Simple Summary
Invasive species are a leading hazard to marine ecosystems worldwide, coupled with climate change. Tackling the emerging biodiversity threat to maintain the ecological balance of the largest biome in the world has now become a pivotal part of the Sustainable Development Goals (SDGs). Marine herbivores are generally regarded as biological agents that restrict invasive species, and their efficiency depends on their dietary habits, especially the autotrophs they eat. Many researchers have found contradicting findings on the effects of nutritional attributes and novelty of autotrophs on herbivore eating behaviour. In light of the scattered literature on the mechanistic basis of autotroph-herbivore interactions, we provide a comprehensive review to fill knowledge gaps about synergies based on macroalgae, an important group of photosynthetic organisms in the marine biome that interact strongly with generalist herbivores. We also analyse macroalgal defence measures against herbivores, underlining unique features and potential roles in maintaining marine ecosystems. The nutritional qualities, shape, and novelty of autotrophs can alter herbivore feeding behaviour. Future research should explore aspects that can alter marine autotroph-herbivore interactions to resolve inconsistent results of specific features and the uniqueness of the organisms involved.
Abstract
Species invasion is a leading threat to marine ecosystems worldwide, being deemed as one of the ultimate jeopardies for biodiversity along with climate change. Tackling the emerging biodiversity threat to maintain the ecological balance of the largest biome in the world has now become a pivotal part of the Sustainable Development Goals (SDGs). Marine herbivores are often considered as biological agents that control the spread of invasive species, and their effectiveness depends largely on factors that influence their feeding preferences, including the specific attributes of their food–the autotrophs. While the marine autotroph-herbivore interactions have been substantially discussed globally, many studies have reported contradictory findings on the effects of nutritional attributes and novelty of autotrophs on herbivore feeding behaviour. In view of the scattered literature on the mechanistic basis of autotroph-herbivore interactions, we generate a comprehensive review to furnish insights into critical knowledge gaps about the synergies based largely on the characteristics of macroalgae; an important group of photosynthetic organisms in the marine biome that interact strongly with generalist herbivores. We also discuss the key defence strategies of these macroalgae against the herbivores, highlighting their unique attributes and plausible roles in keeping the marine ecosystems intact. Overall, the feeding behaviour of herbivores can be affected by the nutritional attributes, morphology, and novelty of the autotrophs. We recommend that future research should carefully consider different factors that can potentially affect the dynamics of the marine autotroph-herbivore interactions to resolve the inconsistent results of specific attributes and novelty of the organisms involved.
1. Introduction
In recent decades, mounting evidence suggests that biological invasions by invasive (also called alien or non-native) species are a growing threat to global biodiversity, and is exacerbated by climate warming [1,2]. Globalization, the transformation of technological regimes and expansions of transportation networks which modify the marine habitats are other recognized drivers behind the rapid shifting of invasive species across a broad geographical range [3,4,5]. In a narrower sense, species invasions can adversely influence the dynamics of specific communities, particularly concerning the extirpation of native species [6,7] and the reduction of species richness [8]. Climate, recipient communities, and invaders are considered the prime determinants of invasion impacts, with the characteristics of recipient communities being the most critical determinant [9]. The mechanisms of invasion impact on the diversity of native species, however, are still not well understood and in fact, previous findings on invasion consequences for species richness have been contradictory; viz., either positive, negative, neutral, or multifarious impacts [9]. This invasion paradox has led to many controversial debates over the past two decades [10]. The diversity and impact of invasive species on marine ecosystems are extensively covered in a recent review by Salimi et al. (2021) [11].
Studies on aquatic ecosystems showed that the interactions between marine herbivores and various plants and/or algae (hereinafter referred to as the “autotrophs”) could reduce or even prevent the detrimental impacts of species invasions [12,13]. Lyons and Scheibling [14] reported that the establishment of the invasive green algae Codium fragile was enhanced by sea urchin food preference for kelps under increased water temperature and wave action, leading to increased herbivore pressure on local kelp stands. By and large, generalist marine herbivores such as most fishes and sea urchins that feed on autotrophs are common biological control agents that suppress the establishment and abundance of invasive species in the recipient communities [15,16]. It has been reported that the feeding (or grazing) preferences of the herbivores can determine the relationship between native or invasive autotrophs [17,18]. Recent findings also suggested that mechanisms underlying autotroph palatability could help resolve the inconsistent results of novelty [19,20].
Since the 1980s, efforts have been undertaken to understand the foraging behaviour of generalist marine herbivores [21,22,23,24]. Their selective foraging behaviour, which aims chiefly to regulate their nutritional needs for growth, fecundity, and performance [25,26], has been found to exert a profound impact on the biological structure of many marine ecosystems [27]. As such, theoretic insights on the nutritional relationships between herbivores and autotrophs will assist in the control and management of invasive species [28,29,30]. Generalist herbivores have also been found to make their food selection based on autotroph palatability, which depends primarily on their other unique attributes including, among others, secondary metabolites, morphology and physical stress [31,32,33,34,35]. Significant research has been devoted to examining the role and importance of some secondary metabolites in the survival and adaptation of autotrophs [36,37,38], but less attention has been paid to dissecting the value of their other attributes that may also influence the preferences of herbivores, i.e., whether to feed on native or invasive plants, or both [12]. It is worth noting that autotrophic characteristics may have the opposite effect on autotroph-herbivore interactions in controlled experimental studies where herbivores are restricted to a single autotroph species than in effects seen in field studies where herbivores are free to move around and cause natural autotroph damage. Future research examining the significance of autotroph features in interactions between autotrophs and herbivores must therefore carefully take into account the context in which the relationships have been observed [19].
In this study, relevant research papers are selected based on the pre-determined key search criteria (autotroph-herbivore interactions, feeding behaviour, macroalgae, marine herbivores, nutrient acquisition) and accessed via a reliable online database of reputable journals. One hundred and seven papers have been validated and reviewed comprehensively. At present, the available literature on the interactions between autotroph palatability and their various attributes on the nutritional ecology of marine herbivores is scattered and fragmentary [39,40,41]. It is also important to note that the chemical compounds in autotrophs that attract or deter their feeders are not well-addressed [42,43]. This review provides an overview of the unique attributes of macroalgae, which generally refers to the primary marine autotrophs and photosynthetic eukaryotes other than terrestrial plants that are capable of interacting strongly with marine herbivores [44]. Several key factors that can influence the feeding preferences of herbivores will be discussed, providing a better understanding of the interactions between autotrophs and herbivores that can potentially increase the ecological resilience of the marine biome. These are in line with the global trends in the marine sciences and sustainable development challenges, particularly in achieving the SDGs.
