Plasma Testosterone and Androstenedione Levels Follow the Same Sex-Specific Patterns in the Two Pan Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals and Sample Collection
2.3. Sample Extraction and Analytical Methods
2.4. Statistical Analyses
3. Results
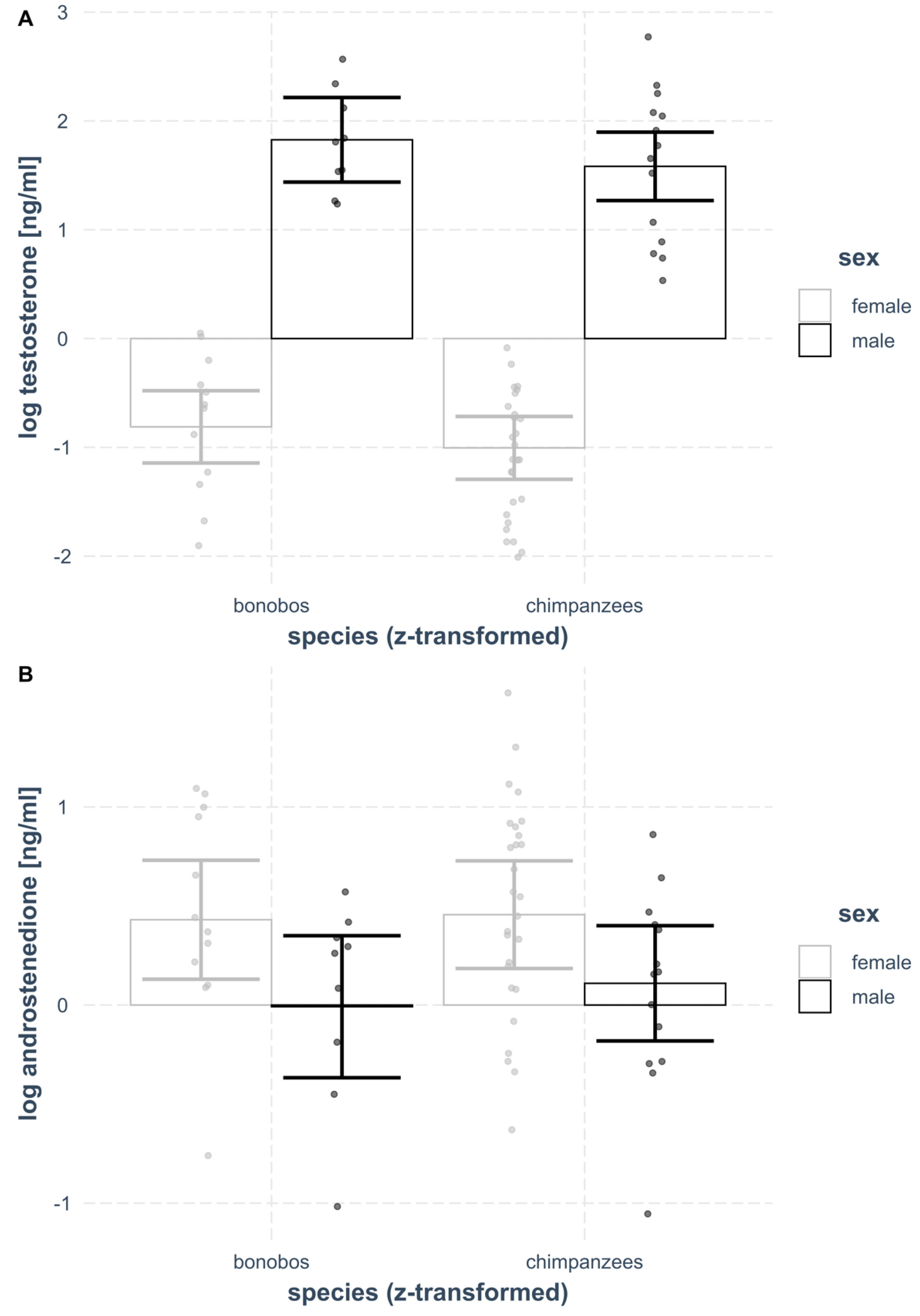
3.1. Plasma Testosterone
3.2. Plasma Androstenedione
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; Murray: London, UK, 1871. [Google Scholar]
- Clutton-Brock, T.H.; Vincent, A.C.J. Sexual Selection and the Potential Reproductive Rates of Males and Females. Nature 1991, 351, 58–60. [Google Scholar] [CrossRef] [PubMed]
- French, J.A.; Mustoe, A.C.; Cavanaugh, J.; Birnie, A.K. The Influence of Androgenic Steroid Hormones on Female Aggression in ‘Atypical’ Mammals. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130084. [Google Scholar] [CrossRef] [PubMed]
- Clutton-Brock, T.H.; Huchard, E. Social Competition and Selection in Males and Females. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130074. [Google Scholar] [CrossRef]
- Ralls, K. Mammals in Which Females Are Larger Than Males. Q. Rev. Biol. 1976, 51, 245–276. [Google Scholar] [CrossRef]
- Wallen, K. Hormonal Influences on Sexually Differentiated Behavior in Nonhuman Primates. Front. Neuroendocrinol. 2005, 26, 7–26. [Google Scholar] [CrossRef]
- Hau, M. Regulation of Male Traits by Testosterone: Implications for the Evolution of Vertebrate Life Histories. BioEssays 2007, 29, 133–144. [Google Scholar] [CrossRef]
- Soma, K.K.; Scotti, M.-A.L.; Newman, A.E.M.; Charlier, T.D.; Demas, G.E. Novel Mechanisms for Neuroendocrine Regulation of Aggression. Front. Neuroendocrinol. 2008, 29, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Glickman, S.E.; Frank, L.G.; Davidson, J.M.; Smith, E.R.; Siiteri, P.K. Androstenedione May Organize or Activate Sex-Reversed Traits in Female Spotted Hyenas. Proc. Natl. Acad. Sci. USA 1987, 84, 3444–3447. [Google Scholar] [CrossRef]
- Goymann, W.; East, M.L.; Hofer, H. Androgens and the Role of Female “Hyperaggressiveness” in Spotted Hyenas (Crocuta Crocuta). Horm. Behav. 2001, 39, 83–92. [Google Scholar] [CrossRef]
- Dloniak, S.M.; French, J.A.; Place, N.J.; Weldele, M.L.; Glickman, S.E.; Holekamp, K.E. Non-Invasive Monitoring of Fecal Androgens in Spotted Hyenas (Crocuta Crocuta). Gen. Comp. Endocrinol. 2004, 135, 51–61. [Google Scholar] [CrossRef]
- Drea, C.M. Sex and Seasonal Differences in Aggression and Steroid Secretion in Lemur Catta: Are Socially Dominant Females Hormonally ‘Masculinized’? Horm. Behav. 2007, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, G.; Fruth, B. Intra- and Inter-Sexual Aggression by Bonobos in the Context of Mating. Behaviour 2003, 140, 1389–1413. [Google Scholar] [CrossRef]
- Tokuyama, N.; Furuichi, T. Do Friends Help Each Other? Patterns of Female Coalition Formation in Wild Bonobos at Wamba. Anim. Behav. 2016, 119, 27–35. [Google Scholar] [CrossRef]
- Wantia, J.; Dätwyler, M.; Hemelrijk, C. Female Co-Dominance in a Virtual World: Ecological, Cognitive, Social and Sexual Causes. Behaviour 2003, 140, 1247–1273. [Google Scholar] [CrossRef]
