A Geometric Morphometric Study on Sexual Dimorphism in Viscerocranium
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
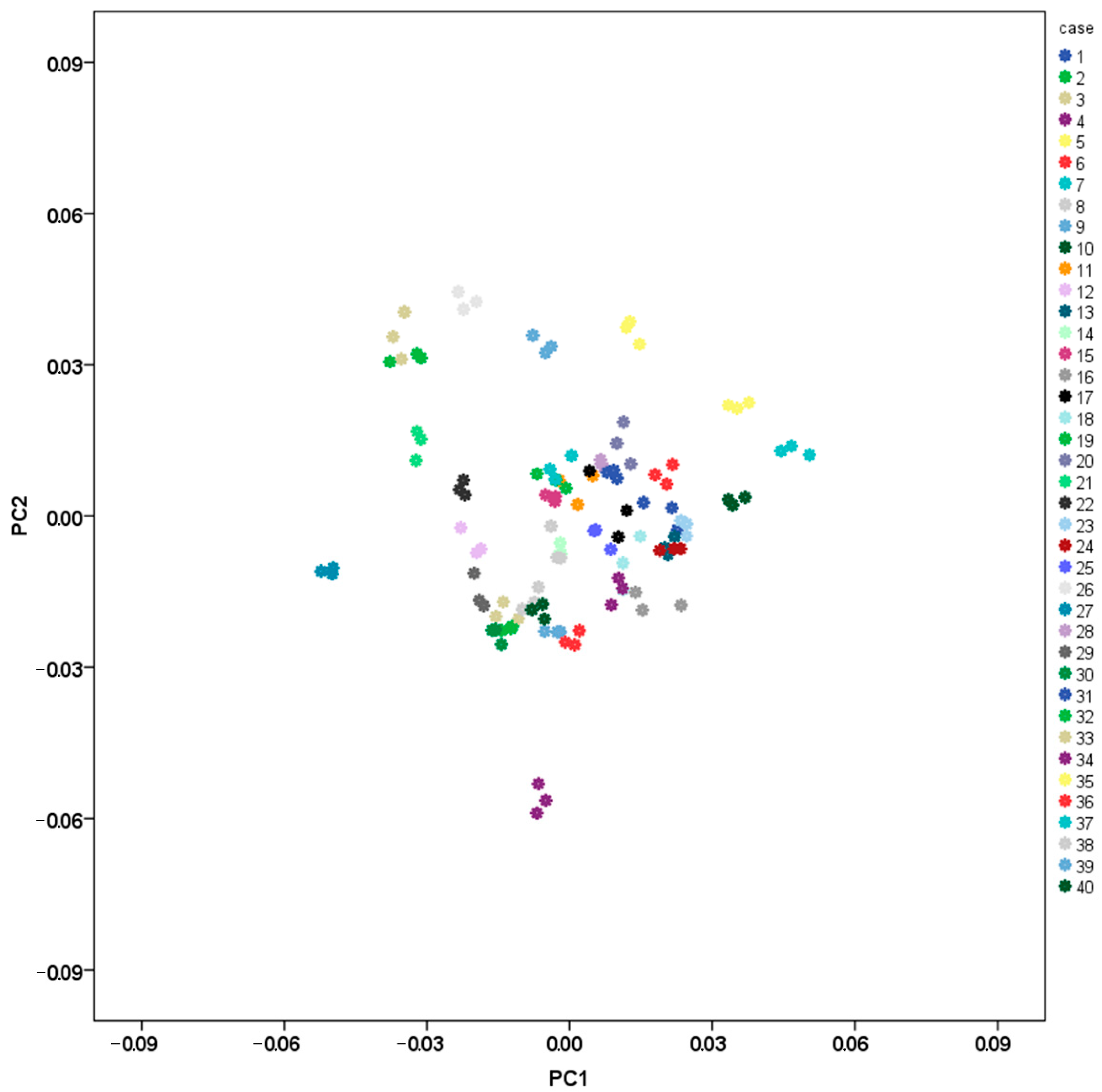
3.1. Intraobserver Error
3.2. Size
3.3. Allometry
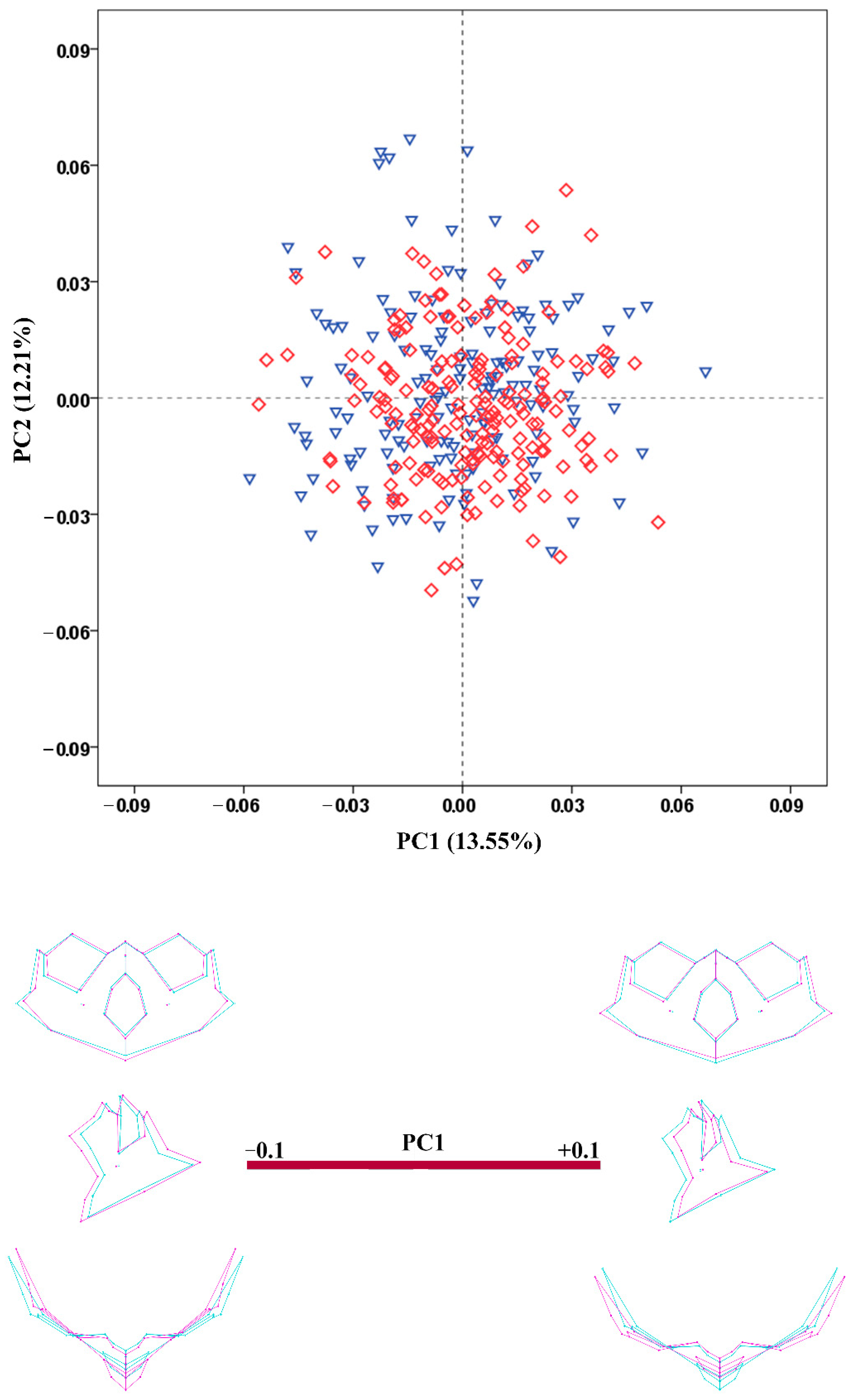
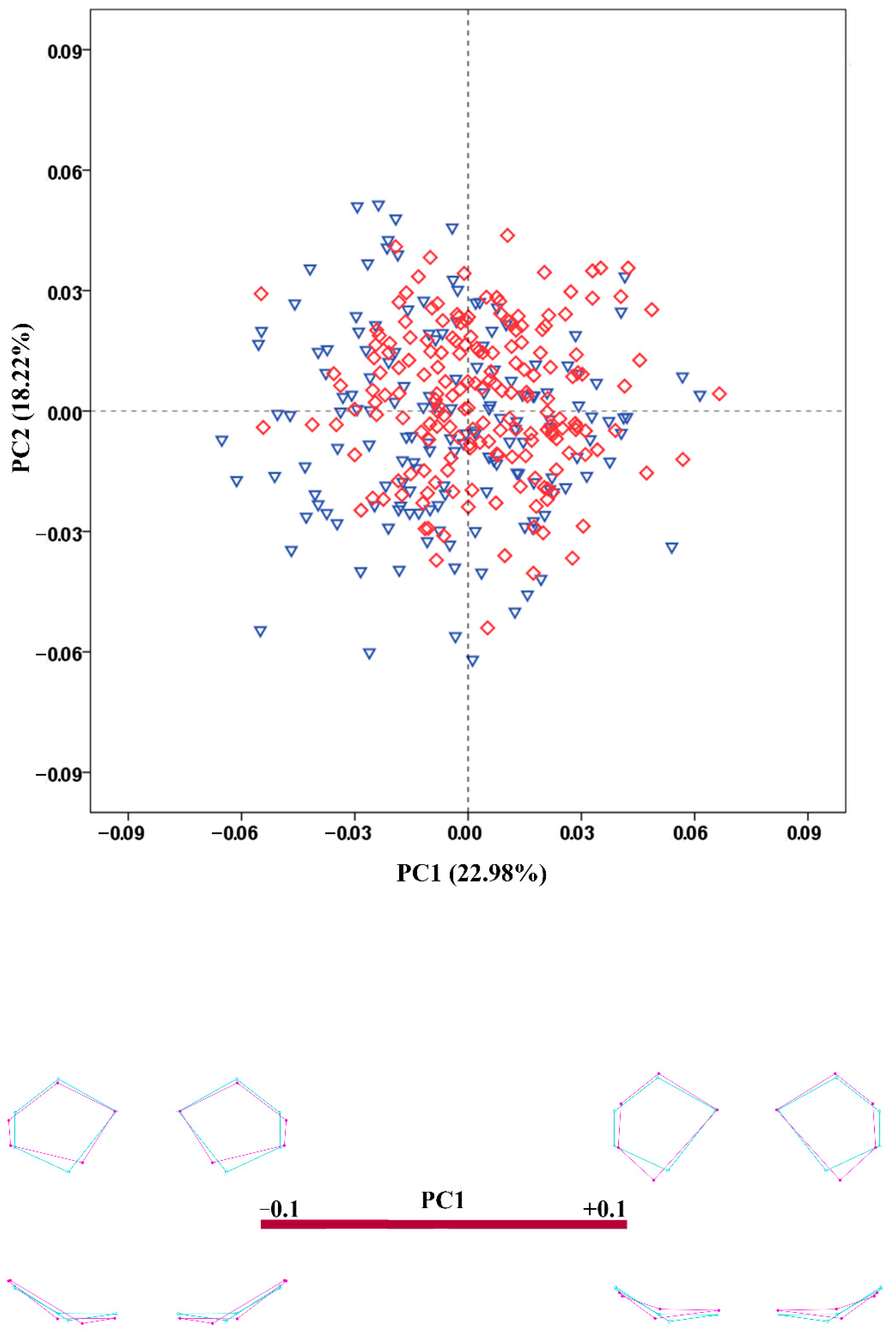
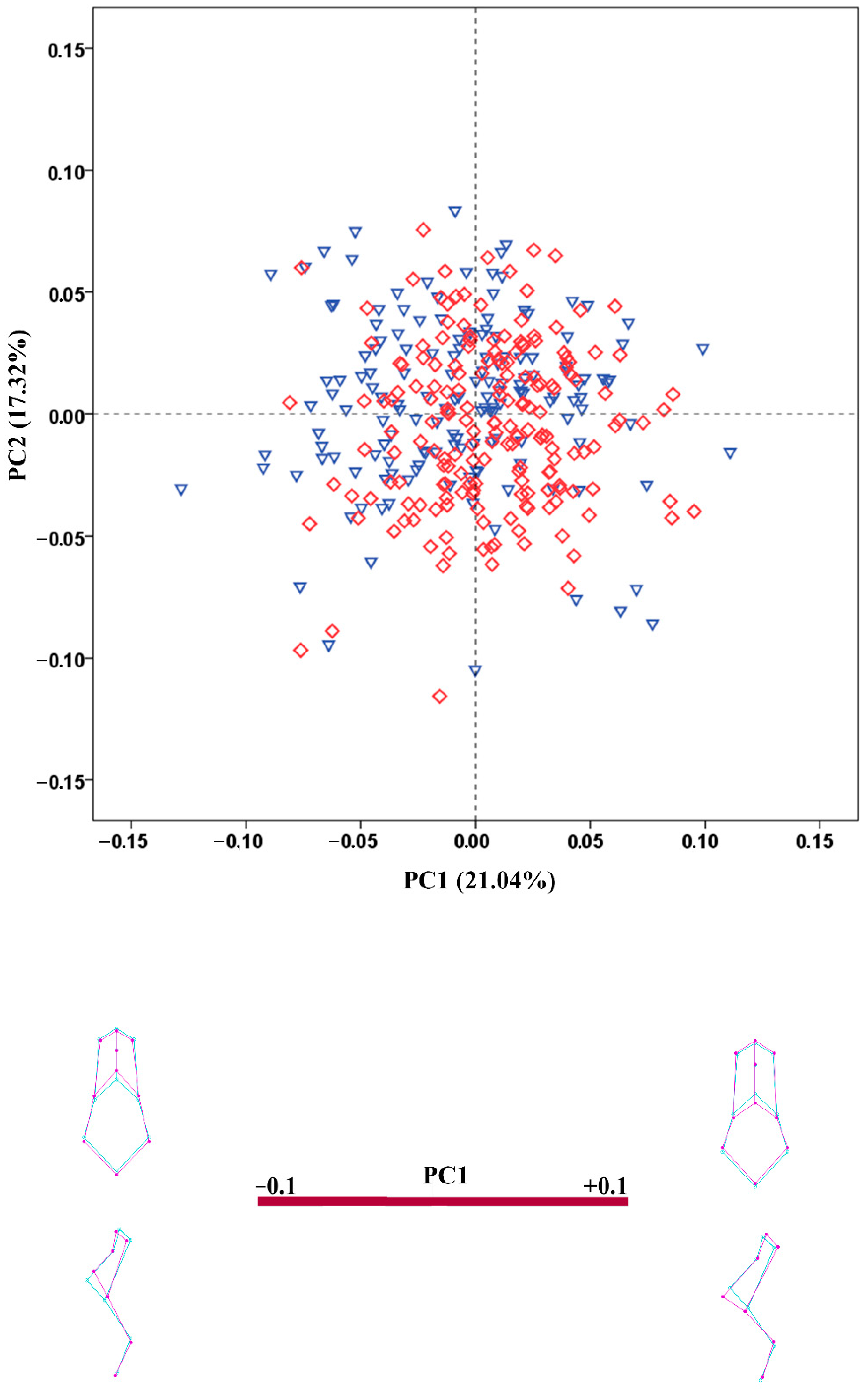
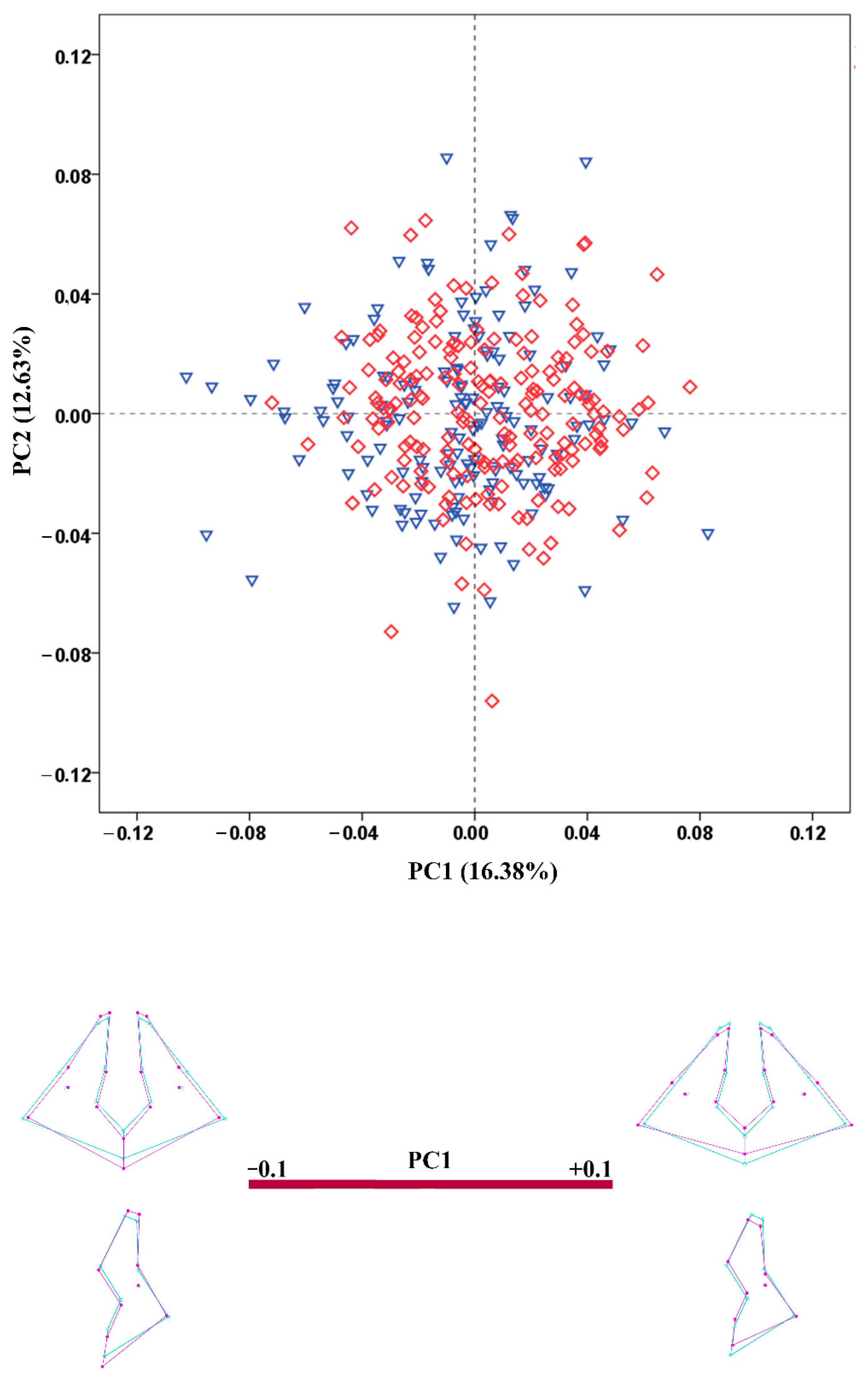
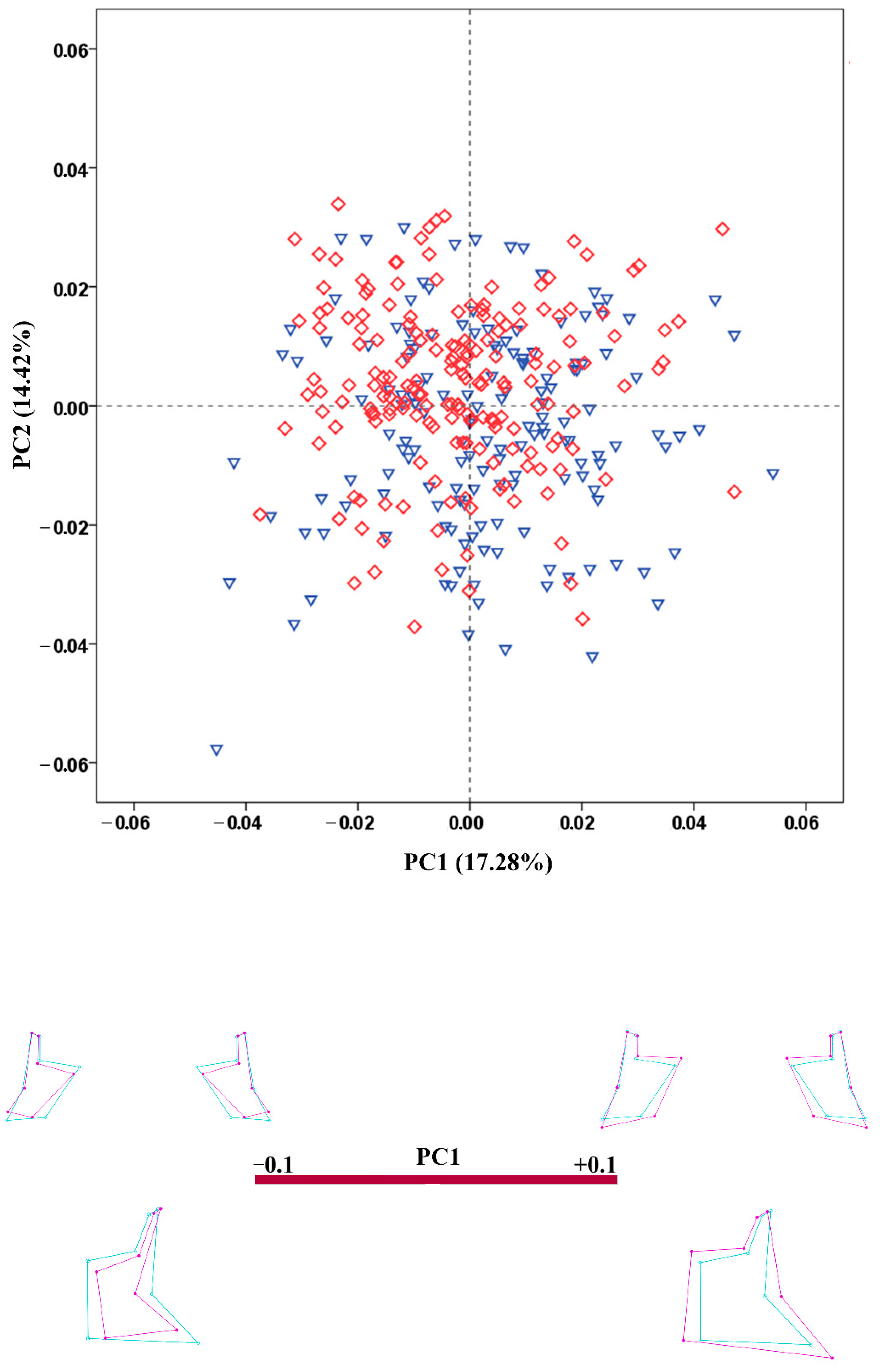
3.4. Shape
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spradley, M.K.; Jantz, R.L. Sex estimation in forensic anthropology: Skull versus postcranial elements. J. Forensic Sci. 2011, 56, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Frayer, D.W.; Wolpoff, M.H. Sexual Dimorphism. Annu. Rev. Anthropol. 1985, 14, 429–473. [Google Scholar] [CrossRef]
- Stinson, S. Sex differences in environmental sensitivity during growth and development. Am. J. Phys. Anthropol. 1985, 28, 123–147. [Google Scholar] [CrossRef]
- Jantz, L.M. The meaning and consequences of morphological variation. In Proceedings of the American Anthropological Association (AAA) Annual Meeting, Chicago, IL, USA, 21 November 2003; American Anthropological Association: Arlington, VA, USA, 2004; pp. 1–17. [Google Scholar]
- Zavando, M.D.A.; Suazo, G.I.C.; Smith, R.L. Sexual dimorphism determination from the lineal dimensions of skulls. Int. J. Morphol. 2009, 27, 133–137. [Google Scholar] [CrossRef]
- Best, K.C.; Garvin, H.M.; Cabo, L.L. An investigation into the relationship between human cranial and pelvic sexual dimorphism. J. Forensic Sci. 2017, 63, 990–1000. [Google Scholar] [CrossRef]
- Dillon, A. Cranial Sexual Dimorphism and the Population Specificity of Anthropological Standards. Master’s Thesis, University of Western Australia, Perth, Australia, 2014. [Google Scholar]
- Hall, R.L. Energetics of nose and mouth breathing, body size, body composition, and nose volume in young adult males and females. Am. J. Hum. Biol. 2005, 17, 321–330. [Google Scholar] [CrossRef]
- Bastir, M.; Godoy, P.; Rosas, A. Common features of sexual dimorphism in the cranial airways of different human populations. Am. J. Phys. Anthropol. 2011, 146, 414–422. [Google Scholar] [CrossRef]
- Harvati, K.; Weaver, T.D. Human cranial anatomy and the differential preservation of population history and climate signatures. Anat. Rec. Part A 2006, 288, 1225–1233. [Google Scholar] [CrossRef]
- Lin, C.; Jiao, B.; Liu, S.; Guan, F.; Chung, N.E.; Han, S.H.; Lee, U.Y. Sex determination from the mandibular ramus flexure of Koreans by discrimination function analysis using three-dimensional mandible models. Forensic Sci. Int. 2014, 236, 191.e1–191.e6. [Google Scholar] [CrossRef]
- Nikita, E.; Michopoulou, E. A quantitative approach for sex estimation based on cranial morphology. Am. J. Phys. Anthropol. 2018, 165, 507–517. [Google Scholar] [CrossRef]
