hemodynamics in heart failure (HF) | 45 pt. with HF. AIT (n = 15): 67.5 ± 1.8 y. MCT (n = 15): 66.3 ± 2.1 y. CON (n = 15): 67.8 ± 2.5 y | AIT: Five cycling intervals of 3 min at 80%, VO2peak interspersed with 5 intervals of 3 min at 30% VO2max. MCT: 30 min at 60% VO2peak. CON: non-exercise | 3 ses/wk/ 3 mo | Vastus lateralis quadriceps | AIT: Higher oxygen extraction muscle during all exercise in a maximal test. CON: without changes |
| Gardner et al., 2014 [47] | Muscle oxygenation in PAD after supervised exercise training (SET), home-exercise program (HEP), or an attention control group (CON) | 180 pt. with PAD. SET (n = 60) 65 ± 11 y. HEP (n = 60) 67 ± 10 y. CON (n = 60) 65 ± 9 y | SET: ITW to mild-to-moderate claudication pain at a speed of 2 mph at 40% maximal power output in maximal treadmill test with increase from 15 to 40 min. HEP: ITW to mild-to-moderate claudication pain at a self-selected pace with a step monitor, increasing 20 to 45 min per session. CON: non-exercise | 3 ses/wk/ 3 mo | Gastrocnemius | SET and HEP: Higher saturation level during submaximal intensity exercise and at half time during resting recovery. CON: without changes |
| Gildea et al., 2021 [48] | Muscle VO2 and oxygenation kinetics after HIIT and MCT in T2D | 28 pt. with T2D. MCT (n = 10): 53 ± 10 y. HIIT (n = 9): 52 ± 10 y. CON (n = 9): 54 ± 9 y | MCT: 50 min moderate-intensity cycling. HIIT: 10 reps of 1 min at 90% HRmax. CON: non-exercise | 3 ses/wk/ 3 mo | Vastus lateralis quadriceps | HIIT and MCT: Improved the VO2 kinetics (↓ tau) and decreased muscle deoxygenation (↓ ∆[HHb + Mb]/dVO2) during exercise. CON: without changes |
| Guimaraes et al., 2021 [49] | AET plus resistance training (ART) on peripheral muscular performance and muscle oxygenation in HF | 24 pt. with HF. HF-ART (n = 16): 49 ± 9 y. HF-CON (n = 8): 46 ± 5.8 y | HF-ART: 30 min of AET on cycle ergometer at CRP and 1 set of 10–15 reps (intensity 13–15 Borg scale) in 5 different resistance exercises. HF-CON: non-exercise | 3 ses/wk/ 3 mo | Vastus lateralis quadriceps | HF-ART: Higher muscle oxygenation (↓Oxy-Hb, ↓ deoxy-Hb, and ↓ tHB) during peak in an exercise test. HF-CON: without changes |
| Kuge et al., 2005 [50] | Vasodilator response, muscle oxygenation, and performance post exercise in hemodialysis patients (HP) by CKD | 15 subjects. HP (n = 8): 61.1 ± 5.8 y. CON (n = 7; healthy subjects): 58.7 ± 5.8 y | HP: Handgrip training for 4 d/wk during 6 wk (15 to 30 min app.). 50 reps at 60% MVC during 1st wk, increasing 20 reps/wk until reaching 150 reps. CON: non-exercise | 4 ses/wk/ 1.5 mo | Flexor digitorum superficialis | HP: Without changes in vasodilator response (↔[tHb]) but a higher muscle reoxygenation (StO2) after 3 min arterial occlusion. CON: without changes |
| Manfredini et al., 2012 [30] | Structured (SW) versus unstructured walking (UW) program exercise on hemodynamic, functional, and muscle VO2 | 45 pt. with PAD. SW (n = 31): 71.9 ± 6.4 y. UW: 70.3 ± 7.4 (n = 14) y. CON: (n = 15, healthy subjects): 38.3 ± 15.3 y | SW: 2 rep/d of 10 min of walking at 20–30% below pain threshold speed. UW: free walking 20 to 30 min/d to a moderate level of pain. CON: non-exercise | 6 ses/wk/ 8.5 mo | Medial gastrocnemius | SW: Increased the mVO2 (↑ the rate of increase in (HHb) during venous occlusion) to healthy subject values and perfusion (ABI) at rest, indicating normalized muscle function. Increased the distance to claudication during exercise. UW: without changes |