2. Marine Algae and Their Unique Attributes
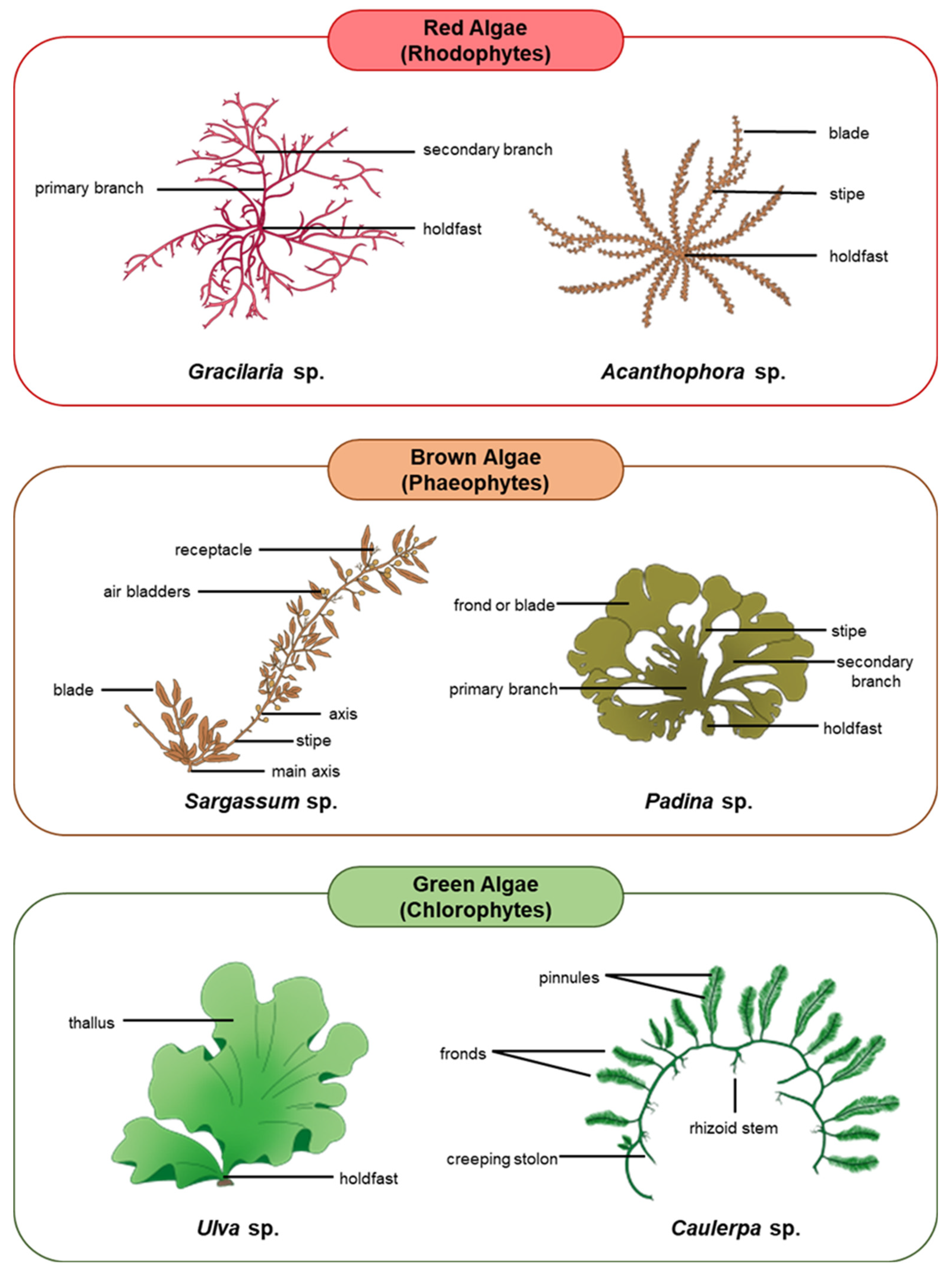
Algae are the ultimate source of nutrients and energy for other organisms living in aquatic ecosystems. Although not considered plants, algae are photosynthetic in nature and produce over 70% of the global oxygen content [45,46,47]. Algae are also effective at sequestering carbon by converting almost 50% of the atmospheric carbon dioxide into organic molecules that build essential cellular constituents and intensify their energy production [48,49,50,51,52]. Macroalgae, being the most important primary producers in the oceans, house a wide range of nutritional quality within and among groups which often influences their palatability to herbivores [25,53]. For the most part, the proteins in macroalgae contain important amino acids, particularly the ones that cannot be synthesized by the animal body [54,55]. Animal hosts can thus obtain all these essential amino acids through symbiosis with the algae [56]. A variety of macroalgae reproduce either exclusively sexually or asexually, whilst some species demonstrate an alternation of generations involving both reproductive strategies in succession [57,58,59]. The following subsections discuss the unique characteristics and ecological relationships of each major group of macroalgae, including red algae (Rhodophytes), brown algae (Phaeophytes), and green algae (Chlorophytes) [51,52]. Figure 1 depicts the three major groups of macroalgae and examples of their common species.
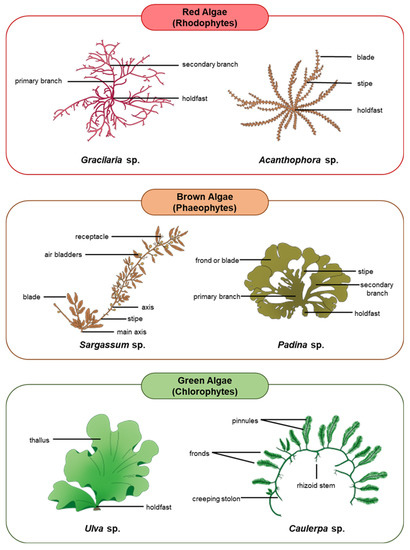
Figure 1.
Major groups of macroalgae and examples of their common species.
2.1. Red Algae (Division Rhodophyta)
The first group is the eukaryotic red algae, or the Rhodophytes, comprising more than 6000 species of primarily marine algae ranging from microscopic to macroscopic in size [60,61]. These algae store their energy as a specialized polysaccharide, known as floridean starch, and their cell walls are made of unique cellulose and polysaccharides, such as agars and carrageenan galactans [62,63,64]. However, some other red algae may adopt sulfated mannans or neutral xylans as the main cell wall components rather than carrageenans [63]. Their photosynthetic pigments include chlorophylls a and d, while their accessory pigments are carotenoids, phycobilins, and xanthophyll [60,65,66] (Table 1). Some notable examples of red algae are, among others, filamentous species like Pleonosporum spp. and coralline algae like Porolithon spp., which contribute significantly to the building of tropical reefs and thalloid species. It is worth noting that the red algae have no flagellated cells or cells with any vestigial structure of flagellation [20]. Irish moss (Chondrus crispus Stackhouse), also known as the carrageen moss, is an example of an economically important red alga which has been used to bind proteins together to stabilize and add texture to various foods and beverages like ice cream, yogurt, and deli meats [67,68]. Another economically and nutritionally important species of red algae is nori (Porphyra umbilicalis Kützing); a high-protein and high-fibre algae which is commonly used in Japanese cuisine as an ingredient to wrap sushi [69]. Porphyra was proved to have the greatest protein content (ca. 35%) among the marine macroalgae, while some members of the brown algae in the order Laminariales have the lowest content (ca. 7%) [70,71].

Table 1.
Major groups of macroalgae and their attributes.
2.2. Brown Algae (Division Chromophyta)
In contrast to other algal groups, brown algae or the Phaeophytes are mostly developed from a secondary endosymbiosis event which involved a non-photosynthetic eukaryote and a unicellular red alga. Resultantly, brown algae exhibit several morphological and metabolic features that make them the most complex macroalgae [72]. Phaeophytes are mostly macroscopic in size, inclusive of the giant kelp (Macrocystis pyrifera (Linnaeus) C.Agardh), which can grow up to 10 m in length [73]. Most of the approximately 1800 species of brown algae live in the marine environment, especially in cool temperate waters located in both the Northern and Southern Hemispheres [74,75]. Fucans and alginates are the specific polysaccharides compounds, which can be found in the cell wall of brown algae [72]. Generally, brown algae consist of three distinctly recognizable parts–the holdfast, stipe, and leaf-like blades [76]. The holdfast is a root-like structure at the bottom, which is often joined by a stipe to one or more leaf-like blades depending on the species. The blades serve as the primary surface for important processes including photosynthesis and nutrient exchange in the algae [77,78]. Although photosynthesis takes place predominantly in the blades, it is crucial that the stipe has the adequate length to place the blades sufficiently close to the light source. Alternatively, algae can absorb sufficient light by swelling the body (thallus) or increasing their growth rate [79]. The photosynthetic pigments in brown algae are chlorophylls a and c, and their accessory pigments include carotenoids and xanthophylls [80] (Table 1). Fucoxanthin contains brown-coloured pigment and the unique xanthophyll in brown algae which gives them their characteristic dark colour [81]. Unlike red algae, most of the brown algae have two flagella which help them achieve locomotion [82]. Some examples of brown algae include the rockweeds (Ascophyllum spp. and Fucus spp.) and the giant kelps (Macrocystis sp.). These algae usually contain laminarin and mannitol, storage sugars which can be fermented to make alcohol [83]. Some brown algae possess the ability to take up certain important substances from seawater. For instance, the iodine concentration in an edible kelp, kombu, can be thousands of times as great in the cells of the species as in its surrounding water [84].
2.3. Green Algae (Division Chlorophyta)
On the other hand, green algae or the Chlorophytes are generally more closely related to the higher plants in comparison to brown and red algae, in particular their chloroplast structure [85,86]. The cell walls of most species of green algae are built mainly by cellulose, with some incorporation of glycans (hemicelluloses) [87]. Their photosynthetic pigments in the chloroplast are chlorophylls a and b, while their accessory pigments are carotenoids and xanthophylls, found in embryophytes [87] (Table 1). Green algae comprise of 9000 to 12,000 species, with the majority of them occurring in freshwater rather than the marine environments [86,87]. Most green algae are microscopic, except for a small number of species in some specific genera such as those in Cladophora which are multicellular and macroscopic [87,88,89]. The unicellular genera Chlamydomonas and Chlorella are some common examples of green algae in both marine and freshwater ecosystems worldwide, which consist of species that disperse in a wide range of habitats [90]. An example of more complex green algae includes Volvox, which forms large hollow-spherical colonies that consist of thousands of cells [91]. The green algae Ulva spp., Caulerpa spp., Enteromorpha spp., and Codium spp. are commonly used as a food source for humans. The Ulva spp., known generally as sea lettuce, are extensively consumed in many Asian countries especially in Japan, China, and the Republic of Korea [86,92]. Access to nitrogen is one of the major limiting factors in the growth of green algae on the grounds that most of them thrive in shallow water [93]. Nevertheless, the increased runoff of fertilizer-related nitrogen into the oceans, mainly from agriculture has created favourable conditions for the growth of green algae and also other groups of algae in the past few decades [94]. According to Lee (2018), the majority of green algae form zoogametes, which are motile flagellated gametes [20]. The review by Moreira et al. (2021) details how macroalgae from various divisions differ in their flagellal construction, orientation, and life cycle in general [89].
