CRABP-I Expression Patterns in the Developing Chick Inner Ear
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Processing
2.2. In Situ Hybridization and Immunohistochemistry Staining Procedures
2.3. Imaging
3. Results
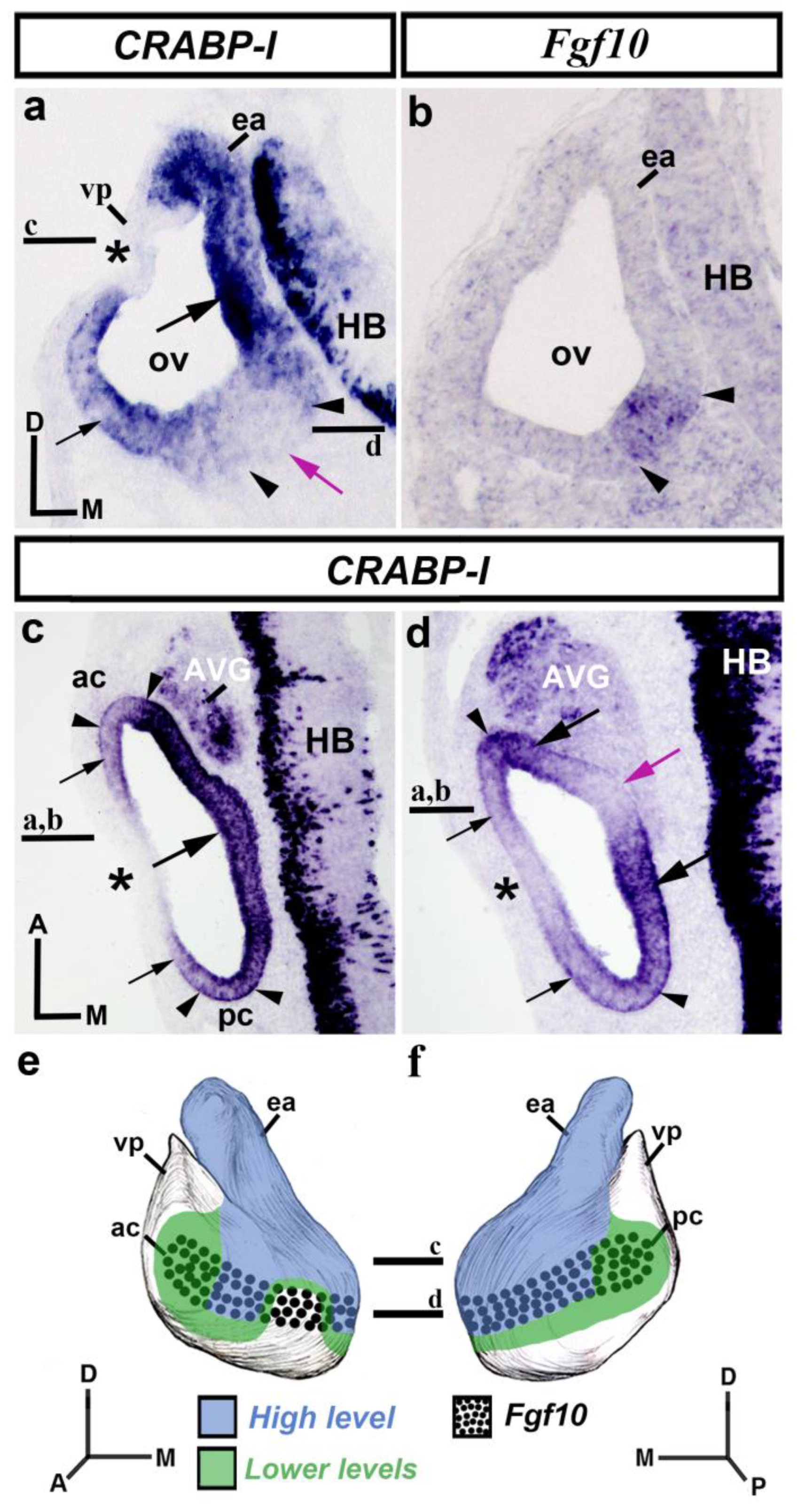
3.1. CRABP-I Expression Pattern at the Otic Vesicle Stage (HH18-20)
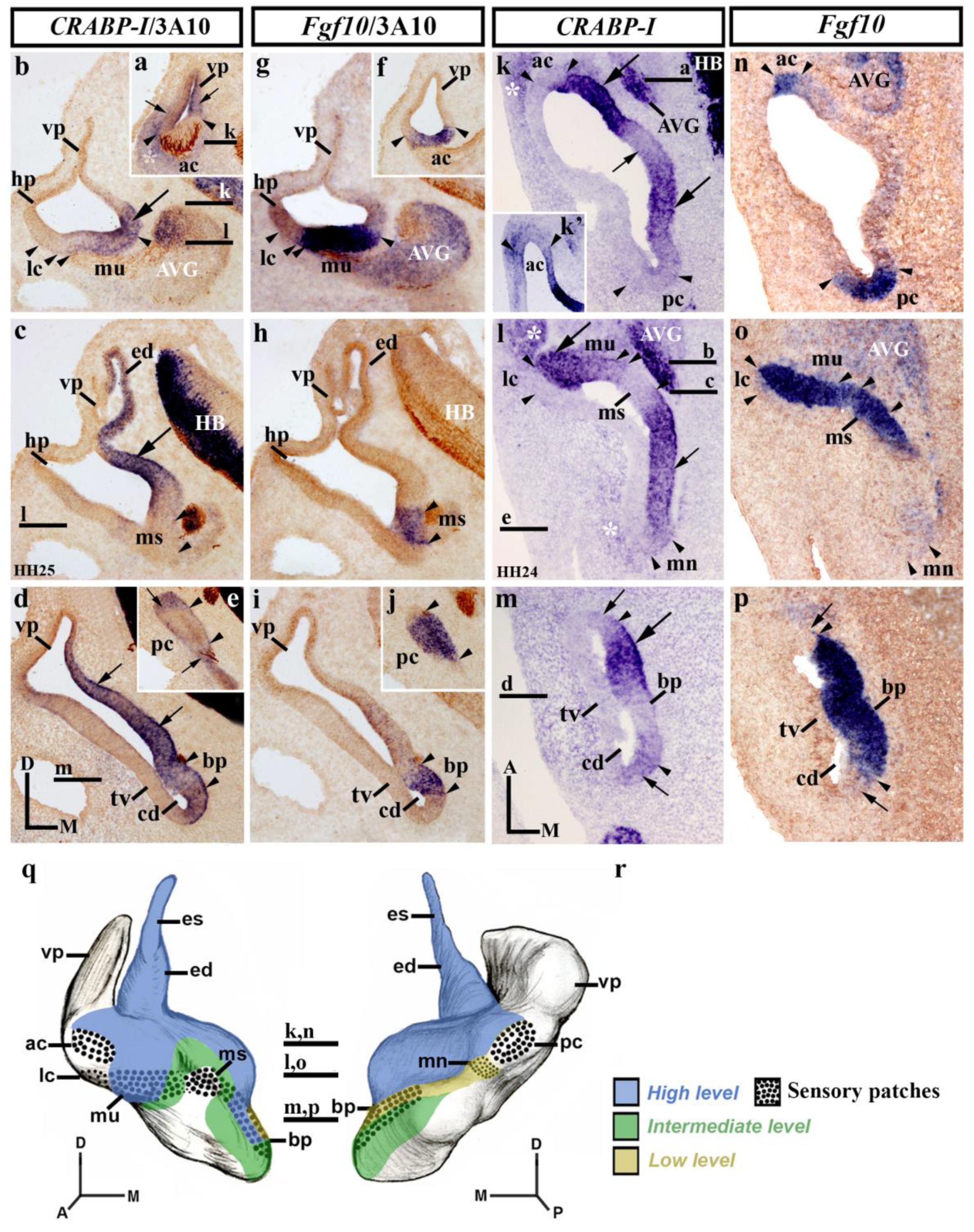
3.2. CRABP-I Expression Patterns at Stages HH24/25
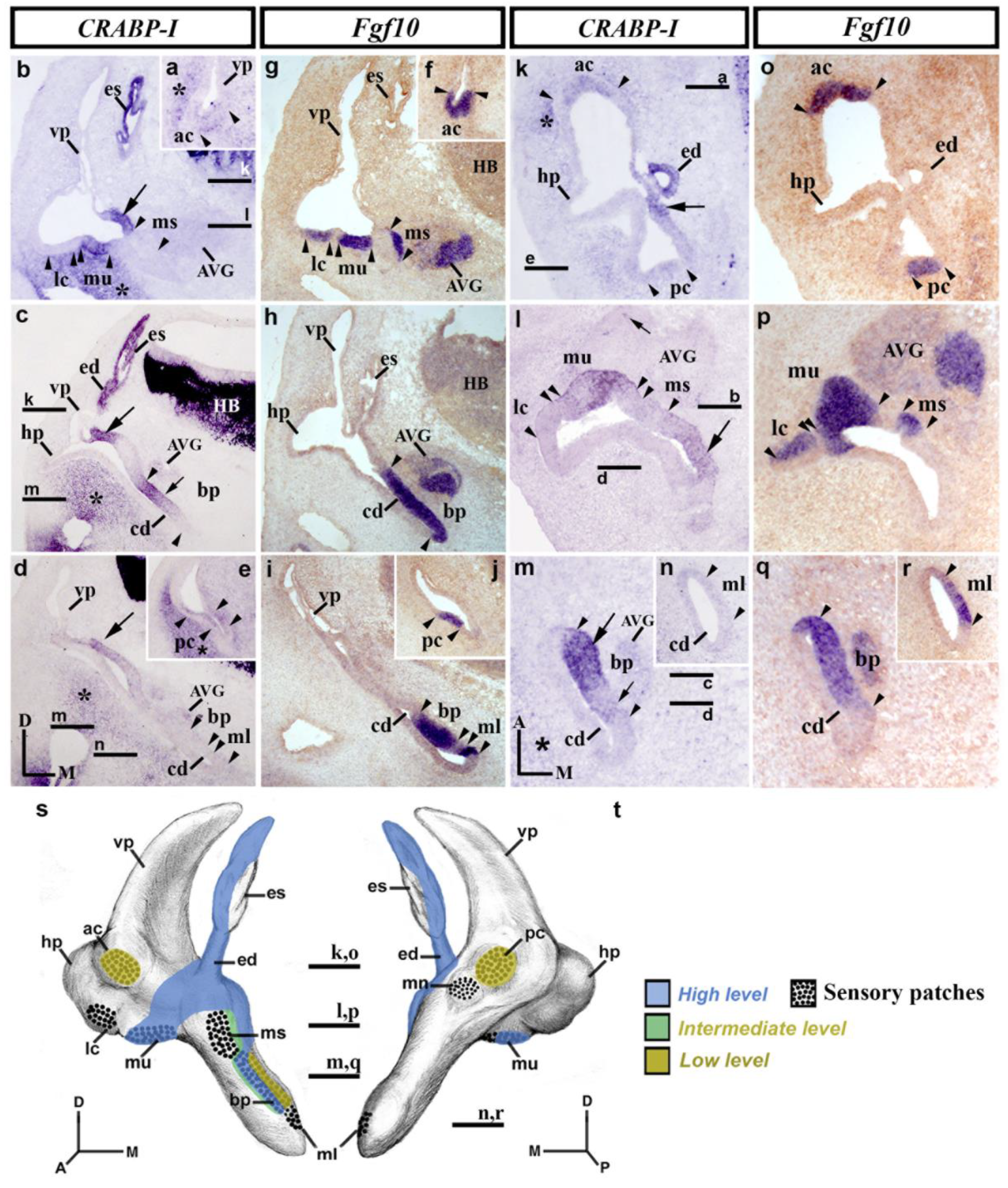
3.3. CRABP-I Expression Patterns at Stage HH27
3.4. CRABP-I Expression Patterns at Stage HH32
4. Discussion
4.1. Specification of the Chick Otic Vesicle by CRABP-I
4.2. Specification of the Vestibular System by CRABP-I
4.3. Specification of the Basilar Papilla by CRABP-I
4.4. CRABP-I and Otic Neurogenesis
4.5. CRABP-I in Periotic Mesenchyme Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ac | anterior crista |
| AG | acoustic ganglion |
| asc | anterior semicircular canal |
| AVG | acoustic-vestibular ganglion |
| bp | basilar papilla |
| cc | crus commune |
| cd | cochlear duct |
| CRABP-I | Cellular retinoic acid-binding protein I |
| ea | endolymphatic apparatus |
| ed | endolymphatic duct |
| es | endolymphatic sac |
| HB | hindbrain |
| hp | horizontal pouch |
| lc | lateral crista |
| lsc | lateral semicircular canal |
| ml | macula lagena |
| mn | macula neglecta |
| ms | macula sacculi |
| mu | macula utriculi |
| ov | otic vesicle |
| pc | posterior crista |
| psc | posterior semicircular canal |
| RA | Retinoic acid |
| s | saccule |
| tv | tegmentum vasculosum |
| u | utricle |
| vp | vertical pouch |
References
- Fekete, D.M.; Wu, D.K. Revisiting Cell Fate Specification in the Inner Ear. Curr. Opin. Neurobiol. 2002, 12, 35–42. [Google Scholar] [CrossRef]
- Wu, D.K.; Kelley, M.W. Molecular Mechanisms of Inner Ear Development. Cold Spring Harb. Perspect. Biol. 2012, 4, a008409. [Google Scholar] [CrossRef]
- Sánchez-Guardado, L.Ó.; Puelles, L.; Hidalgo-Sánchez, M. Fate Map of the Chicken Otic Placode. Dev. Camb. Engl. 2014, 141, 2302–2312. [Google Scholar] [CrossRef] [Green Version]
- Romand, R.; Dollé, P.; Hashino, E. Retinoid Signaling in Inner Ear Development. J. Neurobiol. 2006, 66, 687–704. [Google Scholar] [CrossRef]
- Abello, G.; Alsina, B. Establishment of a Proneural Field in the Inner Ear. Int. J. Dev. Biol. 2007, 51, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Bok, J.; Chang, W.; Wu, D.K. Patterning and Morphogenesis of the Vertebrate Inner Ear. Int. J. Dev. Biol. 2007, 51, 521–533. [Google Scholar] [CrossRef]
- Ohyama, T.; Groves, A.K.; Martin, K. The First Steps towards Hearing: Mechanisms of Otic Placode Induction. Int. J. Dev. Biol. 2007, 51, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Calderon, H.; Milo, M.; Leon, Y.; Varela-Nieto, I. A Network of Growth and Transcription Factors Controls Neuronal Differentation and Survival in the Developing Ear. Int. J. Dev. Biol. 2007, 51, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Schimmang, T. Expression and Functions of FGF Ligands during Early Otic Development. Int. J. Dev. Biol. 2007, 51, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Schneider-Maunoury, S.; Pujades, C. Hindbrain Signals in Otic Regionalization: Walk on the Wild Side. Int. J. Dev. Biol. 2007, 51, 495–506. [Google Scholar] [CrossRef]
- Whitfield, T.T.; Hammond, K.L. Axial Patterning in the Developing Vertebrate Inner Ear. Int. J. Dev. Biol. 2007, 51, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.C.; Chen, P. Development of Form and Function in the Mammalian Cochlea. Curr. Opin. Neurobiol. 2009, 19, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Frenz, D.A.; Liu, W.; Cvekl, A.; Xie, Q.; Wassef, L.; Quadro, L.; Niederreither, K.; Maconochie, M.; Shanske, A. Retinoid Signaling in Inner Ear Development: A “Goldilocks” Phenomenon. Am. J. Med. Genet. A 2010, 152A, 2947–2961. [Google Scholar] [CrossRef] [Green Version]
