Simple Summary
Bacterial infections are considered one of the main challenges to global health. Bacterial virulence is controlled by interplayed systems to regulate bacterial invasion and infection in host tissues. Quorum sensing (QS) plays a crucial role in regulating virulence factor production, thus could be considered as the bacterial communication system in the bacterial population. The current study aimed to assess the anti-QS and anti-virulence activities of α-adrenoreceptor prazosin against three virulent Gram-negative bacteria. It was demonstrated that prazosin significantly downregulates the expression of QS-encoding genes and shows considered ability to compete on QS proteins in tested strains. Prazosin can significantly diminish biofilm formation and production of virulent enzymes and mitigate the virulence factors of tested strains. However, more testing is required alongside pharmacological and toxicological studies to assure the potential clinical use of prazosin as an adjuvant anti-QS and anti-virulence agent.
Abstract
Quorum sensing (QS) controls the production of several bacterial virulence factors. There is accumulative evidence to support that targeting QS can ensure a significant diminishing of bacterial virulence. Lessening bacterial virulence has been approved as an efficient strategy to overcome the development of antimicrobial resistance. The current study aimed to assess the anti-QS and anti-virulence activities of α-adrenoreceptor prazosin against three virulent Gram-negative bacteria Pseudomonades aeruginosa, Proteus mirabilis, and Serratia marcescens. The evaluation of anti-QS was carried out on a series of in vitro experiments, while the anti-virulence activities of prazosin were tested in an in vivo animal model. The prazosin anti-QS activity was assessed on the production of QS-controlled Chromobacterium violaceum pigment violacein and the expression of QS-encoding genes in P. aeruginosa. In vitro tests were performed to evaluate the prazosin effects on biofilm formation and production of extracellular enzymes by P. aeruginosa, P. mirabilis, and S. marcescens. A protective assay was conducted to evaluate the in vivo anti-virulence activity of prazosin against P. aeruginosa, P. mirabilis, and S. marcescens. Moreover, precise in silico molecular docking was performed to test the prazosin affinity to different QS receptors. The results revealed that prazosin significantly decreased the production of violacein and the virulent enzymes, protease and hemolysins, in the tested strains. Prazosin significantly diminished biofilm formation in vitro and bacterial virulence in vivo. The prazosin anti-QS activity was proven by its downregulation of QS-encoding genes and its obvious binding affinity to QS receptors. In conclusion, prazosin could be considered an efficient anti-virulence agent to be used as an adjuvant to antibiotics, however, it requires further pharmacological evaluations prior to clinical application.
1. Introduction
Bacterial infections constitute one of the major challenges to global health, despite the huge achievements in diagnosis and treatments [1]. What makes it worse, is the ability of bacteria to horizontally gain virulence genes from the same species or even from different species [2,3]. The bacterial virulence expands to include specific bacterial structures such as capsules, pili, and flagella, and production of virulent agents such as enzymes, dyes, and others [4,5], in addition to the formation of biofilms [6,7]. Virulence factors play a key role in the establishment of bacterial infection, guarantee bacterial spreading, escape from the immune system, and even enhance the resistance to antibiotics [6,8]. Bacterial virulence is controlled by interplayed systems to regulate bacterial invasion and accommodation in the host tissues [3,9]. Quorum sensing (QS) plays a crucial role in regulating virulence factor production. Simply, QS can be considered as the bacterial communication system in the bacterial population. Specific QS receptors can sense their cognate inducers that are produced by the same bacterial species or even other species to alter the expression of virulence factors [10,11]. Both Gram-positive and Gram-negative bacteria utilize QS in controlling the virulence, despite their utilization of different inducers and different QS machinery [12,13]. The development of a wide range of virulence factors, such as biofilm formation, bacterial motility, and the synthesis of enzymes including urease, elastase, protease, hemolysins, and other virulent factors, is orchestrated by QS [10,14].
Bacterial development to antimicrobial resistance is an additional complication that worsens bacterial infections and makes treatment difficult, especially in severe infections [1]. Bacteria has the ability to continuously develop resistance to almost all known antibiotics [15]. In the absence of new antibiotics, the innovation of new approaches to fight bacterial resistance is urgently required [16]. One of the innovative approaches is to mitigate bacterial virulence that facilitates the task of the immune system in the eradication of infecting bacteria without affecting bacterial growth so they are not stressed to develop resistance [17,18]. In the light of understanding the QS roles in controlling virulence, targeting QS affords curtailing bacterial virulence [14]. In this context, several chemical moieties and natural products were analyzed for their anti-QS activities that have been proven to have significant effects on bacterial virulence [19,20,21,22,23].
Furthermore, repurposing approved safe drugs was tested as showing efficient anti-QS and anti-virulence agents [24,25,26]. Drug repurposing is a favored strategy because of several advantages, mostly saving time and costs [17].
In a leading study [26], the most clinically prescribed α-adrenoreceptor blockers were in silico screened for their anti-QS activities. The study showed that terazosin and prazosin had the most significant ability to interfere with several QS receptors [26]. Furthermore, terazosin showed significant anti-virulence activities and diminished the virulence of Pseudomonas aeruginosa [26] and Salmonella Typhimurium [9]. These findings encouraged us to assess the anti-QS and anti-virulence activities of prazosin on Gram-negative bacteria while Chromobacterium violaceum as well as, Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens were chosen due to their famed involvement in aggressive infections and significant multidrug resistance profiles. Prazosin works by relaxing the blood vessels so that blood can flow more easily through the body while it is mainly used in treatment of high blood pressure. Prazosin is also useful in treating urinary hesitancy associated with benign prostatic hyperplasia and also effective in improving sleep quality and treating nightmares related to post-traumatic stress disorder [27]. The anti-QS activity of prazosin and its effect on the expression of QS-encoding genes were also evaluated in silico. Moreover, the anti-biofilm and anti-virulence activities of prazosin were evaluated in vitro and in vivo.
2. Materials and Methods
2.1. Chemicals, Microbiological Media, and Bacterial Strains
Prazosin (CAS number: 19237-84-4) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All media were ordered from Oxoid (Hampshire, UK). The chemicals were of pharmaceutical grade. The bacterial strains Chromobacterium violaceum CV026 (ATCC 31532), and Pseudomonas aeruginosa PAO1 (ATCC BAA-47-B1), as well as clinical isolates Serratia marcescens [21] and Proteus mirabilis [28] were fully characterized earlier.
2.2. Determination of Minimum Inhibitory Concentration (MIC) and Effect on Bacterial Growth
The Clinical Laboratory and Standards Institute Guidelines were followed in determining the MICs of prazosin against the tested strains using the broth microdilution technique. The effect of prazosin at sub-MIC (1/4 MIC) on bacterial growth was examined as described earlier [9]. Briefly, the viable count of bacterial cultures with or without prazosin at 1/4 MIC was performed at different time points 4, 16, and 24 h.