3. At a Glance: Key Defence Strategies of Marine Macroalgae against Herbivores
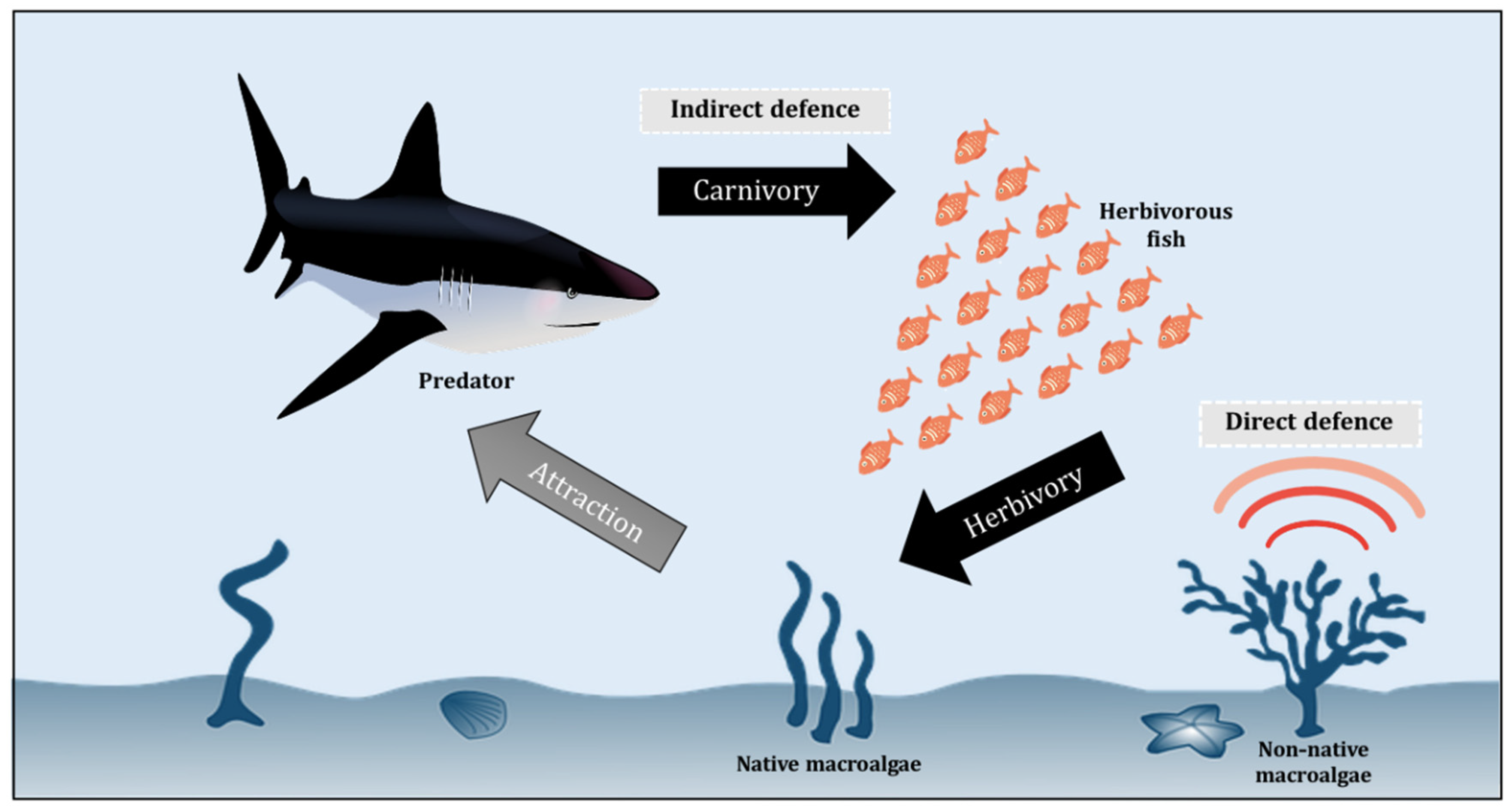
The base of a marine food web is dominated by photosynthetic autotrophs, notably macroalgae and microalgae (phytoplankton), which are the main producers of half of the Earth’s oxygen and also the organic carbon required by all marine animals to survive [95,96,97,98]. The next level of the marine food web is made up of herbivores, from small zooplankton to larger animals (such as herbivorous fishes and manatees) that eat up a huge number of macroalgae [99,100,101]. Figure 2 illustrates a simplified conceptual model of the interaction between different levels of the marine food web, including macroalgae, herbivore, and predator, with the non-native autotroph having direct defences against herbivores. The interactions between herbivores and macroalgae are indeed one of the key drivers of marine ecosystem dynamics, gaining increasing scientific attention in recent decades (Table 2). Unfortunately, the synergies are currently being altered by climate change, affecting macroalgae growth rates and phenology, expression of chemical defenses, and herbivore behaviour and metabolism [102,103,104,105,106,107,108,109,110,111]. These macroalgae, however, have developed a variety of defence mechanisms to help them avoid herbivory and ensure their survival and abundance, as discussed in Section 3.1 and Section 3.2.
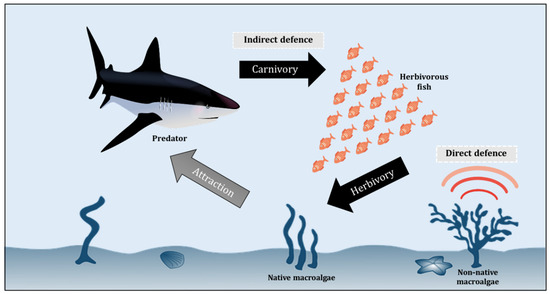
Figure 2.
Conceptual model of macroalgae-herbivore-predator interaction.

Table 2.
Key findings in autotroph-herbivore studies conducted since 2000.
3.1. Physical Defences
A multitude of scientific research indicated that ocean warming has caused ecological impacts on various marine flora and fauna species across the globe, with a range of species marching away from their native homes in search of cooler climes [120,121,122,123,124,125,126,127,128,129,130,131,132,133]. With heatwaves sweeping through oceans twice as much as they did in the early 1990s, many biodiversity hotspots around the world are on the verge of imminent collapse. Many marine algae exhibit morphological plasticity that allows them to thrive in diverse habitats with various environmental pressures [134]. The study conducted by Diaz-Pulido et al. [134] showed that the morphology of different species of brown algae (Padina boergesenii) was significantly affected not only by herbivory but also by climatic and oceanographic factors, and this suggested that algal response to herbivory could also be a seasonal process [135,136,137,138,139]. Populations from more variable environments are considered to be more plastic [140], and algal phenotypic plasticity is potentially another pivotal mechanism that enables algae to respond to either fluctuating environments [141] or species invasion [142]. According to Fordyce [143], ecological interactions mediated by phenotypic plasticity, which are typical in nature, depend heavily on the morphological responses of the interacting organisms.
Hard encrusting calcified algae are common in the tropics where grazing is severe. Calcification of the coralline algal thallus is thought to have evolved as an adaptation to protect reproductive structures from herbivory by developing a multi-layered thallus in which reproductive structures are sunken beneath the calcareous surface cells and are thus protected from grazer access [144]. The calcified thalli may also decrease digestibility and in herbivorous herbivores (such as crabs) cause wear of chelipeds, mandibles, and the teeth of the gastric mill [145]. Increasing anthropogenic CO2 emissions have led to elevated oceanic pCO2 which may impact the structural integrity and protective function of the calcified thallus by decreasing calcification rates, and thus increasing the vulnerability of the coralline algae to bioerosion and grazing by excavating herbivores such as sea urchins and parrotfishes [146]. Non-calcifying macroalgae, on the other hand, typically use thallus toughness or mechanical strength as means of physical defence [136]. It is important to note that herbivore foraging is not essentially detrimental to the marine autotrophs. For example, limpets and chitons reportedly encourage coralline growth by regularly removing algal epiphytes from the surface of the coralline algae, which is necessary to avoid eventual overgrowing and killing of the coralline crust [147].
