Behavioural Responses of Defended and Undefended Prey to Their Predator—A Case Study of Rotifera
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Organisms
2.2. Video Tracking Setup and Settings
2.3. Experimental Design
2.3.1. Predator and Prey Behaviour with Unspined Prey
2.3.2. Predator and Prey Behaviour with Spined Prey (Transgenerational)
Predator and Prey Behaviour
Predator Cues (Kairomones) Treatment
2.4. Video Analysis and Calculation of Swimming Speed and Directional Persistence
2.5. Statistical Analysis
3. Results
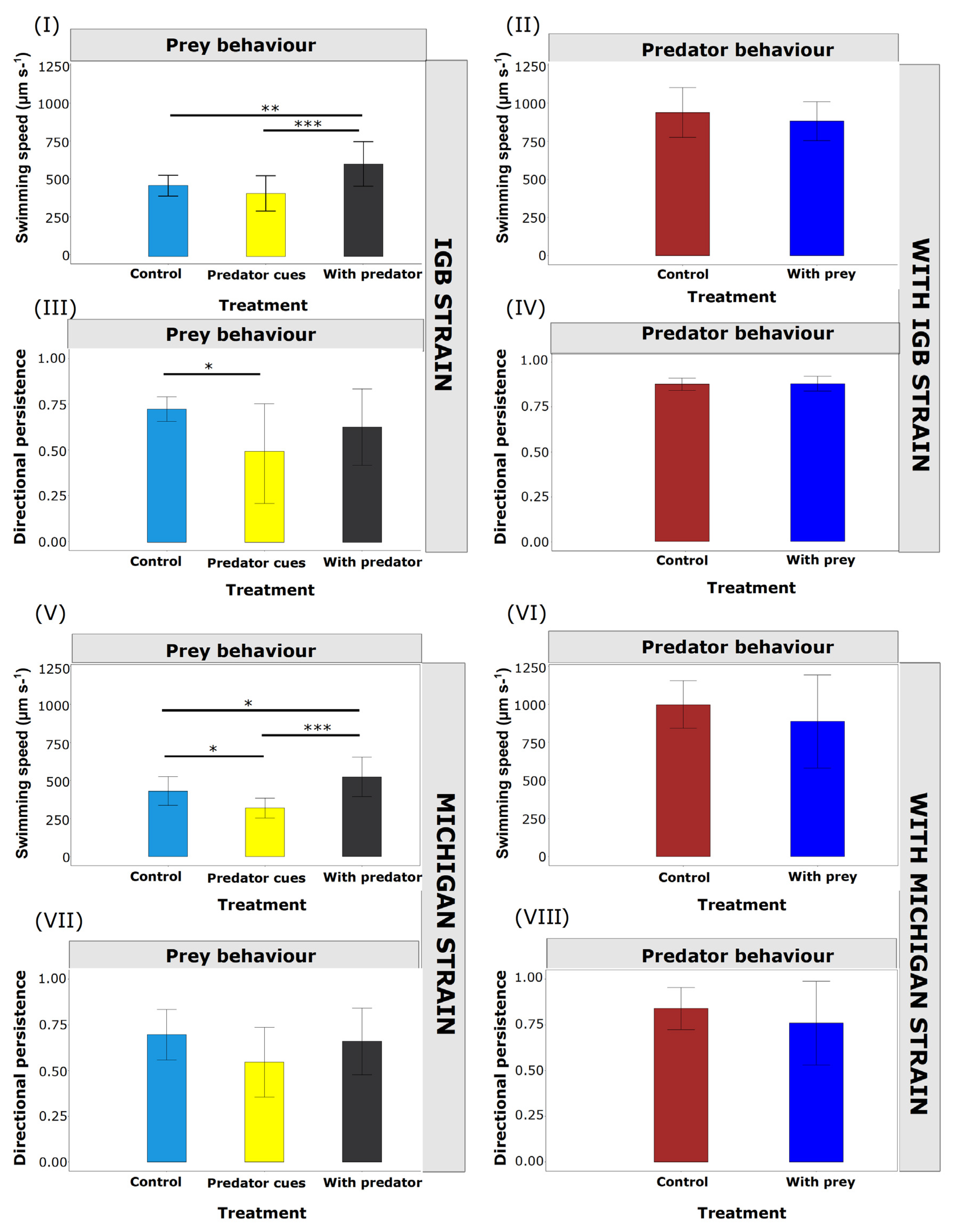
3.1. Behavioural Responses with Unspined Prey
3.1.1. Prey Behaviour
3.1.2. Predator Behaviour
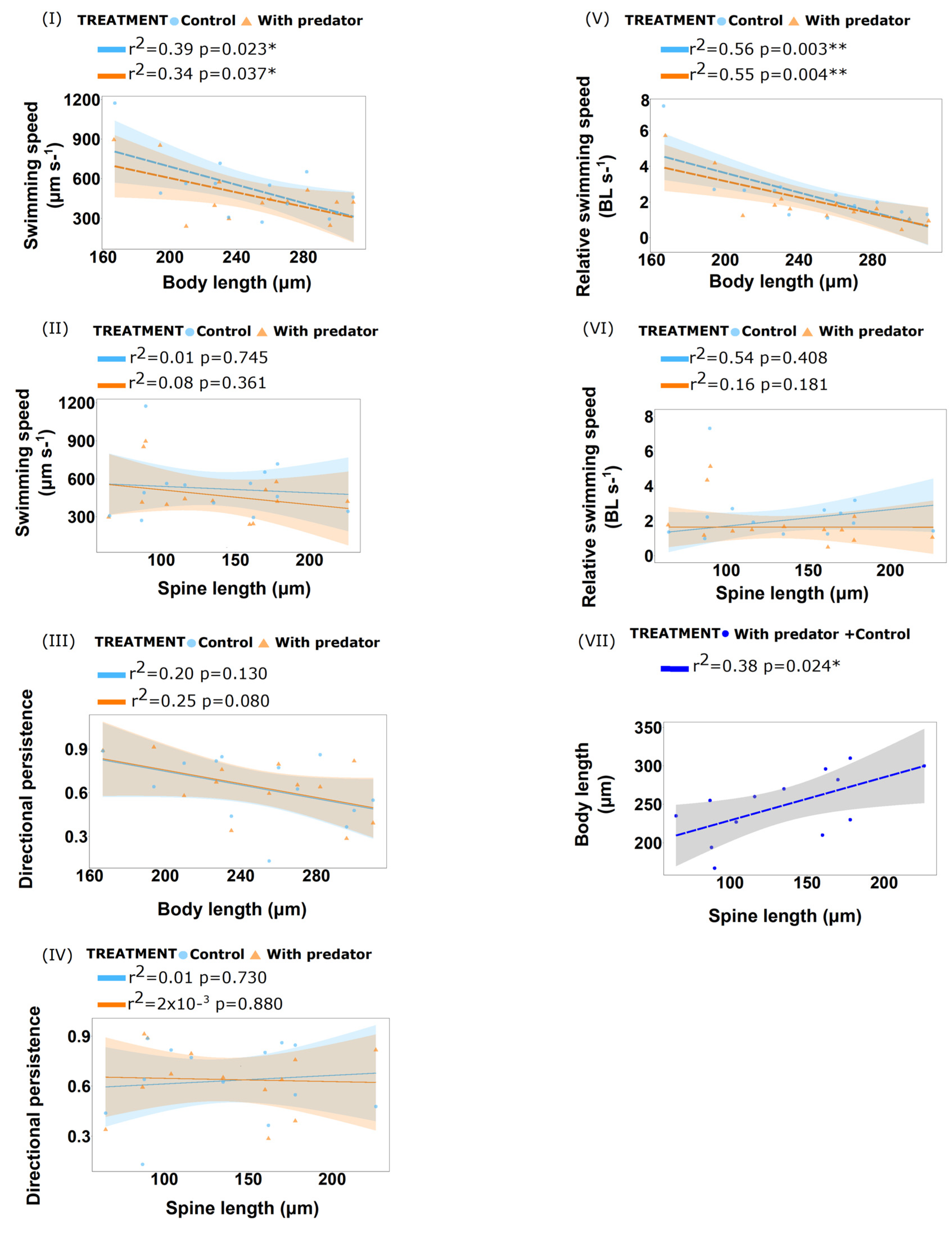
3.2. Transgenerational Behavioural Responses with Spine Prey
3.2.1. Prey Behaviour
Live Predator Treatment
Predator Cues (Kairomones) Treatment
3.2.2. Predator Behaviour
4. Discussion
4.1. Behavioural Responses of Unspined Prey
4.2. Transgenerational Behavioural Responses with Spined Prey
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preston, B.L.; Cecchine, G.; Snell, T.W. Effects of pentachlorophenol on predator avoidance behavior of the rotifer Brachionus calyciflorus. Aquat. Toxicol. 1998, 44, 201–212. [Google Scholar] [CrossRef]
- De Meester, L.; Dawidowicz, P.; Van Gool, E.; Loose, C.J. Ecology and evolution of predator-induced behavior of zooplankton: Depth selection behavior and diel vertical migration. In The Ecology and Evolution of Inducible Defenses; Princeton University Press: Princeton, NJ, USA, 1999; pp. 160–176. [Google Scholar]
- Hawlena, D.; Schmitz, O.J. Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am. Nat. 2010, 176, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Klecka, J.; Boukal, D.S. Foraging and vulnerability traits modify predator–prey body mass allometry: Freshwater macroinvertebrates as a case study. J. Anim. Ecol. 2013, 82, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Laforsch, C.; Tollrian, R. Inducible defenses in multipredator environments: Cyclomorphosis in Daphnia cucullata. Ecology 2004, 85, 2302–2311. [Google Scholar] [CrossRef]
- Tollrian, R. Inducible defenses in Cladocera: Constraints, costs, and multipredator environments. In The Ecology and Evolution of Inducible Defenses; Princeton University Press: Princeton, NJ, USA, 1999; pp. 177–202. [Google Scholar]