- Giles, E.; Elliot, O. Sex determination by discriminant function analysis of crania. Am. J. Phys. Anthropol. 1963, 21, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Steyn, M.; Iscan, M.Y. Sexual dimorphism in the crania and mandibles of South African whites. Forensic Sci. Int. 1998, 98, 9–16. [Google Scholar] [CrossRef]
- Kranioti, E.F.; Işcan, M.Y.; Michalodimitrakis, M. Craniometric analysis of the modern Cretan population. Forensic Sci. Int. 2008, 180, 110.e1–110.e5. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Imaizumi, K.; Miyasaka, S.; Yoshino, M. Discriminant functions for sex estimation of modern Japanese skulls. J. Forensic Leg. Med. 2013, 20, 234–238. [Google Scholar] [CrossRef]
- Franklin, D.; Freedman, L.; Milne, N. Sexual dimorphism and discriminant function sexing in indigenous South African crania. Homo 2005, 55, 213–228. [Google Scholar] [CrossRef]
- von Cramon-Taubadel, N. Evolutionary insights into global patterns of human cranial diversity: Population history, climatic and dietary effects. J. Anthropol. Sci. 2014, 92, 43–77. [Google Scholar] [CrossRef] [PubMed]
- Cabo, L.; Brewster, C.; Azpiazu, J. Sexual dimorphism: Interpreting sex markers. In A Companian to Forensic Anthropology; Dirkmaat, D., Ed.; Wiley-Blackwell: Chichester, UK, 2012; pp. 248–286. [Google Scholar]
- Franklin, D.; Cardini, A.; Flavel, A.; Kuliukas, A. The application of traditional and geometric morphometric analyses for forensic quantification of sexual dimorphism: Preliminary investigations in a Western Australian population. Int. J. Leg. Med. 2012, 126, 549–558. [Google Scholar] [CrossRef]
- Franklin, D.; Cardini, A.; Flavel, A.; Kuliukas, A. Estimation of sex from cranial measurements in a Western Australian population. Forensic Sci. Int. 2013, 229, 158e1–158e8. [Google Scholar] [CrossRef]
- Ekizoglu, O.; Hocaoglu, E.; Inci, E.; Can, I.O.; Solmaz, D.; Aksoy, S.; Buran, C.F.; Sayin, I. Assessment of sex in a modern Turkish population using cranial anthropometric parameters. Leg. Med. 2016, 21, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Alias, A.; Nor, F.M.; Swarhib, M.; Bakar, A.N.; Das, S. Study of sexual dimorphism of Malaysian crania: An important step in identification of the skeletal remains. Anat. Cell Biol. 2017, 50, 86–92. [Google Scholar] [CrossRef]
- Zaafrane, M.; Ben, K.M.; Naccache, I.; Ezzedine, E.; Savall, F.; Telmon, N.; Mnif, N.; Hamdoun, M. Sex determination of a Tunisian population by, C.T.scan analysis of the skull. Int. J. Leg. Med. 2017, 132, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Bejdová, Š.; Dupej, J.; Krajíček, V.; Velemínská, J.; Velemínský, P. Stability of upper face sexual dimorphism in central European populations (Czech Republic) during the modern age. Int. J. Leg. Med. 2018, 132, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Geng, G.; Yang, W. Sex determination of 3D skull based on a novel unsupervised learning method. Comput. Math. Methods Med. 2018, 2018, 4567267. [Google Scholar] [CrossRef] [PubMed]