3.2. Chemical Defences
The chemical strategies of defence against herbivore are complex and generally assigned to two defence mechanisms–the direct and indirect defences [103,109]. In response to herbivory, direct defences are biologically mediated by autotroph chemistry and thus these defences can change the biological functions of the herbivores, including their feeding patterns, growth, and survival. In contrast, indirect defences against herbivory depend upon other species such as the natural enemies of the herbivores [110,111] (Figure 2). The chemical ecology of macroalgae has been widely elucidated in various regions and habitats, focusing primarily on herbivore offence and oxidative burst responses, which are chemical defences activated against pathogens and biofuels (Potin, 2008) [121,122,123]. An enormous diversity of secondary metabolites is regularly produced by autotrophs in response to herbivory in aquatic ecosystems [124,125]. Marine algae are known to be a viable source of specialized metabolites that play a crucial role in the ecosystem and climate functioning [126,127]. Tropical macroalgal taxa have been reported to produce a higher diversity of metabolites compared to their temperate counterparts, dominated by halogenated metabolites, terpenoids, acetogenins, and phenolics [127,128]. Mainly regulated by developmental, genetic, and environmental factors, these metabolites play diverse ecological functions in macroalgae, from being deterrent against herbivores to defenders to fight against specific pathogens and competitors for space with other marine organisms [127,129,130].
Over the past decade, considerable attention has been devoted to understanding the interactions between algal halogenated compound production and the environment, which includes global and anthropogenic climate changes [124,131]. Given that macroalgae produce a range of halogenated secondary metabolites, particularly chlorinated and brominated compounds that are predominant in red (90%) and green (7%) macroalgae, many studies have been conducted using these macroalgae to aid biosorption of pollutants in both industry and agriculture [124,132]. It is worth noting that halogenation of macroalgal components is involved in chemical defence mechanisms because halogenated metabolites are often associated with antibacterial, antifungal, antibacterial, and antioxidant properties [132,133].
4. Does Nutrient Acquisition in Algae Determine the Feeding Preferences of Marine Herbivores?
Palatability can be broadly defined as the characteristics and conditions of autotrophs that stimulate the animal to feed on them [33]. These include their structure, physical, and chemical attributes [148]. It has been long recognized that macronutrient composition influences palatability and foods that are higher in fat and protein content usually have higher palatability in terrestrial and aquatic ecosystems [149,150]. In marine communities, the preference and performance of the herbivores often relate directly to the nutritional value of algae or some other autotrophs, which is driven mostly by the protein and nitrogen content [151,152,153,154,155]. For example, several studies on the high-value marine abalone (Haliotis asinine) suggested that their diverse preferences are primarily influenced by the protein and nitrogen content of macroalgae [156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172]. Table 3 shows some examples of studies involving the interactions between marine autotrophs and herbivores based on the herbivore nutrient acquisition since the 2010s.

Table 3.
Examples of studies involving marine autotroph-herbivore interactions based on herbivore nutrient acquisition since the 2010s.
Living organisms require nitrogen to synthesize amino acids, the basic building blocks of protein that serve essential functions in virtually all biological processes [153]. Many previous studies have pointed out that low nitrogen consumption is associated with reduced food intake in generalist marine herbivores [39,148,160,161,162,163]. The study by Barile, Lapointe and Capo [156] on California sea hare (Aplysia californica) showed that this herbivorous gastropod preferred to feed on gracilarioid algae (Gracilaria ferox) with high levels of nitrogen. The lack of preference for protein-enriched algae (i.e., high-nutrient algae) can be explained by the compensatory feeding behaviour of some herbivore species. Previous studies reported the optimal growth rate and adequate intakes of limiting nutrients by testing the consumption rates of different herbivores on the low nutritional quality of algae foods [39,43,164,165]. According to Bradley et al. (2021), herbivores may avoid species that are less palatable or have lower nutritional value, which may affect their distribution and abundance [164]. However, the distinct nutritional drivers underlying the feeding preferences of specific marine herbivores are still a frontier that needs to be further explored.
5. Conclusions
One of the SDGs is uniquely dedicated to life below water, which is to conserve and sustainably use the oceans, seas, and marine resources for sustainable development. Nevertheless, maintaining the ecological balance of the largest biome in the world has become more challenging, especially when human-induced climate change continues to rapidly affect the diversity of marine life in an adverse way. To tackle the major threats to biodiversity, such as invasive species and habitat loss, it is worthwhile to dive into the unknown interactions between autotrophs and herbivores–those organisms that rule the base of the food chain. Theoretic insights on the synergies of autotrophs and herbivores in the marine biome are therefore crucial in controlling and managing invasive species, which almost always do more harm than good. A more concerted effort to test the major hypotheses in invasion biology, for example, the biotic resistance and enemy release hypotheses, is required to ensure the sustainability of the current marine ecosystems. Future research that aims to develop theories of marine ecology should be carefully designed, looking into various factors that can potentially affect the dynamics of different trophic levels within one or several food webs, including geographic variation and important attributes of the organisms involved. We strongly recommend the integration of evolutionary novelty theory with autotroph attributes and novelty in future studies to provide a better understanding of the consequences of biological invasions in the marine biome.
Author Contributions
Conceptualization, A.C. and Z.I.; data curation, W.Y.L.; funding acquisition, A.C., S.-L.S. and Z.I.; investigation, A.C., Z.I. and W.Y.L.; methodology, A.C. and W.Y.L.; project administration, A.C. and Z.I.; resources, A.C. and Z.I.; software, Z.I.; supervision, A.C.; validation, A.C. and Z.I.; visualization, A.Y.A., P.-E.L., S.-W.P. and S.-L.S.; writing—original draft, A.C., Z.I. and W.Y.L.; writing—review and editing, A.C., Z.I., S.-L.S. and W.Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Universiti Malaya [Grant Numbers: H-5620009 and ST007-2021]. The funders had no role in the preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All the authors would like to acknowledge all the financial supports for this research project and the collaboration works between all the authors. A.C. would like to thank Usaha Fadzilat (M) Sdn. Bhd. for providing information on herbivorous fishes in Malaysia. Z.I. is Fulbright Visiting Research Scholar at Cornell University during the preparation of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thuiller, W.; Richardson, D.M.; Midgley, G.F. Will climate change promote alien plant invasions? In Biological Invasions; Springer: Berlin, Germany, 2008; pp. 197–211. [Google Scholar]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Weber, E.; Winter, M.; et al. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Kühn, I.; Wild, J.; Arianoutsou, M.; Bacher, S.; Chiron, F.; Didžiulis, V.; Essl, F.; et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc. Natl. Acad. Sci. USA 2010, 107, 12157–12162. [Google Scholar] [CrossRef]