- Gilbert, J. Attachment behavior in the rotifer Brachionus rubens: Induction by Asplanchna and effect on sexual reproduction. Hydrobiologia 2019, 844, 9–20. [Google Scholar] [CrossRef]
- Gilbert, J.J.; Kirk, K.L. Escape response of the rotifer Keratella: Description, stimulation, fluid dynamics, and ecological significance. Limnol. Oceanogr. 1988, 33, 1440–1450. [Google Scholar] [CrossRef]
- Kirk, K.L.; Gilbert, J.J. Escape behavior of Polyarthra in response to artificial flow stimuli. Bull. Mar. Sci. 1988, 43, 551–560. [Google Scholar]
- Gilbert, J.J. Predator-specific Inducible defenses in the rotifer Keratella tropica. Freshw. Biol. 2009, 54, 1933–1946. [Google Scholar] [CrossRef]
- Stemberger, R.S.; Gilbert, J.J. Multiple-species induction of morphological defenses in the rotifer Keratella testudo. Ecology 1987, 68, 370–378. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; He, L.; Yao, H.; Xu, J. Behavioural response of Brachionus calyciflorus to the predator Asplanchna sieboldii. Freshw. Biol. 2021, 66, 562–569. [Google Scholar] [CrossRef]
- Åbjörnsson, K.; Wagner, B.; Axelsson, A.; Bjerselius, R.; Olsén, K.H. Responses of Acilius sulcatus (Coleoptera: Dytiscidae) to chemical cues from perch (Perca fluviatilis). Oecologia 1997, 111, 166–171. [Google Scholar] [CrossRef]
- De Meester, L. Genotype, Fish-mediated chemical, and phototactic behavior in Daphnia magna. Ecology 1993, 74, 1467–1474. [Google Scholar] [CrossRef]
- Iyer, N.; Rao, T. Responses of the predatory rotifer Asplanchna intermedia to prey species differing in vulnerability: Laboratory and field studies. Freshw. Biol. 1996, 36, 521–533. [Google Scholar] [CrossRef]
- Garza-Mourino, G.; Silva-Briano, M.; Nandini, S.; Sarma, S.S.S.; Castellanos-Paez, M.E. Morphological and morphometrical variations of selected rotifer species in response to predation: A seasonal study of selected brachionid species from Lake Xochimilco (Mexico). Hydrobiologia 2005, 546, 169–179. [Google Scholar] [CrossRef]
- Conde-Porcuna, J.M.; Sarma, S.S.S. Prey selection by Asplanchna girodi (Rotifera): The importance of prey defence mechanisms. Freshw. Biol. 1995, 33, 341–348. [Google Scholar] [CrossRef]
- Gilbert, J.J. The cost of predator-induced morphological defense in rotifers: Experimental studies and synthesis. J. Plankton Res. 2013, 35, 461–472. [Google Scholar] [CrossRef]
