The Variety of Applications of Hermetia illucens in Industrial and Agricultural Areas—Review
Abstract
:Simple Summary
Abstract
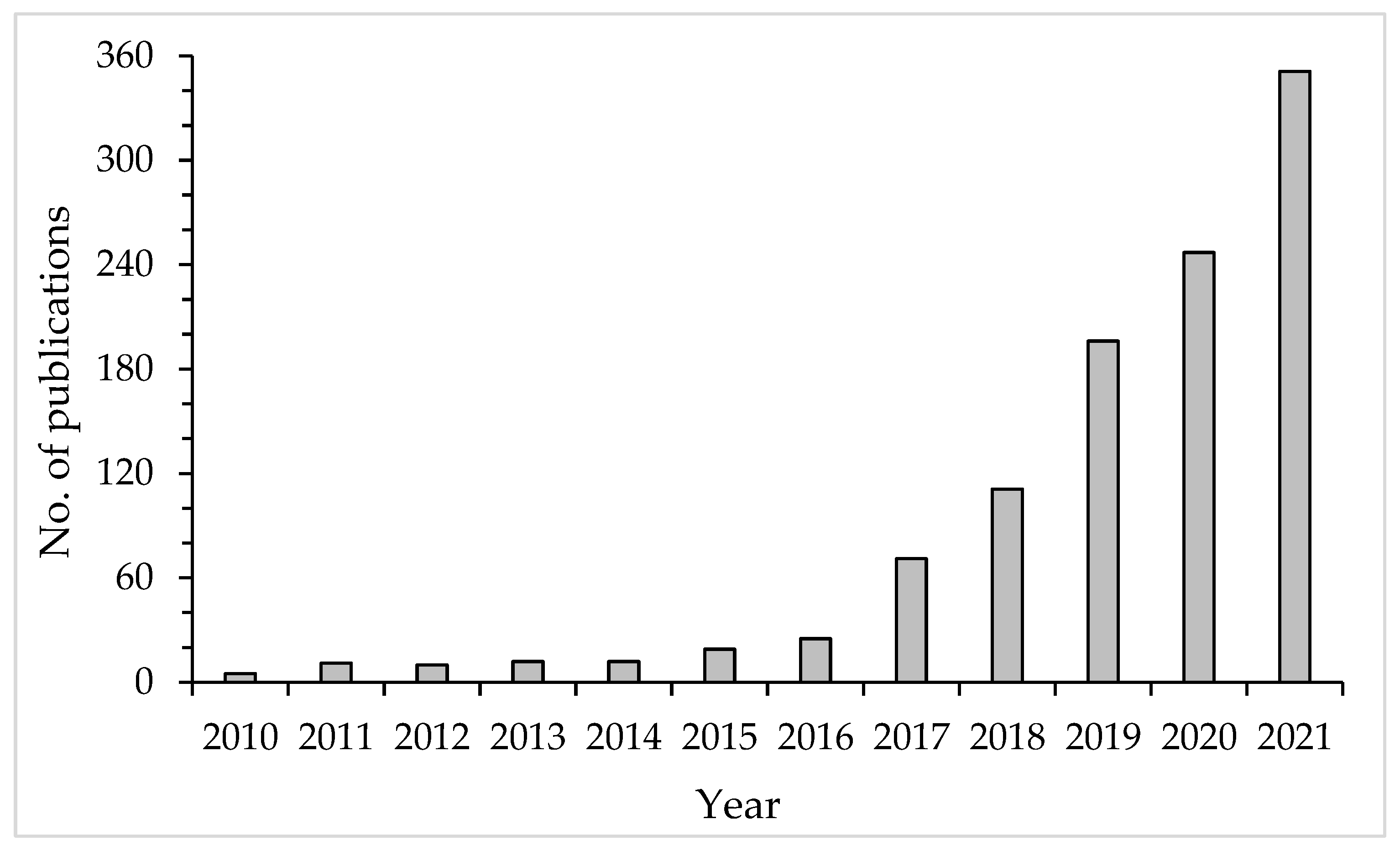
1. Introduction
2. Applications of H. illucens
2.1. Bioconversion of Waste Biomass
2.2. Animal Feed
2.3. Chitin and Chitosan
2.3.1. Chitin
2.3.2. Chitosan
2.4. Antimicrobial Properties
2.5. Biodiesel Production
| Crude Fat Content (%) | Main Acid Residue of FAME | Content of Main Component of FAME (%) | Diet | Development Stage of H. illucens | References |
|---|---|---|---|---|---|
| 33.6 a | lauric acid | 57.4 | chicken feed | prepupae | [18] |
| 21.8 a | lauric acid | 43.7 | biogas digestate | prepupae | |
| 37.1 a | lauric acid | 60.9 | vegetable waste | prepupae | |
| 38.6 a | lauric acid | 57.6 | restaurant waste | prepupae | |
| 35.0–40.0 | lauric acid | 65.7 | exo-microbial fermented coconut endosperm waste | larvae | [115] |
| 23.3 | lauric acid | 35.6 | dairy manure | larvae | [117] |
| - | lauric acid | 38.4 | wheat grain | larvae | [119] |
| - | lauric acid | 58.3 | sewage sludge | larvae | [120] |
| - | lauric acid | 76.1 | fruit waste | larvae | |
| - | lauric acid | 48.1 | palm decanters | larvae | |
| 39.2 | oleinic acid | 27.1 | solid residual fraction of restaurant waste | larvae | [121] |
| 57.8 | lauric acid | 51.8 | bread | larvae | [122] |
| 46.7 | lauric acid | 28.6 | fish | larvae | |
| 40.7 | lauric acid | 39.9 | food waste | larvae | |
| 33.1 | lauric acid | 52.1 | fresh mussels | larvae | |
| 11.2 | lauric acid | 13.4 | ensiled mussels | larvae | |
| 29.7 | lauric acid | 32.3 | rotten mussels | larvae | |
| 20.4 | lauric acid | 47.4 | bread and mussels 10% | larvae | |
| 19.6 | lauric acid | 47.6 | bread and mussels 20% | larvae | |
| 17.9 | lauric acid | 43.6 | bread and mussels 30% | larvae | |
| 17.9 | lauric acid | 42.0 | bread and mussels 40% | larvae | |
| 16.1 | lauric acid | 35.3 | bread and mussels 50% | larvae | |
| 35.7–39.6 | lauric acid | 27.8 | restaurant solid waste and exo-microbial fermented rice straw | larvae | [123] |
| - | lauric acid | 44.9 | food wastes from cafeteria | prepupae | [124] |
| 31.2 | lauric acid | 87.46 | chicken manure mixed with rapeseed straw | larvae | [125] |
2.6. Biogas Production
| Feedstock | Cumulative Biomethane Potential (mL CH4 g−1 VS) | CH4 Concentration (% vol.) | Reference | |
|---|---|---|---|---|
| Whole insect or its parts | H. illucens larvae-food waste | 675 ± 118 | n.d | [140] |
| H. illucens larvae-chicken feed | 661 ± 29 | n.d. | ||
| dead flies | 570 ± 51 | n.d. | ||
| lipid extracted H. illucens larvae-food waste | 363 ± 32 | n.d. | ||
| larval cuticule | 343 ± 7 | n.d. | ||
| lipid extracted H. illucens larvae-chicken feed | 306 ± 23 | n.d. | ||
| whole H. illucens larvae | 108 ± 65 | n.d. | ||
| whole H. illucens larvae | 455.87 ± n.d. | 64.27 | [141] | |
| H. illucens frass | H. illucens larvae post-breeding waste | 207.9 ± 21.5 | 53.2 ± 3.2 | [136] |
| H. illucens larvae-treated human faeces | 178.9 ± 7.1 | 55.2 ± 0.7 | [138] | |
| H. illucens larvae-treated food waste | 322.6 ± 6.4 | 61.4 ± 0.4 | ||
| H. illucens residues | 502 ± 9 | n.d. | [140] |