- Bewes, J.; Low, A.; Morphett, A.; Pate, F.; Henneberg, M. Artificial intelligence for sex determination of skeletal remains: Application of a deep learning artificial neural network to human skulls. J. Forensic Leg. Med. 2019, 62, 40–43. [Google Scholar] [CrossRef]
- Cechová, M.; Dupej, J.; Bružek, J.; Bejdová, Š.; Horák, M.; Velemínská, J. Sex estimation using external morphology of the frontal bone and frontal sinuses in a contemporary Czech population. Int. J. Leg. Med. 2019, 133, 1285–1294. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Wang, K.; Hu, J.; Geng, G.; Feng, J. Sex determination of three-dimensional skull based on improved backpropagation neural network. Comput. Math. Methods Med. 2019, 2019, 9163547–9163548. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Zhang, P.; Geng, G.; Liu, X.; Zhang, H. Skull sex estimation based on wavelet transform and Fourier transform. Biomed. Res. Int. 2020, 2020, 8608209–8608210. [Google Scholar] [CrossRef]
- Inoue, M.; Inoue, T.; Fushimi, Y.; Okada, K. Sex determination by discriminant function analysis of lateral cranial form. Forensic Sci. Int. 1992, 57, 109–117. [Google Scholar] [CrossRef]
- Isçan, M.Y.; Yoshino, M.; Kato, S. Sexual dimorphism in modem Japanese crania. Am. J. Hum. Biol. 1995, 7, 459–464. [Google Scholar] [CrossRef]
- Dayal, M.R.; Spocter, M.A.; Bidmos, M.A. An assessment of sex using the skull of black south Africans by discriminant function analysis. Homo 2008, 59, 209–221. [Google Scholar] [CrossRef]
- Saini, V.; Srivastava, R.; Rai, R.K.; Shamal, S.N.; Singh, T.B.; Tripathi, S.K. An osteometric study of northern Indian populations for sexual dimorphism in craniofacial region. J. Forensic Sci. 2011, 56, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.; Panaitescu, V.; Rosu, M.; Maru, N.; Punga, A. Sexual dimorphism of crania in a Romanian population: Discriminant function analysis approach for sex estimation. Rom. J. Leg. Med. 2014, 22, 21–26. [Google Scholar] [CrossRef]
- Bertsatos, A.; Papageorgopoulou, C.; Yalakos, E.; Chovalopoulou, M. Investigating the sex-related geometric variation of the human cranium. Int. J. Leg. Med. 2018, 132, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Isaza, J.; Dıaz, C.A.; Bedoya, J.F.; Monsalve, T.; Botella, M.C. Assessment of sex from endocranial cavity using volume-rendered, C.T.scans in a sample from Medellín, Colombia. Forensic Sci. Int. 2014, 234, 186.e1–186.e10. [Google Scholar] [CrossRef] [PubMed]
