Transcriptional Dynamics Induced by Diapause Hormone in the Silkworm, Bombyx mori
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Preparation
2.2. RNA Extraction and cDNA Library Construction
2.3. RNA Sequencing (RNA-Seq) and De Novo Assembly of Sequences
2.4. Differentially Expressed Genes (DEGs) Analysis
2.5. Quantitative Real-Time PCR (qRT-PCR) Verification
2.6. Statistical Analysis
3. Results
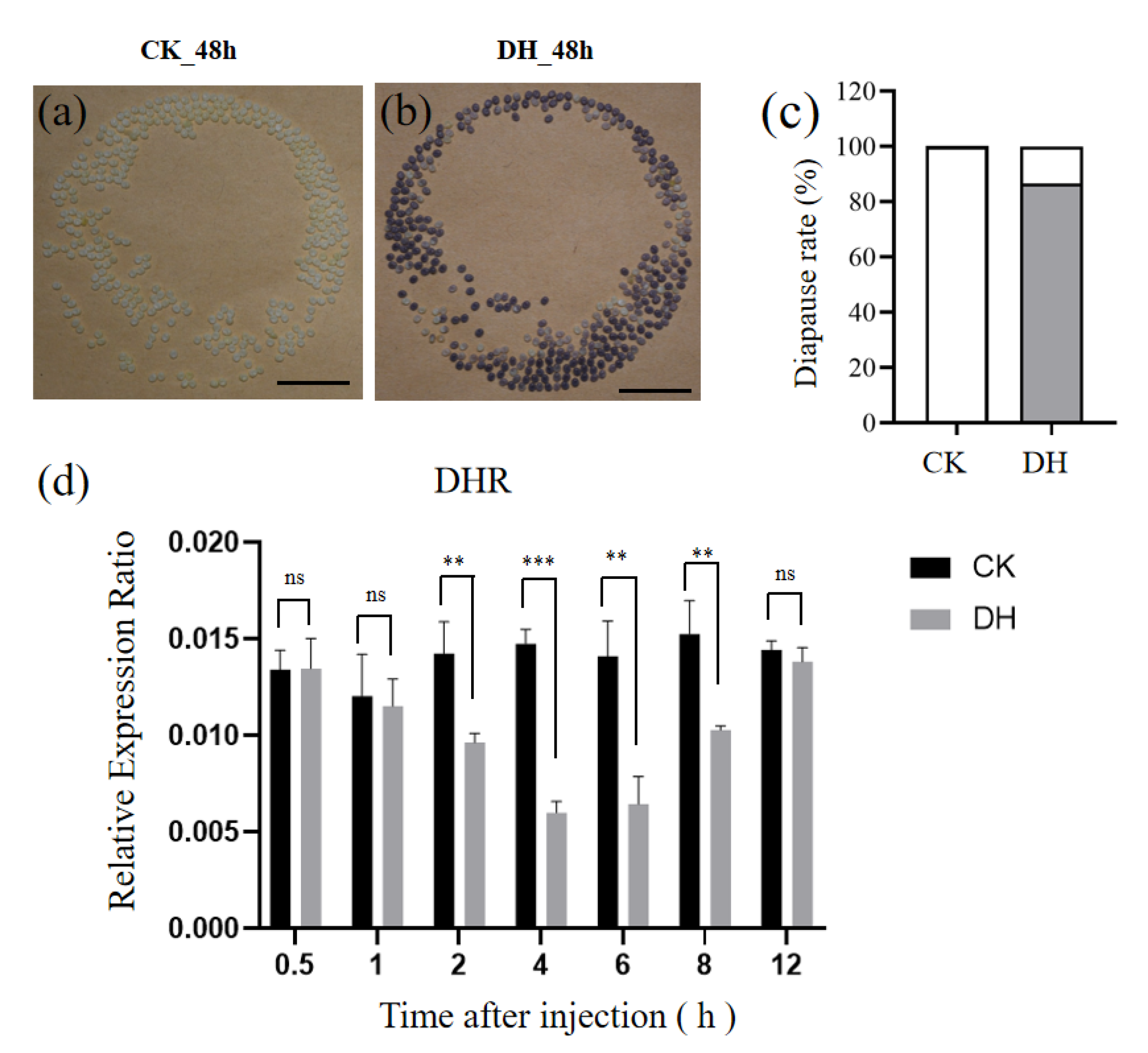
3.1. The Injection of DH Induces Progeny Embryonic Diapause in a Non-Diapause Silkworm Strain
3.2. Overview of Transcriptome Sequencing Data
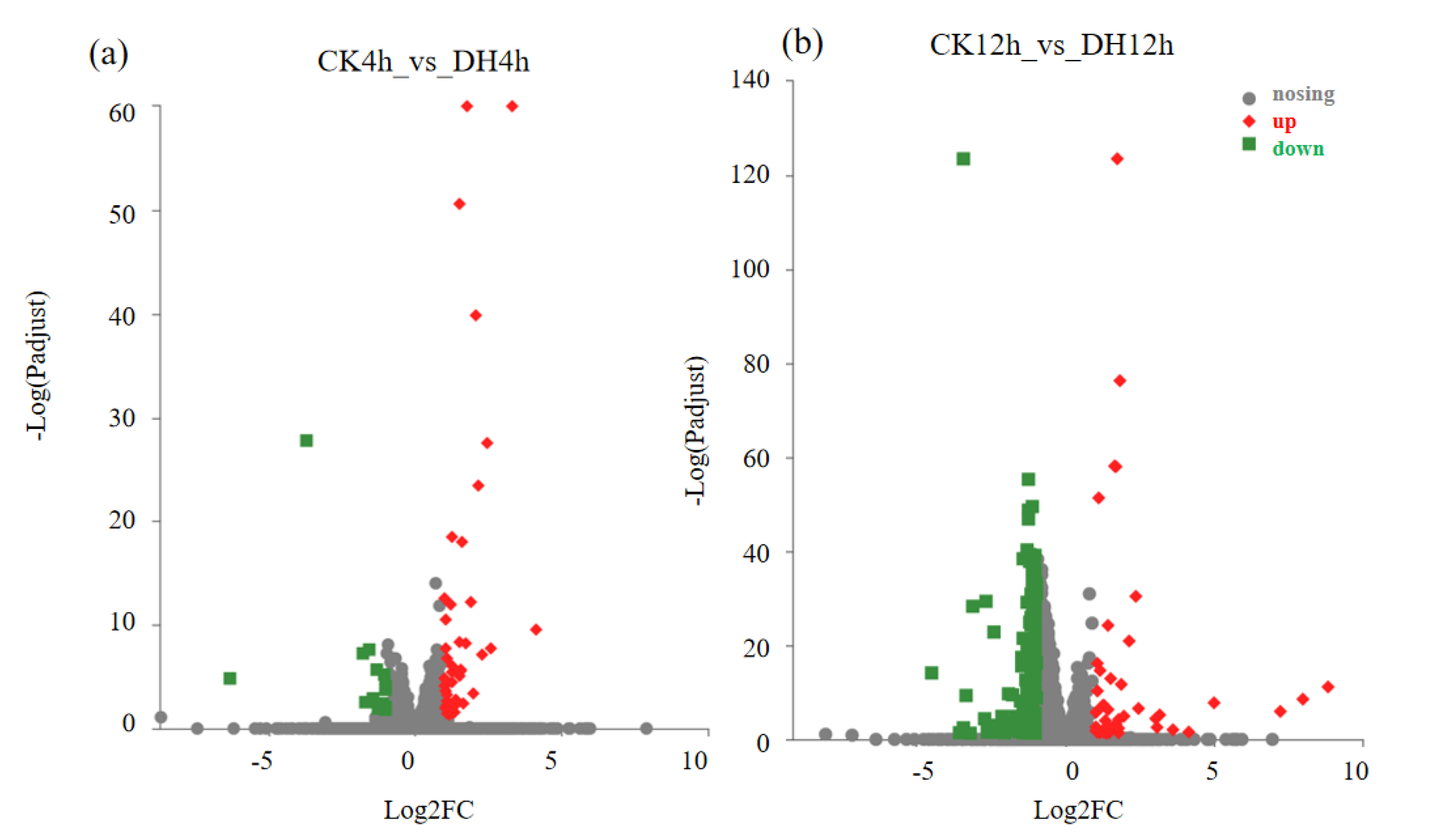
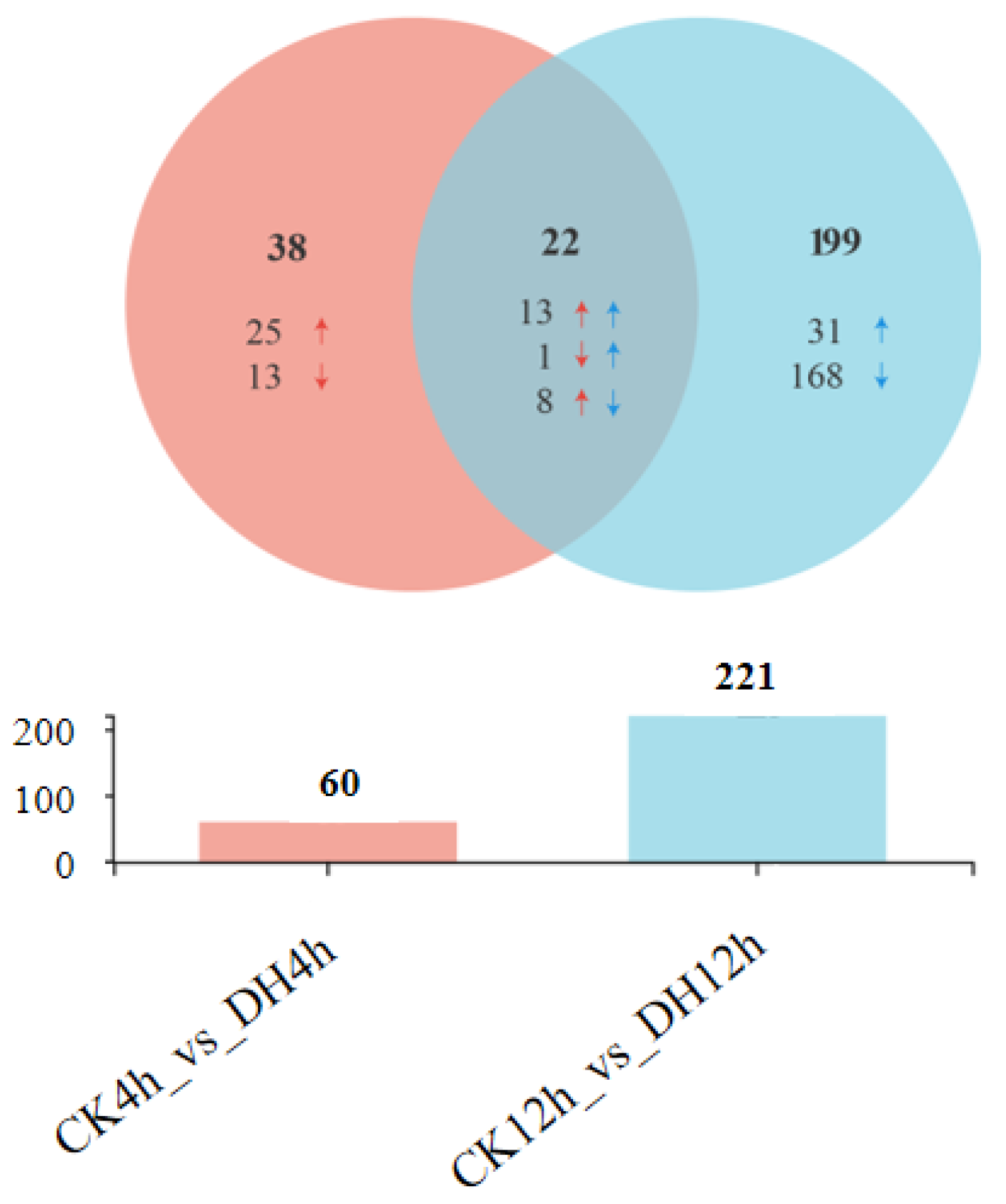
3.3. DH-Induced Transcriptional Changes
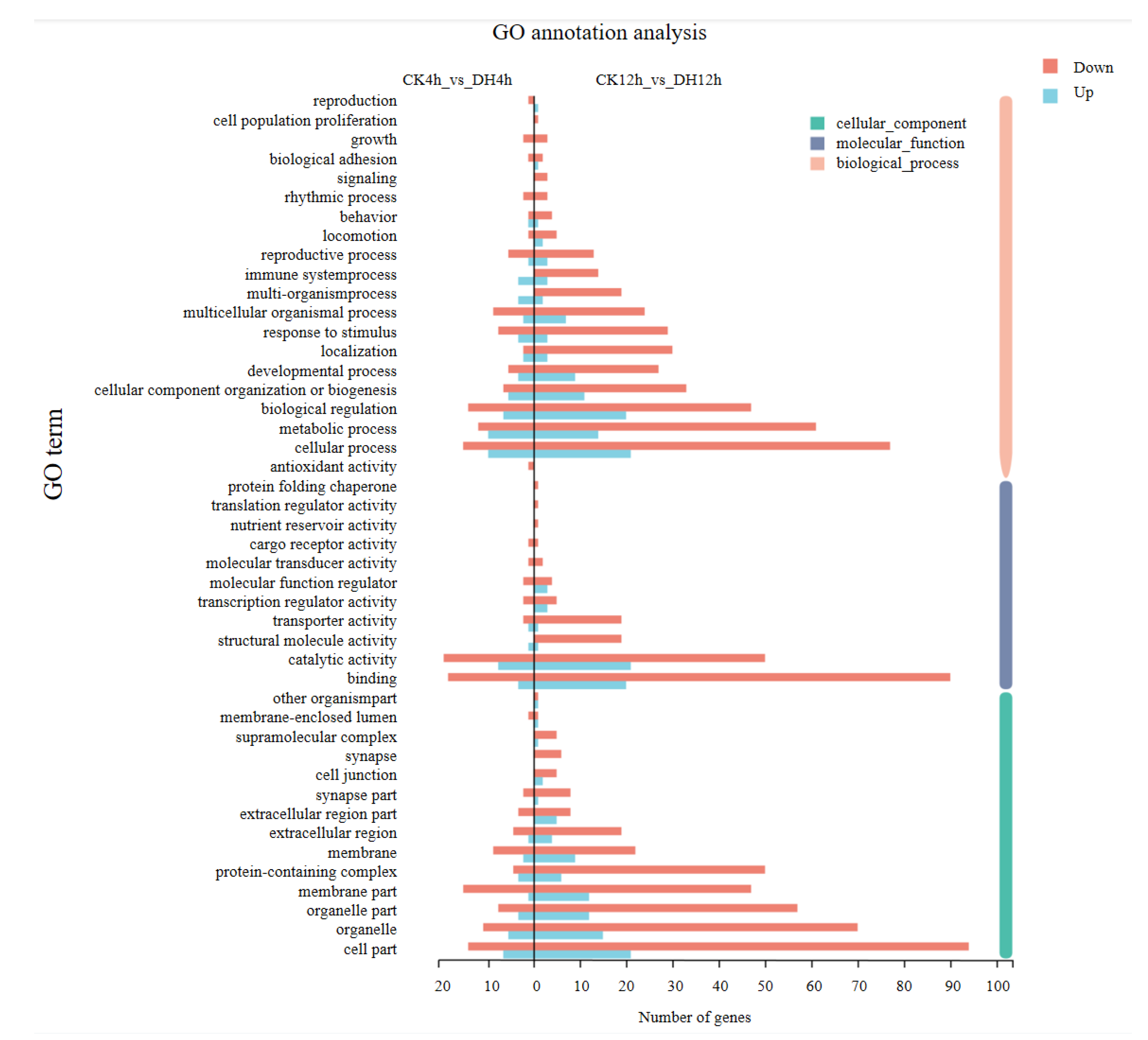
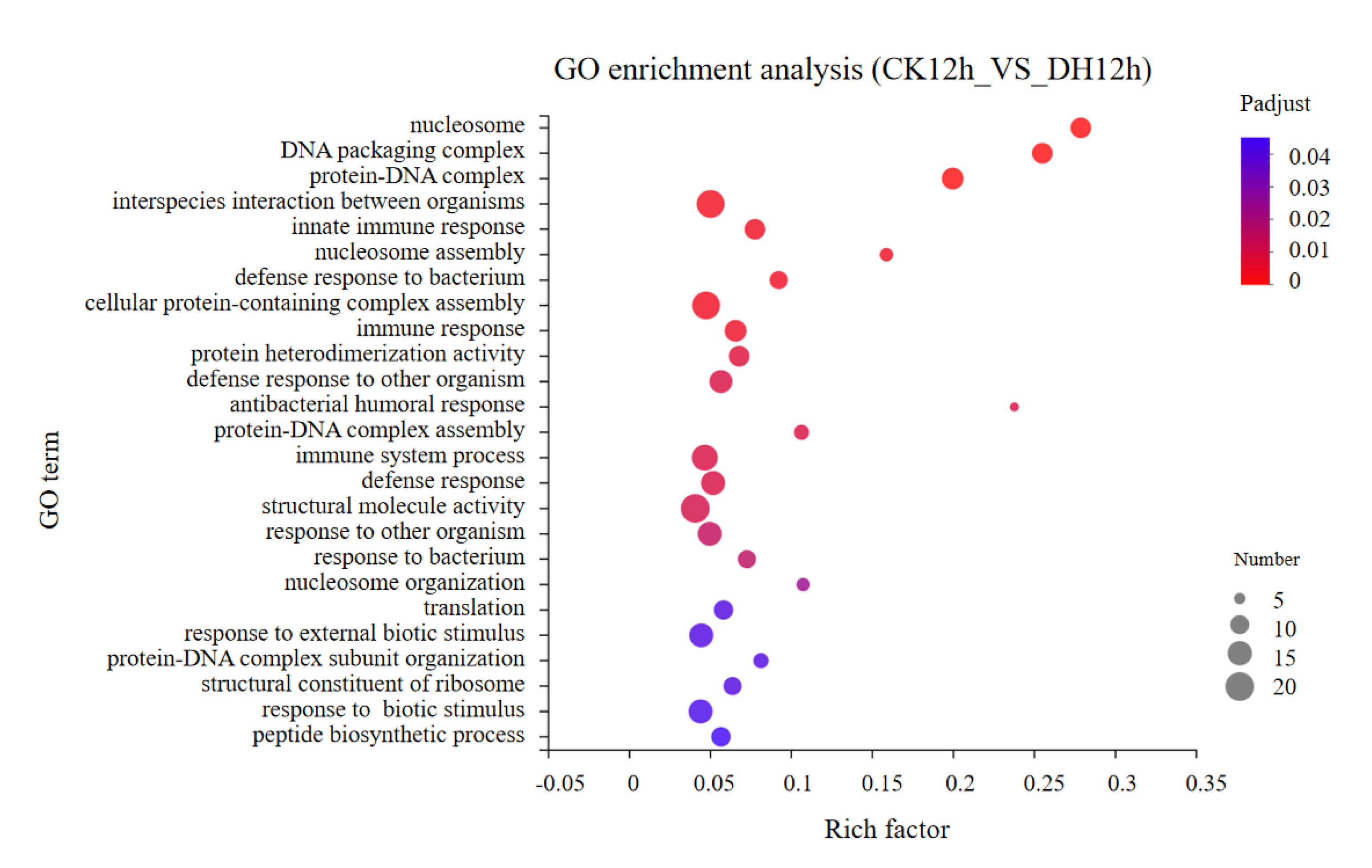
3.4. Gene Ontology (GO) Classification of DEGs
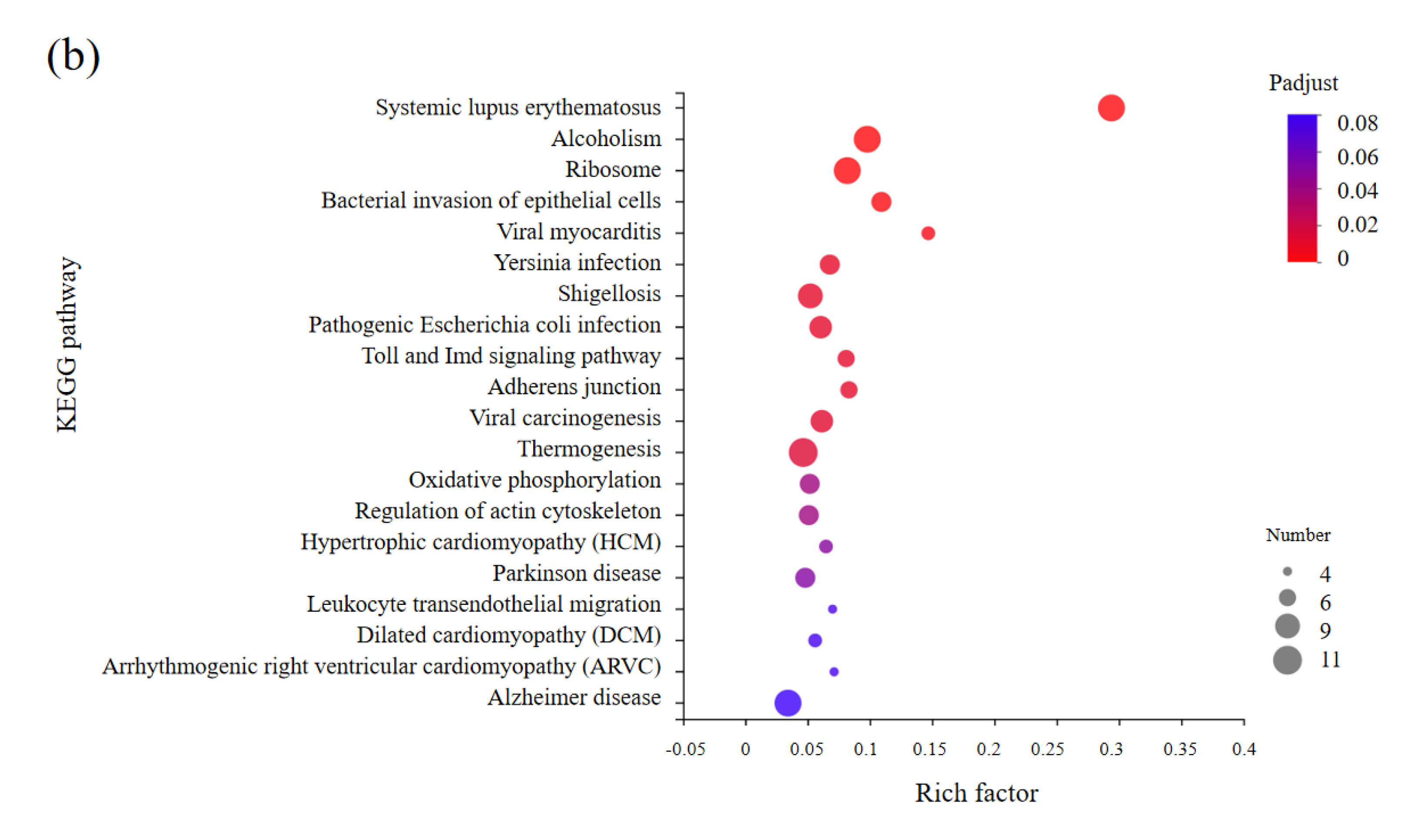
3.5. KEGG Enrichments Analysis of DEGs
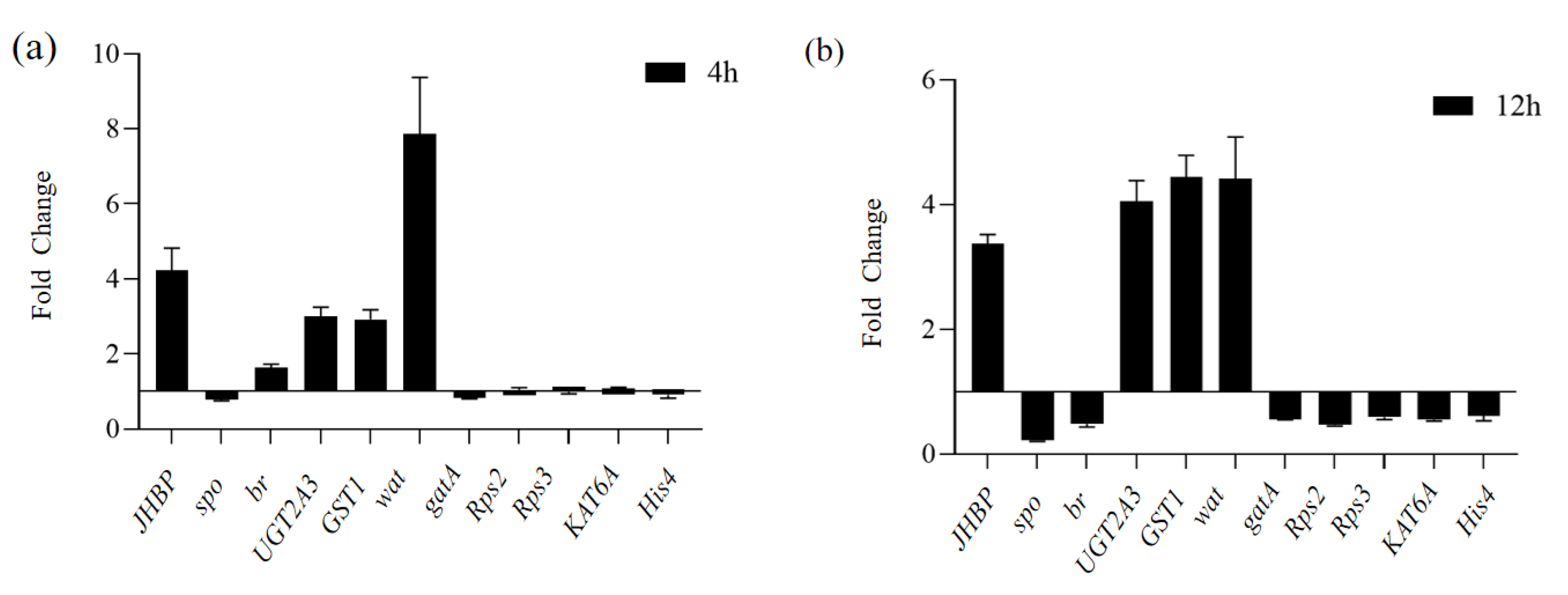
3.6. Validation of DEGs by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragland, G.J.; Armbruster, P.A.; Meuti, M.E. Evolutionary and functional genetics of insect diapause: A call for greater integration. Curr. Opin. Insect Sci. 2019, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Delinger, D. Regulation of diapauses. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, M.; Takei, R.; Nagashima, E.; Yaginuma, T. Cell cycles in embryos of the silkworm, Bombyx mori: G2-arrest at diapause stage. Dev. Genes Evol. 1991, 200, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bean, D.W.; Beck, S.D.; Goodman, W.G. Juvenile hormone esterases in diapause and nondiapause larvae of the European corn borer, Ostrinia nubilalis. J. Insect Physiol. 1982, 28, 485–492. [Google Scholar] [CrossRef]
- Fraenkel, G.; Hsiao, C. Manifestations of a pupal diapause in two species of flies, Sarcophaga argyrostoma and S. bullata. J. Insect Physiol. 1968, 14, 689–705. [Google Scholar] [CrossRef]
- Kotaki, T. Relationships between JH-biosynthetic activity of the corpora allata in vitro, their size and adult diapause in a stink bug, Plautia crossota stali Scott. Entomol. Sci. 1999, 2, 307–313. [Google Scholar]