2.3. Estimation of C. violaceum Violacein Production
To examine the prazosin anti-QS activity, the effect of prazosin at sub-MIC on the QS-controlled C. violaceum violacein. Luria-Bertani (LB) agar plates provided with the autoinducer N-hexanoyl homoserine lactone (C6HSL) were seeded with C. violaceum. Prazosin at sub-MIC was added to wells made in the agar plates. The white or cream color formed around the well indicated QS inhibition, while a clear halo indicated antimicrobial activity [29].
The prazosin effect on the production of violacein was quantified as previously described [9,30]. Briefly, LB broth aliquots provided with C6HSL in the absence or presence of prazosin at-sub-MIC were added to equal volumes of C. violaceum suspensions (O.D600 1) at room temperature for 24 h. The violacein pigment was extracted with dimethyl sulfoxide (DMSO) and the absorbances were measured at 590 nm.
2.4. Anti-Biofilm Activity Evaluation
Strong biofilm-forming bacterial strains P. aeruginosa, P. mirabilis, and S. marcescens [18] were used to evaluate the prazosin anti-biofilm activity. The crystal violet method was employed to evaluate anti-biofilm activity, and the absorbances of extracted crystal violet at 600 nm [31]. Briefly, bacterial suspensions of tested strains were prepared from fresh overnight cultures and their optical densities were adjusted to OD600 of 0.4 (1 × 108 CFU/mL). Ten μL aliquots of prepared suspensions were mixed with 1 mL of fresh Tryptic soy broth (TSB) in the presence or absence of prazosin at sub-MIC. TSB aliquots (100 μL) with and without prazosin were transferred into the wells of 96-wells microtiter plate and incubated at 37 °C for 24 h. The planktonic cells were aspirated and the wells washed several times with distilled water and left to air dry. The adhered cells were fixed with methanol for 25 min and stained with crystal violet (1%) for 25 min. The wells were washed, the attached dye eluted by 33% glacial acetic acid, and the absorbance measured. The prazosin anti-biofilm activity was visualized by allowing the formation of bacterial biofilms on cover slips in the presence or absence of prazosin, as described earlier [21]. The same procedure as described above was followed except that the biofilms of the tested strains were formed on glass slides placed in polystyrene petri plates in the presence and absence of prazosin at sub-MIC. Light microscope images were captured for the formed biofilms in the presence or absence of prazosin using a Leica DM750 HD digital microscope (Mannheim, Germany).
2.5. Protease Production Evaluation
The prazosin effect on protease production by the tested strains was evaluated by applying the skim milk agar method as previously mentioned [18]. Briefly, the supernatants from bacterial cultures with or without prazosin at sub-MIC were obtained. The diameters of the clear zones were measured in millimeters after adding 50 µL to the wells made in 5% skim milk agar plates and incubating them for 24 h at 37 °C.
2.6. Hemolysin Production Evaluation
As previously mentioned, prazosin’s anti-hemolytic action was evaluated. Briefly, the supernatants from bacterial cultures grown with or without prazosin at sub-MIC were obtained. Fresh 2% rabbit blood suspensions were combined evenly with the supernatants. The mixtures were centrifuged and the absorbances at 540 nm were measured after a two-hour incubation at 37 °C. Blood suspensions were treated with sodium dodecyl sulfate (SDS) (0.1%) to provide positive and negative controls of hemolyzed blood.
2.7. In Vivo Anti-Virulence Evaluation
The in vivo anti-virulence effect of prazosin was assessed using the mice survival model, as previously described [25,26]. Briefly, sub-MIC (1 × 106 CFU/mL) fresh overnight bacterial cultures in LB broth with or without prazosin in phosphate-buffered saline (PBS) were used. Three-week-old female Mus musculus mice were divided into four groups (n = 10). One positive control group received an intraperitoneal (ip) injection of bacterial cultures not treated with prazosin, two negative control groups received either no injections or injections of sterile PBS, and the test group received an intraperitoneal injection of 100 µL of prazosin-treated bacterial cultures. The Kaplan–Meier method was employed to plot and record the mice survival over 5 days.
2.8. Real-Time Quantitative Reverse Transcription PCR (RTq-PCR) for QS-Encoding Genes
The RNA of prazosin (at sub-MIC) treated or untreated P. aeruginosa was extracted as previously described (Table 1) [26]. The primers used to amplify the QS-encoding genes lasI, lasR, rhlI, rhlR, pqsA, and pqsR, were indicated previously. The extracted RNA was used to synthesize cDNA, and RTq-PCR was performed to evaluate the QS-encoding gene expression, and the relative expression was evaluated by the comparative threshold cycle (2−∆∆Ct) method [32].

Table 1.
Sequences of the used primers in this study.
2.9. In Silico Study
PubChem database (https://pubchem.ncbi.nlm.nih.gov/; accessed on 4 May 2022) was used for retrieving the prazosin SMILES string. Over drug repurposing, the compound went through the complete consequence of the drug development cycle [33]. SWISSADME tool (https://www.expasy.org/resources/swissadme; accessed on 4 May 2022) was used for analysis of the prazosin for molecular properties. The compound energy was minimized to 0.1 Kcal/mol/Å2 gradient RMS on Molecular Operating Environment (MOE 2019.012) for the docking process.
Molecular Operating Environment (MOE) 2019.0102 was used for the molecular docking processes on the targeted bacterial QS proteins. P. aeruginosa QS control repressor (PDB ID: 3SZT) and P. mirabilis adhesion MrpH (PDB ID: 6Y4F) crystal structures were downloaded from the RCSB Protein Data Bank (https://www.rcsb.org/; accessed on 4 May 2022). S. marcescens QS transcriptional regulator SmaR (Uniprot Entry: Q14RS3) has no resolved crystal structure, so a SWISS MODEL (https://www.expasy.org/resources/swiss-model; accessed on 6 May 2022) was used and active site architecture analyzed for its validation. Protein structures were prepared by using the MOE QuickPrep protocol. The active pocket was validated by re-docking of the co-crystallized ligand and measuring the root-mean square deviation (RMSD) values. Prazosin was docked into the protein active site through Alpha triangle placement through Amber10:EHT force-field using two stages; rigid-receptor protocol and induced-fit protocol. Furthermore, the Computed Atlas for Surface Topography of Proteins (CASTp; http://sts.bioe.uic.edu/castp/index.html; accessed on 8 May 2022) server was used for the active pocket prediction [34].
2.10. Statistical Analysis
The findings of the experiments, which were carried out in triplicate, are shown as means ± standard error. Except where otherwise noted, statistical significance was determined by the student’s t-test, with a significance when p value < 0.05 (GraphPad Prism Software, v.8, San Diego, CA, USA).
3. Results
3.1. Determination of Minimum Inhibitory Concentration (MIC) of Prazosin and Its Effect on Bacterial Growth
The prazosin concentrations that inhibited the C. violaceum, P. aeruginosa, P. mirabilis, and S. marcescens growth were 2, 2, 1, and 1 mg/mL, respectively. The anti-QS and anti-virulence effects of prazosin were evaluated at sub-MIC (1/4 MIC) to rule out any impact on bacterial growth. The tested bacterial strains were grown in the presence of prazosin at a sub-MIC (1/4 MIC) level and their growth was compared to that of the strains grown in the absence of prazosin to demonstrate that prazosin had no effect on bacterial growth. The bacterial counts in the presence or absence of prazosin at various time periods did not change significantly (Figure 1).