- Chovalopoulou, M.E.; Bertsatos, A.; Manolis, S.K. Landmark based sex discrimination on the crania of archaeological Greek populations. A comparative study based on the cranial sexual dimorphism of a modern Greek population. Mediter. Archaeol. Archaeom. 2017, 17, 37–46. [Google Scholar]
- Santos, F.; Guyomarc’h, P.; Bruzek, J. Statistical sex determination from craniometrics: Comparison of linear discriminant analysis, logistic regression, and support vector machines. Forensic Sci. Int. 2014, 245, 204.e1–204.e8. [Google Scholar] [CrossRef]
- Musilová, B.; Dupej, J.; Velemínská, J.; Chaumoitre, K.; Bruzek, J. Exocranial surfaces for sex assessment of the human cranium. Forensic Sci. Int. 2016, 269, 70–77. [Google Scholar] [CrossRef]
- Toneva, D.; Nikolova, S.; Agre, G.; Zlatareva, D.; Hadjidekov, V.; Lazarov, N. Machine learning approaches for sex estimation using cranial measurements. Int. J. Leg. Med. 2021, 135, 951–966. [Google Scholar] [CrossRef]
- Yang, W.; Reziwanguli, X.; Xu, J.; Wang, P.; Hu, J.; Liu, X. Sex determination of skull based on fuzzy decision tree. Proceedings of the 4th Workshop on Advanced Research and Technology in Industry (WARTIA 2018). Adv. Eng. Res. 2018, 173, 14–20. [Google Scholar] [CrossRef][Green Version]
- Toneva, D.H.; Nikolova, S.Y.; Agre, G.P.; Zlatareva, D.K.; Hadjidekov, V.G.; Lazarov, N.E. Data mining for sex estimation based on cranial measurements. Forensic Sci. Int. 2020, 315, 110441. [Google Scholar] [CrossRef]
- Sierp, I.; Henneberg, M. The Difficulty of Sexing Skeletons from Unknown Populations. J. Anthropol. 2015, 2015, 908535. [Google Scholar] [CrossRef]
- Spiegel, J.H. Facial determinants of female gender and feminizing forehead cranioplasty. Laryngoscope 2011, 121, 250–261. [Google Scholar] [CrossRef]
- Carlson, B. Human Embryology and Developmental Biology, 5th ed.; Elsevier: Philadelphia, PA, USA, 2014. [Google Scholar]
- Scheuer, L.; Black, S. Developmental Juvenile Osteology; Elsevier: Amsterdam, The Netherlands; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Lieberman, D.E.; Krovitz, G.E.; McBratney-Owen, B. Testing hypotheses about tinkering in the fossil record: The case of the human skull. J. Exp. Zool. B Mol. Dev. Evol. 2004, 302, 284–301. [Google Scholar] [CrossRef] [PubMed]
- Lacruz, R.S.; Stringer, C.B.; Kimbel, W.H.; Wood, B.; Harvati, K.; O’Higgins, P.; Bromage, T.G.; Arsuaga, J.L. The evolutionary history of the human face. Nat. Ecol. Evol. 2019, 3, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.M.E. Morphometrics of the Juvenile Skull: Analysis of Ontogenetic Growth Patterns. Master’s thesis, University of Western Australia, Perth, Australia, 2015. [Google Scholar]
- Baer, M.J.; Harris, J.E. A commentary on the growth of the human brain and skull. Am. J. Phys. Anthropol. 1969, 30, 39–44. [Google Scholar] [CrossRef]