- Ladher, R.K.; O’Neill, P.; Begbie, J. From Shared Lineage to Distinct Functions: The Development of the Inner Ear and Epibranchial Placodes. Dev. Camb. Engl. 2010, 137, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Groves, A.K.; Zhang, K.D.; Fekete, D.M. The Genetics of Hair Cell Development and Regeneration. Annu. Rev. Neurosci. 2013, 36, 361–381. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Streit, A. Induction of the Inner Ear: Stepwise Specification of Otic Fate from Multipotent Progenitors. Hear. Res. 2013, 297, 3–12. [Google Scholar] [CrossRef]
- Bushue, N.; Wan, Y.-J.Y. Retinoid Pathway and Cancer Therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298. [Google Scholar] [CrossRef] [Green Version]
- Pennimpede, T.; Cameron, D.A.; MacLean, G.A.; Li, H.; Abu-Abed, S.; Petkovich, M. The Role of CYP26 Enzymes in Defining Appropriate Retinoic Acid Exposure during Embryogenesis. Birt. Defects Res. A Clin. Mol. Teratol. 2010, 88, 883–894. [Google Scholar] [CrossRef]
- Gudas, L.J.; Wagner, J.A. Retinoids Regulate Stem Cell Differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Lara-Ramírez, R.; Zieger, E.; Schubert, M. Retinoic Acid Signaling in Spinal Cord Development. Int. J. Biochem. Cell Biol. 2013, 45, 1302–1313. [Google Scholar] [CrossRef]
- Alizadeh, F.; Bolhassani, A.; Khavari, A.; Bathaie, S.Z.; Naji, T.; Bidgoli, S.A. Retinoids and Their Biological Effects against Cancer. Int. Immunopharmacol. 2014, 18, 43–49. [Google Scholar] [CrossRef]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.-C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic Acid Signaling Pathways in Development and Diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Tonk, E.C.M.; Pennings, J.L.A.; Piersma, A.H. An Adverse Outcome Pathway Framework for Neural Tube and Axial Defects Mediated by Modulation of Retinoic Acid Homeostasis. Reprod. Toxicol. Elmsford N 2015, 55, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Willaredt, M.A.; Schlüter, T.; Nothwang, H.G. The Gene Regulatory Networks Underlying Formation of the Auditory Hindbrain. Cell. Mol. Life Sci. CMLS 2015, 72, 519–535. [Google Scholar] [CrossRef]
- Xavier-Neto, J.; Sousa Costa, Â.M.; Figueira, A.C.M.; Caiaffa, C.D.; do Amaral, F.N.; Peres, L.M.C.; da Silva, B.S.P.; Santos, L.N.; Moise, A.R.; Castillo, H.A. Signaling through Retinoic Acid Receptors in Cardiac Development: Doing the Right Things at the Right Times. Biochim. Biophys. Acta 2015, 1849, 94–111. [Google Scholar] [CrossRef] [Green Version]
- Ealy, M.; Ellwanger, D.C.; Kosaric, N.; Stapper, A.P.; Heller, S. Single-Cell Analysis Delineates a Trajectory toward the Human Early Otic Lineage. Proc. Natl. Acad. Sci. USA 2016, 113, 8508–8513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammadova, A.; Zhou, H.; Carels, C.E.L.; Von den Hoff, J.W. Retinoic Acid Signalling in the Development of the Epidermis, the Limbs and the Secondary Palate. Differ. Res. Biol. Divers. 2016, 92, 326–335. [Google Scholar] [CrossRef]
- Tanaka, M. Developmental Mechanism of Limb Field Specification along the Anterior-Posterior Axis during Vertebrate Evolution. J. Dev. Biol. 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanovic, S.; Zaffran, S. Mechanisms of Retinoic Acid Signaling during Cardiogenesis. Mech. Dev. 2017, 143, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Piersma, A.H.; Hessel, E.V.; Staal, Y.C. Retinoic Acid in Developmental Toxicology: Teratogen, Morphogen and Biomarker. Reprod. Toxicol. Elmsford N 2017, 72, 53–61. [Google Scholar] [CrossRef]