- Gilbert, J.J.; Stemberger, R.S. Prey capture in the rotifer Asplanchna girodi: With 1 figure in the text. Int. Ver. Für Theor. Und Angew. Limnol. Verh. 1985, 22, 2997–3000. [Google Scholar] [CrossRef]
- Gómez, A.; Cecchine, G.; Snell, T.W. Effect of pentachlorophenol on predator-prey interaction of two rotifers. Aquat. Toxicol. 1997, 37, 271–282. [Google Scholar] [CrossRef]
- Salt, G.W. The components of feeding behavior in rotifers. Hydrobiologia 1987, 147, 271–281. [Google Scholar] [CrossRef]
- Gilbert, J.J. Rotifer ecology and embryological induction. Science 1966, 151, 1234–1237. [Google Scholar] [CrossRef]
- Gilbert, J.J. Kairomone-induced morphological defenses in rotifers. In The Ecology and Evolution of Inducible Defenses; Princeton University Press: Princeton, NJ, USA, 1999; pp. 127–141. [Google Scholar]
- Gilbert, J.J. Non-genetic polymorphisms in rotifers: Environmental and endogenous controls, development, and features for predictable or unpredictable environments. Biol. Rev. 2017, 92, 964–992. [Google Scholar] [CrossRef]
- Marinone, M.C.; Zagarese, H.E. A field and laboratory study on factors affecting polymorphism in the rotifer Keratella tropica. Oecologia 1991, 86, 372–377. [Google Scholar] [CrossRef]
- Xue, Y.-H.; Yang, X.-X.; Zhang, G.; Xi, Y.-L. Morphological differentiation of Brachionus calyciflorus caused by predation and coal ash pollution. Sci. Rep. 2017, 7, 15779. [Google Scholar] [CrossRef]
- Zhang, H.; Brönmark, C.; Hansson, L.A. Predator Ontogeny Affects Expression of Inducible Defense Morphology in Rotifers; Wiley Online Library: Hoboken, NJ, USA, 2017; ISBN 0012-9658. [Google Scholar]
- Seifert, L.I.; de Castro, F.; Marquart, A.; Gaedke, U.; Weithoff, G.; Vos, M. Heated relations: Temperature-mediated shifts in consumption across trophic levels. PLoS ONE 2014, 9, e95046. [Google Scholar] [CrossRef]
- Nandini, S.; Pérez-Chávez, R.; Sarma, S.S.S. The effect of prey morphology on the feeding behaviour and population growth of the predatory rotifer Asplanchna sieboldii: A case study using five species of Brachionus (Rotifera). Freshw. Biol. 2003, 48, 2131–2140. [Google Scholar] [CrossRef]
- Wurdak, E.; Clément, P.; Amsellem, J. Sensory receptors involved in the feeding behaviour of the rotifer Asplanchna brightwellii. Hydrobiologia 1983, 104, 203–212. [Google Scholar] [CrossRef]
