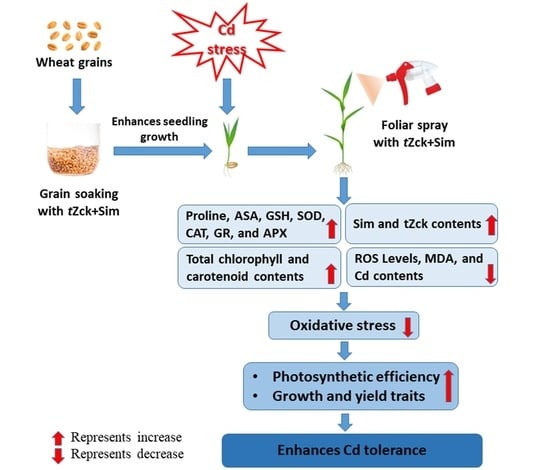
Exploring the Potential Enhancing Effects of Trans-Zeatin and Silymarin on the Productivity and Antioxidant Defense Capacity of Cadmium-Stressed Wheat
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Description and Layout
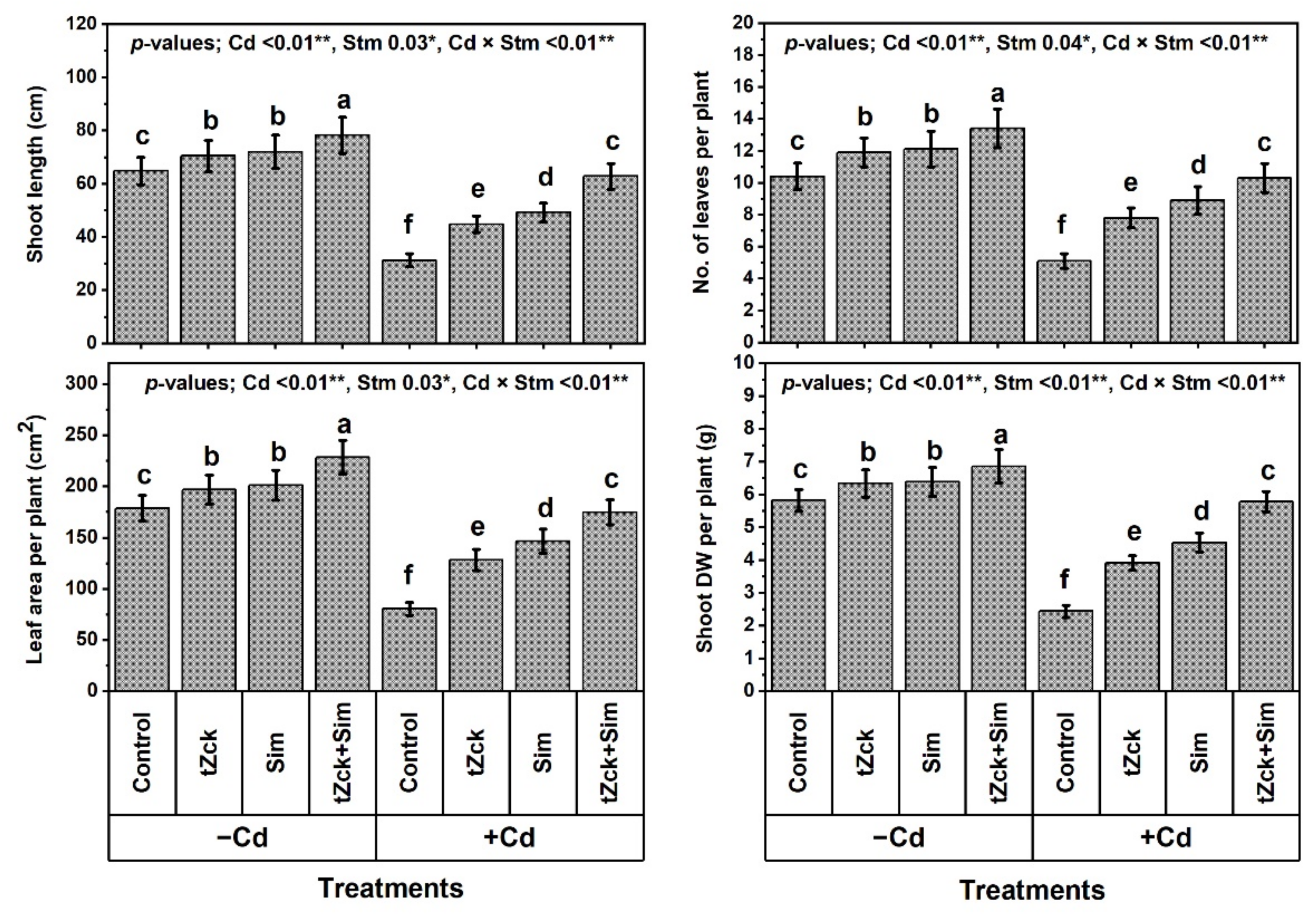
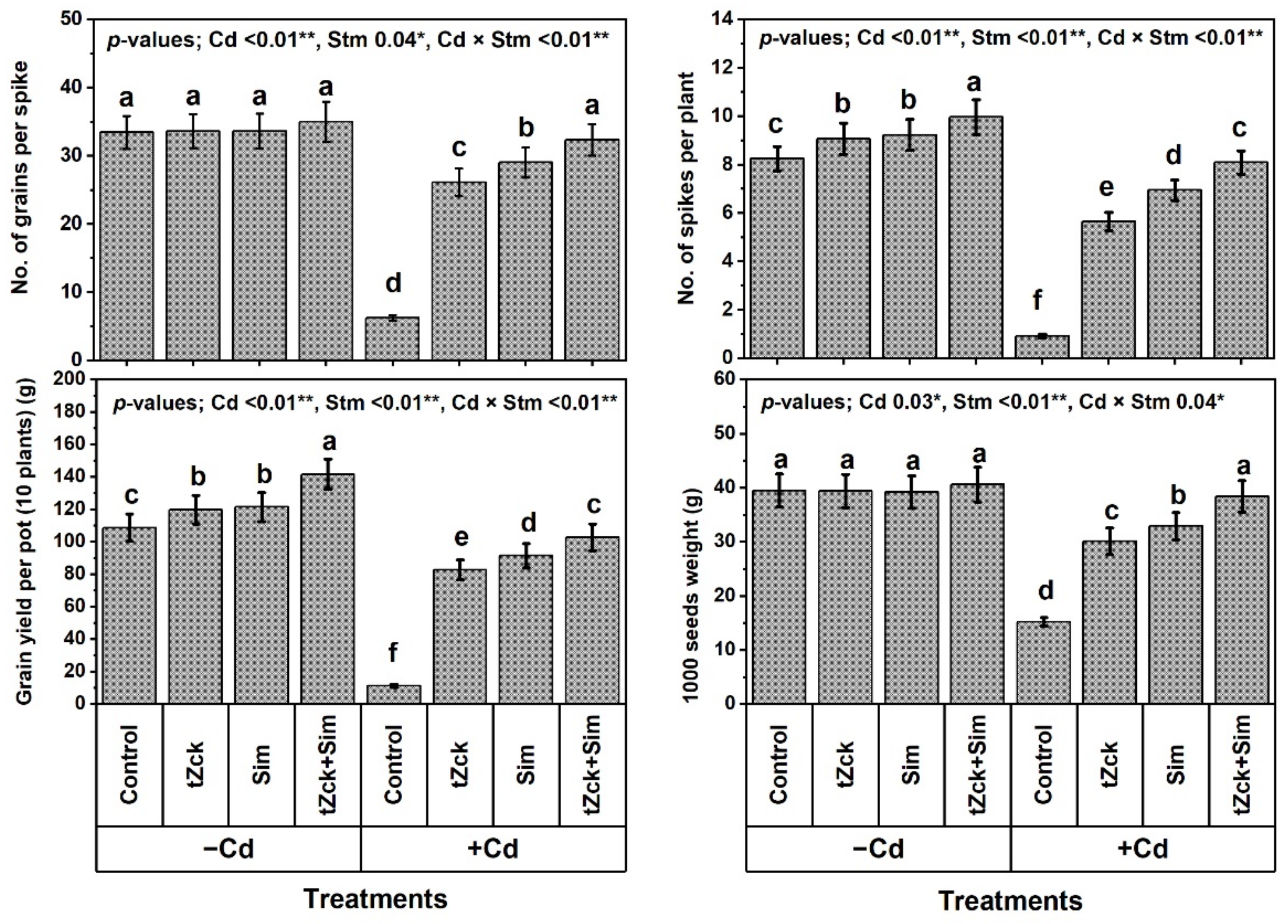
2.2. Traits of Wheat Growth and Yield
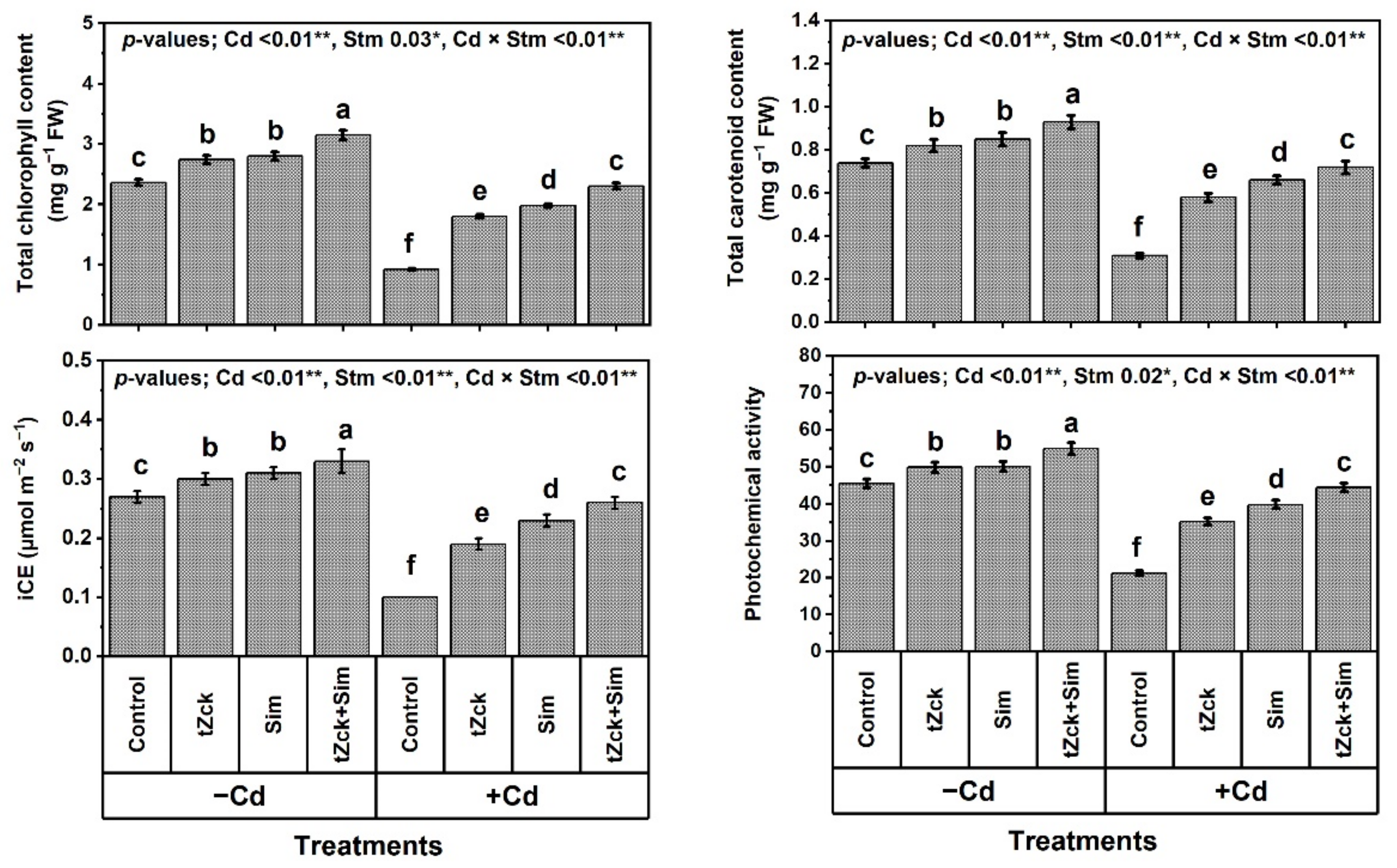
2.3. Determinations of Physiological-Biochemical Indices
2.4. Oxidative Stress Biomarker Levels and Their Consequences and Antioxidant System Components
2.5. Data Analysis
3. Results
3.1. Wheat Growth and Yield, and Leaf Photosynthetic Efficiency in Response to tZck and/or Sim
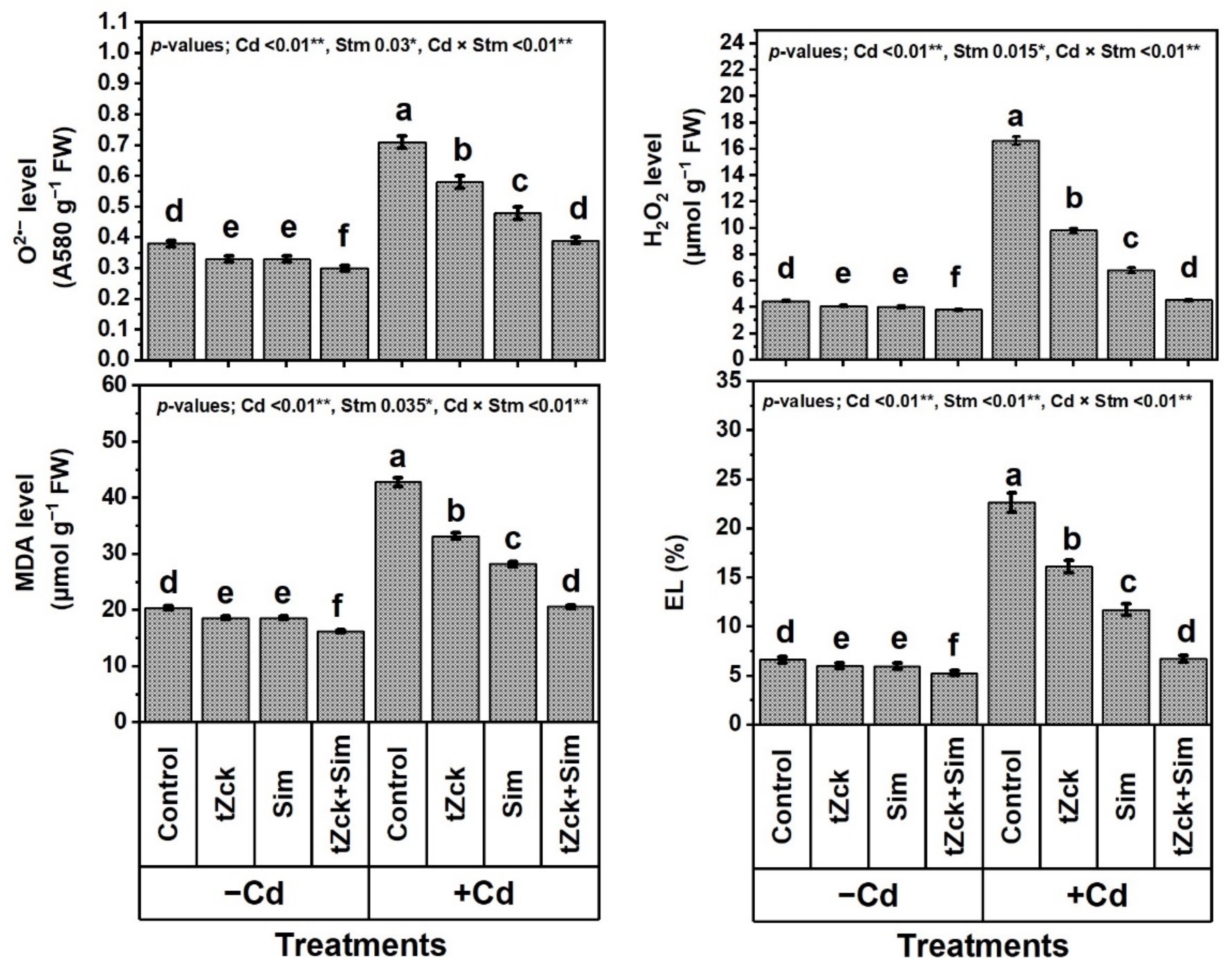
3.2. Markers of Oxidative Stress Levels and Their Consequences in Response to tZck and/or Sim
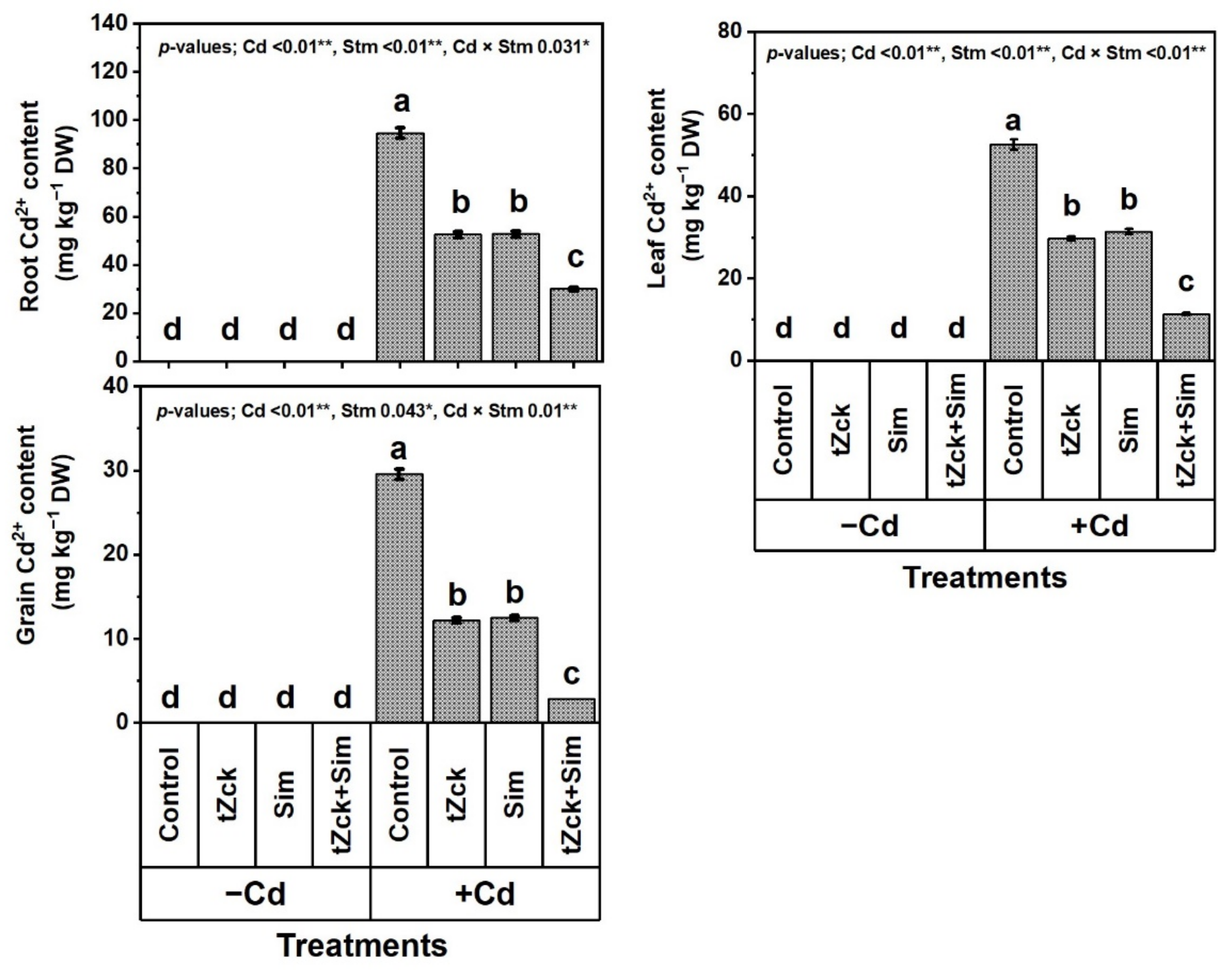
3.3. Root, Leaf and Yielded Grain Cd2+ Contents in Response to tZck and/or Sim
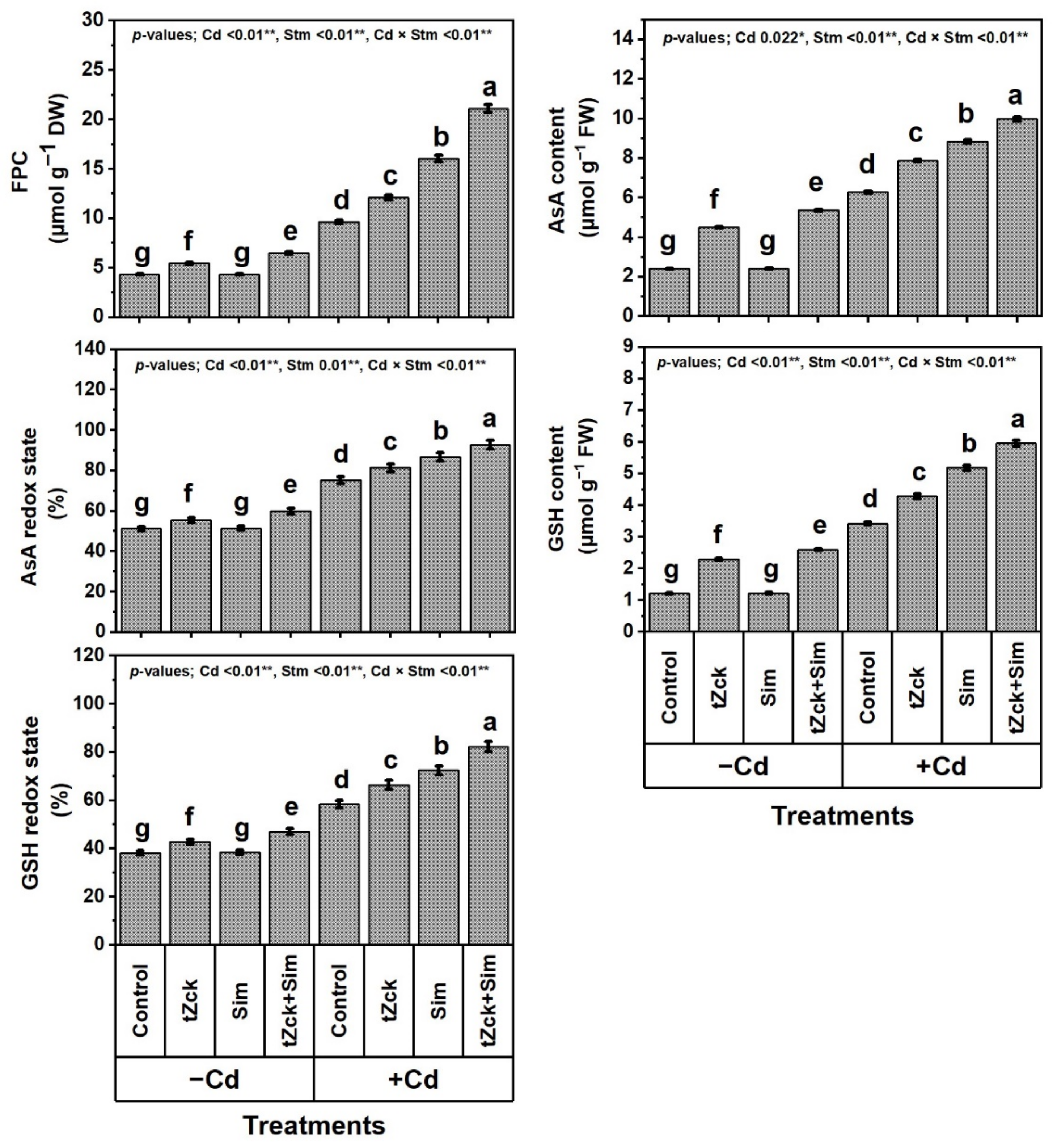
3.4. Free Proline Content (FPC), Capacity of Ascorbate (AsA) and Glutathione (GSH), Silymarin (Sim) and Trans-Zeatin-Type Cytokinin (tZck) Contents in Response to tZck and/or Sim
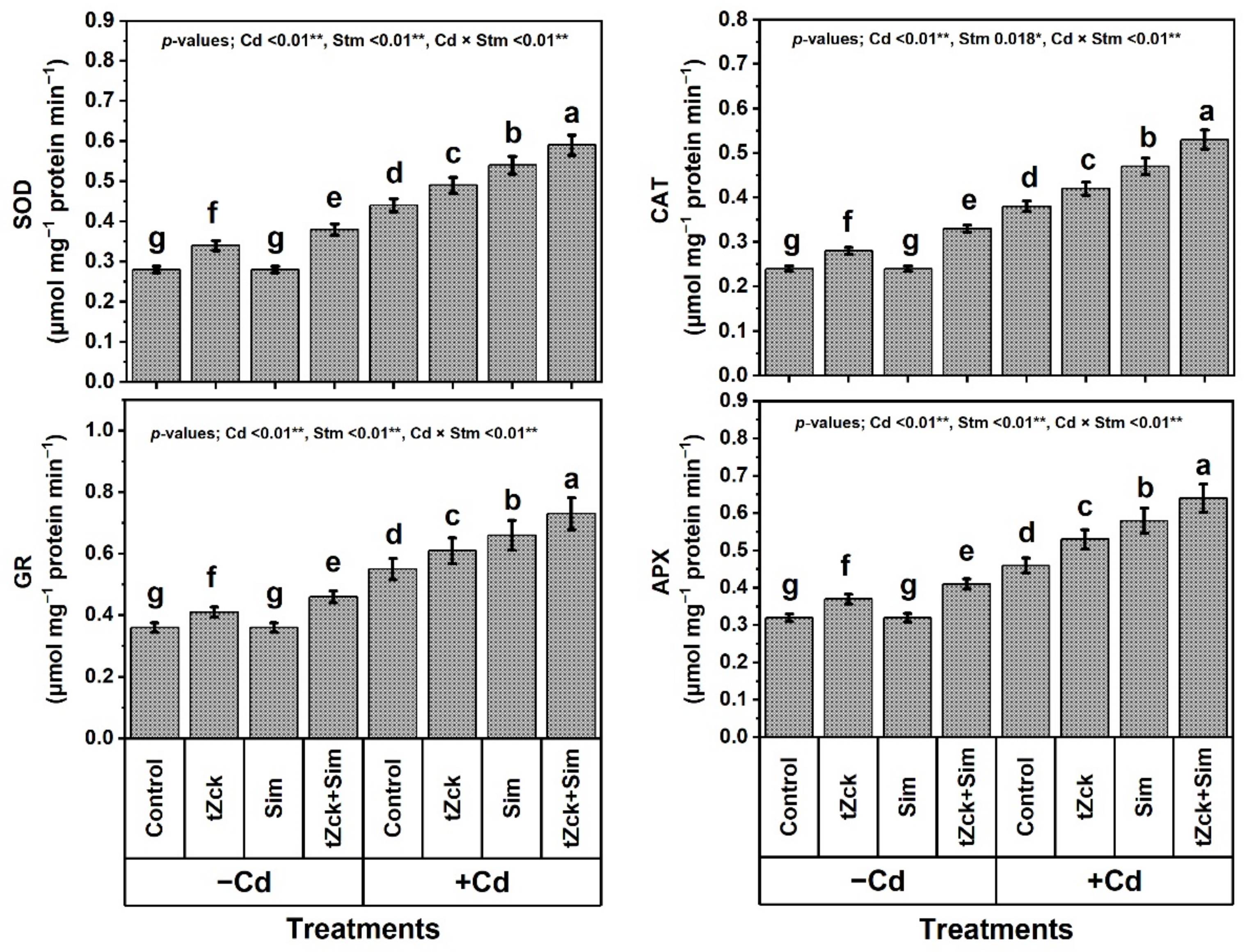
3.5. Enzymatic Activities in Response to tZck and/or Sim
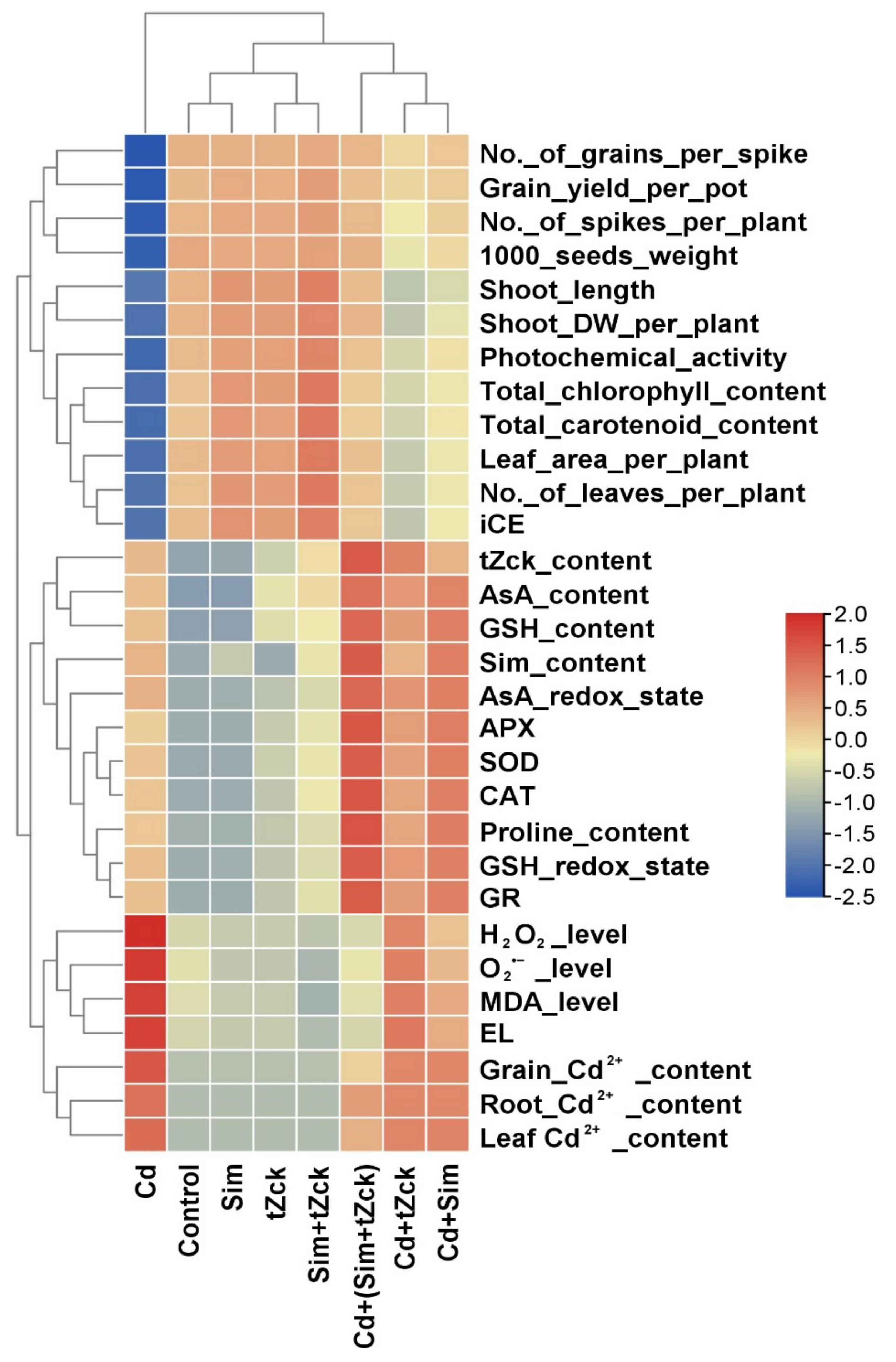
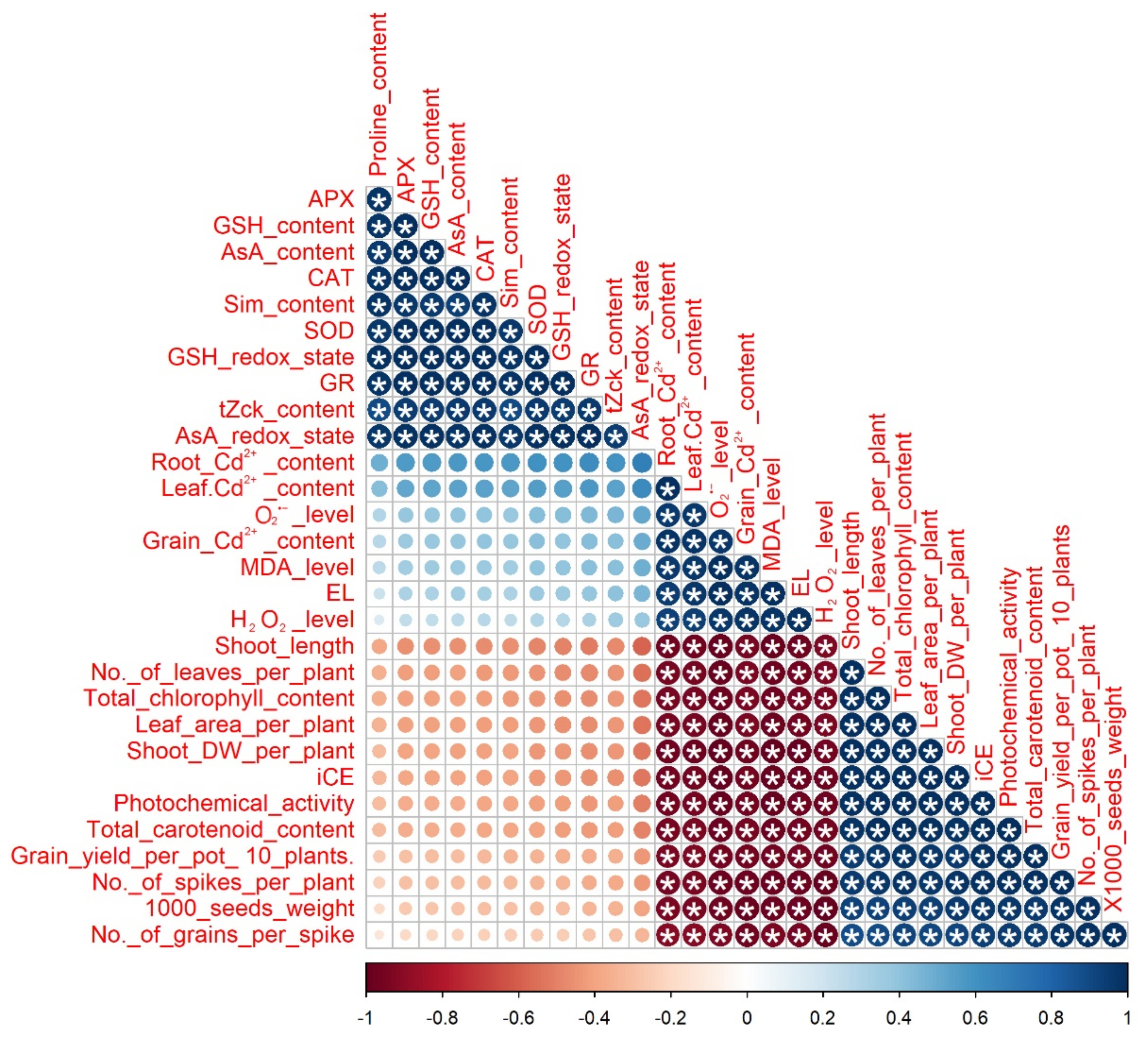
3.6. Relationship between Treatments and the Parameters Studied and Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanumihardjo, S.A.; McCulley, L.; Roh, R.; Lopez-Ridaura, S.; Palacios-Rojas, N.; Gunaratna, N.S. Maize agro-food systems to ensure food and nutrition security in reference to the Sustainable Development Goals. Glob. Food Secur. 2020, 25, 100327. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Taie, H.A.A.; Seif El-Yazal, M.A.; Ahmed, S.M.A.; Rady, M.M. Polyamines modulate growth, antioxidant activity and genomic DNA in heavy metals-stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 26, 22338–22350. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Al-Zahrani, H.S.; Hakeem, K.R.; Alsamadany, H.; Desoky, E.-S.M.; Rady, M.M. Silymarin-enriched biostimulant foliar application minimizes the toxicity of cadmium in maize by suppressing oxidative stress and elevating antioxidant gene expression. Biomolecules 2021, 11, 465. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, P.-F.; Wang, C.; Hou, J.; Miao, L.-Z. The evaluation on the cadmium net concentration for soil ecosystems. Int. J. Environ. Res. Public Health 2017, 14, 297. [Google Scholar] [CrossRef] [Green Version]