- Yamashita, O. Diapause hormone of the silkworm, Bombyx mori: Structure, gene expression and function. Insect Physiol. 1996, 42, 669–679. [Google Scholar] [CrossRef]
- Hasegawa, K. Studies on the voltinism in the silkworm, Bombyx mori L., with special reference to the organs concerning determination of voltinism (a preliminary note). Proc. Jpn. Acad. 1951, 1, 83–124. [Google Scholar] [CrossRef]
- Morita, A.; Niimi, T.; Yamashita, O. Physiological differentiation of DH-PBAN-producing neurosecretory cells in the silkworm embryo. J. Insect Physiol. 2003, 49, 1093–1102. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Effect of Diapause-inducing Temperature and Photoperiod on the Expression of Circadian Rhythm Gene per in the Silkworm, Bombyx mori. Sci. Sericult. 2010, 36, 0052–0059, (In Chinese with English abstract). [Google Scholar]
- Yamashita, O.; Hasegawa, K. Embryonic diapause. Compr. Insect Physiol. Biochem. Pharmacol. 1985, 1, 407–434. [Google Scholar]
- Shiomi, K.; Takasu, Y.; Kunii, M.; Tsuchiya, R.; Mukaida, M.; Kobayashi, M.; Sezutsu, H.; Ichida Takahama, M.; Mizoguchi, A. Disruption of diapause induction by TALEN-based gene mutagenesis in relation to a unique neuropeptide signaling pathway in Bombyx. Sci. Rep. 2015, 5, 15566. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Konno, T.; Nakazawa, Y.; Komiya, T.; Yamashita, O. Isolation and Structure of Diapause Hormone of the Silkworm, Bombyx mori. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1991, 67, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, O.; Hasegawa, K.; Seki, M. Effect of the diapause hormone on trehalase activity in pupal ovaries of the silkworm, Bombyx mori L. Gen. Comp. Endocrinol. 1972, 18, 515–523. [Google Scholar] [CrossRef]
- Huang, J.Z.; Xia, J.W.; Xiang, Z. Complete Works of Sericultural Technology in China; Sichuan Publishing House of Science & Technology Press: Chengdu, China, 1996; pp. 519–521. [Google Scholar]