- Pennekamp, F.; Schtickzelle, N.; Petchey, O.L. BEMOVI, Software for extracting behavior and morphology from videos, illustrated with analyses of microbes. Ecol. Evol. 2015, 5, 2584–2595. [Google Scholar] [CrossRef]
- Paraskevopoulou, S.; Tiedemann, R.; Weithoff, G. Differential response to heat stress among evolutionary lineages of an aquatic invertebrate species complex. Biol. Lett. 2018, 14, 20180498. [Google Scholar] [CrossRef]
- Guillard, R.R.; Lorenzen, C.J. Yellow-green algae with chlorophyllide C 1, 2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Colangeli, P.; Schlägel, U.E.; Obertegger, U.; Petermann, J.S.; Tiedemann, R.; Weithoff, G. Negative phototactic response to UVR in three cosmopolitan rotifers: A video analysis approach. Hydrobiologia 2019, 844, 43–54. [Google Scholar] [CrossRef]
- Parysek, M.; Pietrzak, B. Weak swimming response of a bdelloid rotifer to chemical cues of a native copepod predator. Journal of Ethology 2021, 39, 135–139. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; Version 3.5. 2; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Eliceiri, K.W.; Berthold, M.R.; Goldberg, I.G.; Ibáñez, L.; Manjunath, B.S.; Martone, M.E.; Murphy, R.F.; Peng, H.; Plant, A.L.; Roysam, B. Biological imaging software tools. Nat. Methods 2012, 9, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S. Converting video formats with FFmpeg. Linux J. 2006, 2006, 10. [Google Scholar]
- Obertegger, U.; Cieplinski, A.; Raatz, M.; Colangeli, P. Switching between swimming states in rotifers–case study Keratella cochlearis. Mar. Freshw. Behav. Physiol. 2018, 51, 159–173. [Google Scholar] [CrossRef]
- Lund, U.; Agostinelli, C.; Agostinelli, M.C. Package ‘circular.’ Repos. CRAN 2017, 775, 134–135. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Selker, R.; Love, J.; Dropmann, D.; Moreno, V. jmv: The “jamovi” Analyses. R Package Version 2.3.4.. 2021. Available online: https://CRAN.R-project.org/package=jmv (accessed on 16 June 2022).
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B. Car: Companion to Applied Regression; R Package Version 3.0-2; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Gerritsen, J.; Strickler, J.R. Encounter probabilities and community structure in zooplankton: A mathematical model. J. Fish. Board Can. 1977, 34, 73–82. [Google Scholar] [CrossRef]