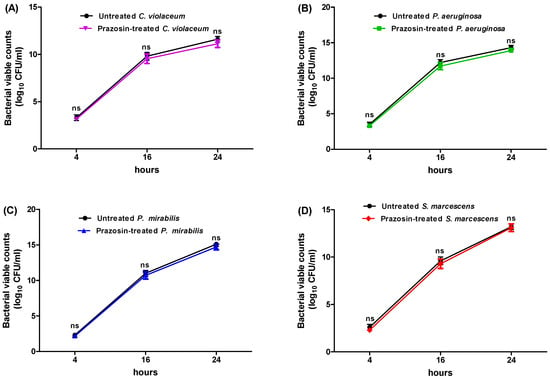
Figure 1.
Effect of prazosin on the growth of (A) C. violaceum, (B) P. aeruginosa, (C) P. mirabilis, and (D) S. marcescens. There were no significant differences between the viable counts of untreated cultures and prazosin-treated cultures. ns: non-significant.
3.2. Prazosin Decreased the Production of QS-Controlled C. violaceum Violacein
Prazosin was examined for its action on the biosensor C. violaceum synthesis of the QS-controlled pigment violacein in order to do a preliminary evaluation of its anti-QS activity. White (creamy) zones were formed around the wells containing prazosin at sub-MIC indicate QS inhibition (Figure 2A). Furthermore, the effect of prazosin at sub-MIC on violacein production was quantified by comparing the pigment production in its presence or absence spectrophotometrically. Prazosin significantly reduced the production of QS-controlled violacein (Figure 2B).
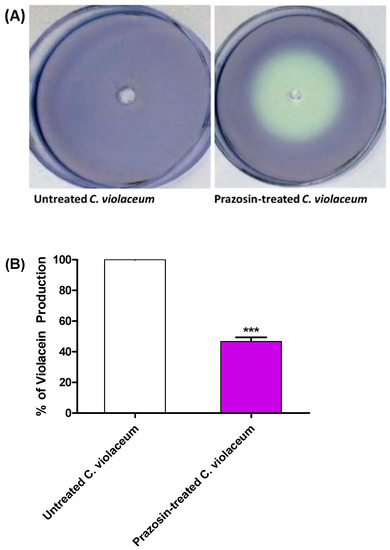
Figure 2.
Prazosin decreased the production of C. violaceum QS-controlled pigment. (A) A white (creamy) zone was formed around the well containing prazosin at sub-MIC, that indicates anti-QS activity. (B) The DMSO extracted violacein was quantified in the cultures treated or not with prazosin at sub-MIC. Prazosin significantly diminished the violacein (***: p ≤ 0.001).
3.3. Prazosin Showed Significant Anti-Biofilm Activities
The production of biofilm in bacterial cultures treated or untreated with prazosin at sub-MIC was evaluated using the crystal violet technique. Interestingly, this work shows that prazosin significantly diminished the biofilm formation by the tested strains (Figure 3).
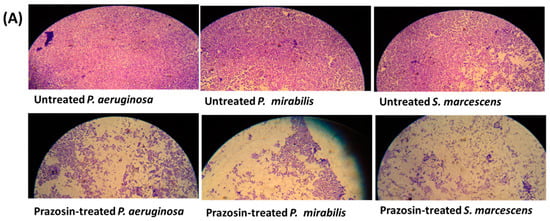
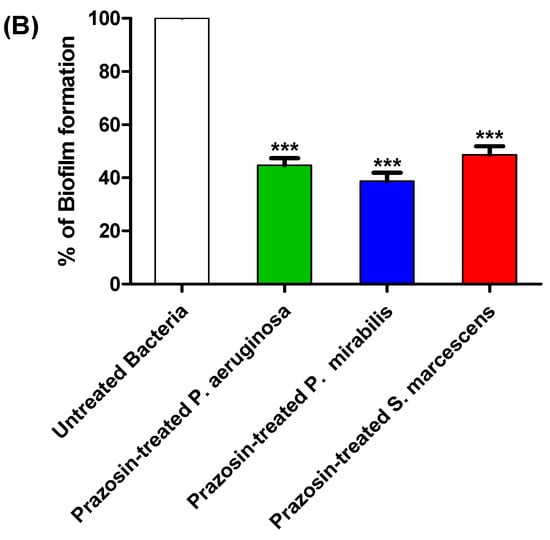
Figure 3.
Prazosin diminished the biofilm formation. (A) Representative microscopic images showed prazosin effect on diminishing the biofilm formation by P. aeruginosa, P. mirabilis, and S. marcescens. (B) The absorbances of crystal violet stained P. aeruginosa, P. mirabilis, and S. marcescens adhered cells. The data are expressed as percentage change from the untreated bacterial strains. Prazosin significantly decreased the biofilm formation (***: p ≤ 0.001).
3.4. Prazosin Decreased the Production of Virulence Factors
The effects of prazosin at sub-MIC on the production of protease and hemolysin by P. aeruginosa, P. mirabilis, and S. marcescens were assayed. Prazosin significantly decreased the production of protease and hemolysin (Figure 4).
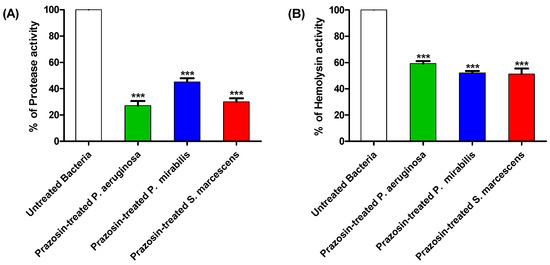
Figure 4.
Prazosin decreased the production of virulence factors. Prazosin significantly decreased the production of (A) protease, and (B) hemolysins (***: p ≤ 0.001). The data are expressed as percentage change from the untreated bacterial strains.
3.5. Prazosin In Vivo Mitigates Bacterial Virulence
The anti-virulence efficacy of prazosin at sub-MIC against P. aeruginosa, P. mirabilis, and S. marcescens was assessed using an in vivo protection assay. All mice survived in the negative control groups injected with sterile PBS or un-injected. On the other hand, only 2 out of 10, 4 out of 10, and 5 out of 10 mice survived when injected with P. aeruginosa, P. mirabilis, and S. marcescens, respectively. Prazosin protected 5 out of 10, 8 out of 10, and 8 out of 10 mice against P. aeruginosa, P. mirabilis, and S. marcescens, respectively. The mice death was recorded by the Kaplan–Meier method and the log-rank test was applied to test the significance. Prazosin significantly reduced the P. aeruginosa, P. mirabilis, and S. marcescens capacity to kill mice, the log rank test for trend (p = 0.0009, =0.0261, and =0.0267, respectively) (Figure 5).