- Staes, N.; Eens, M.; Weiss, A.; Stevens, J.M.G. Bonobo Personality: Age and Sex Effects and Links with Behavior and Dominance. In Bonobos; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-872851-1. [Google Scholar]
- Foerster, S.; Franz, M.; Murray, C.M.; Gilby, I.C.; Feldblum, J.T.; Walker, K.K.; Pusey, A.E. Chimpanzee Females Queue but Males Compete for Social Status. Sci. Rep. 2016, 6, 35404. [Google Scholar] [CrossRef]
- De Waal, F.B.M.; Hoekstra, J.A. Contexts and Predictability of Aggression in Chimpanzees. Anim. Behav. 1980, 28, 929–937. [Google Scholar] [CrossRef]
- Hohmann, G.; Vigilant, L.; Mundry, R.; Behringer, V.; Surbeck, M. Aggression by Male Bonobos against Immature Individuals Does Not Fit with Predictions of Infanticide. Aggress. Behav. 2019, 45, 300–309. [Google Scholar] [CrossRef]
- Wilson, M.L.; Boesch, C.; Fruth, B.; Furuichi, T.; Gilby, I.C.; Hashimoto, C.; Hobaiter, C.L.; Hohmann, G.; Itoh, N.; Koops, K.; et al. Lethal Aggression in Pan Is Better Explained by Adaptive Strategies than Human Impacts. Nature 2014, 513, 414–417. [Google Scholar] [CrossRef]
- Surbeck, M.; Boesch, C.; Girard-Buttoz, C.; Crockford, C.; Hohmann, G.; Wittig, R.M. Comparison of Male Conflict Behavior in Chimpanzees (Pan Troglodytes) and Bonobos (Pan Paniscus), with Specific Regard to Coalition and Post-Conflict Behavior. Am. J. Primatol. 2017, 79, e22641. [Google Scholar] [CrossRef]
- Cramer, D.; Zihlman, A. Sexual Dimorphism in the Pygmy Chimpanzee, Pan Paniscus. In Recent Advances in Primatology; Volume 3. Evolution; Chivers, D.J., Joysey, K.A., Eds.; Academic Press: London, UK, 1978; Volume 3, pp. 487–490. [Google Scholar]
- Plavcan, J.M.; van Schaik, C.P. Intrasexual Competition and Canine Dimorphism in Anthropoid Primates. Am. J. Phys. Anthropol. 1992, 87, 461–477. [Google Scholar] [CrossRef]
- Zihlman, A.L.; Bolter, D.R. Body Composition in Pan Paniscus Compared with Homo Sapiens Has Implications for Changes during Human Evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 7466–7471. [Google Scholar] [CrossRef] [PubMed]
- Sannen, A.; Heistermann, M.; Elsacker, L.V.; Möhle, U.; Eens, M. Urinary Testosterone Metabolite Levels in Bonobos: A Comparison with Chimpanzees in Relation to Social System. Behaviour 2003, 140, 683–696. [Google Scholar] [CrossRef]
- Wobber, V.; Hare, B.; Lipson, S.; Wrangham, R.; Ellison, P. Different Ontogenetic Patterns of Testosterone Production Reflect Divergent Male Reproductive Strategies in Chimpanzees and Bonobos. Physiol. Behav. 2013, 116–117, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Book, A.S.; Starzyk, K.B.; Quinsey, V.L. The Relationship between Testosterone and Aggression: A Meta-Analysis. Aggress. Violent Behav. 2001, 6, 579–599. [Google Scholar] [CrossRef]
- Simon, N.G.; McKenna, S.E.; Lu, S.F.; Cologer-Clifford, A. Development and Expression of Hormonal Systems Regulating Aggression. Ann. N. Y. Acad. Sci. 1996, 794, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.; Wobber, V.; Wrangham, R. The Self-Domestication Hypothesis: Evolution of Bonobo Psychology Is Due to Selection against Aggression. Anim. Behav. 2012, 83, 573–585. [Google Scholar] [CrossRef]