2.7. Entomoremediation
2.8. Insect Frass
2.9. H. illucens Larvae as a Food
2.10. H. illucens in Cosmetic, Cosmeuticals and Personal Care Products
2.11. Bioplastics
3. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lancaster, J.; Downes, B.J. Aquatic versus terrestrial insects: Real or presumed differences in population dynamics? Insects 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigel, E. Stork How many species of insects and other terrestrial arthropods are there on earth ? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar]
- Schaefer, C.W. The insects: Beneficial and harmful aspects. Proc. Entomol. Soc. Washingt. 2009, 111, 544–545. [Google Scholar] [CrossRef]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Larvae mediated valorization of industrial, agriculture and food wastes: Biorefinery concept and products. Processes 2020, 8, 857. [Google Scholar] [CrossRef]
- Kaya, C.; Generalovic, T.N.; Ståhls, G.; Hauser, M.; Samayoa, A.C.; Nunes-Silva, C.G.; Roxburgh, H.; Wohlfahrt, J.; Ewusie, E.A.; Kenis, M.; et al. Global population genetic structure and demographic trajectories of the black soldier fly, Hermetia Illucens. BMC Biol. 2021, 19, 94. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarkar, S. Sustainable waste management using black soldier fly larva: A review. Int. J. Environ. Sci. Technol. 2022, 19, 12701–12726. [Google Scholar] [CrossRef]
- Bertinetti, C.; Samayoa, A.; Hwang, S.-Y. Effects of Feeding Adults of Hermetia illucens (Diptera: Stratiomyidae) on longevity, oviposition, and egg hatchability: Insights into optimizing egg production. J. Insect Sci. 2019, 19, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Huis, A. Edible insects: Non-food and non-feed industrial applications. J. Insects Food Feed 2022, 8, 447–450. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion performance and life table of black soldier fly (Hermetia illucens) on fermented maize straw. J. Clean. Prod. 2019, 230, 974–980. [Google Scholar] [CrossRef]
- Barbi, S.; Macavei, L.I.; Fuso, A.; Luparelli, A.V.; Caligiani, A.; Ferrari, A.M.; Maistrello, L.; Montorsi, M. Valorization of seasonal agri-food leftovers through insects. Sci. Total Environ. 2020, 709, 136209. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, X.; Sun, Y.; Zhao, J.; Awasthi, M.K.; Liu, T.; Li, R.; Zhang, Z. Improvement of the composition and humification of different animal manures by black soldier fly bioconversion. J. Clean. Prod. 2021, 278, 123397. [Google Scholar] [CrossRef]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, K. Life-history traits of the black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), reared on three manure types. Animals 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, A.; Scieuzo, C.; Salvia, R.; Mancini, I.M.; Caniani, D.; Masi, S.; Falabella, P. A mobile black soldier fly farm for on-site disposal of animal dairy manure. Bull. Insectology 2022, 75, 75–82. [Google Scholar]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Awasthi, S.K.; Duan, Y.; Zhang, Z. Effects of black soldier fly larvae (Diptera: Stratiomyidae) on food waste and sewage sludge composting. J. Environ. Manage. 2020, 256, 109967. [Google Scholar] [CrossRef] [PubMed]