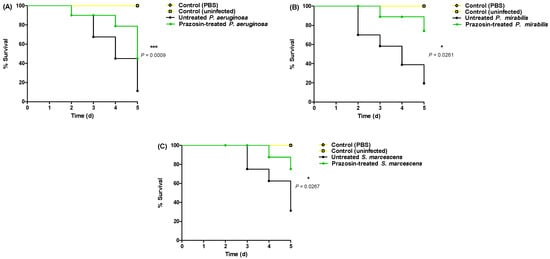
Figure 5.
Prazosin protected mice against virulence of (A) P. aeruginosa, (B) P. mirabilis, and (C) S. marcescens. Prazosin showed significant ability to protect the mice from P. aeruginosa, P. mirabilis, and S. marcescens pathogenesis (the log rank test for trend p = 0.0009, 0.0261, and =0.0267, respectively).
3.6. Prazosin Downregulated the Expression of P. aeruginosa QS-Encoding Genes
In control untreated P. aeruginosa and in P. aeruginosa treated with prazosin (at sub-MIC), the expressions of the P. aeruginosa QS-encoding genes LasI/R, RhlI/R, and PQS were assessed. When compared to the control untreated culture, the expression levels of the genes encoding the autoinducers lasI, rhlI, and pqsA and their receptors lasR, rhlR, and pqsR were markedly downregulated (Figure 6).
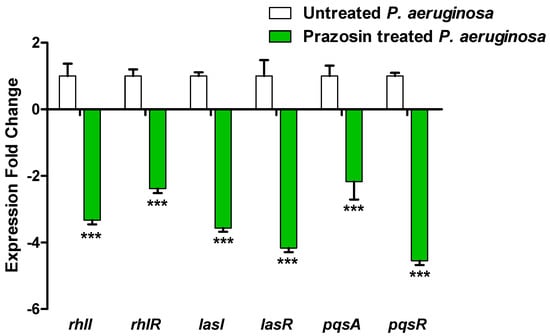
Figure 6.
The QS genes of P. aeruginosa were less expressed when prazosin was present. From cultures that were either treated or not treated with prazosin at sub-MIC, P. aeruginosa RNA was extracted by using RTq-PCR to quantify the expression levels and normalize them to the housekeeping gene ropD using the comparative threshold cycle (2−∆∆Ct) method. The findings were shown as means ± standard error. Prazosin significantly decreased the expressions of all tested genes, ***: p ≤ 0.001.
3.7. Prazosin Binding Affinity to QS Receptors
3.7.1. Prazosin Molecular Descriptors and ADME Properties
Prazosin, as a marketed drug, is a suitable target for repurposing. Figure 7 generated by the SwissADME tool shows that prazosin owes suitable physicochemical properties for oral bioavailability [33].
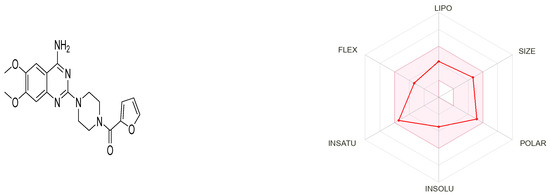
Figure 7.
Physicochemical descriptors of prazosin; LIPO (lipophility): −0.7 < XLOGP3 < +5.0, SIZE: 150 g/mol < MV < 500 g/mol, POLAR (polarity): 20 Å2 < TPSA < 130 Å2, INSOLU (insolubility): −6 < Log S (ESOL) < 0, INSATU (insaturation): 0.25 < Fraction Csp3 < 1, FLEX (flexibility): 0 < Num. rotatable bonds < 9.
The compound possesses molecular weight below 500, hydrogen bond donor < 5, hydrogen bond acceptor < 10, and logP value < 5, rotatable bond count < 10 and topological polar surface area (TPSA) < 140 Ų. Hence, the compound obeys Lipinski’s rule of five and Veber’s rule. The PubChem CID, 3D structures, Lipinski’s rule of five rule, and Veber’s rule are shown in Table 2.

Table 2.
Prazosin molecular descriptors as obtained from PubChem.
3.7.2. Inter-Species Docking Analysis on QS Biotargets
To further investigate the interaction of prazosin with quorum sensing mechanisms, a validated two-stage docking simulation was performed. The first stage is a rigid receptor protocol, and the second stage is an induced-fit protocol. The in silico molecular approach allowed the reliable study of the potential interactions between prazosin with three bacterial targets regulating virulence genes of three different bacteria. The P. aeruginosa quorum-sensing control repressor (PDB ID: 3SZT) and the P. mirabilis adhesin MrpH (PDB ID: 6Y4F), along with a SWISS model for the S. marcescens SmaR (Uniprot ID: Q14RS3) represented the targets for this in silico study.
The P. aeruginosa quorum-sensing transcription factor (QscR) was co-crystallized with N- 3′oxo-octanoyl-L-homoserine lactone (3OC12-HSL, strong activating ligand) as homodimer at 2.55 Å resolution. The general topology of the QscR exhibited an α/β/α sandwich. The ligand-binding domain (LBD) at the N-terminus and the DNA-binding domain (DBD) located at the C-terminus are also connected by a short linker of residues from 165 to 174 [35].
The P. mirabilis adhesin MrpH structure was solved at 1.75 Å. The architecture demonstrated seven β-strands and two α-helices. The N-terminus is located relatively close to the C-terminus. Cys 128 and Cys 152 form a disulfide bond which is important for maintaining structural integrity and function. The protein structure possesses a tetrahedrally coordinated Zn ion bound by three histidines (His 72, His 74, His 117) and by an external ligand [36].
S. marcescens Quorum-sensing transcriptional regulator (SmaR) (Uniprot Entry: Q14RS3, www.uniprot.org; accessed on 4 May 2022) was identified as a target sequence. C. violaceum CviR transcriptional regulator (PDB: 3QP5, 3.25 Å) served as a template [37], as predicted using SWISS model (https://www.expasy.org/resources/swiss-model, accessed on 25 May 2022) [38,39,40,41,42]. C. violaceum CviR transcriptional regulator is the top-ranked template according to the sequence identity from seven suggesting templates. The binding site in the SWISS model was located by the site finder module in the MOE which matched the co-crystallised ligand (HLC) binding site of the template. Moreover, CASTp was used to verify the binding pocket.
The Site Finder module is used to find potential 3D pockets that have potential as active sites for the protein. Figure 8 shows the protein structure and putative pockets of the binding site topology at the five bacterial targets. The calculated Richard’s solvent accessible surface area and volume were estimated as; 381.528 Å2/681.245 Å3, 23.620 Å2/3.608 Å3, and 664.317/358.797 Å2/Å3 for the binding sites of P. aeruginosa QscR (PDB 3SZT), P. mirabilis adhesin MrpH (PDB: 6Y4F), and S. marcescens SmaR model, respectively using the Computed Atlas for Surface Topography of Proteins (CASTp). CASTp is built on recent theoretical and algorithmic results of computational geometry. The pockets and cavities are identified analytically, the boundary between the bulk solvent and the pocket is defined precisely and the parameters are calculated rotationally.