- Zhang, Q.; Denlinger, D.L. Dynamics of diapause hormone and prothoracicotropic hormone transcript expression at diapause termination in pupae of the corn earworm, Helicoverpa zea. Peptides 2012, 34, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nachman, R.J.; Denlinger, D.L. Diapause hormone in the Helicoverpa/Heliothis complex: A review of gene expression, peptide structure and activity, analog and antagonist development, and the receptor. Peptides 2015, 72, 196–201. [Google Scholar] [CrossRef]
- Gong, C.; Zeng, W.; Zhang, T.; Liu, R.; Ou, Y.; Ai, J.; Xiang, Z.; Xu, H. Effects of transgenic overexpression of diapause hormone and diapause hormone receptor genes on non-diapause silkworm. Transgenic Res. 2017, 26, 807–815. [Google Scholar] [CrossRef]
- Sato, A.; Sokabe, T.; Kashio, M.; Yasukochi, Y.; Tominaga, M.; Shiomi, K. Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2014, 111, E1249–E1255. [Google Scholar] [CrossRef]
- Tsuchiya, R.; Kaneshima, A.; Kobayashi, M.; Yamazaki, M.; Takasu, Y.; Sezutsu, H.; Tanaka, Y.; Mizoguchi, A.; Shiomi, K. Maternal GABAergic and GnRH/corazonin pathway modulates egg diapause phenotype of the silkworm Bombyx mori. Proc. Natl. Acad. Sci. USA 2021, 118, e2020028118. [Google Scholar] [CrossRef]
- Cui, W.Z.; Qiu, J.F.; Dai, T.M.; Chen, Z.; Li, J.L.; Liu, K.; Wang, Y.J.; Sima, Y.H.; Xu, S.Q. Circadian Clock Gene Period Contributes to Diapause via GABAeric-Diapause Hormone Pathway in Bombyx mori. Biology 2021, 10, 842. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.N.; Li, B. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramirez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Houten, Y.M.V.; Veenendaal, R.L. Effects of photoperiod, temperature, food and relative humidity on the induction of diapause in the predatory miteAmblyseius potentillae. Exp. Appl. Acarol. 1990, 10, 111–128. [Google Scholar] [CrossRef]
- Yamashita, O.; Hasegawa, K. Ocyte age sensitive to the diapause hormone from the standpoint of glycogen synthesis in the silkworm, Bombyx mori. J. Insect Physiol. 1970, 16, 2377–2383. [Google Scholar] [CrossRef]
- Kim, M.; Denlinger, D.L. A potential role for ribosomal protein S2 in the gene network regulating reproductive diapause in the mosquito Culex pipiens. J. Comp. Physiol. B 2010, 180, 171–178. [Google Scholar] [CrossRef]