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pyšek, P.; Hobbs, R.J. Riparian vegetation: Degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- Seebens, H.; Gastner, M.T.; Blasius, B. The risk of marine bioinvasion caused by global shipping. Ecol. Lett. 2013, 16, 782–790. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Hutchison, M.A.; Ewers, R.M.; Gemmell, N.J. Are invasive species the drivers of ecological change? Trends Ecol. Evol. 2005, 20, 470–474. [Google Scholar] [CrossRef]
- Gilbert, B.; Levine, J.M. Plant invasions and extinction debts. Proc. Natl. Acad. Sci. USA 2013, 110, 1744–1749. [Google Scholar]
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef]
- Dong, L.J.; Yu, H.W.; He, W.M. What determines positive, neutral, and negative impacts of Solidago canadensis invasion on native plant species richness? Sci. Rep. UK 2015, 5, 16804. [Google Scholar]
- Stohlgren, T.J.; Rejmánek, M. No universal scale-dependent impacts of invasive species on native plant species richness. Biol. Lett. 2014, 10, 20130939. [Google Scholar] [CrossRef]
- Salimi, P.A.; Creed, J.C.; Esch, M.M.; Fenner, D.; Jaafar, Z.; Levesque, J.C.; Montgomery, A.D.; Salimi, M.A.; Edward, J.K.P.; Raj, K.D.; et al. A review of the diversity and impact of invasive non-native species in tropical marine ecosystems. Mar. Biodivers. Rec. 2021, 14, 11. [Google Scholar] [CrossRef]
- Grutters, B.M.C.; Roijendijk, Y.O.A.; Verberk, W.; Bakker, E. Plant traits and plant biogeography control the biotic resistance provided by generalist herbivores. Funct. Ecol. 2017, 31, 1184–1192. [Google Scholar] [CrossRef]
- Parker, J.D.; Burkepile, D.E.; Hay, M.E. Opposing Effects of Native and Exotic Herbivores on Plant Invasions. Science 2006, 311, 1459–1461. [Google Scholar] [CrossRef]
- Lyons, D.; Scheibling, R. Context-dependant survival of the invasive seaweed Codium fragile ssp. tomentosoides in kelp bed and urchin barren habitats off Nova Scotia. Aquat. Biol. 2008, 2, 17–27. [Google Scholar] [CrossRef]
- Cebrian, E.; Ballesteros, E.; Linares, C.; Tomas, F. Do native herbivores provide resistance to Mediterranean marine bioinvasions? A seaweed example. Biol. Invasions 2010, 13, 1397–1408. [Google Scholar] [CrossRef]
- Seastedt, T.R. Biological control of invasive plant species: A reassessment for the Anthropocene. New Phytol. 2015, 205, 490–502. [Google Scholar]
- Joshi, J.; Vrieling, K. The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecol. Lett. 2005, 8, 704–714. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar]
- Münzbergová, Z.; Skuhrovec, J. Data on Herbivore Performance and Plant Herbivore Damage Identify the Same Plant Traits as the Key Drivers of Plant–Herbivore Interaction. Insects 2020, 11, 865. [Google Scholar] [CrossRef]
- Lee, R.E. Phycology; Cambridge University Press: Cambridge, UK, 2018; pp. 510–546. [Google Scholar]
- Behmer, S.T.; Simpson, S.J.; Raubenheimer, D. Herbivore foraging in chemically heterogeneous environments: Nutrients and secondary metabolites. Ecology 2002, 83, 2489–2501. [Google Scholar]
- Martinez, A.S.; Byrne, M.; Coleman, R.A. What and when to eat? Investigating the feeding habits of an intertidal herbivorous starfish. Mar. Biol. 2016, 163, 166. [Google Scholar] [CrossRef]
- Senft, R.L.; Coughenour, M.B.; Bailey, D.W.; Rittenhouse, L.R.; Sala, O.; Swift, D.M. Large Herbivore Foraging and Ecological Hierarchies. BioScience 1987, 37, 789–799. [Google Scholar] [CrossRef]
- Wahl, M.; Hay, M. Associational resistance and shared doom: Effects of epibiosis on herbivory. Oecologia 1995, 102, 329–340. [Google Scholar] [CrossRef]
- Duarte, C.; Navarro, J.; Acuña, K.; Gómez, I. Feeding preferences of the sandhopper Orchestoidea tuberculata: The importance of algal traits. Hydrobiologia 2010, 651, 291–303. [Google Scholar] [CrossRef]
- Johnson, J.S.; Clements, K.D.; Raubenheimer, D. The nutritional basis of seasonal selective feeding by a marine herbivorous fish. Mar. Biol. 2017, 164, 201. [Google Scholar] [CrossRef]
- Taylor, D.I.; Schiel, D.R. Algal populations controlled by fish herbivory across a wave exposure gradient on southern temperate shores. Ecology 2010, 91, 201–211. [Google Scholar] [CrossRef]
- Sagerman, J.; Enge, S.; Pavia, H.; Wikström, S.A. Low feeding preference of native herbivores for the successful non-native seaweed Heterosiphonia japonica. Mar. Biol. 2015, 162, 2471–2479. [Google Scholar] [CrossRef]
- Schwartz, N.; Rohde, S.; Hiromori, S.; Schupp, P.J. Understanding the invasion success of Sargassum muticum: Herbivore preferences for native and invasive Sargassum spp. Mar. Biol. 2016, 163, 181. [Google Scholar] [CrossRef]
- Thomas, M.B.; Reid, A.M. Are exotic natural enemies an effective way of controlling invasive plants? Trends Ecol. Evol. 2007, 22, 447–453. [Google Scholar] [CrossRef]
- Chavanich, S.; Harris, L.G. The influence of macroalgae on seasonal abundance and feeding preference of a subtidal snail, lacuna vincta (montagu) (littorinidae) in the gulf of maine. J. Molluscan Stud. 2002, 68, 73–78. [Google Scholar] [CrossRef]
- Molis, M.; Scrosati, R.A.; El-Belely, E.; Lesniowski, T.J.; Wahl, M. Wave-induced changes in seaweed toughness entail plastic modifications in snail traits maintaining consumption efficacy. J. Ecol. 2015, 103, 851–859. [Google Scholar] [CrossRef]
- Pennings, S.C.; Siska, E.L.; Bertness, M.D. Latitudinal differences in plant palatability in Atlantic coast salt marshes. Ecology 2001, 82, 1344–1359. [Google Scholar]
- Rodríguez, A.; Clemente, S.; Hernández, J.C.; Brito, A.; García, I.; Becerro, M.A. Nutritional, structural and chemical defenses of common algae species against juvenile sea urchins. Mar. Biol. 2017, 164, 127. [Google Scholar] [CrossRef]
- Sudatti, D.B.; Fujii, M.; Rodrigues, S.V.; Turra, A.; Pereira, R.C. Prompt induction of chemical defenses in the red seaweed Laurencia dendroidea: The role of herbivory and epibiosis. J. Sea Res. 2018, 138, 48–55. [Google Scholar] [CrossRef]
- Ianora, A.; Boersma, M.; Casotti, R.; Fontana, A.; Harder, J.; Hoffmann, F.; Pavia, H.; Potin, P.; Poulet, S.A.; Toth, G. New trends in marine chemical ecology. Estuaries Coasts 2006, 29, 531–551. [Google Scholar] [CrossRef]
- Nylund, G.M.; Enge, S.; Pavia, H. Costs and Benefits of Chemical Defence in the Red Alga Bonnemaisonia hamifera. PLoS ONE 2013, 8, e61291. [Google Scholar] [CrossRef]
- Maschek, J.A.; Baker, B.J. The Chemistry of Algal Secondary Metabolism. In Algal Chemical Ecology; Springer: Berlin, Germany, 2008; pp. 1–24. [Google Scholar] [CrossRef]
- Yin, L.W.; Eem, L.P.; Amri, A.Y.; Looi, S.S.; Cheng, A. Exploring the role of macroalgal traits on the feeding behaviour of a generalist herbivore in Malaysian waters. Bot. Mar. 2020, 63, 407–417. [Google Scholar] [CrossRef]
- Boyer, K.E.; Fong, P.; Armitage, A.R.; Cohen, R.A. Elevated nutrient content of tropical macroalgae increases rates of herbivory in coral, seagrass, and mangrove habitats. Coral Reefs 2004, 23, 530–538. [Google Scholar] [CrossRef]
- Clements, K.D.; Raubenheimer, D.; Choat, J.H. Nutritional ecology of marine herbivorous fishes: Ten years on. Funct. Ecol. 2009, 23, 79–92. [Google Scholar] [CrossRef]
- Wong, P.K.; Liang, Y.; Liu, N.Y.; Qiu, J.-W. Palatability of macrophytes to the invasive freshwater snail Pomacea canaliculata: Differential effects of multiple plant traits. Freshw. Biol. 2010, 55, 2023–2031. [Google Scholar] [CrossRef]