| Manfredini et al., 2015 [31] | Walking exercise on resting mVO2 and vascular function in myopathy for end-stage renal disease (ESRD) | 54 pt. myopathy by ESRD. EXP (n = 28): 66 ± 14 y. CON (n = 26): 68 ± 13 y | EXP: 2 rep/d of 10 min of walking at 70–120% of maximum walking speed. CON: recommendations for maintaining an active lifestyle | 4 ses/wk/ 6 mo | Medial gastrocnemius | EXP: Decreased the mVO2 (idem [30]) at rest, indicating lower muscle dysfunction. CON: without changes |
| Manfredini et al., 2020 [51] | Robot (RO)- and physiotherapist (PT)- assisted walking on mVO2 in multiple sclerosis (MS) | 46 pt. with MS and 10 control healthy subjects. MS-RO (n = 23). MS-PT (n = 23). CON (n = 10) | MS-RO: 40 min of robot-assisted walking. MS-PT: 40 min of walking assisted by physiotherapists. CON: non-exercise | 2 ses/wk/ 1.5 mo | Medial gastrocnemius | MS-RO: Decreased the mVO2 rest (idem [30]). MS-PT and CON: without changes |
| Mezzani et al., 2013 [52] | AET effects on pulmonary and muscle VO2 kinetics in heart failure | 30 pt. with HF and 7 healthy subjects. HF-AET (n = 15): 65 ± 7 y. HF-CON (n = 15): 63 ± 7 y. CON (n = 7): 66 ± 4 y | HF-EXP: 30 min cycling exercise at ventilatory threshold 1. HF-CON: habitual lifestyle and activities without a formal training protocol. H-CON: non-exercise | 5 ses/wk/ 3 mo | Vastus lateralis quadriceps | HF-EXP: Decreased pulmonary time delay of VO2 kinetics during submaximal steady-state exercise and increased peak oxygen extraction in muscle during maximal exercise test (↑ peak ∆[HHB]). HF-CON: without changes |
| Monteiro et al., 2019 [53] | Muscle oxygenation in PAD after MCT versus modified aerobic training (MAT) with a load on the lower limbs | 40 pt. with PADMCT (n = 20): 65.4 ± 10.6 y. MAT (n = 20) 63.1 ± 10.5 y | MCT: 30 min of walking on the floor and 30 min on treadmill at floor walking speed without inclination (increase of 0.2 km/h with the cessation of symptoms). MAT: 15 min of walking on the floor with ankle weights (increase progressively from 0.5 to 2 kg). Both trainings were symptoms controlled | 3 ses/wk/ 3 mo | Medial gastrocnemius | MCT and MAT: Decrease the rate of muscle desaturation (StO2) with MCT > MAT. MCT: Higher muscle saturation during maximal exercise test (↑ in exercise test duration) |
| Olivier et al., 2010 [54] | One leg cycling training on leg muscle oxygenation (LMO2) in soccer players with anterior cruciate ligament reconstruction | 24 regional-level soccer players with ACLR. EXP (n = 12): 25.1 ± 3.4 y. CON (n = 12): 23.2 ± 3.1 y | EXP: 21 min alternating 3 min at 70% HRmax and 3 min at 85% HRmax. CON: familiarization training during 10 min at 30 W | 3 ses (CON: 1 ses)/wk/ 1.5 mo | Vastus lateralis quadriceps | EXP: Increased in LMO2 (relative change in the oxy/deoxy hemoglobin/myoglobin) and blood flow (changes in tHB) during one leg maximal graded test. CON: without changes |