- Guo, S.; Tian, Z.; Wu, Q.W.; King-Jones, K.; Wang, X.P. Steroid hormone ecdysone deficiency stimulates preparation for photoperiodic reproductive diapause. PLoS Genet. 2021, 17, e1009352. [Google Scholar] [CrossRef]
- Zhe, S.; Yang, Y.P.; Xu, W.H. PTEN expression responds to transcription factor POU and regulates p-AKT levels during diapause initiation in the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 2018, 100, 48–58. [Google Scholar]
- Sonobe, H.; Yamada, R. Ecdysteroids during early embryonic development in silkworm Bombyx mori: Metabolism and functions. Zool. Sci. 2004, 21, 503. [Google Scholar] [CrossRef] [PubMed]
- Namiki, T.; Niwa, R.; Sakudoh, T.; Shirai, K.; Takeuchi, H.; Kataoka, H. Cytochrome P450 CYP307A1/Spook: A regulator for ecdysone synthesis in insects. Biochem. Biophys. Res. Commun. 2005, 337, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Matsuda, T.; Yoshiyama, T.; Namiki, T.; Mita, K.; Fujimoto, Y.; Kataoka, H. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J. Biol. Chem. 2004, 279, 35942–35949. [Google Scholar] [CrossRef]
- Pondeville, E.; David, J.P.; Guittard, E.; Maria, A.; Jacques, J.C.; Ranson, H.; Bourgouin, C.; Dauphin-Villemant, C. Microarray and RNAi analysis of P450s in Anopheles gambiae male and female steroidogenic tissues: CYP307A1 is required for ecdysteroid synthesis. PLoS ONE 2013, 8, e79861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, I.; Beaton, A.H.; Tardiff, J.; Fristrom, D.; Fristrom, J.W. Interactions and developmental effects of mutations in the Broad-Complex of Drosophila melanogaster. Genetics 1988, 118, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Piulachs, M.D.; Pagone, V.; Belles, X. Key roles of the Broad-Complex gene in insect embryogenesis. Insect Biochem. Mol. Biol. 2010, 40, 468–475. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.; Sun, G.; Raikhel, A.S. The early gene Broad is involved in the ecdysteroid hierarchy governing vitellogenesis of the mosquito Aedes aegypti. J. Mol. Endocrinol. 2004, 33, 743–761. [Google Scholar] [CrossRef]