- Machado, G.B.; Leite, F.P.; Sotka, E.E. Nutrition of marine mesograzers: Integrating feeding behavior, nutrient intake and performance of an herbivorous amphipod. PeerJ 2018, 6, e5929. [Google Scholar] [CrossRef]
- Cock, J.M.; Coelho, S.M. Algal models in plant biology. J. Exp. Bot. 2011, 62, 2425–2430. [Google Scholar] [CrossRef]
- Gislason, S. Air and Breathing; Environmed Research Inc.: Vancouver, BC, Canada, 2018; Volume 5. [Google Scholar]
- Greenbaum, E.; Guillard, R.R.L.; Sunda, W.G. Hydrogen and Oxygen Photoproduction by Marine Algae. Photochem. Photobiol. 1983, 37, 649–655. [Google Scholar] [CrossRef]
- Souvorov, A.V. Marine Ecologonomics: The Ecology and Economics of Marine Natural Resources Management, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1999; Volume 6. [Google Scholar]
- Arrigo, K.R. Carbon cycle: Marine manipulations. Nature 2007, 450, 491. [Google Scholar]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2010, 23, 877–886. [Google Scholar] [CrossRef]
- Moreira, D.; Pires, J.C. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol. 2016, 215, 371–379. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedi, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and Nutritional and Cardiovascular-Health Properties of Seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Rothäusler, E.; Macaya, E.; Molis, M.; Wahl, M.; Thiel, M. Laboratory experiments examining inducible defense show variable responses of temperate brown and red macroalgae. Rev. Chil. Hist. Nat. 2005, 78, 603–614. [Google Scholar] [CrossRef][Green Version]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Wang, J.T.; Douglas, A.E. Essential amino acid synthesis and nitrogen recycling in an alga-invertebrate symbiosis. Mar. Biol. 1999, 135, 219–222. [Google Scholar] [CrossRef]
- Douglas, A.E. Host benefit and the evolution of specialization in symbiosis. Heredity 1998, 81, 599. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Frenkel, J.; Vyverman, W.; Pohnert, G. Pheromone signaling during sexual reproduction in algae. Plant J. 2014, 79, 632–644. [Google Scholar] [CrossRef]
- Clifton, K.E.; Clifton, L.M. The Phenology of Sexual Reproduction by Green Algae (Bryopsidales) on Caribbean Coral Reefs. J. Phycol. 1999, 35, 24–34. [Google Scholar] [CrossRef]
- Gantt, E.; Grabowski, B.; Cunningham, F.X. Antenna Systems of Red Algae: Phycobilisomes with Photosystem ll and Chlorophyll Complexes with Photosystem I. In Light-Harvesting Antennas in Photosynthesis; Springer: Dordrecht, The Netherlands, 2003; pp. 307–322. [Google Scholar] [CrossRef]
- Masarin, F.; Cedeno, F.R.P.; Chavez, E.G.S.; De Oliveira, L.E.; Gelli, V.C.; Monti, R. Chemical analysis and biorefinery of red algae Kappaphycus alvarezii for efficient production of glucose from residue of carrageenan extraction process. Biotechnol. Biofuels 2016, 9, 122. [Google Scholar] [CrossRef]
- Michel, G.; Helbert, W.; Kahn, R.; Dideberg, O.; Kloareg, B. The structural bases of the processive degradation of ι-carrageenan, a main cell wall polysaccharide of red algae. J. Mol. Biol. 2003, 334, 421–433. [Google Scholar]
- Usov, A.I. Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 65, pp. 115–217. [Google Scholar]
- Vreeland, V.; Kloareg, B. Cell wall biology in red algae: Divide and conquer. J. Phycol. 2000, 36, 793–797. [Google Scholar]
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotrnoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar]
- Squires, A.H.; Moerner, W.E. Direct single-molecule measurements of phycocyanobilin photophysics in monomeric C-phycocyanin. Proc. Natl. Acad. Sci. USA 2017, 114, 9779–9784. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A traditional ingredients for new gastronomic sensation. Food Hydrocoll. 2017, 68, 255–265. [Google Scholar] [CrossRef]
- Trius, A.; Sebranek, J.G.; Lanier, T. Carrageenans and their use in meat products. Crit. Rev. Food Sci. Nutr. 1996, 36, 69–85. [Google Scholar]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2011, 16, 29–37. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed proteins. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–262. [Google Scholar]
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.-M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef]
- Westermeier, R.; Patiño, D.J.; Müller, H.; Müller, D.G. Towards domestication of giant kelp (Macrocystis pyrifera) in Chile: Selection of haploid parent genotypes, outbreeding, and heterosis. J. Appl. Phycol. 2009, 22, 357–361. [Google Scholar] [CrossRef]
- Aven, J.A.R.; Johnston, A.M.; Kübler, J.E.; Orb, R.E.K.; Cinroy, S.G.M.; Andley, L.I.L.H.; Crimgeour, C.H.M.S.; Alker, D.I.W.; Beardall, J.; Layton, M.N.C.; et al. Seaweeds in Cold Seas: Evolution and Carbon Acquisition. Ann. Bot. 2002, 90, 525–536. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef]
- Wernberg, T.; Thomsen, M.S. The effect of wave exposure on the morphology of Ecklonia radiata. Aquat. Bot. 2005, 83, 61–70. [Google Scholar] [CrossRef]
- Koehl, M.A.R.; Silk, W.K.; Liang, H.; Mahadevan, L. How kelp produce blade shapes suited to different flow regimes: A new wrinkle. Integr. Comp. Biol. 2008, 48, 834–851. [Google Scholar] [CrossRef]
- Stewart, H.L.; Carpenter, R.C. The Effects of Morphology and Water Flow on Photosynthesis of Marine Macroalgae. Ecology 2003, 84, 2999–3012. [Google Scholar] [CrossRef]
- Toohey, B.; Kendrick, G.A.; Wernberg, T.; Phillips, J.C.; Malkin, S.; Prince, J. The effects of light and thallus scour from Ecklonia radiata canopy on an associated foliose algal assemblage: The importance of photoacclimation. Mar. Biol. 2004, 144, 1019–1027. [Google Scholar] [CrossRef]
- Bidigare, R.R. Photosynthetic pigment composition of the brown tide alga: Unique chlorophyll and carotenoid derivatives. In Novel Phytoplankton Blooms; Springer: Berlin/Heidelberg, Germany, 1989; pp. 57–75. [Google Scholar]
- Maria, A.G.; Graziano, R.; Nicolantonio, D.O. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Fu, G.; Nagasato, C.; Oka, S.; Cock, J.M.; Motomura, T. Proteomics Analysis of Heterogeneous Flagella in Brown Algae (Stramenopiles). Protist 2014, 165, 662–675. [Google Scholar] [CrossRef]
- Horn, S.J.; Aasen, I.M.; Østgaard, K. Production of ethanol from mannitol by Zymobacter palmae. J. Ind. Microbiol. Biotechnol. 2000, 24, 51–57. [Google Scholar]
- Amachi, S. Microbial Contribution to Global Iodine Cycling: Volatilization, Accumulation, Reduction, Oxidation, and Sorption of Iodine. Microbes Environ. 2008, 23, 269–276. [Google Scholar] [CrossRef]
- Patron, N.J.; Keeling, P.J. Common evolutionary origin of starch biosynthetic enzymes in green and red algae1. J. Phycol. 2005, 41, 1131–1141. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for Food and Industrial Applications; IntechOpen: London, UK, 2013. [Google Scholar]
- Lewis, L.A.; McCourt, R.M. Green algae and the origin of land plants. Am. J. Bot. 2004, 91, 1535–1556. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2021, 14, 5–26. [Google Scholar] [CrossRef]
- Pinheiro, C.; Azevedo, J.; Campos, A.; Loureiro, S.; Vasconcelos, V. Absence of negative allelopathic effects of cylindrospermopsin and microcystin-LR on selected marine and freshwater phytoplankton species. Hydrobiologia 2012, 705, 27–42. [Google Scholar] [CrossRef]
- Nozaki, H.; Mahakham, W.; Heman, W.; Matsuzaki, R.; Kawachi, M. A new preferentially outcrossing monoicous species of Volvox sect. Volvox (Chlorophyta) from Thailand. PLoS ONE 2020, 15, e0235622. [Google Scholar] [CrossRef]
- García-Casal, M.N.; Ramirez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br. J. Nutr. 2008, 101, 79–85. [Google Scholar]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloğlu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef]
