Growth and Nutrient Utilization in Basil Plant as Affected by Applied Nutrient Quantity in Nutrient Solution and Light Spectrum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Plant Cultivation
2.2. Measurements
2.2.1. Plant Growth Parameters
2.2.2. Gas Exchange Parameters
2.2.3. Chlorophyll Fluorescence Parameters
2.2.4. NA, NUE, NAE, and Nutrient Waste (NW) from Producing 1 g of Shoot Dry Weight
2.3. Statistical Analysis
3. Results
3.1. Experiment 1
Daily Absorption of Nutrients by Basil Plants
3.2. Experiment 2
3.2.1. Comparison of Plant Growth between Experiment 1 and the Control Group (1T under RB7:3) in Experiment 2
3.2.2. Plant Growth under Four ANQ Treatments and Three R:B Ratios
3.2.3. Gas Exchange and Chlorophyll Fluorescence Parameters
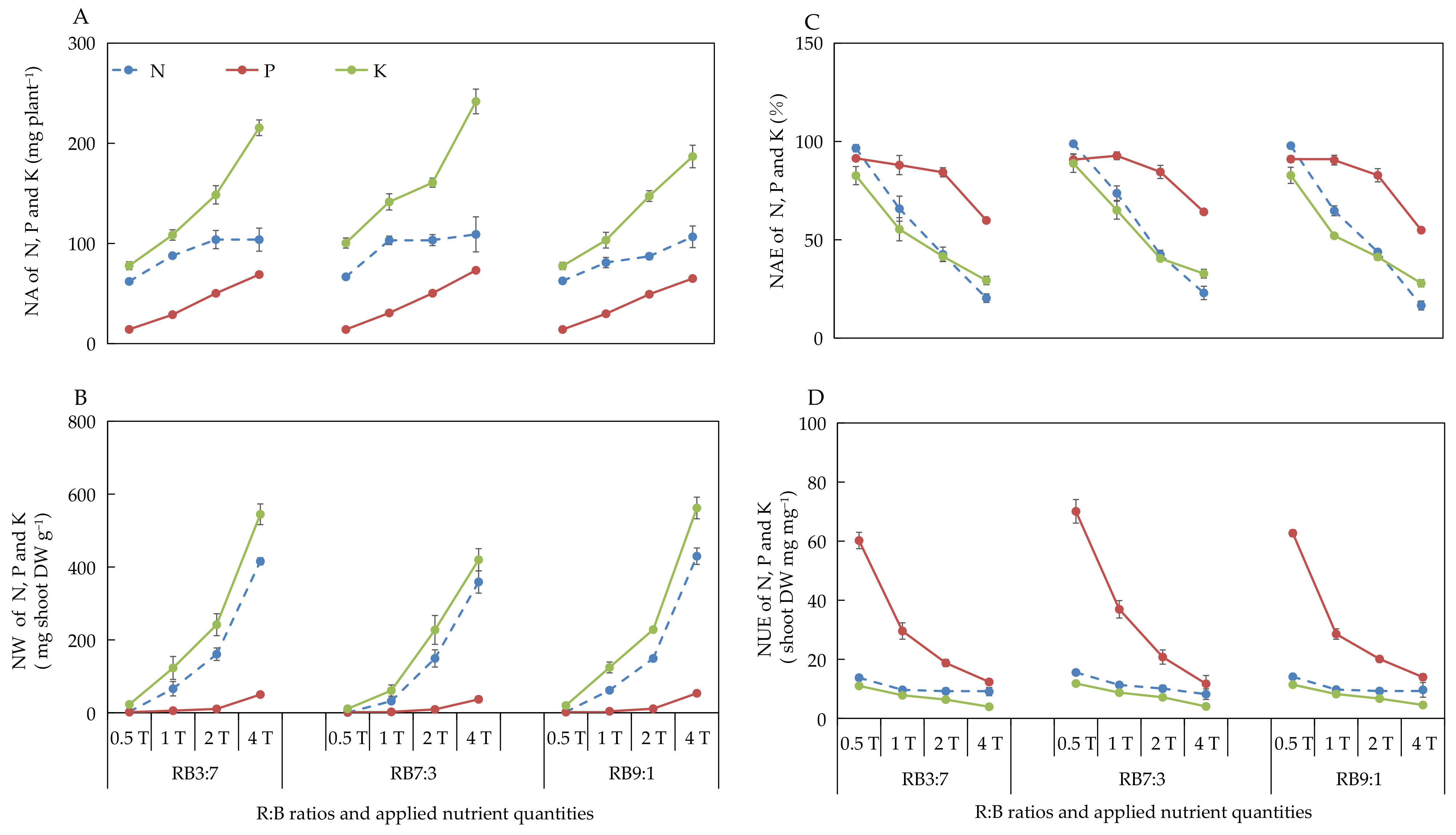
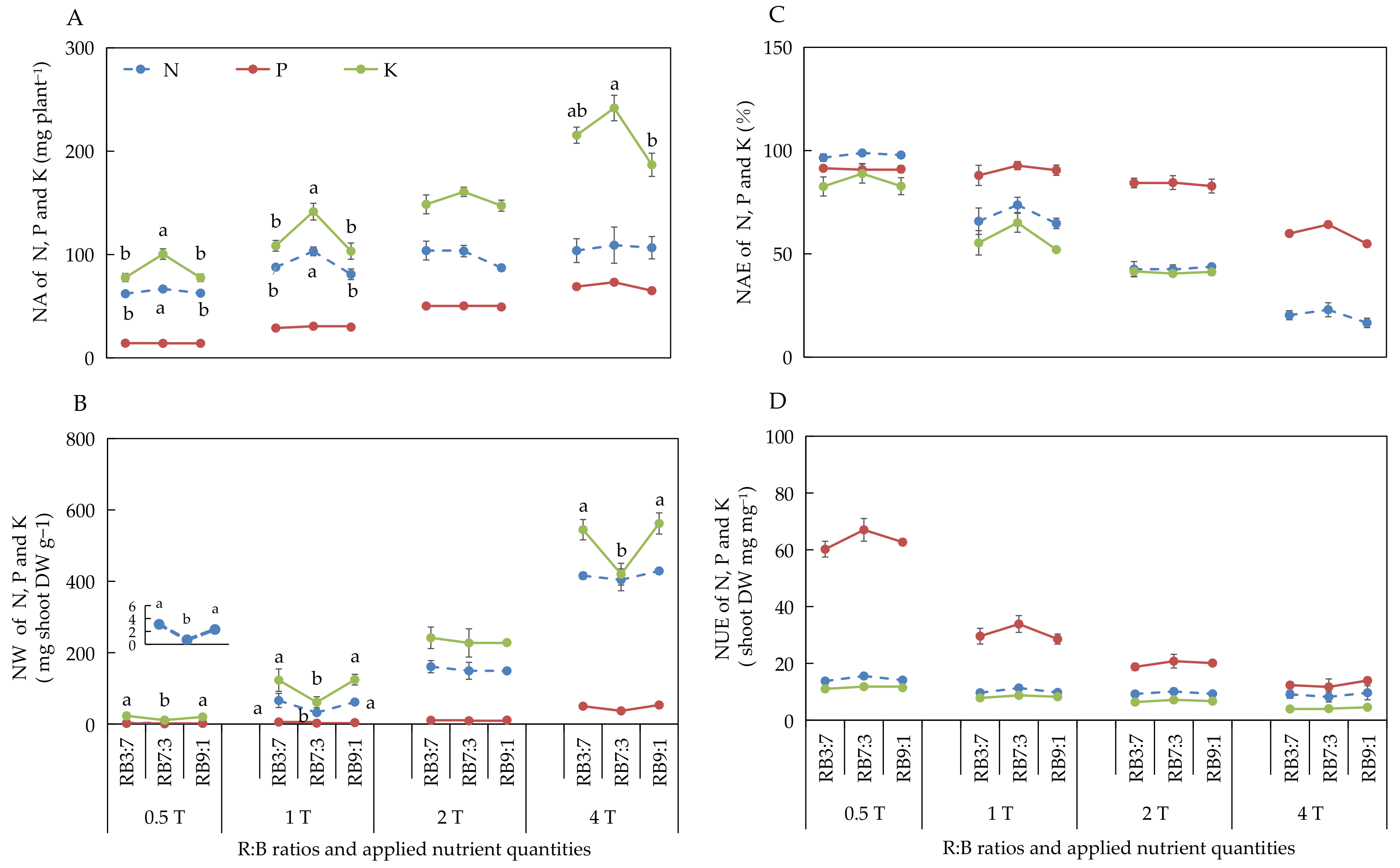
3.2.4. Absorption and Utilization of N, P, and K under Different ANQ Treatments
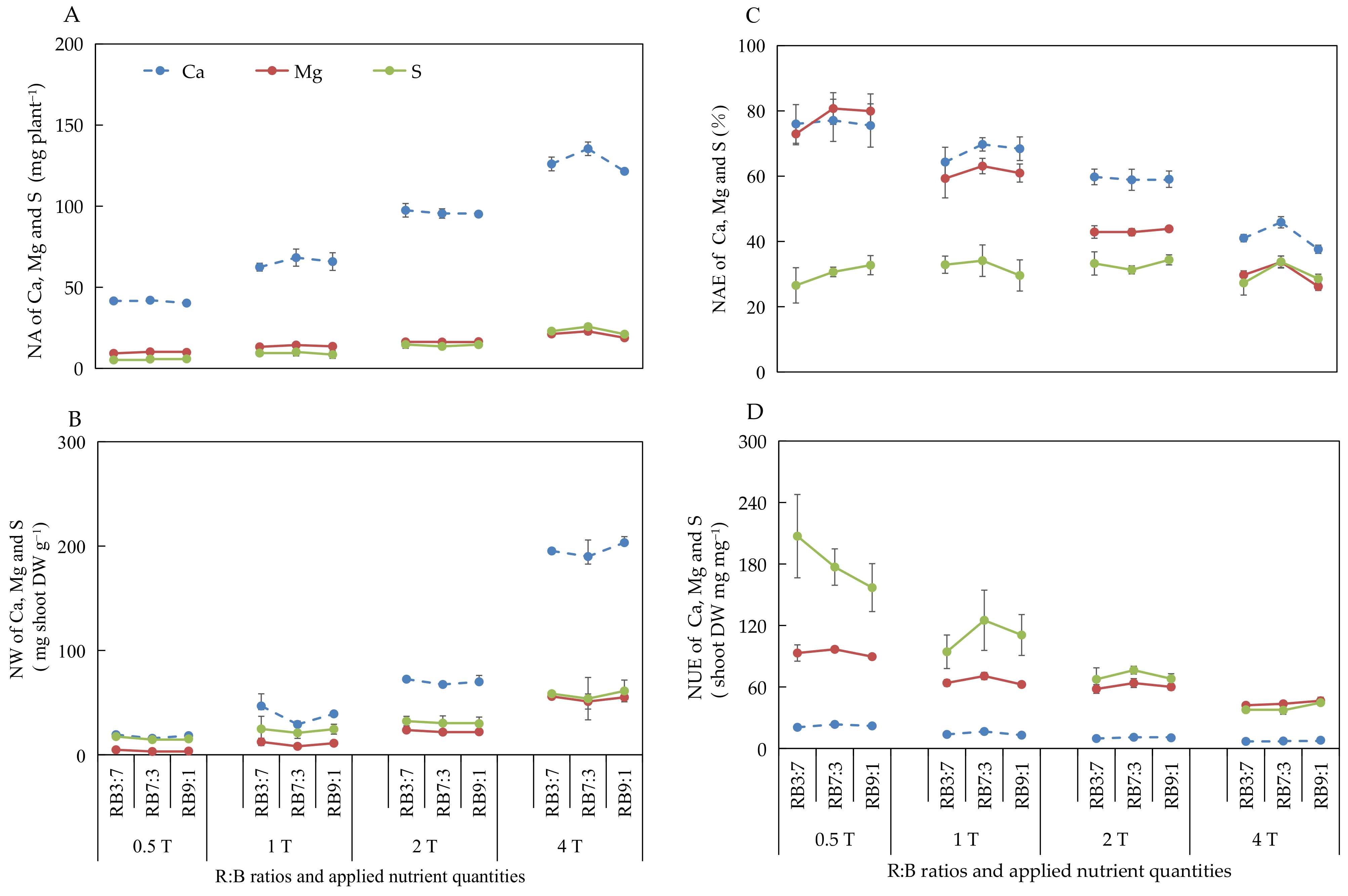
3.2.5. Absorption and Utilization of Ca, Mg, and S under Different ANQ Treatments
3.2.6. Absorption and Utilization of N, P, and K at Different R:B Ratios
3.2.7. Absorption and Utilization of Ca, Mg, and S at Different R:B Ratios
4. Discussion
4.1. Growth Response of Basil Plant to ANQ Treatments and R:B Ratios
4.2. Nutrient Utilization under ANQ Treatments and R:B Ratios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, R. Saving global land resources by enhancing eco-efficiency of agroecosystems. J. Soil Water Conserv. 2018, 73, 100A–106A. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.; Adhikari, R.; Nemali, K. Recycling nutrient solution can reduce growth due to nutrient deficiencies in hydroponic production. Front. Plant Sci. 2020, 11, 607643. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Lee, K.S.; Matsui, Y. Effect of the addition of activated charcoal to the nutrient solution on the growth of tomato in hydroponic culture. Soil Sci. Plant Nutr. 1993, 39, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Sago, Y.; Shigemura, A. Quantitative nutrient management reduces nitrate accumulation in hydroponic butterhead lettuces grown under artificial lighting. HortScience 2018, 53, 963–967. [Google Scholar] [CrossRef]