- Surbeck, M.; Girard-Buttoz, C.; Boesch, C.; Crockford, C.; Fruth, B.; Hohmann, G.; Langergraber, K.E.; Zuberbühler, K.; Wittig, R.M.; Mundry, R. Sex-Specific Association Patterns in Bonobos and Chimpanzees Reflect Species Differences in Cooperation. R. Soc. Open Sci. 2017, 4, 161081. [Google Scholar] [CrossRef]
- Surbeck, M.; Deschner, T.; Schubert, G.; Weltring, A.; Hohmann, G. Mate Competition, Testosterone and Intersexual Relationships in Bonobos, Pan Paniscus. Anim. Behav. 2012, 83, 659–669. [Google Scholar] [CrossRef]
- von Engelhard, N.; Kappeler, P.M.; Heistermann, M. Androgen Levels and Female Social Dominance in Lemur Catta. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1533–1539. [Google Scholar] [CrossRef]
- Dixson, A.F. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Humans; Oxford University Press: Oxford, UK, 2012; ISBN 0-19-162418-7. [Google Scholar]
- Surbeck, M.; Mundry, R.; Hohmann, G. Mothers Matter! Maternal Support, Dominance Status and Mating Success in Male Bonobos (Pan Paniscus). Proc. R. Soc. Lond. B Biol. Sci. 2011, 278, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Surbeck, M.; Langergraber, K.E.; Fruth, B.; Vigilant, L.; Hohmann, G. Male Reproductive Skew Is Higher in Bonobos than Chimpanzees. Curr. Biol. 2017, 27, R640–R641. [Google Scholar] [CrossRef] [PubMed]
- Kaburu, S.S.K.; Newton-Fisher, N.E. Egalitarian Despots: Hierarchy Steepness, Reciprocity and the Grooming-Trade Model in Wild Chimpanzees, Pan Troglodytes. Anim. Behav. 2015, 99, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Behringer, V.; Deschner, T.; Deimel, C.; Stevens, J.M.G.; Hohmann, G. Age-Related Changes in Urinary Testosterone Levels Suggest Differences in Puberty Onset and Divergent Life History Strategies in Bonobos and Chimpanzees. Horm. Behav. 2014, 66, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Higham, J.P. Field Endocrinology of Nonhuman Primates: Past, Present, and Future. Horm. Behav. 2016, 84, 145–155. [Google Scholar] [CrossRef]
- Preis, A.; Mugisha, L.; Hauser, B.; Weltring, A.; Deschner, T. Androgen and Androgen Metabolite Levels in Serum and Urine of East African Chimpanzees (Pan Troglodytes Schweinfurthii): Comparison of EIA and LC–MS Analyses. Gen. Comp. Endocrinol. 2011, 174, 335–343. [Google Scholar] [CrossRef]
- Granger, D.A.; Shirtcliff, E.A.; Booth, A.; Kivlighan, K.T.; Schwartz, E.B. The “Trouble” with Salivary Testosterone. Psychoneuroendocrinology 2004, 29, 1229–1240. [Google Scholar] [CrossRef]
- Kutsukake, N.; Ikeda, K.; Honma, S.; Teramoto, M.; Mori, Y.; Hayasaka, I.; Yamamoto, R.; Ishida, T.; Yoshikawa, Y.; Hasegawa, T. Validation of Salivary Cortisol and Testosterone Assays in Chimpanzees by Liquid Chromatography-Tandem Mass Spectrometry. Am. J. Primatol. 2009, 71, 696–706. [Google Scholar] [CrossRef]
- Shirtcliff, E.; Granger, D.; Schwartz, E.; Curran, M.J. Use of Salivary Biomarkers in Biobehavioral Research: Cotton-Based Sample Collection Methods Can Interfere with Salivary Immunoassay Results. Psychoneuroendocrinology 2001, 26, 165–173. [Google Scholar] [CrossRef]
- Murtagh, R.; Behringer, V.; Deschner, T. LC-MS as a Method for Non-Invasive Measurement of Steroid Hormones and Their Metabolites in Urine and Faeces of Animals. Vet. Med. Austria 2013, 100, 247–254. [Google Scholar]