- Gilbert, J.J.; Williamson, C.E. Predator-prey behavior and its effect on rotifer survival in associations of Mesocyclops edax, Asplanchna girodi, Polyarthra vulgaris, and Keratella cochlearis. Oecologia 1978, 37, 13–22. [Google Scholar] [CrossRef]
- Åbjörnsson, K.; Dahl, J.; Nyström, P.; Brönmark, C. Influence of predator and dietary chemical cues on the behaviour and shredding efficiency of Gammarus pulex. Aquat. Ecol. 2000, 34, 379–387. [Google Scholar] [CrossRef]
- Kats, L.B.; Dill, L.M. The scent of death: Chemosensory assessment of predation risk by prey animals. Ecoscience 1998, 5, 361–394. [Google Scholar] [CrossRef]
- Gilbert, J.J. Morphological and behavioral responses of a rotifer to the predator Asplanchna. J. Plankton Res. 2014, 36, 1576–1584. [Google Scholar] [CrossRef]
- Yúfera, M. Swimming behaviour of Brachionus plicatilis in relation to food concentration and feeding rates. Hydrobiologia 2007, 593, 13–18. [Google Scholar] [CrossRef]
- Horstmann, M.; Weiss, L.C.; Tollrian, R. Specific turbulence and Chaoborus-induced morphotypes affect the streamlining properties of Daphnia cucullata. Front. Ecol. Evol. 2022, 9, 3389. [Google Scholar] [CrossRef]
- Epp, R.W.; Lewis, W.M. Cost and speed of locomotion for rotifers. Oecologia 1984, 61, 289–292. [Google Scholar] [CrossRef]
- Stemberger, R.S.; Gilbert, J.J. Rotifer threshold food concentrations and the size-efficiency hypothesis. Ecology 1987, 68, 181–187. [Google Scholar] [CrossRef]
- Yúfera, M.; Pascual, E.; Olivares, J.M. Factors affecting swimming speed in the rotifer Brachionus plicatilis. In Rotifera X; Springer: Dordrecht, The Netherlands, 2005; pp. 375–380. [Google Scholar]
- Tollrian, R.; Harvell, C.D. The Evolution of inducible defenses: Current ideas. Ecol. Evol. Inducible Def. 1999, 306, 321. [Google Scholar]
- Boersma, M.; Spaak, P.; De Meester, L. Predator-mediated plasticity in morphology, life history, and behavior of Daphnia: The uncoupling of responses. Am. Nat. 1998, 152, 237–248. [Google Scholar] [CrossRef]
- Bourdeau, P.E.; Johansson, F. Predator-induced morphological defences as by-products of prey behaviour: A review and prospectus. Oikos 2012, 121, 1175–1190. [Google Scholar] [CrossRef]