- Desoky, E.M.; Merwad, A.M.; Semida, W.M.; Ibrahim, S.A.; El-Saadony, M.T.; Rady, M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators for heavy metals-stressed spinach plants. Ecotoxicol. Environ. Saf. 2020, 198, 110685. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Rehman, M.Z.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Zhong, Q.; Yin, M.; Zhang, Q.; Beiyuan, J.; Liu, J.; Yang, X.; Wang, J.; Wang, L.; Jiang, Y.; Xiao, T.; et al. Cadmium isotopic fractionation in lead-zinc smelting process and signatures in fluvial sediments. J. Hazard. Mater. 2021, 411, 125015. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M.; Abd El-Mageed, T.A.; Howladar, S.M.; Abdelhamid, M.T. Alleviation of cadmium toxicity in common bean (Phaseolus vulgaris L.) plants by the exogenous application of salicylic acid. J. Hortic. Sci. Biotechnol. 2015, 90, 83–91. [Google Scholar]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Assessment of sulforaphane-induced protective mechanisms against cadmium toxicity in human mesenchymal stem cells. Environ. Sci. Pollut. Res. 2018, 25, 10080–10089. [Google Scholar] [CrossRef]
- Filippini, T.; Cilloni, S.; Malavolti, M.; Violi, F.; Malagoli, C.; Tesauro, M.; Bottecchi, I.; Ferrari, A.; Vescovi, L.; Vinceti, M. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018, 50, 508–517. [Google Scholar] [CrossRef]
- Qiao, K.; Liang, S.; Wang, F.; Wang, H.; Hu, Z.; Chai, T. Effects of cadmium toxicity on diploid wheat (Triticum urartu) and the molecular mechanism of the cadmium response. J. Hazard. Mater. 2019, 374, 1–10. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Shaaban, A. Integrative applications of nitrogen, zinc, and boron to nutrients-deficient soil improves sugar beet productivity and technological sugar contents under semi-arid conditions. J. Plant Nutr. 2020, 43, 1935–1950. [Google Scholar] [CrossRef]
- Zeshan, A.; Abdullah, M.; Adil, M.F.; Wei, D.; Noman, M.; Ahmed, T.; Sehar, S.; Ouyang, Y.; Shamsi, I.H. Improvement of morpho-physiological, ultrastructural and nutritional profiles in wheat seedlings through astaxanthin nanoparticles alleviating the cadmium toxicity. J. Hazard. Mater. 2022, 424, 126511. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Hou, L.L.; Tong, T.; Tian, B.; Xue, D.W. Crop yield and quality under cadmium stress. In Cadmium Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 1–18. [Google Scholar]