- Dubey, A.; Rose, R.E.; Jones, D.R.; Saint-Jeannet, J.-P. Generating Retinoic Acid Gradients by Local Degradation during Craniofacial Development: One Cell’s Cue Is Another Cell’s Poison. Genesis 2018, 56, e23091. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, H.; Lopez-Juarez, A.; Fontbonne, A.; Nivet, E.; Zine, A. Modeling Human Early Otic Sensory Cell Development with Induced Pluripotent Stem Cells. PLoS ONE 2018, 13, e0198954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, S.A.; McCracken, K.W.; Luedeke, D.M.; Han, L.; Wells, J.M.; Shannon, J.M.; Zorn, A.M. Timing Is Everything: Reiterative Wnt, BMP and RA Signaling Regulate Developmental Competence during Endoderm Organogenesis. Dev. Biol. 2018, 434, 121–132. [Google Scholar] [CrossRef]
- Frank, D.; Sela-Donenfeld, D. Hindbrain Induction and Patterning during Early Vertebrate Development. Cell. Mol. Life Sci. CMLS 2019, 76, 941–960. [Google Scholar] [CrossRef]
- Boylan, J.F.; Gudas, L.J. Overexpression of the Cellular Retinoic Acid Binding Protein-I (CRABP- I) Results in a Reduction in Differentiation-Specific Gene Expression in F9 Teratocarcinoma Cells. J. Cell Biol. 1991, 112, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Nam, E.-C.; Yoo, S.J.; Kwon, H.J.; Um, S.J.; Han, H.S.; Kim, S.H.; Byun, Y.; Kim, S.Y. The Effect of Cellular Retinoic Acid Binding Protein-I Expression on the CYP26-Mediated Catabolism of All-Trans Retinoic Acid and Cell Proliferation in Head and Neck Squamous Cell Carcinoma. Metabolism. 2004, 53, 1007–1012. [Google Scholar] [CrossRef]
- Tanaka, K.; Imoto, I.; Inoue, J.; Kozaki, K.; Tsuda, H.; Shimada, Y.; Aiko, S.; Yoshizumi, Y.; Iwai, T.; Kawano, T.; et al. Frequent Methylation-Associated Silencing of a Candidate Tumor-Suppressor, CRABP1, in Esophageal Squamous-Cell Carcinoma. Oncogene 2007, 26, 6456–6468. [Google Scholar] [CrossRef]
- White, R.J.; Schilling, T.F. How Degrading: Cyp26s in Hindbrain Development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 2775–2790. [Google Scholar] [CrossRef] [Green Version]
- Thatcher, J.E.; Isoherranen, N. The Role of CYP26 Enzymes in Retinoic Acid Clearance. Expert Opin. Drug Metab. Toxicol. 2009, 5, 875–886. [Google Scholar] [CrossRef] [Green Version]
- Cai, A.Q.; Radtke, K.; Linville, A.; Lander, A.D.; Nie, Q.; Schilling, T.F. Cellular Retinoic Acid-Binding Proteins Are Essential for Hindbrain Patterning and Signal Robustness in Zebrafish. Dev. Camb. Engl. 2012, 139, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Y.; Fu, Z.; Xu, N.; Chen, F.; Yin, H.; Lu, X.; Shen, R.; Lu, C. Differential DNA Methylation Status between Breast Carcinomatous and Normal Tissues. Biomed. Pharmacother. Biomedecine Pharmacother. 2014, 68, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L. Cellular Retinoid Binding-Proteins, CRBP, CRABP, FABP5: Effects on Retinoid Metabolism, Function and Related Diseases. Pharmacol. Ther. 2017, 173, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.-N. Cellular Retinoic Acid Binding Proteins: Genomic and Non-Genomic Functions and Their Regulation. Subcell. Biochem. 2016, 81, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.J.; Vaessen, M.J.; van den Berg, C.; Timmermans, A.; Godsave, S.; Holling, T.; Nieuwkoop, P.; Geurts van Kessel, A.; Durston, A. Overexpression of a Cellular Retinoic Acid Binding Protein (XCRABP) Causes Anteroposterior Defects in Developing Xenopus Embryos. Dev. Camb. Engl. 1994, 120, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Mercola, M.; Gudas, L.J. Xenopus Laevis Cellular Retinoic Acid-Binding Protein: Temporal and Spatial Expression Pattern during Early Embryogenesis. Mech. Dev. 1994, 47, 53–64. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Sharma, M.K.; Sun, Q.; Thisse, C.; Thisse, B.; Denovan-Wright, E.M.; Wright, J.M. Retention of the Duplicated Cellular Retinoic Acid-Binding Protein 1 Genes (Crabp1a and Crabp1b) in the Zebrafish Genome by Subfunctionalization of Tissue-Specific Expression. FEBS J. 2005, 272, 3561–3571. [Google Scholar] [CrossRef]