- Tollrian, R. Predator-induced morphological defenses: Costs, life history shifts, and maternal effects in Daphnia pulex. Ecology 1995, 76, 1691–1705. [Google Scholar] [CrossRef]
- Aránguiz-Acuña, A.; Ramos-Jiliberto, R.; Sarma, N.; Sarma, S.S.S.; Bustamante, R.O.; Toledo, V. Benefits, costs and reactivity of inducible defences: An experimental test with rotifers. Freshw. Biol. 2010, 55, 2114–2122. [Google Scholar] [CrossRef]
- Gilbert, J.J. Predator-induced defense in rotifers: Developmental lags for morph transformations, and effect on population growth. Aquat. Ecol. 2012, 46, 475–486. [Google Scholar] [CrossRef]
- Pavón-Meza, E.L.; Sarma, S.S.S.; Nandini, S. Combined effects of temperature, food availability and predator’s (Asplanchna girodi) allelochemicals on the demography and population growth of Brachionus havanaensis (Rotifera). Allelopath. J. 2008, 21, 95–106. [Google Scholar]
- Sarma, S.S.S.; Resendiz, R.A.L.; Nandini, S. Morphometric and demographic responses of brachionid prey (Brachionus calyciflorus, Pallas and Plationus macracanthus (Daday)) in the presence of different densities of the predator Asplanchna brightwellii (Rotifera: Asplanchnidae). Hydrobiologia 2011, 662, 179–187. [Google Scholar] [CrossRef]

| Swimming Speed (µm s−1) | |||||
|---|---|---|---|---|---|
| Treatment | Strain | Variable | df | F | p-Value |
| Live predator | Brachionus calyciflorus—“Michigan” | Treatment | 1 | 0.58 | 0.456 |
| Body length (µm) | 1 | 15.84 | <0.001 *** | ||
| Spine length (µm) | 1 | 2.43 | 0.137 | ||
| Treatment × Body length | 1 | 0.89 | 0.359 | ||
| Treatment × Spine length | 1 | 0.88 | 0.359 | ||
| Body length × Spine length | 1 | 3.75 | 0.069 | ||
| Treatment × Body length × Spine length | 1 | 0.70 | 0.413 | ||
| Predator cues (Kairomones) | Brachionus calyciflorus—“Michigan” | Treatment | 1 | 1.22 | 0.284 |
| Body length (µm) | 1 | 0.08 | 0.781 | ||
| Spine length (µm) | 1 | 1.23 | 0.282 | ||
| Treatment × Body length | 1 | 7.84 | 0.012 * | ||
| Treatment × Spine length | 1 | 0.08 | 0.782 | ||
| Body length × Spine length | 1 | 0.02 | 0.890 | ||
| Treatment × Body length × Spine length | 1 | 6.28 | 0.022 * | ||
| Relative Swimming Speed (BL s−1) | |||||
|---|---|---|---|---|---|
| Treatment | Strain | Variable | df | F | p-Value |
| Live predator | Brachionus calyciflorus—“Michigan” | Treatment | 1 | 0.46 | 0.506 |
| Body length (µm) | 1 | 31.53 | <0.001 *** | ||