- Scieuzo, C.; Franco, A.; Salvia, R.; Triunfo, M.; Addeo, N.F.; Vozzo, S.; Piccolo, G.; Bovera, F.; Ritieni, A.; Francia, A.D.; et al. Enhancement of fruit byproducts through bioconversion by Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2022. [Google Scholar] [CrossRef]
- Giannetto, A.; Oliva, S.; Riolo, K.; Savastano, D.; Parrino, V.; Cappello, T.; Maisano, M.; Fasulo, S.; Mauceri, A. Waste valorization via Hermetia illucens to produce protein-rich biomass for feed: Insight into the critical nutrient taurine. Animals 2020, 10, 1710. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Singh, A.; Kumari, K. An inclusive approach for organic waste treatment and valorisation using black soldier fly larvae: A review. J. Environ. Manag. 2019, 251, 109569. [Google Scholar] [CrossRef] [PubMed]
- Surendra, K.C.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef]
- Diener, S.; Studt Solano, N.M.; Roa Gutiérrez, F.; Zurbrügg, C.; Tockner, K. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valorization 2011, 2, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Tomberlin, J.K.; Zheng, L.; Yu, Z.; Zhang, J. Developmental and waste reduction plasticity of three black soldier fly strains (Diptera: Stratiomyidae) raised on different livestock manures. J. Med. Entomol. 2013, 50, 1224–1230. [Google Scholar] [CrossRef]
- Dortmans, B.; Diener, S.; Verstappen, B.; Zurbrügg, C. Black Soldier Fly Biowaste Processing: A Step by Step Guide; Eawag-Swiss Federal Institute of Aquatic Science and Technology: Dübendorf, Switzerland, 2017; ISBN 978-3-906484-66-2. [Google Scholar]
- Liu, Z.; Minor, M.; Morel, P.C.H.; Najar-Rodriguez, A.J. Bioconversion of three organic wastes by black soldier fly (Diptera: Stratiomyidae) larvae. Environ. Entomol. 2018, 47, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, R.R.; El-Dakar, M.A.; Wang, D.; Ji, H. Conversion efficiency of lignin-rich olive pomace to produce nutrient-rich insect biomass by black soldier fly larvae, Hermetia illucens. Waste Biomass Valorization 2022, 13, 893–903. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Bastrakov, A.I.; Karagodin, V.P.; Pavlov, D.S. Specific features of organic waste bioconversion by Hermetia illucens fly larvae (Diptera: Stratiomyidae, Linnaeus, 1758). Biol. Bull. Rev. 2018, 8, 533–541. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Yao, H. Comprehensive resource utilization of waste using the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae). Animals 2019, 9, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, G.D.P.; Hesselberg, T. A Review of the use of black soldier fly larvae, Hermetia illucens (Diptera: Stratiomyidae), to compost organic waste in tropical regions. Neotrop. Entomol. 2020, 49, 151–162. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; Shafi, M.E.; Alghamdi, W.Y.; Abdelnour, S.A.; Shehata, A.M.; Noreldin, A.E.; Ashour, E.A.; Swelum, A.A.; Al-sagan, A.A.; Alkhateeb, M.; et al. Black soldier fly (Hermetia illucens) meal as a promising feed ingredient for poultry: A comprehensive review. Agriculture 2020, 10, 339. [Google Scholar] [CrossRef]