- Sharma, M.K.; Denovan-Wright, E.M.; Boudreau, M.E.R.; Wright, J.M. A Cellular Retinoic Acid-Binding Protein from Zebrafish (Danio Rerio): CDNA Sequence, Phylogenetic Analysis, MRNA Expression, and Gene Linkage Mapping. Gene 2003, 311, 119–128. [Google Scholar] [CrossRef]
- Sharma, M.K.; Saxena, V.; Liu, R.-Z.; Thisse, C.; Thisse, B.; Denovan-Wright, E.M.; Wright, J.M. Differential Expression of the Duplicated Cellular Retinoic Acid-Binding Protein 2 Genes (Crabp2a and Crabp2b) during Zebrafish Embryonic Development. Gene Expr. Patterns GEP 2005, 5, 371–379. [Google Scholar] [CrossRef]
- Clouthier, D.E.; Williams, S.C.; Yanagisawa, H.; Wieduwilt, M.; Richardson, J.A.; Yanagisawa, M. Signaling Pathways Crucial for Craniofacial Development Revealed by Endothelin-A Receptor-Deficient Mice. Dev. Biol. 2000, 217, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Hu, X.; Zhou, F.C.; Wei, L.-N. Upregulation of Cellular Retinoic Acid-Binding Protein I Expression by Ethanol. Dev. Growth Differ. 2001, 43, 553–561. [Google Scholar] [CrossRef]
- Mey, J.; McCaffery, P.; Klemeit, M. Sources and Sink of Retinoic Acid in the Embryonic Chick Retina: Distribution of Aldehyde Dehydrogenase Activities, CRABP-I, and Sites of Retinoic Acid Inactivation. Brain Res. Dev. Brain Res. 2001, 127, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Hind, M.; Corcoran, J.; Maden, M. Temporal/Spatial Expression of Retinoid Binding Proteins and RAR Isoforms in the Postnatal Lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L468–L476. [Google Scholar] [CrossRef] [PubMed]
- Deak, K.L.; Dickerson, M.E.; Linney, E.; Enterline, D.S.; George, T.M.; Melvin, E.C.; Graham, F.L.; Siegel, D.G.; Hammock, P.; Mehltretter, L.; et al. Analysis of ALDH1A2, CYP26A1, CYP26B1, CRABP1, and CRABP2 in Human Neural Tube Defects Suggests a Possible Association with Alleles in ALDH1A2. Birt. Defects Res. A Clin. Mol. Teratol. 2005, 73, 868–875. [Google Scholar] [CrossRef]
- Propping, C.; Mönig, B.; Luksch, H.; Mey, J. Distribution of the Cellular Retinoic Acid Binding Protein CRABP-I in the Developing Chick Optic Tectum. Brain Res. 2007, 1168, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Watt, F.M. Dynamic Regulation of Retinoic Acid-Binding Proteins in Developing, Adult and Neoplastic Skin Reveals Roles for Beta-Catenin and Notch Signalling. Dev. Biol. 2008, 324, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, F.; Corsini, E.; Broccia, M.L.; Marinovich, M.; Galli, C.L.; Giavini, E.; Menegola, E. Molecular Mechanism of Teratogenic Effects Induced by the Fungicide Triadimefon: Study of the Expression of TGF-β MRNA and TGF-β and CRABPI Proteins during Rat in Vitro Development. Toxicol. Appl. Pharmacol. 2009, 234, 107–116. [Google Scholar] [CrossRef]
- Inman, K.E.; Caiaffa, C.D.; Melton, K.R.; Sandell, L.L.; Achilleos, A.; Kume, T.; Trainor, P.A. Foxc2 Is Required for Proper Cardiac Neural Crest Cell Migration, Outflow Tract Septation, and Ventricle Expansion. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2018, 247, 1286–1296. [Google Scholar] [CrossRef] [Green Version]
- Thompson Haskell, G.; Maynard, T.M.; Shatzmiller, R.A.; Lamantia, A.-S. Retinoic Acid Signaling at Sites of Plasticity in the Mature Central Nervous System. J. Comp. Neurol. 2002, 452, 228–241. [Google Scholar] [CrossRef]
- Parenti, R.; Cicirata, F. Retinoids and Binding Proteins in the Cerebellum during Lifetime. Cerebellum Lond. Engl. 2004, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Arias, A.C.; Liu, L.; Chen, Y.-B.; Bronner, M.E.; Maxson, R.E. A Stable Cranial Neural Crest Cell Line from Mouse. Stem Cells Dev. 2012, 21, 3069–3080. [Google Scholar] [CrossRef]