- Kumar, A.; Chanderman, A.; Makolomakwa, M.; Perumal, K.; Singh, S. Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Crit. Rev. Environ. Sci. Technol. 2016, 46, 556–591. [Google Scholar] [CrossRef] [Green Version]
- Karak, T.; Bhagat, R.M.; Bhattacharyya, P. Municipal solid waste generation, composition, and management: The world scenario. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1509–1630. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Savci, S. An agricultural pollutant: Chemical nutrient. Int. J. Environ. Sci. Dev. 2012, 3, 73. [Google Scholar] [CrossRef] [Green Version]
- Michalsky, R.; Pfromm, P.H. Thermodynamics of metal reactants for ammonia synthesis from steam, nitrogen and biomass at atmospheric pressure. AIChE J. 2012, 58, 3203–3213. [Google Scholar] [CrossRef]
- Buvaneshwari, S.; Riotte, J.; Sekhar, M.; Sharma, A.K.; Helliwell, R.; Kumar, M.S.; Ruiz, L. Potash nutrient promotes incipient salinization in groundwater irrigated semi-arid agriculture. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Nutrient solutions for hydroponic systems. In Hydroponics-A Standard Methodology for Plant Biological Researches; Asao, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 1–22. [Google Scholar]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef] [Green Version]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Hosseini, H.; Mozafari, V.; Roosta, H.R.; Shirani, H.; van de Vlasakker, P.C.; Farhangi, M. Nutrient use in vertical farming: Optimal electrical conductivity of nutrient solution for growth of lettuce and basil in hydroponic cultivation. Horticulturae 2021, 7, 283. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F. Vertical farming: Moving from genetic to environmental modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Samarakoon, U.C.; Weerasinghe, P.A.; Weerakkody, W.A.P. Effect of electrical conductivity [EC] of the nutrient solution on nutrient uptake, growth and yield of leaf lettuce (Lactuca sativa L.) in stationary culture. Trop. Agric. Res. 2006, 18, 13–21. [Google Scholar]
- Tsukagoshi, S.; Shinohara, Y. Nutrition and nutrient uptake in soilless culture systems In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Elsevier: San Diego, CA, USA, 2016; Volume 11, pp. 165–172. [Google Scholar]
- Bugbee, B. Nutrient management in recirculating hydroponic culture. Acta Hortic. 2004, 648, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, H.; Hohjo, M.; Shinohara, Y.; Ito, T.; Maruo, T. Quantitative nutrient management at low concentration condition in NFT spinach culture. Acta Hortic. 1999, 548, 133–140. [Google Scholar]
- Terabayashi, S.; Asaka, T.; Tomaturi, A.; Fujime, Y. Effect of the limited supply of nitrate and phosphate on nutrient uptake and fruit production of tomato (Lycopersicon esculentum Mill) in hydroponic culture. Hortic. Res. 2004, 3, 195–200. [Google Scholar] [CrossRef]
- Maneejantra, N.; Tsukagoshi, S.; Lu, N.; Supoaibulwatana, K.; Takagaki, M.; Yamori, W. A quantitative analysis of nutrient requirements for hydroponics Spinach (Spinacia oleracea L.) production under artificial light in a plant factory. J. Fertil. Pestic. 2016, 7, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Tsukagoshi, S.; Aoki, M.; Johkan, M.; Hohjo, M.; Maruo, T. A quantitative management of potassium supply for hydroponic production of low-potassium cherry-type tomato fruit for chronic kidney disease patients. Horticulturae 2021, 7, 87. [Google Scholar] [CrossRef]