- Vidarsdottir, U.S.; O’Higgins, P.; Stringer, C. A geometric morphometrics study of regional differences in the ontogeny of the modern human facial skeleton. J. Anat. 2002, 201, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Bastir, M.; Rosas, A.; O’Higgins, P. Craniofacial levels and the morphological maturation of the human skull. J. Anat. 2006, 209, 637–654. [Google Scholar] [CrossRef]
- Rosas, A.; Bastir, M. Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am. J. Phys. Anthropol. 2002, 117, 236–245. [Google Scholar] [CrossRef]
- Green, H.; Curnoe, D. Sexual dimorphism in Southeast Asian crania: A geometric morphometric approach. Homo 2009, 60, 517–534. [Google Scholar] [CrossRef]
- Bigoni, L.; Velemínská, J.; Bružek, J. Three-dimensional geometric morphometric analysis of cranio-facial sexual dimorphism in a Central European sample of known sex. Homo 2010, 61, 16–32. [Google Scholar] [CrossRef]
- Chovalopoulou, M.E.; Bertsatos, A. Exploring the shape variation of the human cranium. A geometric morphometrics study on a modern Greek population sample. In Geometric Morphometrics. Trends in Biology, Paleobiology and Archaeology; Rissech, C., Lloveras, L., Nadal, J., Fullola, J.M., Eds.; Monografies del, S.ERP; Universitat de Barcelona: Barcelona, Spain, 2018; pp. 25–39. [Google Scholar]
- Milella, M.; Franklin, D.; Belcastro, M.G.; Cardini, A. Sexual differences in human cranial morphology: Is one sex more variable or one region more dimorphic? Anat. Rec. 2021, 304, 2789–2810. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Steyn, M.; Scholtz, Y. Investigation into the usability of geometric morphometric analysis in assessment of sexual dimorphism. Am. J. Phys. Anthropol. 2006, 129, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.L. Determination of human remains through cranial morphology. J. Forensic Sci. 2005, 50, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An open-source mesh processing tool. In Proceedings of the Sixth Eurographics Italian Chapter Conference, Salerno, Italy, 2–4 July 2008. [Google Scholar]
- Martin, R.; Sal1er, K. Lehrbuch Der Anthropologie in Systematicsher Darstellung. Band I; Gustav Fischer Verlag: Stuttgart, Germany, 1957. [Google Scholar]
- Alekseev, V.P.; Debets, G.F. Craniometry: Methods of Anthropological Study; Nauka: Moscow, Russia, 1964. (In Russia) [Google Scholar]
- Howells, W.W. Cranial Variation in Man: A Study by Multivariate Analysis of Patterns of Difference among Recent Human Populations. Pap. Peabody Mus. Archaeol. Ethnol. 1973, 67, 1–259. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, K.-H. Practical Statistical Power Analysis Using Webpower and R; ISDSA Press: Granger, IN, USA, 2018; Available online: https://webpower.psychstat.org/models/kurtosis/ (accessed on 1 December 2021).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9–18. [Google Scholar]