- Yang, C.W.; Lin, Y.; Liu, H.L.; Shen, G.W.; Luo, J.; Zhang, H.Y.; Peng, Z.X.; Chen, E.X.; Xing, R.M.; Han, C.S.; et al. The Broad Complex isoform 2 (BrC-Z2) transcriptional factor plays a critical role in vitellogenin transcription in the silkworm Bombyx mori. BBA-Gen. Subj. 2014, 1840, 2674–2684. [Google Scholar] [CrossRef]
- Henrich, V.C.; Rybczynski, R.; Gilbert, L.I. Peptide hormones, steroid hormones, and puffs: Mechanisms and models in insect development. Vitam. Horm. 1999, 55, 73–125. [Google Scholar]
- Dubrovsky, E.B. Hormonal cross talk in insect development. Trends Endocrin. Met. 2005, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, M.; Ozyhar, A.; Kochman, M. Identification of specific interaction of juvenile hormone binding protein with isocitrate dehydrogenase. Acta Biochim. Pol. 2011, 58, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.; Sanburg, L.L.; Kezdy, F.J.; Law, J.H. The Juvenile Hormone Binding Protein in the Hemolymph of Manduca sexta Johannson (Lepidoptera: Sphingidae). Proc. Natl. Acad. Sci. USA 1974, 71, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. Juvenile hormone action: A 2007 perspective. J. Insect Physiol. 2008, 54, 895–901. [Google Scholar] [CrossRef]
- Zhou, S.T.; Guo, W.; Song, J.S. The research of molecular mechanism of juvenile hormone. Appl. Entomol. 2012, 49, 1087–1094. (In Chinese) [Google Scholar]
- Wang, Z.T.; Zheng, W.H.; Guo, F. Determination of juvenile hormone titer in hemolymph of Philosamia cymhia ricini. Chin. Sci. Bull. 1998, 15, 1182. (In Chinese) [Google Scholar]
- Plantevin, G.; Reggi, M.; Nardon, C. Changes in ecdysteroid and juvenile hormone titers in the hemolymph of Galleria mellonella larvae and pupae. Gen. Comp. Endocrinol. 1984, 56, 218–230. [Google Scholar] [CrossRef]
- Strambi, C.; Strambi, A.; Reggi, M.L.; Hirn, M.H.; Delaage, M.A. Radioimmunoassay of Insect Juvenile Hormones and of Their Diol Derivatives. FEBS J. 1981, 118, 401–406. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Huang, L.Q. Studies on the Oviposition Mechanism of Bombyx mori regulated by Hormones i. Effects of juvenile hormone analogized during larval stage of Bombyx mori and ecdysone on reproduction. Sericult. Sci. 1991, 1, 28–32. (In Chinese) [Google Scholar]