| Porcelli et al., 2016 [55] | Home-based AET on muscle oxygen uptake and fractional O2 extraction in mitochondrial myopathies (MM) and McArdle’s disease (McA) | 13 patients with mitochondrial myopathies. MM (n = 6): 51 ± 1.6 y. McA (n = 7): 41 ± 1.3 y | MM and McA: 30 min of cycling (wk 1–6) and 45 min (wk 7–12) at 65–70% of HRmax | 4 ses/wk/ 3 mo | Vastus lateralis quadriceps | MM and McA: Higher changes in skeletal muscle fractional O2 extraction (deoxy(Hb + Mb)) during exercise |
| Søgaard et al., 2012 [56] | General fitness training (GFT) performed as leg bicycling versus strength training (ST) on muscle oxygenation in trapezius with chronic myalgia | 39 pt. with trapezius myalgia. GAT (n = 15): 45.5 ± 8.0 y. ST (n = 16): 44.6 ± 8.5 y. CON (n = 8): 42.5 ± 11.1 y | GFT: 20′ at 50–70% of VO2max. ST: 8–12 rep at 70–80% MR. CON: non-exercise | 3 ses/wk/ 2.5 mo | Trapezius muscle | GFT: Higher blood flow (↑ in O2Hb and tHB) during pegboard exercise. CON: without changes |
| Takagi et al., 2016 [57] | AET on muscle deoxygenation and VO2peak in post-myocardial infarction (AMI) | 16 pt. with AMI. AET (n = 10): 59 ± 10 y. CON (n = 6): 61 ± 9 y | AET: 10 W below LT, 30’ x 10′ warm up and 10′ cool down. CON: non-exercise | 2 ses/wk/ 3 mo | Vastus lateralis quadriceps | AET: Higher muscle oxigenation (↓ SmO2 and ↑ deoxy-Hb) during submaximal and peak intensity in a maximal exercise test. CON: without changes |
| Tew et al., 2009 [58] | Arm-crank exercise (ACE) training on lower-limb O2 delivery in patients with intermittent claudication | 57 pt. with PAD. ACE (n = 27): 69 ± 9 y. CON (n = 24): 70 ± 8 y | ACE: Cycles of 2 min exercise at a crank rate of 50 rev/min at 60–70% of the peak work rate in an incremental arm-crank test followed by 2 min of rest for a total exercise time of 20 min in a 40 min session. CON: non-exercise | 2 ses/wk/ 3 mo | Gastrocnemius | ACE: Higher submaximal oxygenation (↑ StO2) during maximal exercise testing. CON: without changes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuesta, M.; Yáñez-Sepúlveda, R.; Verdugo-Marchese, H.; Mateluna, C.; Alvear-Ordenes, I. Near-Infrared Spectroscopy Used to Assess Physiological Muscle Adaptations in Exercise Clinical Trials: A Systematic Review. Biology 2022, 11, 1073. https://doi.org/10.3390/biology11071073
Tuesta M, Yáñez-Sepúlveda R, Verdugo-Marchese H, Mateluna C, Alvear-Ordenes I. Near-Infrared Spectroscopy Used to Assess Physiological Muscle Adaptations in Exercise Clinical Trials: A Systematic Review. Biology. 2022; 11(7):1073. https://doi.org/10.3390/biology11071073
Chicago/Turabian StyleTuesta, Marcelo, Rodrigo Yáñez-Sepúlveda, Humberto Verdugo-Marchese, Cristián Mateluna, and Ildefonso Alvear-Ordenes. 2022. "Near-Infrared Spectroscopy Used to Assess Physiological Muscle Adaptations in Exercise Clinical Trials: A Systematic Review" Biology 11, no. 7: 1073. https://doi.org/10.3390/biology11071073
APA StyleTuesta, M., Yáñez-Sepúlveda, R., Verdugo-Marchese, H., Mateluna, C., & Alvear-Ordenes, I. (2022). Near-Infrared Spectroscopy Used to Assess Physiological Muscle Adaptations in Exercise Clinical Trials: A Systematic Review. Biology, 11(7), 1073. https://doi.org/10.3390/biology11071073