- Matsuda, R.; Suzuki, K.; Nakano, Y.; Sasaki, H.; Takaichi, M. Daily based quantitative nutrient management in rockwool hydroponics: Growth and yield of tomato and nutrient use at elevated CO2. J. Agric. Meteorol. 2010, 66, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Gianquinto, G. Unraveling the role of red: Blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [Green Version]
- Graamans, L.; Baeza, E.; Van Den Dobbelsteen, A.; TsANQras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Mudrilov, M.; Nerush, V.; Pecherina, A.; Smirnov, A.A.; Sukhov, V. Ratio of intensities of blue and red light at cultivation influences photosynthetic light reactions, respiration, growth, and reflectance indices in lettuce. Biology 2022, 11, 60. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light regulation of horticultural crop nutrient uptake and utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Marcelis, L.F. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: Blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Pinho, P.; Jokinen, K.; Halonen, L. The influence of the LED light spectrum on the growth and nutrient uptake of hydroponically grown lettuce. Light. Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light-emitting diode lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Ren, X.; Lu, N.; Xu, W.; Zhuang, Y.; Takagaki, M. Optimization of the yield, total phenolic content, and antioxidant capacity of basil by controlling the electrical conductivity of the nutrient solution. Horticulturae 2022, 8, 216. [Google Scholar] [CrossRef]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red: Blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Continuous lighting and high daily light integral enhance yield and quality of mass-produced nasturtium (Tropaeolum majus L.) in plant factories. Plants 2021, 10, 1203. [Google Scholar] [CrossRef]
- Dobermann, A. Nutrient use efficiency—measurement and management. In Nutrient Best Management Practices: General Principles, Strategy for Their Adoption and Voluntary Initiatives Versus Regulations; Krauss, A., Isherwood, K., Heffer, P., Eds.; International Nutrient Industry Association: Paris, France, 2007; pp. 1–28. [Google Scholar]