- Çatav, Ş.S.; Genç, T.O.; Oktay, M.K.; Küçükakyüz, K. Cadmium toxicity in wheat: Impacts on element contents, antioxidant enzyme activities, oxidative stress, and genotoxicity. Bull. Environ. Contam. Toxicol. 2020, 104, 71–77. [Google Scholar] [CrossRef]
- Abou Tahoun, A.M.; El-Enin, M.M.A.; Mancy, A.G.; Sheta, M.H.; Shaaban, A. Integrative soil application of humic acid and foliar plant growth stimulants improves soil properties and wheat yield and quality in nutrient-poor sandy soil of a semiarid region. J. Soil Sci. Plant Nutr. 2022, 1–15. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef]
- FAO. Food Outlook-Biannual Report on Global Food Markets; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Ali, S.; Rizwan, M.; Hussain, A.; Zia ur Rehman, M.; Ali, B.; Yousaf, B.; Wijayae, L.; Alyemenie, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Rady, M.M.; Elrys, E.S.; Abo El-Maati, M.E.; Desoky, E.M. Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol. Biochem. 2019, 139, 558–568. [Google Scholar] [CrossRef]
- Semida, W.M.; Hemida, K.A.; Rady, M.M. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol. Environ. Saf. 2018, 154, 171–179. [Google Scholar] [CrossRef]
- Rady, M.M.; Seif El-Yazal, M.A.; Taie, H.A.A.; Ahmed Safia, M.A. Response of Triticum aestivum (L.) Plants Grown Under Cadmium Stress to Polyamines Pretreatments. Plant 2016, 4, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Gaballah, M.S.; Rady, M.M. Salicylic acid mitigated cadmium toxicity by attenuating the oxidative stress in pea (Pisum sativum L.) plants. Int. J. Biol. Ecol. Environ. Sci. 2012, 1, 159–165. [Google Scholar]
- Wang, L.; Zhang, Q.; Liao, X.; Li, X.; Zheng, S.; Zhao, F. Phytoexclusion of heavy metals using low heavy metal accumulating cultivars: A green technology. J. Hazard. Mater. 2021, 413, 125427. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y. Immobilization remediation of Cd-polluted soil with different water condition. J. Environ. Manag. 2017, 193, 607–612. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; El-Sherif, A.M.; Abd El-Mageed, S.A.; Abdou, N.M. A novel compost alleviate drought stress for sugar beet production grown in Cd-contaminated saline soil. Agric. Water Manag. 2019, 226, 105831. [Google Scholar] [CrossRef]