- Anestis, S.F. Testosterone in Juvenile and Adolescent Male Chimpanzees (Pan Troglodytes): Effects of Dominance Rank, Aggression, and Behavioral Style. Am. J. Phys. Anthropol. 2006, 130, 536–545. [Google Scholar] [CrossRef]
- Hauser, B.; Mugisha, L.; Preis, A.; Deschner, T. LC–MS Analysis of Androgen Metabolites in Serum and Urine from East African Chimpanzees (Pan Troglodytes Schweinfurthii). Gen. Comp. Endocrinol. 2011, 170, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, R.; Stevens, J.M.G.; Hohmann, G.; Deschner, T.; Behringer, V. Blood Testosterone Levels in Sickness and in Health: Male Chimpanzee Testosterone Levels Decrease in Face of an Immune Challenge. Am. J. Primatol. 2022, 84, e23334. [Google Scholar] [CrossRef] [PubMed]
- Hauser, B.; Deschner, T.; Boesch, C. Development of a Liquid Chromatography–Tandem Mass Spectrometry Method for the Determination of 23 Endogenous Steroids in Small Quantities of Primate Urine. J. Chromatogr. B 2008, 862, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Stroup, W.W. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-1512-0. [Google Scholar]
- Gilks, W.R.; Richardson, S.; Spiegelhalter, D. Markov Chain Monte Carlo in Practice; CRC Press: Boca Raton, FL, USA, 1995; ISBN 978-1-4822-1497-0. [Google Scholar]
- Bribiescas, R.G. Aging, Life History, and Human Evolution. Annu. Rev. Anthropol. 2020, 49, 101–121. [Google Scholar] [CrossRef]
- Schaebs, F.S.; Perry, S.E.; Cohen, D.; Mundry, R.; Deschner, T. Social and Demographic Correlates of Male Androgen Levels in Wild White-Faced Capuchin Monkeys (Cebus Capucinus). Am. J. Primatol. 2017, 79, e22653. [Google Scholar] [CrossRef]
- Bassindale, T.; Cowan, D.A.; Dale, S.; Hutt, A.J.; Leeds, A.R.; Wheeler, M.J.; Kicman, A.T. Effects of Oral Administration of Androstenedione on Plasma Androgens in Young Women Using Hormonal Contraception. J. Clin. Endocrinol. Metab. 2004, 89, 6030–6038. [Google Scholar] [CrossRef] [PubMed]
- Swinkels, L.M.J.W.; Meulenberg, P.M.M.; Ross, H.A.; Benraad, T.J. Salivary and Plasma Free Testosterone and Androstenedione Levels in Women Using Oral Contraceptives Containing Desogestrel or Levonorgestrel. Ann. Clin. Biochem. 1988, 25, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, R.H.; Morris, C.V. Effect of an Oral Contraceptive on Adrenal and Ovarian Androgenic Steroids. Obstet. Gynecol. 1984, 63, 12–14. [Google Scholar]
- Schielzeth, H. Simple Means to Improve the Interpretability of Regression Coefficients. Methods Ecol. Evol. 2010, 1, 103–113. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; SAGE Publications: Thousand Oaks, CA, USA, 2018; ISBN 978-1-5443-3648-0. [Google Scholar]
- Dobson, A.J.; Barnett, A.G. An Introduction to Generalized Linear Models; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-72622-1. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Wrangham, R.W. Two Types of Aggression in Human Evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.R.; Shender, M.A. Daily Travel Distances of Zoo-Housed Chimpanzees and Gorillas: Implications for Welfare Assessments and Space Requirements. Primates 2016, 57, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Bribiescas, R.G. An Evolutionary and Life History Perspective on Human Male Reproductive Senescence. Ann. N. Y. Acad. Sci. 2010, 1204, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.M.; Blamires, T.; Rockwood, A.L.; Roberts, W.L.; Yue, B.; Erdogan, E.; Bunker, A.M.; Meikle, A.W. Liquid Chromatography-Tandem Mass Spectrometry Assay for Androstenedione, Dehydroepiandrosterone, and Testosterone with Pediatric and Adult Reference Intervals. Clin. Chem. 2010, 56, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Büttler, R.M.; Martens, F.; Fanelli, F.; Pham, H.T.; Kushnir, M.M.; Janssen, M.J.W.; Owen, L.; Taylor, A.E.; Soeborg, T.; Blankenstein, M.A.; et al. Comparison of 7 Published LC-MS/MS Methods for the Simultaneous Measurement of Testosterone, Androstenedione, and Dehydroepiandrosterone in Serum. Clin. Chem. 2015, 61, 1475–1483. [Google Scholar] [CrossRef]
- Phillips, G.B. Relationship between Serum Dehydroepiandrosterone Sulfate, Androstenedione, and Sex Hormones in Men and Women. Eur. J. Endocrinol. 1996, 134, 201–206. [Google Scholar] [CrossRef]
- Puri, C.P.; Puri, V.; Kumar, T.C.A. Serum Levels of Testosterone, Cortisol, Prolactin and Bioactive Luteinizing Hormone in Adult Male Rhesus Monkeys Following Cage-Restraint or Anaesthetizing with Ketamine Hydrochloride. Eur. J. Endocrinol. 1981, 97, 118–124. [Google Scholar] [CrossRef]
- Zaidi, P.; Wickings, E.J.; Nieschlag, E. The Effects of Ketamine HC1 and Barbiturate Anaesthesia on the Metabolic Clearance and Production Rates of Testosterone in the Male Rhesus Monkey, Macaca Mulatta. J. Steroid Biochem. 1982, 16, 463–466. [Google Scholar] [CrossRef]
- Malaivijitnond, S.; Takenaka, O.; Sankai, T.; Yoshida, T.; Cho, F.; Yoshikawa, Y. Effects of Single and Multiple Injections of Ketamine Hydrochloride on Serum Hormone Concentrations in Male Cynomolgus Monkeys. Comp. Med. 1998, 48, 270–274. [Google Scholar]
- Gould, E.M. The Effect of Ketamine/Xylazine and Carbon Dioxide on Plasma Luteinizing Hormone Releasing Hormone and Testosterone Concentrations in the Male Norway Rat. Lab. Anim. 2008, 42, 483–488. [Google Scholar] [CrossRef]
- Mitani, J.C. Cooperation and Competition in Chimpanzees: Current Understanding and Future Challenges. Evol. Anthropol. Issues News Rev. 2009, 18, 215–227. [Google Scholar] [CrossRef]
- Wobber, V.; Hare, B.; Maboto, J.; Lipson, S.; Wrangham, R.; Ellison, P.T. Differential Changes in Steroid Hormones before Competition in Bonobos and Chimpanzees. Proc. Natl. Acad. Sci. USA 2010, 107, 12457–12462. [Google Scholar] [CrossRef] [PubMed]
- Sannen, A.; Van Elsacker, L.; Heistermann, M.; Eens, M. Urinary Testosterone-Metabolite Levels and Dominance Rank in Male and Female Bonobos (Pan Paniscus). Primates 2004, 45, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Cordes, M.A.; Stevenson, S.A.; Riters, L.V. Status-Appropriate Singing Behavior, Testosterone and Androgen Receptor Immunolabeling in Male European Starlings (Sturnus Vulgaris). Horm. Behav. 2014, 65, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Juntti, S.A.; Tollkuhn, J.; Wu, M.V.; Fraser, E.J.; Soderborg, T.; Tan, S.; Honda, S.-I.; Harada, N.; Shah, N.M. The Androgen Receptor Governs the Execution, but Not Programming, of Male Sexual and Territorial Behaviors. Neuron 2010, 66, 260–272. [Google Scholar] [CrossRef]