- de Bruijn, D.R.H.; Oerlemans, F.; Hendriks, W.; Baats, E.; Ploemacher, R.; Wieringa, B.; van Kessel, A.G. Normal Development, Growth and Reproduction in Cellular Retinoic Acid Binding Protein-I (CRABPI) Null Mutant Mice. Differentiation 1995, 58, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Gorry, P.; Lufkin, T.; Dierich, A.; Rochette-Egly, C.; Décimo, D.; Dollé, P.; Mark, M.; Durand, B.; Chambon, P. The Cellular Retinoic Acid Binding Protein I Is Dispensable. Proc. Natl. Acad. Sci. USA 1994, 91, 9032–9036. [Google Scholar] [CrossRef] [Green Version]
- Lampron, C.; Rochette-Egly, C.; Gorry, P.; Dollé, P.; Mark, M.; Lufkin, T.; LeMeur, M.; Chambon, P. Mice Deficient in Cellular Retinoic Acid Binding Protein II (CRABPII) or in Both CRABPI and CRABPII Are Essentially Normal. Dev. Camb. Engl. 1995, 121, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.; Pasceri, P.; Fraser, R.; Colbert, M.; Rossant, J.; Giguère, V. Postaxial Polydactyly in Forelimbs of CRABP-II Mutant Mice. Dev. Camb. Engl. 1995, 121, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, E.; Friederich, V.; Morriss-Kay, G.; Chambon, P. Differential Distribution Patterns of CRABP I and CRABP II Transcripts during Mouse Embryogenesis. Dev. Camb. Engl. 1992, 115, 973–987. [Google Scholar] [CrossRef]
- Maden, M.; Hunt, P.; Eriksson, U.; Kuroiwa, A.; Krumlauf, R.; Summerbell, D. Retinoic Acid-Binding Protein, Rhombomeres and the Neural Crest. Dev. Camb. Engl. 1991, 111, 35–43. [Google Scholar] [CrossRef]
- Kleinjan, D.A.; Dekker, S.; Vaessen, M.J.; Grosveld, F. Regulation of the CRABP-I Gene during Mouse Embryogenesis. Mech. Dev. 1997, 67, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Romand, R.; Sapin, V.; Ghyselinck, N.B.; Avan, P.; Le Calvez, S.; Dollé, P.; Chambon, P.; Mark, M. Spatio-Temporal Distribution of Cellular Retinoid Binding Protein Gene Transcripts in the Developing and the Adult Cochlea. Morphological and Functional Consequences in CRABP- and CRBPI-Null Mutant Mice. Eur. J. Neurosci. 2000, 12, 2793–2804. [Google Scholar] [CrossRef]
- Kelley, M.W.; Xu, X.M.; Wagner, M.A.; Warchol, M.E.; Corwin, J.T. The Developing Organ of Corti Contains Retinoic Acid and Forms Supernumerary Hair Cells in Response to Exogenous Retinoic Acid in Culture. Dev. Camb. Engl. 1993, 119, 1041–1053. [Google Scholar] [CrossRef]
- Ylikoski, J.; Pirvola, U.; Eriksson, U. Cellular Retinol-Binding Protein Type I Is Prominently and Differentially Expressed in the Sensory Epithelium of the Rat Cochlea and Vestibular Organs. J. Comp. Neurol. 1994, 349, 596–602. [Google Scholar] [CrossRef]
- Deng, X.; Wu, D.K. Temporal Coupling between Specifications of Neuronal and Macular Fates of the Inner Ear. Dev. Biol. 2016, 414, 21–33. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A Series of Normal Stages in the Development of the Chick Embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Sánchez-Guardado, L.Ó.; Puelles, L.; Hidalgo-Sánchez, M. Fgf10 Expression Patterns in the Developing Chick Inner Ear. J. Comp. Neurol. 2013, 521, 1136–1164. [Google Scholar] [CrossRef] [PubMed]
- Ferran, J.L.; Ayad, A.; Merchán, P.; Morales-Delgado, N.; Sánchez-Arrones, L.; Alonso, A.; SandovalSandoval, J.E.; Bardet, S.M.; Corral-San-Miguel, R.; Sánchez-Guardado, L.Ó.; et al. Exploring Brain Genoarchitecture by Single and Double Chromogenic In Situ Hybridization (ISH) and Immunohistochemistry (IHC) on Cryostat, Paraffin, or Floating Sections. In In Situ Hybridization Methods; Neuromethods; Hauptmann, G., Ed.; Springer: New York, NY, USA, 2015; pp. 83–107. ISBN 978-1-4939-2303-8. [Google Scholar]