- Abdul Majeed Niaz, A.; Rizwan, M.; Imran, M.; Alsahli, A.A.; Alyemeni, M.N.; Ali, S. Effects of biochar, farm manure, and pressmud on mineral nutrients and cadmium availability to wheat (Triticum aestivum L.) in Cd-contaminated soil. Physiol. Plant. 2021, 173, 191–200. [Google Scholar]
- Minari, G.D.; Saran, L.M.; Constancio, M.T.L.; da Silva, R.C.; Rosalen, D.L.; de Melo, W.J.; Alves, L.M.C. Bioremediation potential of new cadmium, chromium, and nickel-resistant bacteria isolated from tropical agricultural soil. Ecotoxicol. Environ. Saf. 2020, 204, 111038. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Mbarki, S.; Hossain, A.; Iqbal, M.A.; Islam, M.S.; Raza, A.; Llanes, A.; Reginato, M.; Rahman, M.A.; Mahboob, W.; et al. Potential Role of Plant Growth Regulators in Administering Crucial Processes Against Abiotic Stresses. Front. Agron. 2021, 3, 648694. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [Green Version]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book Am. Soc. Plant Biol. 2014, 12, e0168. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3894907/ (accessed on 2 January 2014). [CrossRef] [Green Version]
- Sakakibara, H. Cytokinins: Activity, Biosynthesis, and Translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [Green Version]
- Osugi, A.; Kojima, M.; Takebayashi, Y.; Ueda, N.; Kiba, T.; Sakakibara, H. Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nat. Plants 2017, 3, 17112. [Google Scholar] [CrossRef]
- Hirose, N.; Takei, K.; Kuroha, T.; Kamada-Nobusada, T.; Hayashi, H.; Sakakibara, H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2008, 59, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Alharby, H.F.; Alzahrani, Y.M.; Rady, M.M. Seeds pretreatment with zeatins or maize grain-derived organic biostimulant improved hormonal contents, polyamine gene expression, and salinity and drought tolerance of wheat. Int. J. Agric. Biol. 2020, 24, 714–724. [Google Scholar]
- Azzam, C.R.; Zaki, S.N.S.; Bamagoos, A.A.; Rady, M.M.; Alharby, H.F. Soaking maize seeds in zeatin-type cytokinin biostimulators improves salt tolerance by enhancing the antioxidant system and photosynthetic efficiency. Plants 2022, 11, 1004. [Google Scholar] [CrossRef]
- Mishra, D.C.; Arora, D.; Budhlakoti, N.; Solanke, A.U.; Mithra, S.A.C.; Kumar, A.; Pandey, P.S.; Srivastava, S.; Kumar, S.; Farooqi, M.S.; et al. Identification of potential cytokinin responsive key genes in rice treated with trans-zeatin through systems biology approach. Front. Genet. 2022, 12, 780599. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Erinle, K.O.; Wani, S.H.; Okon, O.G.; Nadeem, M.A.; Nawaz, M.; Bodlah, M.A.; Waqas, M.M.; Iqba, J.; et al. Inoculation of Azospirillum brasilense and exogenous application of trans-zeatin riboside alleviates arsenic induced physiological damages in wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 33909–33919. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H.J. Silymarin as supportive treatment in liver diseases: A narrative review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [Green Version]
- Salman, E.K.; Badr, E.S.; Ghoniem, K.E.; Aboulila, A.A.; Emeran, A.A. Role of silymarin induced rice immunity against blast pathogen Magnaporthe oryzae through regulation of resistance genes expression. Physiol. Mol. Plant Pathol. 2021, 115, 101678. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The food plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and clinical evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Cultivation of milk thistle (Silybum marianum L. Gaertn.), a medicinal weed. Ind. Crop. Prod. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Konrad, M.L.F.; Silva, J.A.B.; Furlani, P.R.; Machado, E.C. Gas exchange and chlorophyll fluorescence in six coffee cultivars under aluminum stress. Bragantia 2005, 64, 339–347. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total Carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Avron, M. Photophosphorylation by swiss-chard chloroplasts. Biochim. Biophys. Acta. 1960, 40, 257–272. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soil, Plants and Water; University of California, Division of Agricultural Science: Berkeley, CA, USA, 1961; pp. 56–63. [Google Scholar]
- Arampatzis, D.A.; Karkanis, A.C.; Tsiropoulos, N.G. Impact of plant density and mepiquat chloride on growth, yield, and silymarin content of Silybum marianum grown under Mediterranean semi-arid conditions. Agronomy 2019, 9, 669. [Google Scholar] [CrossRef] [Green Version]
- Arampatzis, D.A.; Karkanis, A.C.; Tsiropoulos, N.G. Silymarin content and antioxidant activity of seeds of wild Silybum marianum populations growing in Greece. Ann. Appl. Biol. 2019, 174, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Novák, O.; Hauserová, E.; Amakorová, P.; Doležal, K.; Strnad, M. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 2008, 69, 2214–2224. [Google Scholar] [CrossRef]