- Giammanco, M.; Tabacchi, G.; Giammanco, S.; Majo, D.D.; Guardia, M.L. Testosterone and Aggressiveness. Med. Sci. Monit. 2005, 11, RA136–RA145. [Google Scholar]
- Eisenegger, C.; Haushofer, J.; Fehr, E. The Role of Testosterone in Social Interaction. Trends Cogn. Sci. 2011, 15, 263–271. [Google Scholar] [CrossRef]
- Garai, C.; Furuichi, T.; Kawamoto, Y.; Ryu, H.; Inoue-Murayama, M. Androgen Receptor and Monoamine Oxidase Polymorphism in Wild Bonobos. Meta Gene 2014, 2, 831–843. [Google Scholar] [CrossRef]
- Hong, K.-W.; Hibino, E.; Takenaka, O.; Hayasaka, I.; Murayama, Y.; Ito, S.; Inoue-Murayama, M. Comparison of Androgen Receptor CAG and GGN Repeat Length Polymorphism in Humans and Apes. Primates 2006, 47, 248–254. [Google Scholar] [CrossRef]
- Fivizzani, A.J.; Oring, L.W. Plasma Steroid Hormones in Relation to Behavioral Sex Role Reversal in the Spotted Sandpiper, Actitis Macularia. Biol. Reprod. 1986, 35, 1195–1201. [Google Scholar] [CrossRef]
- Goymann, W.; Wingfield, J.C. Competing Females and Caring Males. Sex Steroids in African Black Coucals, Centropus Grillii. Anim. Behav. 2004, 68, 733–740. [Google Scholar] [CrossRef]
- Lipshutz, S.E.; Rosvall, K.A. Neuroendocrinology of Sex-Role Reversal. Integr. Comp. Biol. 2020, 60, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Rissman, E.F.; Wingfield, J.C. Hormonal Correlates of Polyandry in the Spotted Sandpiper, Actitis Macularia. Gen. Comp. Endocrinol. 1984, 56, 401–405. [Google Scholar] [CrossRef]
- Voigt, C.; Goymann, W. Sex-Role Reversal Is Reflected in the Brain of African Black Coucals (Centropus Grillii). Dev. Neurobiol. 2007, 67, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Surbeck, M.; Hohmann, G. Intersexual Dominance Relationships and the Influence of Leverage on the Outcome of Conflicts in Wild Bonobos (Pan Paniscus). Behav. Ecol. Sociobiol. 2013, 67, 1767–1780. [Google Scholar] [CrossRef]
- Muller, M.N. Agonistic Relations among Kanyawara Chimpanzees. In Behavioural Diversity in Chimpanzees and Bonobos; Boesch, C., Hohmann, G., Marchant, L., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 112–124. ISBN 978-0-521-00613-2. [Google Scholar]
- Pusey, A.; Murray, C.; Wallauer, W.; Wilson, M.; Wroblewski, E.; Goodall, J. Severe Aggression Among Female Pan Troglodytes Schweinfurthii at Gombe National Park, Tanzania. Int. J. Primatol. 2008, 29, 949. [Google Scholar] [CrossRef]
- Pusey, A.E.; Schroepfer-Walker, K. Female Competition in Chimpanzees. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013, 368, 20130077. [Google Scholar] [CrossRef]
- Newton-Fisher, N.E. Female Coalitions Against Male Aggression in Wild Chimpanzees of the Budongo Forest. Int. J. Primatol. 2006, 27, 1589–1599. [Google Scholar] [CrossRef]
- Cashdan, E. Hormones and Competitive Aggression in Women. Aggress. Behav. 2003, 29, 107–115. [Google Scholar] [CrossRef]
- Somit, A. Humans, Chimps, and Bonobos: The Biological Bases of Aggression, War, and Peacemaking. J. Confl. Resolut. 1990, 34, 553–582. [Google Scholar] [CrossRef]