- Zeng, H.; Wang, G.; Hu, X.; Wang, H.; Du, L.; Zhu, Y. Role of microRNAs in plant responses to nutrient stress. Plant Soil 2014, 374, 1005–1021. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Hsu, M.H. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green, and blue LED lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Ciriello, M.; Pannico, A.; El-Nakhel, C.; Formisano, L.; Cristofano, F.; Duri, L.G.; Rouphael, Y. Sweet basil functional quality as shaped by genotype and macronutrient concentration reciprocal action. Plants 2020, 9, 1786. [Google Scholar] [CrossRef]
- Iglesias, A.; Estrella, M.; Pieckenstain, F. Nitrogen assimilation and carbon metabolism. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; CRC Press: New York, NY, USA, 2005; pp. 679–687. [Google Scholar]
- Rychter, A.M.; Rao, I.M. Role of phosphorus in photosynthetic carbon metabolism. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; CRC Press: New York, NY, USA, 2005; pp. 123–148. [Google Scholar]
- Reid, R.; Hayes, J. Mechanisms and control of nutrient uptake in plants. Int. Rev. Cytol. 2003, 229, 73–114. [Google Scholar]
- Yang, H.; Zhang, G.; Cui, J. BK channels: Multiple sensors, one activation gate. Front. Physiol. 2015, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2015; pp. 143–145. [Google Scholar]
- Li, H. Modern Plant Physiology, 1st ed.; Higher Education Press: Beijing, China, 2002; pp. 210–212. [Google Scholar]
- Siddiqi, M.Y.; Glass, A.D. Utilization index: A modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J. Plant Nutr. 1981, 4, 289–302. [Google Scholar] [CrossRef]
- An, Y.; Wan, S.; Zhou, X.; Subedar, A.A.; Wallace, L.L.; Luo, Y. Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Glob. Chang. Biol. 2005, 11, 1733–1744. [Google Scholar] [CrossRef]