- Kubis, J. Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J. Plant Physiol. 2008, 165, 397–406. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photo peroxidation isolated chloroplasts: Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Griffth, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinyl pyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Rao, M.V.; Paliyath, G.; Ormrod, P. Ultraviolet-9- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill, Book International Co.: Singapore, 1997. [Google Scholar]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Chen, X.X.; Liu, Y.M.; Zhao, Q.Y.; Cao, W.Q.; Chen, X.P.; Zou, C.Q. Health risk assessment associated with heavy metal accumulation in wheat after long-term phosphorus fertilizer application. Environ. Pollut. 2020, 262, 114348. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.; Selem, E.; Rady, M.M.; Ali, E.F.; Mersal, G.A.M.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Xuebin, Q.; Yatao, X.; Ahmad, M.I.; Shehzad, M.; Zain, M. Silicon and its application methods improve physiological traits and antioxidants in Triticum aestivum (L.) under cadmium stress. J. Soil Sci. Plant Nutr. 2020, 20, 1110–1121. [Google Scholar]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.J.; Shimono, M.; Sugano, S.; Kojima, M.; Liu, X.; Inoue, H.; Sakakibara, H.; Takatsuji, H. Cytokinins act synergistically with salicylic acid to activate defense gene expression in rice. Mol. Plant Microb. Interact. 2013, 26, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Großkinsky, D.; Edelsbrunner, K.; Pfeifhofer, H.; Graaff, E.V.D.; Roitsch, T. Cis- and trans-zeatin differentially modulate plant immunity. Plant Signal Behav. 2013, 8, e24798. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications—A review. Front. Plant Sci. 2012, 3, 222. [Google Scholar]
- Wu, F.B.; Chen, F.; Wei, K.; Zhang, G.P. Effect of cadmium on free amino acid, glutathione and ascorbic acid concentrations in two barley genotypes (Hordeum vulgare L.) differing in cadmium tolerance. Chemosphere 2004, 57, 447–454. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [Green Version]
- Li, G.Z.; Chen, S.J.; Li, N.Y.; Wang, Y.Y.; Kang, G.Z. Exogenous glutathione alleviates cadmium toxicity in wheat by influencing the absorption and translocation of cadmium. Bull. Environ. Contam. Toxicol. 2021, 107, 320–326. [Google Scholar] [CrossRef]