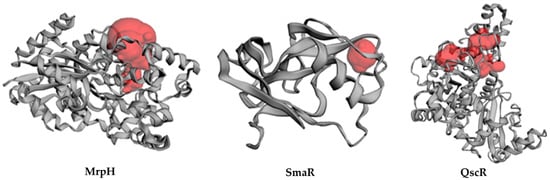
Figure 8.
Cartoon representation of the binding site topology at the three bacterial targets, Putative pockets; red color, were calculated via the on-line Computed Atlas of Surface Topography of proteins (CASTp; http://sts.bioe.uic.edu/castp/index.html, accessed on 30 May 2022).
3.7.3. Docking Simulations on P. aeruginosa QS Control Repressor
Docking simulations of prazosin with QScR (PDB ID: 3SZT) showed comparable results to the docking of the co-crystallized ligand (3OC12-HSL, strong activating ligand) in terms of 3D fitting and filling of the same space in the binding site as shown in Figure 9A, also in terms of hydrophobic interactions with Ser38, Phe54, Tyr58, Tyr66, Val78, and Met127 Figure 9B.
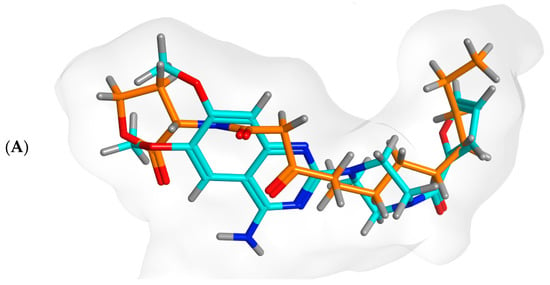
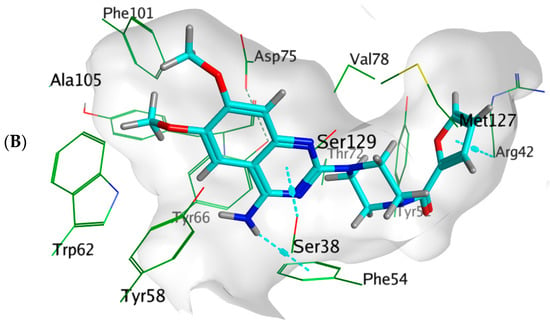
Figure 9.
Arg42 and Phe54. However, the co-crystallized ligand was stabilized with different hydrogen bonds with Tyr58, Trp62, and Asp75. These differential binding modes resulted in a slight difference in score S = −9.9177 and −10.1774 for prazosin and 3OC12-HSL, respectively Table 3.

Table 3.
Docking results for both 3OC12-HSL and prazosin with P. aeruginosa QS control repressor.
Table 3.
Docking results for both 3OC12-HSL and prazosin with P. aeruginosa QS control repressor.
| Ligand | Rigid Receptor Protocol | Induced-Fit Protocol | H-bond Interactions | Hydrophobic Interactions | pi-Interactions | ||
|---|---|---|---|---|---|---|---|
| S score Kcal/mol | RMSD | S score Kcal/mol | RMSD | ||||
| Prazosin | −9.5648 | 1.4357 | −9.9177 | 1.2806 | - | Ser38, Phe54, Tyr58, Tyr66, Val78, Met127 | Ser38(pi-H), Arg42 (pi-H), Phe54(H-pi) |
| 3OC12-HSL | −10.4256 | 1.2495 | −10.1774 | 1.1676 | Tyr58, Trp62, Asp75 | Ser38, Tyr52, Phe54, Tyr58, Tyr66, Val78, Met127 | - |
3.7.4. Docking Simulations on P. Mirabilis Adhesin MrpH
Docking of prazosin with P. mirabilis adhesin MrpH revealed the ability of prazosin to act as the external ligand for Zn+2 binding, which is crucial for biofilm formation, Figure 10. This is in addition to the comparable hydrophobic interactions with glutamate (co-crystallized ligand).
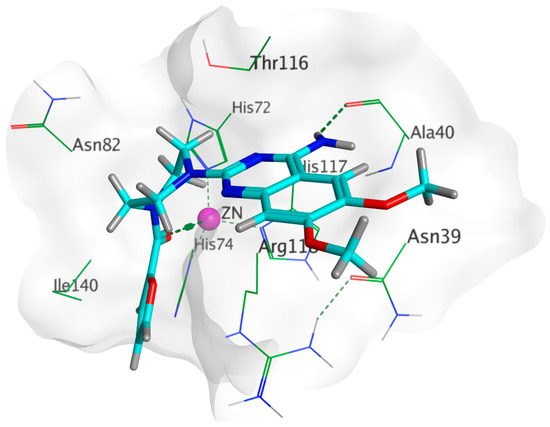
Figure 10.
3D binding of prazosin (Cyan) with MrpH active site showing the key amino acid interactions along with the crucial Zn+2 binding (purple).
The docking results summarized in Table 4 showed the inferiority of prazosin in terms of hydrogen bond formation that was reflected in glutamate having a better score = −8.4332 when compared to −6.0361 achieved by prazosin. However, this may be attributed to the much smaller size of glutamate that enables the molecule to move and bind more freely than a much bigger molecule like prazosin as demonstrated by the superimposition of both molecules in the MrpH pocket Figure 11.

Table 4.
Docking results for both glutamate and prazosin with P. mirabilis adhesin MrpH.
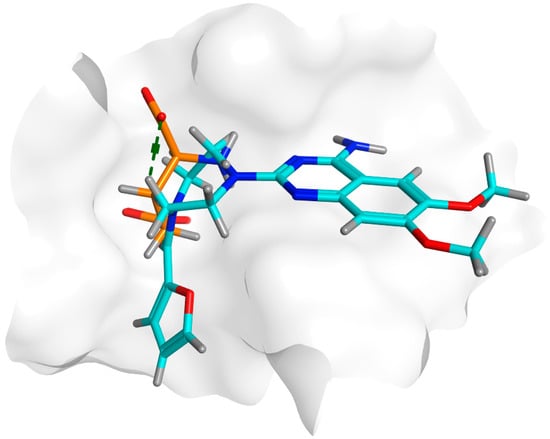
Figure 11.
A: 3D alignment of prazosin (cyan) and glutamate (orange) in the binding pocket of MrpH showing the significant difference in size between both.
3.7.5. Docking Simulations on S. marcescens SmaR Model
4-(4-chlorophenoxy)-N-[(3S)-2-oxotetrahydrofuran-3-yl] butanamide (HLC) as the co-crystallized ligand of the template; C. violaceum CviR transcriptional regulator, was docked in the active site for the SWISS model of S. marcescens SmaR for its validation. Docking HLC identified key amino acid residues with reasonable RMSD (1.3923 Å) and good binding affinity where the S score was −7.1761 Kcal/mol. Carbonyl group forms H- bonding interaction with Trp53 and hydrophobic contacts with Ala32, Phe44, Tyr57, Trp81, Ile105, Val122, Ser124 amino acid residues Figure 12.