- Goudarzi, K.M.; Lindstrom, M.S. Role of ribosomal protein mutations in tumor development (Review). Int. J. Oncol. 2016, 48, 1313–1324. [Google Scholar] [CrossRef]
- Reynaud, E.; Bolshakov, V.N.; Barajas, V.; Kafatos, F.C.; Zurita, M. Antisense suppression of the putative ribosomal protein S3A gene disrupts ovarian development in Drosophila melanogaster. Mol. Gen. Genet. 1997, 256, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Zurita, M.; Reynaud, E.; Kafatos, F.C. Cloning and characterization of cDNAs preferentially expressed in the ovary of the mosquito, Anopheles gambiae. Insect Mol. Biol. 1997, 6, 55–62. [Google Scholar] [CrossRef]
- Niu, L.L.; Fallon, A.M. Differential regulation of ribosomal protein gene expression in Aedes aegypti mosquitoes before and after the blood meal. Insect Mol. Biol. 2000, 9, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sim, C.; Denlinger, D.L. RNA interference directed against ribosomal protein S3a suggests a link between this gene and arrested ovarian development during adult diapause in Culex pipiens. Insect Mol. Biol. 2010, 19, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cramton, S.E.; Laski, F.A. string of pearls encodes Drosophila ribosomal protein S2, has Minute-like characteristics, and is required during oogenesis. Genetics 1994, 137, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Jing, G.; Tian, S.; Zhou, X.; Yang, H.; Yong, Z.; Yong, H. Transcriptional Response of Silkworm (Bombyx mori) Eggs to O2 or HCl Treatment. Int. J. Mol. Sci. 2016, 17, 1838. [Google Scholar]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef]
- Hofvander, P.; Doan, T.T.; Hamberg, M. A prokaryotic acyl-CoA reductase performing reduction of fatty acyl-CoA to fatty alcohol. FEBS Lett. 2011, 585, 3538–3543. [Google Scholar] [CrossRef]
- Cheng, J.B.; Russell, D.W. Mammalian wax biosynthesis—II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J. Biol. Chem. 2004, 279, 37798–37807. [Google Scholar] [CrossRef]