- Szepesi, Á.; Szőllősi, R. Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 337–353. [Google Scholar]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Rasheed, R.; Ashraf, M.A.; Hussain, I.; Haider, M.Z.; Kanwal, U.; Iqbal, M. Exogenous proline and glycinebetaine mitigate cadmium stress in two genetically different spring wheat (Triticum aestivum L.) cultivars. Braz. J. Bot. 2014, 37, 399–406. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Al-Hakimi, A.M.; Hamada, A.M. Ascorbic acid, thiamine or salicylic acid induced changes in some physiological parameters in wheat grown under copper stress. Plant. Protect. Sci. 2011, 47, 92–108. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, Q.; Wu, W.G.; Guo, J.; Yang, Y.L. Cadmium stress tolerance in wheat seedlings induced by ascorbic acid was mediated by NO signaling pathways. Ecotoxicol. Environ. Saf. 2017, 135, 75–81. [Google Scholar] [CrossRef]
- Ball, L.; Accotto, G.; Bechtold, U.; Creissen, G.; Funck, D.; Jimenez, A.; Kular, B.; Leyland, N.; Mejia-Carranza, J.; Reynolds, H.; et al. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 2004, 16, 2448–2462. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Sen, A. Oxidative stress studies in plant tissue culture. In Biochemistry, Genetics and Molecular Biology “Antioxidant Enzyme”; Chapter 3; El-Missiry, M.A., Ed.; World’s Largest Science, Technology & Medicine Open Access Book Publisher (INTECH): Rijeka, Croatia, 2012; pp. 59–88. [Google Scholar]
- Guo, J.; Qin, S.; Rengel, Z.; Gao, W.; Nie, Z.; Liu, H.; Li, C.; Zhao, P. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef]
- Varga, B.; Janda, T.; László, E.; Veisz, O. Influence of abiotic stresses on the antioxidant enzyme activity of cereals. Acta Physiol. Plant. 2012, 34, 849–858. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, P.; Arora, P.; Verma, V.; Khanna, K.; Saini, P.; Bhardwaj, R. Role and regulation of ROS and antioxidants as signaling molecules in response to abiotic stresses. In Plant Signaling Molecules; Woodhead Publishing: Sawston, UK, 2019; pp. 141–156. [Google Scholar]
- Foyer, C.H.; Descourvieres, P.; Kunert, K.J. Protection against oxygen radicals: An important defense mechanism studied in transgenic plants. Plant Cell Environ. 1994, 17, 507–523. [Google Scholar] [CrossRef]
- Ghavami, N.; Ramin, A.A. Grain yield and active substances of milk thistle as affected by soil salinity. Commun. Soil Sci. Plant Anal. 2008, 39, 2608–2618. [Google Scholar] [CrossRef]
- Afshar, R.K.; Chaichi, M.R.; Jovini, M.A.; Jahanzad, E.; Hashemi, M. Accumulation of silymarin in milk thistle seeds under drought stress. Planta 2015, 242, 539–543. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]