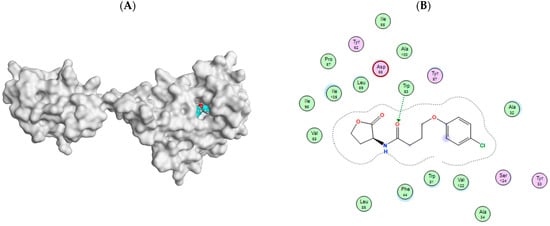
Figure 12.
(A) SWISS model of S. marcescens SmaR with HLC binding site. (B) 2D interaction of HLC with S. marcescens SmaR for active site validation.
Prazosin showed a good ability to bind to S. marcescens SmaR protein, forming two pi-H bond interactions with Leu69 and quinazolinyl moiety in addition to hydrophobic interactions with Ala32, Phe44, Tyr57, Leu69, Trp81, Ile105, Val122, and Ser124 Figure 13. The energy docking score for prazosin and HLC (co-crystallized ligand) Table 5. This indicates the prazosin ability to antagonize receptor function, and this might result in the inhibition of QS and its regulated virulence factors.
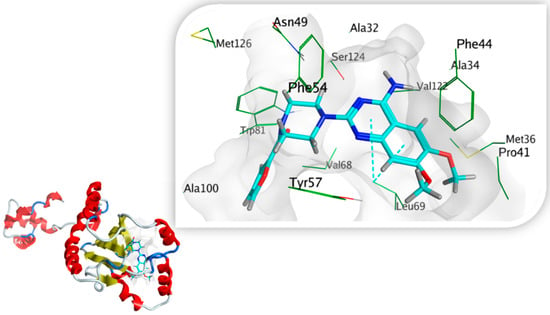
Figure 13.
A 3D prazosin-S. marcescens SmaR model interaction diagram, prazosin is in cyan thick sticks within the molecular surface of the active site, amino acid residues of the active site are shown as green thin sticks. Pi-bond is presented as cyan dots.

Table 5.
Docking results for both HLC and prazosin with S. marcescens SmaR.
4. Discussion
QS amends bacterial virulence via controlling the expression of virulence factors’ encoding genes [25]. Mitigating bacterial virulence controlled by QS is an interesting approach that may be applied to conquer the overstated pathogenesis and resistance development of antibiotics [43]. Despite the considered successes of antibiotics that have been achieved since their first discovery, bacteria have managed to develop resistance to almost all antibiotic classes [1,16]. It is well known that affecting bacterial growth could stress them into developing resistance to the used antibiotics [15]. The main advantage of targeting QS systems is curtailing the bacterial virulence to ease the immune system’s task in facilitating eradication of bacteria without affecting bacterial growth, and hence avoiding stressing bacteria to develop resistance [31,44]. In a previous study, α-adrenoreceptor blockers were in silico screened for their anti-QS activity that sheds light on the considered ability of prazosin to compete on different QS receptors [26].
The main concept of targeting QS is to affect bacterial virulence without affecting growth to avoid resistance development [18,45]. In this context, the prazosin was tested at a sub-MIC concentration to avoid any effect on bacterial growth. Prazosin at sub-MIC did not significantly influence the growth of the tested strains. Our previous in silico findings showed prazosin’s ability to bind to LuxR-type QS receptor C. violaceum CviR (PDB: 3QP5) [26]. C. violaceum is a Gram-negative bacterium and produces its violet pigment violacein in response to QS regulation of violacein-encoding genes vioA-D [30,46]. C. violaceum biosensor strain CV026A is a mutant of the wild type lacking autoinducer synthase and requires exogenous autoinducer (N-acyl homoserine lactones) to release violacein [47]. C. violaceum CV026A confers a suitable tool for screening QS activities, and it has been used routinely to evaluate QS activity in Gram-negative bacteria [9,25,47]. Prazosin at sub-MIC significantly reduced the production of violacein, which indicates the possible prazosin anti-QS activities.
P. aeruginosa is one of the most important human pathogens that can cause serious systemic infections, such as eye, burn, wound, respiratory tract, nosocomial, and blood infections [20]. P. aeruginosa could acquire resistance to nearly all classes of antibiotics which complicates its treatment [48]. P. aeruginosa utilizes several interplayed secretion and QS systems to orchestrate the production of its huge arsenal of virulence factors [30,49]. P. aeruginosa employs mainly three types of QS systems namely Lux-types including RhlI/R and LasI/R, and non-Lux type PQS in addition to Lux analogues QscR [35]. P. mirabilis causes serious infections, such as nosocomial, urinary tract, wound, and burn infections [50]. The P. mirabilis virulence is owing to its inherent capability of peritrichous flagellar motility, formation of biofilm and enzyme production, such as urease, protease, and hemolysins which facilitate the infection spread [28]. P. mirabilis also demonstrates increased multi-drug resistance profiles [50]. S. marcescens is one of the most frequent nosocomial pathogens, causing pneumonia and blood infections [21,51]. S. marcescens QS modulates biofilm formation, swarming and sliding motilities, hemolytic activity, and production of biosurfactant and enzymes such as protease, lipase, nuclease, and chitinase [18,52,53]. Two main QS systems have been identified in Serratia spp. SwrI/R and SmaI/R [53]. It is worthy of mention that both P. mirabilis [28], and S. marcescens used in this study were isolated from macerated diabetic foot wound and endotracheal aspiration, respectively, and demonstrate multi-drug resistant profiles.
Bacteria biofilms are membrane attached communities in which polymer matrixes mainly composed of polysaccharides are produced to hold bacteria together [6,20]. Bacteria resist environmental stress by formation of biofilms in which the bacterial resistance to antibiotics is augmented in comparison to planktonic bacterial cells. So, the bacterial biofilm formation constitutes an additional obstacle, and their eradication from animate or inanimate objects is an essential requirement for efficient antibiotic treatments [6]. QS plays the crucial role in the formation of bacterial biofilms and targeting QS results in diminishing their formation [43]. The tested strains P. aeruginosa, P. mirabilis [28], and S. marcescens [52] were documented as strong-biofilm forming. Interestingly, prazosin significantly diminished the biofilm formation in the three tested strains.
QS modulates the production of diverse virulence extracellular enzymes like lipase, protease, elastase, hemolysins, urease, and other virulence factors [14]. The production of these enzymes facilitates the spread and establishment of bacterial infections into host tissues [21,54,55]. For instance, proteolytic and hemolytic activities significantly enhance bacterial pathogenesis [54,55]. Prazosin significantly decreased P. aeruginosa, P. mirabilis, and S. marcescens production of hemolysins and proteases. In agreement with the in vitro phenotypic prazosin anti-virulence activities; prazosin at sub-MIC showed a significant reduction in the killing capacity of P. aeruginosa, P. mirabilis, and S. marcescens, protecting the tested mice.