- Krempl, C.; Sporer, T.; Reichelt, M.; Ahn, S.J.; Heidel-Fischer, H.; Vogel, H.; Heckel, D.G.; Joußen, N. Potential detoxification of gossypol by UDP-glycosyltransferases in the two Heliothine moth species Helicoverpa armigera and Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 71, 49–57. [Google Scholar] [CrossRef]
- Roncalli, V.; Cieslak, M.C.; Passamaneck, Y.; Christie, A.E.; Lenz, P.H. Glutathione S-Transferase (GST) Gene Diversity in the Crustacean Calanus finmarchicus--Contributors to Cellular Detoxification. PLoS ONE 2015, 10, E0123322. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W. Vertebrate UDP-glucuronosyltransferases: Functional and evolutionary aspects. Biochem. Pharm. 2003, 66, 691–696. [Google Scholar] [CrossRef]
- Josephy, P.D. Genetic variations in human glutathione transferase enzymes: Significance for pharmacology and toxicology. Hum. Genom. Proteom. 2010, 2010, 876940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, W.; Zhang, J.; Zou, Z.; Ruan, C. High-throughput profiling of diapause regulated genes from Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae). BMC Genom. 2020, 21, 864. [Google Scholar] [CrossRef]
- Nakamura, A.; Miyado, K.; Takezawa, Y.; Ohnami, N.; Sato, M.; Ono, C.; Harada, Y.; Yoshida, K.; Kawano, N.; Kanai, S.; et al. Innate immune system still works at diapause, a physiological state of dormancy in insects. Biochem. Biophys. Res. Commun. 2011, 410, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Lebenzon, J.E.; Torson, A.S.; Sinclair, B.J. Diapause differentially modulates the transcriptomes of fat body and flight muscle in the Colorado potato beetle. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100906. [Google Scholar] [CrossRef]
- Santos, P.K.F.; de Souza Araujo, N.; Francoso, E.; Zuntini, A.R.; Arias, M.C. Diapause in a tropical oil-collecting bee: Molecular basis unveiled by RNA-Seq. BMC Genom. 2018, 19, 305. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.W. Epigenetics: The link between nature and nurture. Mol. Asp. Med. 2013, 34, 753–764. [Google Scholar] [CrossRef]