- English, G.; Wanger, G.; Colombo, S.M. A review of advancements in black soldier fly (Hermetia illucens) production for dietary inclusion in salmonid feeds. J. Agric. Food Res. 2021, 5, 100164. [Google Scholar] [CrossRef]
- Kawasaki, K.; Hashimoto, Y.; Hori, A.; Kawasaki, T.; Hirayasu, H.; Iwase, S.I.; Hashizume, A.; Ido, A.; Miura, C.; Miura, T.; et al. Evaluation of black soldier fly (Hermetia illucens) larvae and pre-pupae raised on household organic waste, as potential ingredients for poultry feed. Animals 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Moniello, G.; Ariano, A.; Panettieri, V.; Tulli, F.; Olivotto, I.; Messina, M.; Randazzo, B.; Severino, L.; Piccolo, G.; Musco, N.; et al. Intestinal morphometry, enzymatic and microbial activity in laying hens fed different levels of a Hermetia illucens larvae meal and toxic elements content of the insect meal and diets. Animals 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Ipema, A.F.; Bokkers, E.A.M.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E. Long-term access to live black soldier fly larvae (Hermetia illucens) stimulates activity and reduces fearfulness of broilers, without affecting health. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Boykin, K.L.; Carter, R.T.; Butler-Perez, K.; Buck, C.Q.; Peters, J.W.; Rockwell, K.E.; Mitchell, M.A. Digestibility of black soldier fly larvae (Hermetia illucens) fed to leopard geckos (Eublepharis macularius). PLoS One 2020, 15, e0232496. [Google Scholar] [CrossRef]
- Fisher, H.J.; Collins, S.A.; Hanson, C.; Mason, B.; Colombo, S.M.; Anderson, D.M. Black soldier fly larvae meal as a protein source in low fish meal diets for Atlantic salmon (Salmo salar). Aquaculture 2020, 521, 734978. [Google Scholar] [CrossRef]
- Guerreiro, I.; Castro, C.; Antunes, B.; Coutinho, F.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousão-Ferreira, P.; Matos, E.; et al. Catching black soldier fly for meagre: Growth, whole-body fatty acid profile and metabolic responses. Aquaculture 2020, 516, 734613. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M.R. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Li, Z.; Chen, W.; Wang, G.; Rong, T.; Liu, Z.; Wang, F.; Ma, X. Hermetia illucens larvae as a fishmeal replacement alters intestinal specific bacterial populations and immune homeostasis in weanling piglets. J. Anim. Sci. 2020, 1–13. [Google Scholar] [CrossRef]
- Lei, X.J.; Kim, T.H.; Park, J.H.; Kim, I.H. Evaluation of supplementation of defatted black soldier fly (Hermetia illucens) larvae meal in beagle dogs. Ann. Anim. Sci. 2019, 19, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Dabbou, S.; Ferrocino, I.; Gasco, L.; Schiavone, A.; Trocino, A.; Xiccato, G.; Barroeta, A.C.; Maione, S.; Soglia, D.; Biasato, I.; et al. Antimicrobial effects of black soldier fly and yellow mealworm fats and their impact on gut microbiota of growing rabbits. Animals 2020, 10, 1292. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.G.; Sánchez-Muros, M.J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.L. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Gariglio, M.; Dabbou, S.; Crispo, M.; Biasato, I.; Gai, F.; Gasco, L.; Piacente, F.; Odetti, P.; Bergagna, S.; Plach, I.; et al. Effects of the dietary inclusion of partially defatted black soldier fly (Hermetia illucens) meal on the blood chemistry and Ttssue (spleen, liver, thymus, and bursa of Fabricius) histology of muscovy ducks (Cairina moschata domestica). Animals 2019, 9, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Jung, T.S.; Ha, Y.J.; Gal, S.W.; Noh, C.W.; Kim, I.S.; Lee, J.H.; Yoo, J.H. Removal of fat from crushed black soldier fly larvae by carbon dioxide supercritical extraction. J. Anim. Feed Sci. 2019, 28, 83–88. [Google Scholar] [CrossRef]
- Fawole, F.J.; Adeoye, A.A.; Tiamiyu, L.O.; Ajala, K.I.; Obadara, S.O.; Ganiyu, I.O. Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): Effects on growth, nutrient utilization, haemato-physiological response, and oxidative stress biomarker. Aquaculture 2020, 518, 734849. [Google Scholar] [CrossRef]
- Rimoldi, S.; Antonini, M.; Gasco, L.; Moroni, F.; Terova, G. Intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) may be improved by feeding a Hermetia illucens meal/low-fishmeal diet. Fish Physiol. Biochem. 2021, 47, 365–380. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Hansen, J.; Nøkland, D.; Lagos, L.; Rawski, M.; Øverland, M. Full-fat black soldier fly larvae (Hermetia illucens) meal and paste in extruded diets for Atlantic salmon (Salmo salar): Effect on physical pellet quality, nutrient digestibility, nutrient utilization and growth performances. Aquaculture 2021, 530, 735785. [Google Scholar] [CrossRef]
- Kierończyk, B.; Sypniewski, J.; Mikołajczak, Z.; Rawski, M.; Pruszyńska-Oszmałek, E.; Sassek, M.; Kołodziejski, P.; Józefiak, D. Replacement of soybean oil with cold-extracted fat from Hermetia illucens in young turkey diets: Effects on performance, nutrient digestibility, selected organ measurements, meat and liver tissue traits, intestinal microbiota modulation, and physiological. Anim. Feed Sci. Technol. 2022, 286, 115210. [Google Scholar] [CrossRef]
- Cullere, M.; Schiavone, A.; Dabbou, S.; Gasco, L.; Zotte, A.D. Meat quality and sensory traits of finisher broiler chickens fed with black soldier fly (Hermetia illucens L.) larvae fat as alternative fat source. Animals 2019, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Al-Khalaifah, H.; Al-Nasser, A. Dietary source of polyunsaturated fatty acids influences cell cytotoxicity in broiler chickens. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Randazzo, B.; Cardinaletti, G.; Zarantoniello, M.; Mina, F.; Secci, G.; Tulli, F.; Olivotto, I.; Parisi, G. Dietary inclusion of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss): Lipid metabolism and fillet quality investigations. Aquaculture 2020, 529, 735678. [Google Scholar] [CrossRef]