| Spine length (µm) | 1 | 2.16 | 0.159 | ||
| Treatment × Body length | 1 | 0.95 | 0.342 | ||
| Treatment × Spine length | 1 | 0.78 | 0.389 | ||
| Body length × Spine length | 1 | 7.49 | 0.014 * | ||
| Treatment × Body length × Spine length | 1 | 0.39 | 0.543 | ||
| Predator cues (Kairomones) | Brachionus calyciflorus—“Michigan” | Treatment | 1 | 1.89 | 0.186 |
| Body length (µm) | 1 | 4.50 | 0.048 * | ||
| Spine length (µm) | 1 | 0.89 | 0.357 | ||
| Treatment × Body length | 1 | 6.99 | 0.017 * | ||
| Treatment × Spine length | 1 | 1.7 × 10−3 | 0.968 | ||
| Body length × Spine length | 1 | 0.02 | 0.899 | ||
| Treatment × Body length × Spine length | 1 | 6.29 | 0.022 * | ||
| Directional Persistence | |||||
|---|---|---|---|---|---|
| Treatment | Strain | Variable | df | F | p-Value |
| Live predator | Brachionus calyciflorus—“Michigan” | Treatment | 1 | 0.01 | 0.909 |
| Body length (µm) | 1 | 12.38 | 0.002 ** | ||
| Spine length (µm) | 1 | 3.42 | 0.054 | ||
| Treatment × Body length | 1 | 0.12 | 0.731 | ||
| Treatment × Spine length | 1 | 0.31 | 0.582 | ||
| Body length × Spine length | 1 | 0.05 | 0.823 | ||
| Treatment × Body length × Spine length | 1 | 0.89 | 0.358 | ||
| Predator cues (Kairomones) | Brachionus calyciflorus—“Michigan” | Treatment | 1 | 7.55 | 0.013 * |
| Body length (µm) | 1 | 0.03 | 0.856 | ||
| Spine length (µm) | 1 | 0.67 | 0.425 | ||
| Treatment × Body length | 1 | 1.35 | 0.260 | ||
| Treatment × Spine length | 1 | 3.19 | 0.091 | ||
| Body length × Spine length | 1 | 0.78 | 0.390 | ||
| Treatment × Body length × Spine length | 1 | 0.21 | 0.651 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parry, V.; Schlägel, U.E.; Tiedemann, R.; Weithoff, G. Behavioural Responses of Defended and Undefended Prey to Their Predator—A Case Study of Rotifera. Biology 2022, 11, 1217. https://doi.org/10.3390/biology11081217
Parry V, Schlägel UE, Tiedemann R, Weithoff G. Behavioural Responses of Defended and Undefended Prey to Their Predator—A Case Study of Rotifera. Biology. 2022; 11(8):1217. https://doi.org/10.3390/biology11081217
Chicago/Turabian StyleParry, Victor, Ulrike E. Schlägel, Ralph Tiedemann, and Guntram Weithoff. 2022. "Behavioural Responses of Defended and Undefended Prey to Their Predator—A Case Study of Rotifera" Biology 11, no. 8: 1217. https://doi.org/10.3390/biology11081217