- Rendon, N.M.; Demas, G.E. Bi-Directional Actions of Dehydroepiandrosterone and Aggression in Female Siberian Hamsters. J. Exp. Zool. Part Ecol. Genet. Physiol. 2016, 325, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Soma, K.K.; Rendon, N.M.; Boonstra, R.; Albers, H.E.; Demas, G.E. DHEA Effects on Brain and Behavior: Insights from Comparative Studies of Aggression. J. Steroid Biochem. Mol. Biol. 2015, 145, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Goymann, W.; Wittenzellner, A.; Schwabl, I.; Makomba, M. Progesterone Modulates Aggression in Sex-Role Reversed Female African Black Coucals. Proc. R. Soc. B Biol. Sci. 2008, 275, 1053–1060. [Google Scholar] [CrossRef]
- Terranova, J.I.; Ferris, C.F.; Albers, H.E. Sex Differences in the Regulation of Offensive Aggression and Dominance by Arginine-Vasopressin. Front. Endocrinol. 2017, 8, 308. [Google Scholar] [CrossRef]
- Terranova, J.I.; Song, Z.; Larkin, T.E.; Hardcastle, N.; Norvelle, A.; Riaz, A.; Albers, H.E. Serotonin and Arginine–Vasopressin Mediate Sex Differences in the Regulation of Dominance and Aggression by the Social Brain. Proc. Natl. Acad. Sci. USA 2016, 113, 13233–13238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutzler, S.J.; Karom, M.; Erwin, W.D.; Albers, H.E. Arginine-Vasopressin and the Regulation of Aggression in Female Syrian Hamsters (Mesocricetus Auratus). Eur. J. Neurosci. 2010, 31, 1655–1663. [Google Scholar] [CrossRef]
- Lewis, R.J. Beyond Dominance: The Importance of Leverage. Q. Rev. Biol. 2002, 77, 149–164. [Google Scholar] [CrossRef]
- Hammock, E.A.D.; Young, L.J. Oxytocin, Vasopressin and Pair Bonding: Implications for Autism. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 2187–2198. [Google Scholar] [CrossRef]
- Rogers Flattery, C.N.; Coppeto, D.J.; Inoue, K.; Rilling, J.K.; Preuss, T.M.; Young, L.J. Distribution of Brain Oxytocin and Vasopressin V1a Receptors in Chimpanzees (Pan Troglodytes): Comparison with Humans and Other Primate Species. Brain Struct. Funct. 2021, 227, 1907–1919. [Google Scholar] [CrossRef]
- Staes, N.; Guevara, E.E.; Helsen, P.; Eens, M.; Stevens, J.M.G. The Pan Social Brain: An Evolutionary History of Neurochemical Receptor Genes and Their Potential Impact on Sociocognitive Differences. J. Hum. Evol. 2021, 152, 102949. [Google Scholar] [CrossRef]
- Staes, N.; Stevens, J.M.G.; Helsen, P.; Hillyer, M.; Korody, M.; Eens, M. Oxytocin and Vasopressin Receptor Gene Variation as a Proximate Base for Inter- and Intraspecific Behavioral Differences in Bonobos and Chimpanzees. PLoS ONE 2014, 9, e113364. [Google Scholar] [CrossRef]
- Parish, A.R.; Waal, F.B.M.D.; Haig, D. The Other “Closest Living Relative”: How Bonobos (Pan Paniscus) Challenge Traditional Assumptions about Females, Dominance, Intra- and Intersexual Interactions, and Hominid Evolution. Ann. N. Y. Acad. Sci. 2000, 907, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Stanford, C.B. The Social Behavior of Chimpanzees and Bonobos: Empirical Evidence and Shifting Assumptions. Curr. Anthropol. 1998, 39, 399–420. [Google Scholar] [CrossRef]
| Predictions of Sex Differences in Plasma Androgen (A) Levels in the Two Pan Species | Within Species Bonobos | Within Species Chimpanzees | Species Comparison (Bonobo, b vs. Chimpanzee, c) |