Prazosin significantly downregulated the expression of P. aeruginosa QS receptors RhlR, LasR, and PqsR encoding genes and their synthetase encoding genes rhlI, lasR, and pqsA, respectively. Our detailed molecular docking work was performed to evaluate the prazosin binding affinity to the P. aeruginosa QscR, P. mirabilis MrpH, and S. marcescens SmaR. P. aeruginosa QscR is a Lux analogues QS receptor that does not have a partner LuxI homolog but can sense LasI autoinducers [53]. S. marcescens SmaR is Lux type QS receptor that senses C4- and C6-homoserine lactone to control the expression of virulence genes encoding lipase, nuclease, protease, hemolysin, prodigiosin, and biofilm formation [21,53]. Mannose resistant proteus (MRP) like fimbriae are important for biofilm formation, aggregation, and colonization of P. mirabilis, in bladder and kidney [56]. The Zn-dependent receptor-binding domain of P. mirabilis adhesin MrpH plays a key role in adhesion and biofilm formation and is considered one of the QS proteins [28,36]. In agreement with in vitro and in vivo findings, the docking study showed a considered ability of prazosin to compete on different QS proteins showing possible anti-QS activity.
The current findings show the considered prazosin anti-QS and anti-virulence activity that can potentially be used alone or as adjuvant to antibiotics. Moreover, this study hints at the use of prazosin as a pharmacophore to synthesis new active compounds to be used for anti-virulence. Although, this study suggests the anti-virulence activities of prazosin, larger multicenter studies alongside pharmacological, toxicological, and pharmaceutical studies are very much needed to further investigate its potential use in clinical settings.
5. Conclusions
Mitigation of bacterial virulence is a promising approach to overcoming bacterial resistance development. QS controls the regulation of several virulence factors and hence interfering with QS could guarantee diminishing bacterial virulence. The present study demonstrates that prazosin significantly downregulated the QS-encoding genes and shows considered ability to compete on QS proteins in P. aeruginosa, P. mirabilis, and S. marcescens. Prazosin can significantly diminish the formation of biofilm, virulent enzyme production and mitigate the virulence factors of P. aeruginosa, P. mirabilis, and S. marcescens. However, more testing is required alongside pharmacological and toxicological studies to assure the potential clinical use of prazosin as an adjuvant anti-QS and anti-virulence agent.
Author Contributions
Conceptualization, W.A.H.H., A.K.T. and M.A.E.; methodology, A.G.E., S.S.E. and W.A.H.H.; software, T.S.I.; validation, K.E. and A.Z.; formal analysis, T.S.I.; investigation, A.G.E., S.S.E. and W.A.H.H.; resources, A.K.T. and M.A.E.; data curation, K.E. and A.Z.; writing—original draft preparation, A.G.E., S.S.E. and W.A.H.H.; writing—review and editing, A.K.T. and M.A.E.; visualization, K.E. and A.Z.; supervision, A.K.T. and M.A.E.; project administration, A.K.T. and M.A.E.; funding acquisition, A.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFPRC-002-249-2020 and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Institutional Review Board Statement
The animal study protocol was approved by the Faculty of Pharmacy ethical committee, Zagazig University. The experiments were conducted in compliance with the ARRIVE guidelines, in compliance with the U.K. Animals (Scientific Procedures) Act, 1986, and related guidelines (ECAHZU, December 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included in the main text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohr, K.I. History of antibiotics research. Curr. Top. Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, H.; Su, Y.; Liu, S.; Xu, L.; Guo, Z.; Wu, J.; Cheng, C.; Feng, J. Horizontal gene transfer contributes to virulence and antibiotic resistance of Vibrio harveyi 345 based on complete genome sequence analysis. BMC Genom. 2019, 20, 761. [Google Scholar] [CrossRef] [PubMed]
- Diard, M.; Hardt, W.D. Evolution of bacterial virulence. FEMS Microbiol. Rev. 2017, 41, 679–697. [Google Scholar] [CrossRef]
- Francis, V.I.; Stevenson, E.C.; Porter, S.L. Two-component systems required for virulence in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2017, 364, fnx104. [Google Scholar] [CrossRef] [PubMed]
- Winzer, K.; Williams, P. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int. J. Med. Microbiol. 2001, 291, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Gogoi, M.; Sharma, A.; Hazarika, N.K. Biofilm formation by bacterial isolates from patients on indwelling medical devices. Indian J. Med. Microbiol. 2015, 33, 319–320. [Google Scholar] [CrossRef]
- Heras, B.; Scanlon, M.; Martin, J.L. Targeting virulence not viability in the search for future antibacterials. Br. J. Clin. Pharmacol. 2014, 79, 208–215. [Google Scholar] [CrossRef]
- Hegazy, W.A.H.; Salem, I.M.; Alotaibi, H.F.; Khafagy, E.S.; Ibrahim, D. Terazosin interferes with quorum sensing and type three secretion system and diminishes the bacterial espionage to mitigate the Salmonella typhimurium pathogenesis. Antibiotics 2022, 11, 465. [Google Scholar] [CrossRef]
- Jiang, T.; Li, M. Quorum sensing inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 867–894. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Givskov, M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 2006, 296, 149–161. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. MBio 2018, 9, e02331-17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K.; Song, D. Quorum sensing: A prospective therapeutic target for bacterial diseases. Biomed Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Livermore, D.M.; Development, on behalf of the British Society for Antimicrobial Chemotherapy Working Party on The Urgent Need: Regenerating Antibacterial Drug Discovery and Development; Blaser, M.; Carrs, O.; Cassell, G.; Fishman, N.; Guidos, R.; Levy, S.; Powers, J.; Norrby, R.; et al. Discovery research: The scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 2011, 66, 1941–1944. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Abbas, H.A.; Khayat, M.T.; Shaldam, M.A.; Askoura, M.; Asfour, H.Z.; Khafagy, E.S.; Abu Lila, A.S.; Allam, A.N.; Hegazy, W.A.H. Secnidazole is a promising imidazole mitigator of serratia marcescens virulence. Microorganisms 2021, 9, 2333. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Byun, Y.; Park, H.D. 6-gingerol reduces pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci. Rep. 2015, 5, 8656. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Khafagy, E.S.; Saqr, A.A.; Alalaiwe, A.; Abbas, H.A.; Shaldam, M.A.; Hegazy, W.A.H.; Goda, R.M. Tackling virulence of pseudomonas aeruginosa by the natural furanone sotolon. Antibiotics 2021, 10, 871. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Hegazy, W.A.H.; Shaldam, M.A.; Mosbah, R.; Almalki, A.J.; Ibrahim, T.S.; Khayat, M.T.; Khafagy, E.S.; Soliman, W.E.; Abbas, H.A. Xylitol inhibits growth and blocks virulence in serratia marcescens. Microorganisms 2021, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Almalki, A.J.; Ibrahim, T.S.; Taher, E.S.; Mohamed, M.F.A.; Youns, M.; Hegazy, W.A.H.; Al-Mahmoudy, A.M.M. Synthesis, antimicrobial, anti-virulence and anticancer evaluation of new 5(4H)-oxazolone-based sulfonamides. Molecules 2022, 27, 671. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.H.; Rajab, A.A.H.; Abu Lila, A.S.; Abbas, H.A. Anti-diabetics and antimicrobials: Harmony of mutual interplay. World J. Diabetes 2021, 12, 1832–1855. [Google Scholar] [CrossRef]