- Sánchez-Guardado, L.Ó.; Ferran, J.L.; Rodríguez-Gallardo, L.; Puelles, L.; Hidalgo-Sánchez, M. Meis Gene Expression Patterns in the Developing Chicken Inner Ear. J. Comp. Neurol. 2011, 519, 125–147. [Google Scholar] [CrossRef]
- Cardeña-Núñez, S.; Sánchez-Guardado, L.Ó.; Corral-San-Miguel, R.; Rodríguez-Gallardo, L.; Marín, F.; Puelles, L.; Aroca, P.; Hidalgo-Sánchez, M. Expression Patterns of Irx Genes in the Developing Chick Inner Ear. Brain Struct. Funct. 2017, 222, 2071–2092. [Google Scholar] [CrossRef]
- Olaya-Sánchez, D.; Sánchez-Guardado, L.Ó.; Ohta, S.; Chapman, S.C.; Schoenwolf, G.C.; Puelles, L.; Hidalgo-Sánchez, M. Fgf3 and Fgf16 Expression Patterns Define Spatial and Temporal Domains in the Developing Chick Inner Ear. Brain Struct. Funct. 2017, 222, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Ruuska, S.E.; Levinthal, D.J.; Noy, N. Distinct Roles for Cellular Retinoic Acid-Binding Proteins I and II in Regulating Signaling by Retinoic Acid. J. Biol. Chem. 1999, 274, 23695–23698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalik, L.; Wahli, W. Guiding Ligands to Nuclear Receptors. Cell 2007, 129, 649–651. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Guardado, L.O.; Ferran, J.L.; Mijares, J.; Puelles, L.; Rodríguez-Gallardo, L.; Hidalgo-Sánchez, M. Raldh3 Gene Expression Pattern in the Developing Chicken Inner Ear. J. Comp. Neurol. 2009, 514, 49–65. [Google Scholar] [CrossRef]
- Wassarman, K.M.; Lewandoski, M.; Campbell, K.; Joyner, A.L.; Rubenstein, J.L.; Martinez, S.; Martin, G.R. Specification of the Anterior Hindbrain and Establishment of a Normal Mid/Hindbrain Organizer Is Dependent on Gbx2 Gene Function. Dev. Camb. Engl. 1997, 124, 2923–2934. [Google Scholar] [CrossRef]
- Hidalgo-Sánchez, M.; Alvarado-Mallart, R.; Alvarez, I.S. Pax2, Otx2, Gbx2 and Fgf8 Expression in Early Otic Vesicle Development. Mech. Dev. 2000, 95, 225–229. [Google Scholar] [CrossRef]
- Su, Y.; Meng, A. The Expression of Gbx-2 during Zebrafish Embryogenesis. Mech. Dev. 2002, 113, 107–110. [Google Scholar] [CrossRef]
- Sánchez-Calderón, H.; Martín-Partido, G.; Hidalgo-Sánchez, M. Differential Expression of Otx2, Gbx2, Pax2, and Fgf8 in the Developing Vestibular and Auditory Sensory Organs. Brain Res. Bull. 2002, 57, 321–323. [Google Scholar] [CrossRef]
- Sánchez-Calderón, H.; Martín-Partido, G.; Hidalgo-Sánchez, M. Otx2, Gbx2, and Fgf8 Expression Patterns in the Chick Developing Inner Ear and Their Possible Roles in Otic Specification and Early Innervation. Gene Expr. Patterns GEP 2004, 4, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.K.; Oh, S.-H. Sensory Organ Generation in the Chick Inner Ear. J. Neurosci. 1996, 16, 6454–6462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlach-Bank, L.M.; Cleveland, A.R.; Barald, K.F. DAN Directs Endolymphatic Sac and Duct Outgrowth in the Avian Inner Ear. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2004, 229, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Sienknecht, U.J.; Fekete, D.M. Mapping of Wnt, Frizzled, and Wnt Inhibitor Gene Expression Domains in the Avian Otic Primordium. J. Comp. Neurol. 2009, 517, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Choo, D.; Sanne, J.L.; Wu, D.K. The Differential Sensitivities of Inner Ear Structures to Retinoic Acid during Development. Dev. Biol. 1998, 204, 136–150. [Google Scholar] [CrossRef]
- Riccomagno, M.M.; Takada, S.; Epstein, D.J. Wnt-Dependent Regulation of Inner Ear Morphogenesis Is Balanced by the Opposing and Supporting Roles of Shh. Genes Dev. 2005, 19, 1612–1623. [Google Scholar] [CrossRef] [Green Version]
- Groves, A.K.; Fekete, D.M. Shaping Sound in Space: The Regulation of Inner Ear Patterning. Dev. Camb. Engl. 2012, 139, 245–257. [Google Scholar] [CrossRef]