- Houshmandfar, A.; Fitzgerald, G.J.; O’Leary, G.; Tausz-Posch, S.; Fletcher, A.; Tausz, M. The relationship between transpiration and nutrient uptake in wheat changes under elevated atmospheric CO2. Physiol. Plant. 2018, 163, 516–529. [Google Scholar] [CrossRef] [Green Version]
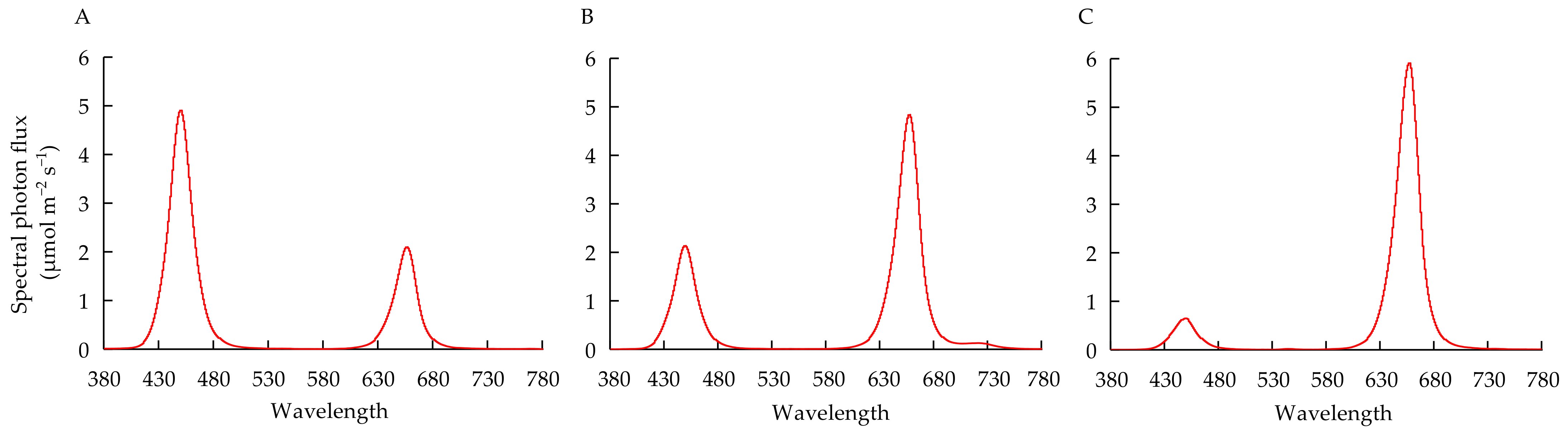


| Growth Stage (Days) | Quantity of Nutrient Supply (mg Plant−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Applied Nutrient Quantity | Ca(NO3)2·4H2O | KNO3 | Mg(NO3)2·6H2O | NaNO3 | K2SO4 | NaH2PO4 | KCl | MgSO4·7H2O | KH2PO4 | |
| 1–10 | 0.5T | 33.50 | 36.23 | 19.22 | 24.00 | 10.45 | 14.57 | 6.17 | 0.00 | 0.00 |
| 1T | 67.00 | 72.46 | 38.44 | 48.00 | 20.90 | 29.13 | 12.33 | 0.00 | 0.00 | |
| 2T | 134.00 | 144.91 | 76.88 | 96.00 | 41.81 | 58.27 | 24.67 | 0.00 | 0.00 | |
| 4T | 268.00 | 289.82 | 153.75 | 192.00 | 83.61 | 116.53 | 49.33 | 0.00 | 0.00 | |
| 11–15 | 0.5T | 51.24 | 54.76 | 3.20 | 12.75 | 0.00 | 0.00 | 0.00 | 19.12 | 15.61 |
| 1T | 102.47 | 109.53 | 6.41 | 25.50 | 0.00 | 0.00 | 0.00 | 38.24 | 31.23 | |
| 2T | 204.94 | 219.05 | 12.81 | 51.00 | 0.00 | 0.00 | 0.00 | 76.48 | 62.46 | |
| 4T | 409.89 | 438.10 | 25.63 | 102.00 | 0.00 | 0.00 | 0.00 | 152.95 | 124.91 | |
| 16–20 | 0.5T | 126.12 | 80.04 | 16.02 | 6.38 | 0.00 | 0.00 | 0.00 | 30.80 | 32.37 |
| 1T | 252.24 | 160.08 | 32.03 | 12.75 | 0.00 | 0.00 | 0.00 | 61.61 | 64.74 | |
| 2T | 504.48 | 320.15 | 64.06 | 25.50 | 0.00 | 0.00 | 0.00 | 123.21 | 129.48 | |
| 4T | 1008.95 | 640.31 | 128.13 | 51.00 | 0.00 | 0.00 | 0.00 | 246.42 | 258.96 | |
| Growth Stage | Days | Unit | Nutrients | |||||
|---|---|---|---|---|---|---|---|---|
| NO3− | PO43− | K+ | Ca2+ | Mg2+ | SO42− | |||
| Stage 1 | 1–10 | mg plant−1 day−1 | 13.33 | 2.31 | 4.38 | 1.12 | 0.36 | 1.14 |
| Stage 2 | 11–15 | 28.6 | 4.35 | 10.32 | 3.5 | 0.86 | 2.86 | |
| Stage 3 | 16–20 | 50.97 | 9.01 | 16.01 | 8.57 | 1.78 | 4.69 | |
| Average | 1–20 | 25.56 | 4.5 | 8.77 | 3.58 | 0.84 | 2.45 | |
| Growth Parameters | Experiment 1 | Control Group in Experiment 2 |
|---|---|---|