- Almalki, A.J.; Ibrahim, T.S.; Elhady, S.S.; Hegazy, W.A.H.; Darwish, K.M. Computational and biological evaluation of β-adrenoreceptor blockers as promising bacterial anti-virulence agents. Pharmaceuticals 2022, 15, 110. [Google Scholar] [CrossRef]
- Almalki, A.J.; Ibrahim, T.S.; Elhady, S.S.; Darwish, K.M.; Hegazy, W.A.H. Repurposing α-adrenoreceptor blockers as promising anti-virulence agents in gram-negative bacteria. Antibiotics 2022, 11, 178. [Google Scholar] [CrossRef]
- Aykac, A.; Şehirli, A.Ö.; Gören, M.Z. Evaluation of the effect of prazosin treatment on α-2c adrenoceptor and apoptosis protein levels in the predator scent-induced rat model of post-traumatic stress disorder. J. Mol. Neurosci. 2020, 70, 1120–1129. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Abbas, H.A.; Mohamed, M.F.A.; Asfour, H.Z.; Khayat, M.T.; Ibrahim, T.S.; Youns, M.; Khafagy, E.S.; Abu Lila, A.S.; Safo, M.K.; et al. Not only antimicrobial: Metronidazole mitigates the virulence of proteus mirabilis isolated from macerated diabetic foot ulcer. Appl. Sci. 2021, 11, 6847. [Google Scholar] [CrossRef]
- Koh, K.H.; Tham, F.Y. Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J. Microbiol. Immunol. Infect. 2011, 44, 144–148. [Google Scholar] [CrossRef]
- Saqr, A.A.; Aldawsari, M.F.; Khafagy, E.S.; Shaldam, M.A.; Hegazy, W.A.H.; Abbas, H.A. A novel use of allopurinol as a quorum-sensing inhibitor in Pseudomonas aeruginosa. Antibiotics 2021, 10, 1385. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Alalaiwe, A.; Khafagy, E.S.; Saqr, A.A.; Alshahrani, S.M.; Alsulays, B.B.; Alshehri, S.; Abu Lila, A.S.; Rizvi, S.M.D.; Hegazy, W.A.H. Efficacy of SPG-ODN 1826 nanovehicles in inducing M1 phenotype through TLR-9 activation in murine alveolar J774A.1 cells: Plausible nano-immunotherapy for lung carcinoma. Int. J. Mol. Sci. 2021, 22, 6833. [Google Scholar] [CrossRef] [PubMed]
- Youns, M.; Askoura, M.; Abbas, H.A.; Attia, G.H.; Khayyat, A.N.; Goda, R.M.; Almalki, A.J.; Khafagy, E.S.; Hegazy, W.A.H. Celastrol modulates multiple signaling pathways to inhibit proliferation of pancreatic cancer via DDIT3 and ATF3 up-regulation and RRM2 and MCM4 down-regulation. Onco Targets Ther. 2021, 14, 3849. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; Chan, D.S.H.; Leung, C.H. Drug repositioning by structure-based virtual screening. Chem. Soc. Rev. 2013, 42, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef]
- Lintz, M.J.; Oinuma, K.I.; Wysoczynski, C.L.; Greenberg, E.P.; Churchill, M.E.A. Crystal structure of QscR, a Pseudomonas aeruginosa quorum sensing signal receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 15763–15768. [Google Scholar] [CrossRef]
- Jiang, W.; Ubhayasekera, W.; Breed, M.C.; Norsworthy, A.N.; Serr, N.; Mobley, H.L.T.; Pearson, M.M.; Knight, S.D. MrpH, a new class of metal-binding adhesin, requires zinc to mediate biofilm formation. PLoS Pathog. 2020, 16, e1008707. [Google Scholar] [CrossRef]
- Chen, G.; Swem, L.R.; Swem, D.L.; Stauff, D.L.; O’Loughlin, C.T.; Jeffrey, P.D.; Bassler, B.L.; Hughson, F.M. A strategy for antagonizing quorum sensing. Mol. Cell 2011, 42, 199–209. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; De Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef]
- Studer, G.; Studer, G.; Rempfer, C.; Rempfer, C.; Waterhouse, A.M.; Waterhouse, A.M.; Gumienny, R.; Gumienny, R.; Haas, J.; Haas, J.; et al. Erratum: QMEANDisCo-distance constraints applied on model quality estimation. Bioinformatics 2020, 36, 2647. [Google Scholar] [CrossRef] [Green Version]
- Studer, G.; Tauriello, G.; Bienert, S.; Biasini, M.; Johner, N.; Schwede, T. ProMod3—A versatile homology modelling toolbox. PLOS Comput. Biol. 2021, 17, e1008667. [Google Scholar] [CrossRef]
- Bertoni, M.; Kiefer, F.; Biasini, M.; Bordoli, L.; Schwede, T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 2017, 7, 10480. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar] [CrossRef]
- Garcïa-Contreras, R. Is quorum sensing interference a viable alternative to treat Pseudomonas aeruginosa infections? Front. Microbiol. 2016, 7, 01454. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The natural antimicrobial carvacrol inhibits quorum sensing in chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef]
- Harrison, A.M.; Soby, S.D. Reclassification of Chromobacterium violaceum ATCC 31532 and its quorum biosensor mutant CV026 to Chromobacterium subtsugae. AMB Express 2020, 10, 202. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Girlich, D.; Bonnin, R.A.; Dortet, L.; Naas, T. Genetics of Acquired Antibiotic Resistance Genes in Proteus spp. Front. Microbiol. 2020, 11, 256. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.; Mohankumar, R.; Kannappan, A.; Raja, V.K.; Archunan, G.; Pandian, S.K.; Ruckmani, K.; Ravi, A.V. Exploring the anti-quorum sensing and antibiofilm efficacy of phytol against Serratia marcescens associated acute pyelonephritis infection in wistar rats. Front. Cell. Infect. Microbiol. 2017, 7, 498. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.H.; Khayat, M.T.; Ibrahim, T.S.; Youns, M.; Mosbah, R.; Soliman, W.E. Repurposing of antidiabetics as Serratia marcescens virulence inhibitors. Braz. J. Microbiol. 2021, 52, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Fischer, B.M.; Zheng, S. Proteases and cystic fibrosis. Int. J. Biochem. Cell Biol. 2008, 40, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, G.; Merieau, A.; Guerillon, J.; Veron, W.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N. Involvement of a phospholipase C in the hemolytic activity of a clinical strain of Pseudomonas fluorescens. BMC Microbiol. 2008, 8, 189. [Google Scholar] [CrossRef]
- Schaffer, J.N.; Norsworthy, A.N.; Sun, T.T.; Pearson, M.M. Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation. Proc. Natl. Acad. Sci. USA 2016, 113, 4494–4499. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).