| Cd | Stm | Description |
|---|---|---|
| −Cd | Control | No stress; grain soaking + 2 foliar sprays using distilled water. |
| tZck | Grain soaking + 2 foliar sprays using 0.05 mM tZck. | |
| Sim | Grain soaking + 2 foliar sprays using 0.5 mM Sim. | |
| tZck + Sim | Grain soaking + 2 foliar sprays using 0.05 mM tZck + 0.5 mM Sim. | |
| +Cd | Control | Irrigating wheat seedlings with nutrient solution containing 0.6 mM Cd. |
| tZck | Irrigating wheat seedlings with nutrient solution containing 0.6 mM Cd + (grain soaking + 2 foliar sprays using 0.05 mM tZck). | |
| Sim | Irrigating wheat seedlings with nutrient solution containing 0.6 mM Cd + (grain soaking + 2 foliar sprays using 0.5 mM Sim). | |
| tZck + Sim | Irrigating wheat seedlings with nutrient solution containing 0.6 mM Cd + (grain soaking + 2 foliar sprays using 0.05 mM tZck + 0.5 mM Sim). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, E.F.; Aljarani, A.M.; Mohammed, F.A.; Desoky, E.-S.M.; Mohamed, I.A.A.; El-Sharnouby, M.; Tammam, S.A.; Hassan, F.A.S.; Rady, M.M.; Shaaban, A. Exploring the Potential Enhancing Effects of Trans-Zeatin and Silymarin on the Productivity and Antioxidant Defense Capacity of Cadmium-Stressed Wheat. Biology 2022, 11, 1173. https://doi.org/10.3390/biology11081173
Ali EF, Aljarani AM, Mohammed FA, Desoky E-SM, Mohamed IAA, El-Sharnouby M, Tammam SA, Hassan FAS, Rady MM, Shaaban A. Exploring the Potential Enhancing Effects of Trans-Zeatin and Silymarin on the Productivity and Antioxidant Defense Capacity of Cadmium-Stressed Wheat. Biology. 2022; 11(8):1173. https://doi.org/10.3390/biology11081173
Chicago/Turabian StyleAli, Esmat F., Alshafei M. Aljarani, Fozia A. Mohammed, El-Sayed M. Desoky, Ibrahim A. A. Mohamed, Mohamed El-Sharnouby, Suzan A. Tammam, Fahmy A. S. Hassan, Mostafa M. Rady, and Ahmed Shaaban. 2022. "Exploring the Potential Enhancing Effects of Trans-Zeatin and Silymarin on the Productivity and Antioxidant Defense Capacity of Cadmium-Stressed Wheat" Biology 11, no. 8: 1173. https://doi.org/10.3390/biology11081173
APA StyleAli, E. F., Aljarani, A. M., Mohammed, F. A., Desoky, E.-S. M., Mohamed, I. A. A., El-Sharnouby, M., Tammam, S. A., Hassan, F. A. S., Rady, M. M., & Shaaban, A. (2022). Exploring the Potential Enhancing Effects of Trans-Zeatin and Silymarin on the Productivity and Antioxidant Defense Capacity of Cadmium-Stressed Wheat. Biology, 11(8), 1173. https://doi.org/10.3390/biology11081173