| Shoot fresh weight | 16.08 a z | 16.02 a |
| Leaf fresh weight | 12.50 a | 12.16 a |
| Shoot dry weight | 1.30 a | 1.16 a |
| Leaf dry weight | 1.00 a | 0.89 a |
| Photosynthesis | Unit | ANQ | R:B Ratios | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RB3:7 | RB7:3 | RB9:1 | |||||||||
| Pn | (μmol CO2 m−2 s−1) | 0.5T | 9.76 | ±0.70 z | a A | 10.32 | ±1.04 | a A | 8.99 | ±0.86 | a A |
| 1T | 10.70 | ±0.30 | a A | 8.59 | ±0.90 | a A | 8.19 | ±1.68 | a A | ||
| 2T | 11.37 | ±0.85 | a A | 9.79 | ±1.67 | a A | 8.49 | ±0.70 | a A | ||
| 4T | 10.00 | ±0.99 | a A | 9.66 | ±1.34 | a A | 8.73 | ±0.88 | a A | ||
| Tr | (mmol m−2 s−1) | 0.5T | 1.58 | ±0.18 | a A | 1.48 | ±0.28 | a A | 1.79 | ±0.08 | a A |
| 1T | 1.47 | ±0.33 | a A | 1.48 | ±0.05 | a A | 2.08 | ±0.12 | a A | ||
| 2T | 1.91 | ±0.18 | a A | 1.55 | ±0.07 | a A | 1.78 | ±0.18 | a A | ||
| 4T | 1.73 | ±0.16 | a A | 1.56 | ±0.25 | a A | 1.36 | ±0.23 | a A | ||
| ETR | (μmol electrons m−2 s−1) | 0.5T | 44.10 | ±4.04 | a A | 44.57 | ±7.33 | a A | 45.47 | ±3.21 | a A |
| 1T | 44.47 | ±4.32 | a A | 37.19 | ±6.65 | a A | 39.87 | ±0.97 | a A | ||
| 2T | 43.19 | ±3.23 | a A | 40.59 | ±3.59 | a A | 41.61 | ±5.30 | a A | ||
| 4T | 43.74 | ±4.69 | a A | 37.81 | ±8.26 | a A | 35.06 | ±5.81 | a A | ||
| Fv/Fm | 0.5T | 0.82 | ±0.01 | a A | 0.81 | ±0.005 | a A | 0.80 | ±0.02 | a A | |
| 1T | 0.82 | ±0.02 | a A | 0.81 | ±0.008 | a A | 0.80 | ±0.004 | a A | ||
| 2T | 0.82 | ±0.01 | a A | 0.81 | ±0.009 | a A | 0.80 | ±0.01 | a A | ||
| 4T | 0.80 | ±0.03 | a A | 0.81 | ±0.013 | a A | 0.80 | ±0.001 | a A | ||
| Fv’/Fm’ | 0.5T | 0.69 | ±0.02 | a A | 0.68 | ±0.07 | a A | 0.68 | ±0.04 | a A | |
| 1T | 0.69 | ±0.04 | a A | 0.66 | ±0.06 | a A | 0.68 | ±0.003 | a A | ||
| 2T | 0.67 | ±0.03 | a A | 0.69 | ±0.02 | a A | 0.70 | ±0.01 | a A | ||
| 4T | 0.70 | ±0.02 | a A | 0.64 | ±0.05 | a A | 0.63 | ±0.02 | a A | ||
| PhiPSII | 0.5T | 0.56 | ±0.05 | a A | 0.51 | ±0.11 | a A | 0.58 | ±0.04 | a A | |