- von Cramon-Taubadel, N.; Frazier, B.C.; Lahr, M.M. The problem of assessing landmark error in geometric morphometrics: Theory, methods, and modifications. Am. J. Phys. Anthropol. 2007, 134, 24–35. [Google Scholar] [CrossRef]
- Weisensee, K.E.; Jantz, R.L. Secular changes in craniofacial morphology of the Portuguese using geometric morphometrics. Am. J. Phys. Anthropol. 2011, 145, 548–559. [Google Scholar] [CrossRef]
- Abdel Fatah, E.E.; Shirley, N.R.; Jantz, R.L.; Mahfouz, M.R. Improving sex estimation from crania using a novel three-dimensional quantitative method. J. Forensic Sci. 2014, 59, 590–600. [Google Scholar] [CrossRef]
- Bulygina, E.; Mitteroecker, P.; Aiello, L. Ontogeny of facial dimorphism and patterns of individual development within one human population. Am. J. Phys. Anthropol. 2006, 131, 432–443. [Google Scholar] [CrossRef]
- Vidarsdottir, U.S. Changes in the Form of the Facial Skeleton during Growth: A Comparative Morphometric Study of Modern Humans and Neanderthals. Ph.D. Thesis, University of London, London, UK, 1999. [Google Scholar]
- Katsube, M.; Yamada, S.; Utsunomiya, N.; Yamaguchi, Y.; Takakuwa, T.; Yamamoto, A.; Imai, H.; Saito, A.; Vora, S.; Morimoto, N. A 3D analysis of growth trajectory and integration during early human prenatal facial growth. Sci. Rep. 2021, 11, 6867. [Google Scholar] [CrossRef] [PubMed]
- Kimmerle, E.H.; Ross, A.; Slice, D.E. Sexual dimorphism in America: Geometric morphometric analysis of the craniofacial region. J. Forensic Sci. 2008, 53, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Lestrel, P.E.; Kanazawa, E.; Wolfe, C.A. Sexual dimorphism using elliptical Fourier analysis: Shape differences in the craniofacial complex. Anthropol. Sci. 2011, 119, 213–229. [Google Scholar] [CrossRef]
- Hennessy, R.J.; Kinsella, A.; Waddington, J.L. 3D laser surface scanning and geometric morphometric analysis of craniofacial shape as an index of cerebro-craniofacial morphogenesis: Initial application to sexual dimorphism. Biol. Psychiatry 2002, 51, 507–514. [Google Scholar] [CrossRef]
- Toneva, D.H.; Nikolova, S.Y.; Tasheva-Terzieva, E.D.; Zlatareva, D.K.; Lazarov, N.E. Sexual dimorphism in shape and size of the neurocranium. Int. J. Leg. Med. 2022; Ahead of print. [Google Scholar] [CrossRef]
- Cunningham, C.; Scheuer, L.; Black, S. Developmental Juvenile Osteology, 2nd ed.; Academic Press: London, UK, 2016. [Google Scholar]
- Kaya, A.; Uygun, S.; Eraslan, C.; Akar, G.C.; Kocak, A.; Aktas, E.; Govsa, F. Sex estimation: 3D CTA-scan based on orbital measurements in Turkish population. Rom. J. Leg. Med. 2014, 22, 257–262. [Google Scholar] [CrossRef]
- Walsh, J.S. Normal bone physiology, remodeling and its hormonal regulation. Surgery 2017, 36, 1–6. [Google Scholar]
- Gonzalez, P.N.; Bernal, V.; Perez, S.I. Analysis of sexual dimorphism of craniofacial traits using geometric morphometric techniques. Int. J. Osteoarchaeol. 2011, 21, 82–91. [Google Scholar] [CrossRef]