- Ohta, S.; Schoenwolf, G.C. Hearing Crosstalk: The Molecular Conversation Orchestrating Inner Ear Dorsoventral Patterning. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e302. [Google Scholar] [CrossRef]
- Alsina, B.; Abelló, G.; Ulloa, E.; Henrique, D.; Pujades, C.; Giraldez, F. FGF Signaling Is Required for Determination of Otic Neuroblasts in the Chick Embryo. Dev. Biol. 2004, 267, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.N.; Blaner, W.S.; Goodman, D.S.; Nguyen-Huu, M.C. Regulation of the Cellular Retinoid-Binding Proteins and Their Messenger Ribonucleic Acids during P19 Embryonal Carcinoma Cell Differentiation Induced by Retinoic Acid. Mol. Endocrinol. Baltim. Md 1989, 3, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Cardeña-Núñez, S.; Sánchez-Guardado, L.Ó.; Hidalgo-Sánchez, M. Cyp1B1 Expression Patterns in the Developing Chick Inner Ear. Dev Dyn 2020, 249, 410–424. [Google Scholar] [CrossRef]
- Nugent, P.; Potchinsky, M.; Lafferty, C.; Greene, R.M. TGF-Beta Modulates the Expression of Retinoic Acid-Induced RAR-Beta in Primary Cultures of Embryonic Palate Cells. Exp. Cell Res. 1995, 220, 495–500. [Google Scholar] [CrossRef]
- Nugent, P.; Greene, R.M. Interactions between the Transforming Growth Factor Beta (TGF Beta) and Retinoic Acid Signal Transduction Pathways in Murine Embryonic Palatal Cells. Differ. Res. Biol. Divers. 1994, 58, 149–155. [Google Scholar] [CrossRef]
- Thompson, D.L.; Gerlach-Bank, L.M.; Barald, K.F.; Koenig, R.J. Retinoic Acid Repression of Bone Morphogenetic Protein 4 in Inner Ear Development. Mol. Cell. Biol. 2003, 23, 2277–2286. [Google Scholar] [CrossRef] [Green Version]
- Dollé, P.; Ruberte, E.; Leroy, P.; Morriss-Kay, G.; Chambon, P. Retinoic Acid Receptors and Cellular Retinoid Binding Proteins. I. A Systematic Study of Their Differential Pattern of Transcription during Mouse Organogenesis. Dev. Camb. Engl. 1990, 110, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- Holder, N.; Hill, J. Retinoic Acid Modifies Development of the Midbrain-Hindbrain Border and Affects Cranial Ganglion Formation in Zebrafish Embryos. Dev. Camb. Engl. 1991, 113, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Lie, D.C.; DeCicco, K.L.; Shi, Y.; DeLuca, L.M.; Gage, F.H.; Evans, R.M. Retinoic Acid Is Required Early during Adult Neurogenesis in the Dentate Gyrus. Proc. Natl. Acad. Sci. USA 2006, 103, 3902–3907. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, E.; Touyarot, K.; Alfos, S.; Pallet, V.; Higueret, P.; Abrous, D.N. Retinoic Acid Restores Adult Hippocampal Neurogenesis and Reverses Spatial Memory Deficit in Vitamin A Deprived Rats. PLoS ONE 2008, 3, e3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radosevic, M.; Robert-Moreno, A.; Coolen, M.; Bally-Cuif, L.; Alsina, B. Her9 Represses Neurogenic Fate Downstream of Tbx1 and Retinoic Acid Signaling in the Inner Ear. Dev. Camb. Engl. 2011, 138, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, R.; Kitanaka, T.; Namba, S.; Kitanaka, N.; Sato, M.; Shibukawa, Y.; Masuhiro, Y.; Kano, K.; Matsumoto, T.; Sugiya, H. All-Trans Retinoic Acid Induces Reprogramming of Canine Dedifferentiated Cells into Neuron-like Cells. PLoS ONE 2020, 15, e0229892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crandall, J.E.; Goodman, T.; McCarthy, D.M.; Duester, G.; Bhide, P.G.; Dräger, U.C.; McCaffery, P. Retinoic Acid Influences Neuronal Migration from the Ganglionic Eminence to the Cerebral Cortex. J. Neurochem. 2011, 119, 723–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritzsch, B. Development of Inner Ear Afferent Connections: Forming Primary Neurons and Connecting Them to the Developing Sensory Epithelia. Brain Res. Bull. 2003, 60, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Guardado, L.Ó.; Puelles, L.; Hidalgo-Sánchez, M. Origin of Acoustic-Vestibular Ganglionic Neuroblasts in Chick Embryos and Their Sensory Connections. Brain Struct. Funct. 2019, 224, 2757–2774. [Google Scholar] [CrossRef]