- Snell-Rood, E. The importance of epigenetics for behavioral ecologists (and vice versa). Behav. Ecol. 2013, 24, 328–329. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Clark, J.; Diakoff, S.J.; Denlinger, D.L. Transcriptional evidence for small RNA regulation of pupal diapause in the flesh fly, Sarcophaga bullata. Insect Biochem. Mol. Biol. 2013, 43, 982–989. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Bautista-Jimenez, R.; Denlinger, D.L. Changes in histone acetylation as potential mediators of pupal diapause in the flesh fly, Sarcophaga bullata. Insect Biochem. Mol. 2016, 76, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Raw Reads | Clean Reads | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|
| DH12h_3 | 42,895,926 | 42,586,708 | 0.0259 | 97.59 | 93.33 | 45.15 |
| DH12h_2 | 42,493,302 | 42,166,558 | 0.0258 | 97.66 | 93.49 | 44.97 |
| DH12h_1 | 43,438,602 | 43,116,974 | 0.0261 | 97.53 | 93.15 | 45.14 |
| CK12h_3 | 42,469,504 | 42,131,634 | 0.0255 | 97.77 | 93.76 | 46.18 |
| CK12h_2 | 46,026,052 | 45,682,102 | 0.0256 | 97.72 | 93.61 | 46.68 |
| CK12h_1 | 45,596,578 | 45,186,554 | 0.026 | 97.57 | 93.3 | 46.73 |
| DH4h_3 | 44,528,428 | 44,154,426 | 0.0256 | 97.72 | 93.62 | 45.96 |
| DH4h_2 | 44,029,406 | 43,654,302 | 0.0258 | 97.63 | 93.43 | 45.94 |
| DH4h_1 | 41,634,522 | 41,229,916 | 0.026 | 97.56 | 93.32 | 46.29 |
| CK4h_3 | 44,979,162 | 44,663,354 | 0.0255 | 97.78 | 93.73 | 45.02 |
| CK4h_2 | 46,506,402 | 46,104,824 | 0.0263 | 97.43 | 92.96 | 45.5 |
| CK4h_1 | 44,441,656 | 44,079,890 | 0.026 | 97.56 | 93.27 | 45.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhang, Z.; Chen, K.; Yu, Y.; Hu, B.; Song, H.; Liu, X. Transcriptional Dynamics Induced by Diapause Hormone in the Silkworm, Bombyx mori. Biology 2022, 11, 1313. https://doi.org/10.3390/biology11091313
Chen L, Zhang Z, Chen K, Yu Y, Hu B, Song H, Liu X. Transcriptional Dynamics Induced by Diapause Hormone in the Silkworm, Bombyx mori. Biology. 2022; 11(9):1313. https://doi.org/10.3390/biology11091313
Chicago/Turabian StyleChen, Lijuan, Zhongjie Zhang, Kai Chen, Ye Yu, Bo Hu, Hongsheng Song, and Xiaojing Liu. 2022. "Transcriptional Dynamics Induced by Diapause Hormone in the Silkworm, Bombyx mori" Biology 11, no. 9: 1313. https://doi.org/10.3390/biology11091313