- Chovalopoulou, M.E.; Valakos, E.D.; Manolis, S.K. Sex determination by three-dimensional geometric morphometrics of the vault and midsagittal curve of the neurocranium in a modern Greek population sample. Homo 2016, 67, 173–187. [Google Scholar] [CrossRef]
- Manthey, L.; Jantz, R.L.; Bohnert, M.; Jellinghaus, K. Secular change of sexually dimorphic cranial variables in Euro-Americans and Germans. Int. J. Leg. Med. 2017, 131, 1113–1118. [Google Scholar] [CrossRef]
- Malina, R.M. Secular changes in growth, maturation, and physical performance. Exerc. Sport Sci. Rev. 1978, 6, 203–255. [Google Scholar]
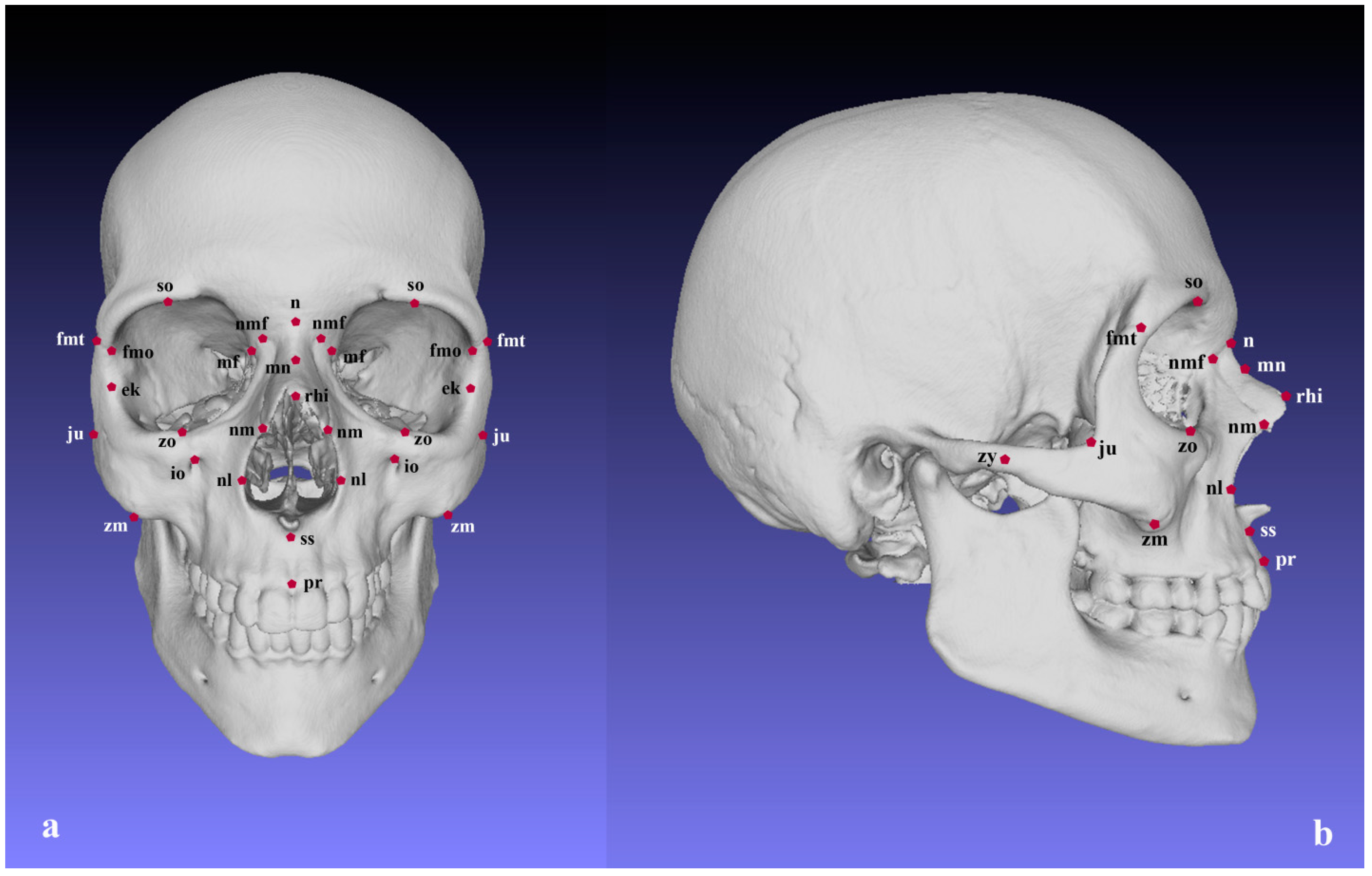
| Landmarks | Description | Configurations * | |
|---|---|---|---|
| 1 | Nasion (n) | The point of intersection of the nasofrontal suture and the midsagittal plane. | V, N |
| 2 | Rhinion (rhi) | The endpoint of the internasal suture at the lower edge of the nasal bones. | V, N |
| 3 | Subspinale (ss) | The deepest midline point below the anterior nasal spine. | V, N, M |
| 4 | Prosthion (pr) | The most anterior midpoint on the alveolar process of maxilla. | V, M |
| 5 | Frontomalare temporale (fmt) | The most lateral point on the frontozygomatic suture. | V, Z |
| 6 | Frontomalare orbitale (fmo) | The point of intersection of the zygomaticofrontal suture and the lateral orbital margin. | V, O, Z |
| 7 | Maxillofrontale (mf) | The point of intersection of the medial orbital margin and the frontomaxillary suture. | V, O, M |
| 8 | Zygomaxillare (zm) | The most inferior point on the zygomaticomaxillary suture. | V, M, Z |
| 9 | Jugale (ju) | The point corresponding to the apex of the angle between the posterior edge of the frontal process and the superior margin of the temporal process of the zygomatic bone. | V, Z |
| 10 | Zygion (zy) | The most lateral point on the zygomatic arch. | V, Z |
| 11 | Ektoconchion (ek) | The point of intersection between the lateral orbital margin and the line originating from maxillofrontale and crossing the orbit parallel to the upper orbital margin. | V, O, Z |
| 12 | Nasolaterale (nl) | The most lateral point on the margin of the piriform aperture. | V, N, M |
| 13 | Zygoorbitale (zo) | The point of intersection of the inferior orbital margin and the zygomaticomaxillary suture. | V, O, M, Z |
| 14 | Supraorbitale (so) | The most superior point on the superior orbital margin. | V, O |
| 15 | Midnasale (mn) | The deepest point on the nasal bones in the midsagittal plane. | V, N |
| 16 | Nasomaxillofrontale (nmf) | The point located on the junction of the frontal, maxillary and nasal bones. | V, N, M |
| 17 | Nasomaxillare (nm) | The most inferior point of the nasomaxillary suture at the intersection with the piriform aperture. | V, N, M |