- Maberly, S.C.; Pitt, J.-A.; Davies, P.S.; Carvalho, L. Nitrogen and phosphorus limitation and the management of small productive lakes. Inland Waters 2020, 10, 159–172. [Google Scholar] [CrossRef]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. In The Ocean and Cryosphere in a Changing Climate: Special Report of the Intergovernmental Panel on Climate Change, Intergovernmental Panel on Climate; Cambridge University Press: Cambridge, UK, 2022; pp. 447–588. [Google Scholar]
- Jorda, G.; Marbà, N.; Bennett, S.; Santana-Garcon, J.; Agusti, S.; Duarte, C.M. Ocean warming compresses the three-dimensional habitat of marine life. Nat. Ecol. Evol. 2019, 4, 109–114. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Williams, S.L.; Carlton, J.T. Marine range shifts and species introductions: Comparative spread rates and community impacts. Glob. Ecol. Biogeogr. 2010, 19, 303–316. [Google Scholar] [CrossRef]
- Wabnitz, C.C.C.; Lam, V.W.Y.; Reygondeau, G.; Teh, L.C.L.; Al-Abdulrazzak, D.; Khalfallah, M.; Pauly, D.; Palomares, M.L.D.; Zeller, D.; Cheung, W.W.L. Climate change impacts on marine biodiversity, fisheries and society in the Arabian Gulf. PLoS ONE 2018, 13, e0194537. [Google Scholar] [CrossRef]
- Currin, C.; Newell, S.; Paerl, H. The role of standing dead Spartina alterniflora and benthic microalgae in salt marsh food webs: Considerations based on multiple stable isotope analysis. Mar. Ecol. Prog. Ser. 1995, 121, 99–116. [Google Scholar] [CrossRef]
- Chapman, R.L. Algae: The world’s most important “plants”—An introduction. Mitig. Adapt. Strat. Glob. Chang. 2010, 18, 5–12. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Parker, J.D. Recent advances in plant-herbivore interactions. F1000Research 2017, 6, 119. [Google Scholar] [CrossRef]
- Jormalainen, V.; Honkanen, T. Macroalgal Chemical Defenses and Their Roles in Structuring Temperate Marine Communities. In Algal Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 57–89. [Google Scholar] [CrossRef]
- Kinlan, B.P.; Gaines, S.D. Propagule dispersal in marine and terrestrial environments: A community perspective. Ecology 2003, 84, 2007–2020. [Google Scholar] [CrossRef]
- Wallentinus, I. Introduced Marine Algae and Vascular Plants in European Aquatic Environments. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Springer: Dordrecht, The Netherlands, 2002; pp. 27–52. [Google Scholar] [CrossRef]
- Van Donk, E.; Ianora, A.; Vos, M. Induced defences in marine and freshwater phytoplankton: A review. Hydrobiologia 2010, 668, 3–19. [Google Scholar] [CrossRef]
- Zamzow, J.; Amsler, C.; McClintock, J.; Baker, B. Habitat choice and predator avoidance by Antarctic amphipods: The roles of algal chemistry and morphology. Mar. Ecol. Prog. Ser. 2010, 400, 155–163. [Google Scholar] [CrossRef]
- Sakanishi, Y.; Tanaka, K.; Kasai, H.; Tanaka, J. Characterization of thallus mechanical and physiological traits of tropical fucoids: A preliminary study. Phycol. Res. 2020, 68, 208–215. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Arimura, G.-I.; Matsui, K.; Takabayashi, J. Chemical and Molecular Ecology of Herbivore-Induced Plant Volatiles: Proximate Factors and Their Ultimate Functions. Plant Cell Physiol. 2009, 50, 911–923. [Google Scholar] [CrossRef]
- Dicke, M.; van Poecke, R.M.; de Boer, J.G. Inducible indirect defence of plants: From mechanisms to ecological functions. Basic Appl. Ecol. 2003, 4, 27–42. [Google Scholar]
- Russ, G.R.; Alcala, A.C. MARINE RESERVES: RATES AND PATTERNS OF RECOVERY AND DECLINE OF PREDATORY FISH, 1983–2000. Ecol. Appl. 2003, 13, 1553–1565. [Google Scholar] [CrossRef]
- Floeter, S.R.; Behrens, M.D.; Ferreira, C.E.L.; Paddack, M.J.; Horn, M.H. Geographical gradients of marine herbivorous fishes: Patterns and processes. Mar. Biol. 2005, 147, 1435–1447. [Google Scholar] [CrossRef]
- Paddack, M.J.; Cowen, R.K.; Sponaugle, S. Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs 2006, 25, 461–472. [Google Scholar] [CrossRef]
- Castellanos-Galindo, G.A.; Giraldo, A. Food resource use in a tropical eastern Pacific tidepool fish assemblage. Mar. Biol. 2008, 153, 1023–1035. [Google Scholar] [CrossRef]
- Kopp, D.; Bouchon-Navaro, Y.; Louis, M.; Mouillot, D.; Bouchon, C. Juvenile Fish Assemblages in Caribbean Seagrass Beds: Does Nearby Habitat Matter? J. Coast. Res. 2010, 26, 1133–1141. [Google Scholar]
- Jessen, C.; Wild, C. Herbivory effects on benthic algal composition and growth on a coral reef flat in the Egyptian Red Sea. Mar. Ecol. Prog. Ser. 2013, 476, 9–21. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L., Jr.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Martínez-Crego, B.; Arteaga, P.; Tomas, F.; Santos, R. The Role of Seagrass Traits in Mediating Zostera noltei Vulnerability to Mesograzers. PLoS ONE 2016, 11, e0156848. [Google Scholar] [CrossRef]
- Kornijów, R.; Karpowicz, M.; Ejsmont-Karabin, J.; Nawrocka, L.; De Eyto, E.; Grzonkowski, K.; Magnuszewski, A.; Jakubowska, A.; Wodzinowski, T.; Woźniczka, A. Patchy distribution of phyto- and zooplankton in large and shallow lagoon under ice cover and resulting trophic interactions. Mar. Freshw. Res. 2020, 71, 1327–1341. [Google Scholar] [CrossRef]
- Amsler, C.D. Induced defenses in macroalgae: The herbivore makes a difference. J. Phycol. 2001, 37, 353–356. [Google Scholar] [CrossRef]
- Borell, E.M.; Foggo, A.; Coleman, R.A. Induced resistance in intertidal macroalgae modifies feeding behaviour of herbivorous snails. Oecologia 2004, 140, 328–334. [Google Scholar] [CrossRef]
- Hemmi, A.; Jormalainen, V. Geographic covariation of chemical quality of the host alga Fucus vesiculosus with fitness of the herbivorous isopod Idotea baltica. Mar. Biol. 2003, 145, 759–768. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated Compounds from Marine Algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.B.; Åberg, P. Optimal defense theory: Elasticity analysis as a tool to predict intraplant variation in defenses. Ecology 2002, 83, 891–897. [Google Scholar]
- Paul, C.; Pohnert, G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011, 28, 186–195. [Google Scholar] [CrossRef]
- Gaubert, J.; Payri, C.E.; Vieira, C.; Solanki, H.; Thomas, O.P. High metabolic variation for seaweeds in response to environmental changes: A case study of the brown algae Lobophora in coral reefs. Sci. Rep. 2019, 9, 993. [Google Scholar] [CrossRef]
- Baker, B.J.; Amsler, C.D.; McClintock, J.B. Macroalgal Chemical Defenses in Polar Marine Communities. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 91–103. [Google Scholar] [CrossRef]
- Kooke, R.; Keurentjes, J.J.B. Multi-dimensional regulation of metabolic networks shaping plant development and performance. J. Exp. Bot. 2011, 63, 3353–3365. [Google Scholar] [CrossRef]
- Dell, C.; Hay, M.E. Induced defence to grazing by vertebrate herbivores: Uncommon or under-investigated? Mar. Ecol. Prog. Ser. 2016, 561, 137–145. [Google Scholar]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef]
- Nielsen, B.; Maneein, S.; Farid, A.; Milledge, J. The Effects of Halogenated Compounds on the Anaerobic Digestion of Macroalgae. Fermentation 2020, 6, 85. [Google Scholar] [CrossRef]
- Küpper, F.C.; Miller, E.P.; Andrews, S.J.; Hughes, C.; Carpenter, L.J.; Meyer-Klaucke, W.; Toyama, C.; Muramatsu, Y.; Feiters, M.C.; Carrano, C.J. Emission of volatile halogenated compounds, speciation and localization of bromine and iodine in the brown algal genome model Ectocarpus siliculosus. JBIC J. Biol. Inorg. Chem. 2018, 23, 1119–1128. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; McCook, L.J.; Larkum, A.W.; Lotze, H.K.; Raven, J.A.; Schaffelke, B.; Smith, J.E.; Steneck, R.S. Vulnerability of macroalgae of the Great Barrier Reef to climate change. Phycologia 2007, 46, 131–136. [Google Scholar]
- Lewis, S.M.; Norris, J.N.; Searles, R.B. The Regulation of Morphological Plasticity in Tropical Reef Algae by Herbivory. Ecology 1987, 68, 636–641. [Google Scholar] [CrossRef]