- Alvarez, I.S.; Martín-Partido, G.; Rodríguez-Gallardo, L.; González-Ramos, C.; Navascués, J. Cell Proliferation during Early Development of the Chick Embryo Otic Anlage: Quantitative Comparison of Migratory and Nonmigratory Regions of the Otic Epithelium. J. Comp. Neurol. 1989, 290, 278–288. [Google Scholar] [CrossRef]
- Bok, J.; Raft, S.; Kong, K.-A.; Koo, S.K.; Dräger, U.C.; Wu, D.K. Transient Retinoic Acid Signaling Confers Anterior-Posterior Polarity to the Inner Ear. Proc. Natl. Acad. Sci. USA 2011, 108, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.; Palmer, T.D.; Gage, F.H. Retinoic Acid and Neurotrophins Collaborate to Regulate Neurogenesis in Adult-Derived Neural Stem Cell Cultures. J. Neurobiol. 1999, 38, 65–81. [Google Scholar] [CrossRef]
- Satoh, T.; Fekete, D.M. Clonal Analysis of the Relationships between Mechanosensory Cells and the Neurons That Innervate Them in the Chicken Ear. Dev. Camb. Engl. 2005, 132, 1687–1697. [Google Scholar] [CrossRef]
- Raft, S.; Groves, A.K. Segregating Neural and Mechanosensory Fates in the Developing Ear: Patterning, Signaling, and Transcriptional Control. Cell Tissue Res. 2015, 359, 315–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bard, J.B.L.; Lam, M.S.; Aitken, S. A Bioinformatics Approach for Identifying Candidate Transcriptional Regulators of Mesenchyme-to-Epithelium Transitions in Mouse Embryos. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 2748–2754. [Google Scholar] [CrossRef]
- Akama, K.; Horikoshi, T.; Nakayama, T.; Otsu, M.; Imaizumi, N.; Nakamura, M.; Toda, T.; Inuma, M.; Hirano, H.; Kondo, Y.; et al. Proteomic Identification of Differentially Expressed Genes during Differentiation of Cynomolgus Monkey (Macaca Fascicularis) Embryonic Stem Cells to Astrocyte Progenitor Cells in Vitro. Biochim. Biophys. Acta 2013, 1834, 601–610. [Google Scholar] [CrossRef]
- Braunstein, E.M.; Monks, D.C.; Aggarwal, V.S.; Arnold, J.S.; Morrow, B.E. Tbx1 and Brn4 Regulate Retinoic Acid Metabolic Genes during Cochlear Morphogenesis. BMC Dev.Biol. 2009, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Monks, D.C.; Morrow, B.E. Identification of Putative Retinoic Acid Target Genes Downstream of Mesenchymal Tbx1 during Inner Ear Development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2012, 241, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Pittlik, S.; Domingues, S.; Meyer, A.; Begemann, G. Expression of Zebrafish Aldh1a3 (Raldh3) and Absence of Aldh1a1 in Teleosts. Gene Expr. Patterns GEP 2008, 8, 141–147. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardeña-Núñez, S.; Callejas-Marín, A.; Villa-Carballar, S.; Rodríguez-Gallardo, L.; Sánchez-Guardado, L.Ó.; Hidalgo-Sánchez, M. CRABP-I Expression Patterns in the Developing Chick Inner Ear. Biology 2023, 12, 104. https://doi.org/10.3390/biology12010104
Cardeña-Núñez S, Callejas-Marín A, Villa-Carballar S, Rodríguez-Gallardo L, Sánchez-Guardado LÓ, Hidalgo-Sánchez M. CRABP-I Expression Patterns in the Developing Chick Inner Ear. Biology. 2023; 12(1):104. https://doi.org/10.3390/biology12010104
Chicago/Turabian StyleCardeña-Núñez, Sheila, Antuca Callejas-Marín, Sergio Villa-Carballar, Lucía Rodríguez-Gallardo, Luis Óscar Sánchez-Guardado, and Matías Hidalgo-Sánchez. 2023. "CRABP-I Expression Patterns in the Developing Chick Inner Ear" Biology 12, no. 1: 104. https://doi.org/10.3390/biology12010104
APA StyleCardeña-Núñez, S., Callejas-Marín, A., Villa-Carballar, S., Rodríguez-Gallardo, L., Sánchez-Guardado, L. Ó., & Hidalgo-Sánchez, M. (2023). CRABP-I Expression Patterns in the Developing Chick Inner Ear. Biology, 12(1), 104. https://doi.org/10.3390/biology12010104