| 18 | Infraorbitale (io) | The most superior point on the margin of the infraorbital foramen. | V, M |
| * Configurations in which the corresponding landmark is included: V–viscerocranium; O–orbital region; N–nasal region; M–maxillary region; Z–zygomatic region. Landmark definition: 1–10–Martin and Saller [62]; 11–12–Alekseev and Debets [63]; 13–Howells [64]; 14–18–not traditionally defined craniometric landmarks. | |||
| Landmark Configuration | Males | Females | t-Test/U-Test /p-Value/ | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Viscerocranium | 249.52 | 8.32 | 235.10 | 6.91 | U = 2653.00, p ≤ 0.001 |
| Orbital region | 125.00 | 5.02 | 120.62 | 4.17 | t = 8.779, p ≤ 0.001 |
| Nasal region | 67.25 | 3.33 | 61.98 | 2.78 | U = 3083.00, p ≤ 0.001 |
| Maxillary region | 126.59 | 5.04 | 118.51 | 3.93 | U = 3118.00, p ≤ 0.001 |
| Zygomatic region | 208.67 | 7.50 | 197.16 | 6.26 | U = 3426.00, p ≤ 0.001 |
| Landmark Configuration | Males | Females | Total |
|---|---|---|---|
| Viscerocranium | 80.8 | 82.6 | 81.8 |
| Orbital region | 70.5 | 69.6 | 70.0 |
| Nasal region | 79.5 | 84.8 | 82.4 |
| Maxillary region | 77.6 | 84.2 | 81.2 |
| Zygomatic region | 78.2 | 80.4 | 79.4 |
| Landmark Configuration | Males | Females | Total |
|---|---|---|---|
| Viscerocranium (PC1-30) | 66.0 | 66.3 | 66.2 |
| Orbital region (PC1-9) | 69.9 | 67.9 | 68.8 |
| Nasal region (PC1-10) | 63.5 | 65.2 | 64.4 |
| Maxillary region (PC1-16) | 59.0 | 62.5 | 60.9 |
| Zygomatic region (PC1-12) | 66.0 | 65.8 | 65.9 |
| Landmark Configuration | Males | Females | Total |
|---|---|---|---|
| Viscerocranium (PC1-27) | 92.9 | 92.9 | 92.9 |
| Orbital region (PC1-9) | 71.8 | 78.8 | 75.6 |
| Nasal region (PC1-10) | 87.8 | 86.4 | 87.1 |
| Maxillary region (PC1-16) | 82.1 | 89.1 | 85.9 |
| Zygomatic region (PC1-11) | 86.5 | 89.7 | 88.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toneva, D.; Nikolova, S.; Tasheva-Terzieva, E.; Zlatareva, D.; Lazarov, N. A Geometric Morphometric Study on Sexual Dimorphism in Viscerocranium. Biology 2022, 11, 1333. https://doi.org/10.3390/biology11091333
Toneva D, Nikolova S, Tasheva-Terzieva E, Zlatareva D, Lazarov N. A Geometric Morphometric Study on Sexual Dimorphism in Viscerocranium. Biology. 2022; 11(9):1333. https://doi.org/10.3390/biology11091333
Chicago/Turabian StyleToneva, Diana, Silviya Nikolova, Elena Tasheva-Terzieva, Dora Zlatareva, and Nikolai Lazarov. 2022. "A Geometric Morphometric Study on Sexual Dimorphism in Viscerocranium" Biology 11, no. 9: 1333. https://doi.org/10.3390/biology11091333
APA StyleToneva, D., Nikolova, S., Tasheva-Terzieva, E., Zlatareva, D., & Lazarov, N. (2022). A Geometric Morphometric Study on Sexual Dimorphism in Viscerocranium. Biology, 11(9), 1333. https://doi.org/10.3390/biology11091333