- Bittick, S.J.; Clausing, R.J.; Fong, C.R.; Fong, P. Bolstered physical defences under nutrient-enriched conditions may facilitate a secondary foundational algal species in the South Pacific. J. Ecol. 2016, 104, 646–653. [Google Scholar] [CrossRef]
- Hay, M. The Functional Morphology of Turf-Forming Seaweeds: Persistence in Stressful Marine Habitats. Ecology 1981, 62, 739–750. [Google Scholar] [CrossRef]
- Yñiguez, A.; McManus, J.; Collado-Vides, L. Capturing the dynamics in benthic structures: Environmental effects on morphology in the macroalgal genera Halimeda and Dictyota. Mar. Ecol. Prog. Ser. 2010, 411, 17–32. [Google Scholar] [CrossRef]
- Charrier, B.; Le Bail, A.; de Reviers, B. Plant Proteus: Brown algal morphological plasticity and underlying developmental mechanisms. Trends Plant Sci. 2012, 17, 468–477. [Google Scholar] [CrossRef]
- Schaum, C.E.; Collins, S. Plasticity predicts evolution in a marine alga. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141486. [Google Scholar]
- Graham, M.H.; Vasquez, J.A.; Buschmann, A.H. Global ecology of the giant kelp Macrocystis: From ecotypes to ecosystems. Oceanogr. Mar. Biol. 2007, 45, 39. [Google Scholar]
- Yun, H.Y.; Molis, M. Comparing the ability of a non-indigenous and a native seaweed to induce anti-herbivory defenses. Mar. Biol. 2012, 159, 1475–1484. [Google Scholar] [CrossRef]
- Fordyce, J.A. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. J. Exp. Biol. 2006, 209, 2377–2383. [Google Scholar] [CrossRef]
- Steneck, R.S. Escalating herbivory and resulting adaptive trends in calcareous algal crusts. Paleobiology 1983, 9, 44–61. [Google Scholar] [CrossRef]
- Kennish, R.; Williams, G.A.; Lee, S.Y. Algal seasonality on an exposed rocky shore in Hong Kong and the dietary implications for the herbivorous crab Grapsus albolineatus. Mar. Biol. 1996, 125, 55–64. [Google Scholar] [CrossRef]
- Johnson, M.D.; Carpenter, R.C. Ocean acidification and warming decrease calcification in the crustose coralline alga Hydrolithon onkodes and increase susceptibility to grazing. J. Exp. Mar. Biol. Ecol. 2012, 434, 94–101. [Google Scholar]
- Borowitzka, M.A.; Vesk, M. Ultrastructure of the corallinaceae. I. The vegetative cells of Corallina officinalis and C. cuvierii. Mar. Biol. 1978, 46, 295–304. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Pelletreau, K.N.; Kirby, A. Nutritional preferences override chemical defenses in determining food choice by a generalist herbivore, Littorina sitkana. J. Exp. Mar. Biol. Ecol. 2009, 379, 85–91. [Google Scholar] [CrossRef]
- Schai-Braun, S.C.; Reichlin, T.S.; Ruf, T.; Klansek, E.; Tataruch, F.; Arnold, W.; Hackländer, K. The European Hare (Lepus europaeus): A Picky Herbivore Searching for Plant Parts Rich in Fat. PLoS ONE 2015, 10, e0134278. [Google Scholar] [CrossRef]
- Cebrian, J.; Shurin, J.B.; Borer, E.; Cardinale, B.J.; Ngai, J.T.; Smith, M.D.; Fagan, W.F. Producer Nutritional Quality Controls Ecosystem Trophic Structure. PLoS ONE 2009, 4, e4929. [Google Scholar] [CrossRef]
- Angell, A.R.; Pirozzi, I.; De Nys, R.; Paul, N.A. Feeding Preferences and the Nutritional Value of Tropical Algae for the Abalone Haliotis asinina. PLoS ONE 2012, 7, e38857. [Google Scholar] [CrossRef]
- Lemoine, N.P.; Giery, S.T.; Burkepile, D.E. Differing nutritional constraints of consumers across ecosystems. Oecologia 2014, 174, 1367–1376. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino Acid Nutrition in Animals: Protein Synthesis and Beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef]
- Shepherd, S.A.; Steinberg, P.D. Food preferences of three abalone species with a review of the food of abalone. In Abalone of The World: Biology, Fisheries and Culture; Shepherd, S.A., Tegner, M.J., Guzman del Proo, S.A., Eds.; Blackwell Scientific: Oxford, UK, 1992; pp. 169–181. [Google Scholar]
- Adin, R.; Riera, P. Preferential food source utilization among stranded macroalgae by Talitrus saltator (Amphipod, Talitridae): A stable isotopes study in the northern coast of Brittany (France). Estuar. Coast. Shelf Sci. 2003, 56, 91–98. [Google Scholar] [CrossRef]
- Barile, P.J.; E Lapointe, B.; Capo, T.R. Dietary nitrogen availability in macroalgae enhances growth of the sea hare Aplysia californica (Opisthobranchia: Anaspidea). J. Exp. Mar. Biol. Ecol. 2004, 303, 65–78. [Google Scholar] [CrossRef]
- Quintanilla-Ahumada, D.; Quijón, P.A.; Navarro, J.M.; Pulgar, J.; Duarte, C. Living on a trophic subsidy: Algal quality drives an upper-shore herbivore’s consumption, preference and absorption but not growth rates. PLoS ONE 2018, 13, e0196121. [Google Scholar]
- Renaud, S.M.; Luong-Van, J.T. Seasonal variation in the chemical composition of tropical Australian marine macroalgae. Proc. Eighteenth Int. Seaweed Symp. 2006, 1, 155–161. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Endo, H.; Suehiro, K.; Kinoshita, J.; Agatsuma, Y. Combined Effects of Temperature and Nutrient Enrichment on Palatability of the Brown Alga Sargassum yezoense (Yamada) Yoshida & T. Konno. Am. J. Plant Sci. 2015, 6, 275–282. [Google Scholar] [CrossRef]
- Hauxwell, J.; McClelland, J.; Behr, P.J.; Valiela, I. Relative Importance of Grazing and Nutrient Controls of Macroalgal Biomass in Three Temperate Shallow Estuaries. Estuaries 1998, 21, 347–360. [Google Scholar] [CrossRef]
- Vergés, A.; Alcoverro, T.; Romero, J. Plant defences and the role of epibiosis in mediating within-plant feeding choices of seagrass consumers. Oecologia 2010, 166, 381–390. [Google Scholar] [CrossRef]
- Jiménez-Ramos, R.; Brun, F.G.; Egea, L.G.; Vergara, J.J. Food choice effects on herbivory: Intra-specific seagrass palatability and inter-specific macrophyte palatability in seagrass communities. Estuarine, Coast. Shelf Sci. 2018, 204, 31–39. [Google Scholar] [CrossRef]
- Bradley, D.J.; Boada, J.; Gladstone, W.; Glasby, T.M.; Gribben, P.E. Sublethal effects of a rapidly spreading native alga on a key herbivore. Ecol. Evol. 2021, 11, 12605–12616. [Google Scholar] [CrossRef]
- Duarte, C.; Acuña, K.; Navarro, J.M.; Gómez, I.; Jaramillo, E.; Quijón, P. Variable feeding behavior in Orchestoidea tuberculata (Nicolet 1849): Exploring the relative importance of macroalgal traits. J. Sea Res. 2014, 87, 1–7. [Google Scholar] [CrossRef]
- Lyons, D.A.; Scheibling, R.E. Effect of dietary history and algal traits on feeding rate and food preference in the green sea urchin Strongylocentrotus droebachiensis. J. Exp. Mar. Biol. Ecol. 2007, 349, 194–204. [Google Scholar] [CrossRef]
- Duarte, C.; Acuña, K.; Navarro, J.M.; Gómez, I. Intra-plant differences in seaweed nutritional quality and chemical defenses: Importance for the feeding behavior of the intertidal amphipod Orchestoidea tuberculata. J. Sea Res. 2011, 66, 215–221. [Google Scholar] [CrossRef]
- You, C.; Zeng, F.; Wang, S.; Li, Y. Preference of the herbivorous marine teleost Siganus canaliculatus for different macroalgae. J. Ocean Univ. China 2014, 13, 516–522. [Google Scholar] [CrossRef]
- Tomas, F.; Box, A.; Terrados, J. Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biol. Invasions 2010, 13, 1559–1570. [Google Scholar] [CrossRef]
- Chan, A.; Lubarsky, K.; Judy, K.; Fong, P. Nutrient addition increases consumption rates of tropical algae with different initial palatabilities. Mar. Ecol. Prog. Ser. 2012, 465, 25–31. [Google Scholar] [CrossRef][Green Version]
- Shantz, A.; Ladd, M.; Burkepile, D. Algal nitrogen and phosphorus content drive inter- and intraspecific differences in herbivore grazing on a Caribbean reef. J. Exp. Mar. Biol. Ecol. 2017, 497, 164–171. [Google Scholar] [CrossRef]
- Cacabelos, E.; Olabarria, C.; Incera, M.; Troncoso, J.S. Do grazers prefer invasive seaweeds? J. Exp. Mar. Biol. Ecol. 2010, 393, 182–187. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).