|---|---|---|---|
| Sex-specific aggression patterns are mediated by female androgen levels (Hypothesis 1) | ♀ < or = or > ♂, because ♀ A are high | ♀ < ♂ | b♂ = c♂ b♀ > c♀ |
| Sex-specific aggression patterns are mediated by male androgen levels (Hypothesis 2) | ♀ = or > ♂, because ♂ A is low | ♀ < ♂ | b♂ < c♂ b♀ = c♀ |
| Males need high androgen levels for agonistic power and to ensure reproductive success, while androgens play a lesser role in females (Hypothesis 3) | ♀ < ♂ | ♀ < ♂ | b♂ = c♂ b♀ = c♀ |
| Androgen | Bonobo | Chimpanzee | |||
|---|---|---|---|---|---|
| Testosterone (ng/mL) | Female | Male | Female | Male | |
| Mean ± SD | 0.5 ±0.3 | 6.7 ± 2.9 | 0.4 ± 0.2 | 5.8 ± 3.6 | |
| Median | 0.5 | 5.9 | 0.3 | 5.7 | |
| Range | 0.2–1.1 | 3.7–12.1 | 0.1–1.0 | 1.57–14.50 | |
| Sex-ratio | 12.6 | 14.6 | |||
| Androstenedione (ng/mL) | |||||
| Mean ± SD | 1.8 ± 0.9 | 1.1 ± 0.4 | 1.8 ± 0.9 | 1.2 ± 0.6 | |
| Median | 1.6 | 1.2 | 1.7 | 1.2 | |
| Range | 0.5–3.2 | 0.4–1.7 | 0.6–4.5 | 0.4–2.4 | |
| Sex-ratio | 0.6 | 0.7 | |||
| Results of the Final Testosterone Model | Estimate | Standard Error | p-Value |
|---|---|---|---|
| Intercept | −0.807 | 0.145 | |
| Species (z-transformed): bonobo | −0.215 | 0.165 | 0.205 |
| Sex (z-transformed): female | 2.610 | 0.161 | <0.001 |
| Individual age (z-transformed) | 0.074 | 0.175 | |
| Contraception (z-transformed): no contraception | −0.195 | 0.226 | |
| Variance | Standard deviation | ||
| Intercept zoo | 0.009 | 0.095 | |
| Residual variance | 0.314 | 0.561 |
| Results of the Final Androstenedione Model | Estimate | Standard Error | p-Value |
|---|---|---|---|
| Intercept | 0.414 | 0.134 | |
| Species (z-transformed): bonobo | 0.063 | 0.152 | 0.692 |
| Sex (z-transformed): female | −0.386 | 0.142 | 0.008 |
| Individual age (z-transformed) | −0.033 | 0.153 | |
| Contraception (z-transformed): no contraception | 0.020 | 0.201 | |
| Variance | Standard deviation | ||
| Intercept zoo | 0.026 | 0.161 | |
| Residual variance | 0.227 | 0.476 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonnweber, R.; Stevens, J.M.G.; Hohmann, G.; Deschner, T.; Behringer, V. Plasma Testosterone and Androstenedione Levels Follow the Same Sex-Specific Patterns in the Two Pan Species. Biology 2022, 11, 1275. https://doi.org/10.3390/biology11091275
Sonnweber R, Stevens JMG, Hohmann G, Deschner T, Behringer V. Plasma Testosterone and Androstenedione Levels Follow the Same Sex-Specific Patterns in the Two Pan Species. Biology. 2022; 11(9):1275. https://doi.org/10.3390/biology11091275
Chicago/Turabian StyleSonnweber, Ruth, Jeroen M. G. Stevens, Gottfried Hohmann, Tobias Deschner, and Verena Behringer. 2022. "Plasma Testosterone and Androstenedione Levels Follow the Same Sex-Specific Patterns in the Two Pan Species" Biology 11, no. 9: 1275. https://doi.org/10.3390/biology11091275
APA StyleSonnweber, R., Stevens, J. M. G., Hohmann, G., Deschner, T., & Behringer, V. (2022). Plasma Testosterone and Androstenedione Levels Follow the Same Sex-Specific Patterns in the Two Pan Species. Biology, 11(9), 1275. https://doi.org/10.3390/biology11091275