| 1T | 0.57 | ±0.05 | a A | 0.48 | ±0.08 | a A | 0.51 | ±0.01 | a A | ||
| 2T | 0.55 | ±0.04 | a A | 0.52 | ±0.05 | a A | 0.53 | ±0.07 | a A | ||
| 4T | 0.64 | ±0.01 | a A | 0.48 | ±0.11 | a A | 0.45 | ±0.07 | a A | ||
| qP | 0.5T | 0.81 | ±0.05 | a A | 0.82 | ±0.09 | a A | 0.85 | ±0.03 | a A | |
| 1T | 0.82 | ±0.04 | a A | 0.72 | ±0.06 | a A | 0.75 | ±0.02 | a A | ||
| 2T | 0.83 | ±0.03 | a A | 0.75 | ±0.05 | a A | 0.76 | ±0.11 | a A | ||
| 4T | 0.89 | ±0.02 | a A | 0.75 | ±0.11 | a A | 0.71 | ±0.13 | a A | ||
| qN | 0.5T | 0.52 | ±0.11 | a A | 0.52 | ±0.14 | a A | 0.53 | ±0.09 | a A | |
| 1T | 0.52 | ±0.11 | a A | 0.61 | ±0.09 | a A | 0.55 | ±0.08 | a A | ||
| 2T | 0.55 | ±0.09 | a A | 0.57 | ±0.02 | a A | 0.53 | ±0.02 | a A | ||
| 4T | 0.45 | ±0.02 | a A | 0.56 | ±0.13 | a A | 0.64 | ±0.04 | a A | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Lu, N.; Xu, W.; Zhuang, Y.; Tsukagoshi, S.; Takagaki, M. Growth and Nutrient Utilization in Basil Plant as Affected by Applied Nutrient Quantity in Nutrient Solution and Light Spectrum. Biology 2022, 11, 991. https://doi.org/10.3390/biology11070991
Ren X, Lu N, Xu W, Zhuang Y, Tsukagoshi S, Takagaki M. Growth and Nutrient Utilization in Basil Plant as Affected by Applied Nutrient Quantity in Nutrient Solution and Light Spectrum. Biology. 2022; 11(7):991. https://doi.org/10.3390/biology11070991
Chicago/Turabian StyleRen, Xiaowei, Na Lu, Wenshuo Xu, Yunfei Zhuang, Satoru Tsukagoshi, and Michiko Takagaki. 2022. "Growth and Nutrient Utilization in Basil Plant as Affected by Applied Nutrient Quantity in Nutrient Solution and Light Spectrum" Biology 11, no. 7: 991. https://doi.org/10.3390/biology11070991
APA StyleRen, X., Lu, N., Xu, W., Zhuang, Y., Tsukagoshi, S., & Takagaki, M. (2022). Growth and Nutrient Utilization in Basil Plant as Affected by Applied Nutrient Quantity in Nutrient Solution and Light Spectrum. Biology, 11(7), 991. https://doi.org/10.3390/biology11070991