- El-Dakar, M.A.; Ramzy, R.R.; Ji, H.; Plath, M. Bioaccumulation of residual omega-3 fatty acids from industrial Schizochytrium microalgal waste using black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2020, 268, 122288. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed - a review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Romano, N.; Sinha, A.; Powell, A.; Fischer, H. Mineral composition in black soldier fly (Hermetia illucens) larvae and resulting frass from fruit and their peels. J. Insects Food Feed 2022, 1–12. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.R.; Lee, S.; Baek, Y.-C.; Jeong, J.Y.; Bang, H.T.; Ji, S.Y.; Park, S.H. Effects of dietary inclusion level of microwave-dried and meal on carcass traits and meat quality in broilers. Animals 2021, 11, 665. [Google Scholar] [CrossRef]
- Guerreiro, I.; Serra, C.R.; Coutinho, F.; Couto, A.; Castro, C.; Rangel, F.; Peres, H.; Pousão-Ferreira, P.; Matos, E.; Gasco, L.; et al. Digestive enzyme activity and nutrient digestibility in meagre (Argyrosomus regius) fed increasing levels of black soldier fly meal (Hermetia illucens). Aquac. Nutr. 2021, 27, 142–152. [Google Scholar] [CrossRef]
- Lin, Y.S.; Liang, S.H.; Lai, W.L.; Lee, J.X.; Wang, Y.P.; Liu, Y.T.; Wang, S.H.; Lee, M.H. Sustainable extraction of chitin from spent pupal shell of black soldier fly. Processes 2021, 9, 976. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; Kady, E.M. El Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43. [Google Scholar]
- Weiss, I.M. Species-specific shells: Chitin synthases and cell mechanics in molluscs. Zeitschrift fur Krist. 2012, 227, 723–738. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef]
- Faria, R.R.; Guerra, R.F.; De Sousa Neto, L.R.; Motta, L.F.; Franca, E.D.F. Computational study of polymorphic structures of α- and β- chitin and chitosan in aqueous solution. J. Mol. Graph. Model. 2016, 63, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Rehman, K.; Feng, W.; Yang, D.; Rehman, R.; Cai, M.; Zhang, J.; Yu, Z.; Zheng, L. Physicochemical structure of chitin in the developing stages of black soldier fly. Int. J. Biol. Macromol. 2020, 149, 901–907. [Google Scholar] [CrossRef] [PubMed]
- D’Hondt, E.; Soetemans, L.; Bastiaens, L.; Maesen, M.; Jespers, V.; Van den Bosch, B.; Voorspoels, S.; Elst, K. Simplified determination of the content and average degree of acetylation of chitin in crude black soldier fly larvae samples. Carbohydr. Res. 2020, 488, 107899. [Google Scholar] [CrossRef]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of chitin extracted from black soldier fly in different life stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J. Clean. Prod. 2020, 275. [Google Scholar] [CrossRef]
- Złotko, K.; Waśko, A.; Kamiński, D.M.; Budziak-Wieczorek, I.; Bulak, P.; Bieganowski, A. Isolation of chitin from black soldier fly (Hermetia illucens) and its usage to metal sorption. Polymers (Basel). 2021, 13, 818. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Özel, N.; Elibol, M. A review on the potential uses of deep eutectic solvents in chitin and chitosan related processes. Carbohydr. Polym. 2021, 262. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Yan, T.; Wang, X.; Huang, J.; Kuang, Z.; Ye, M.; Pan, M. Selectivity of deproteinization and demineralization using natural deep eutectic solvents for production of insect chitin (Hermetia illucens). Carbohydr. Polym. 2019, 225. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Chin, Y.L.; Chen, W.N. Comparison of sustainable lipid and protein removal methods for the isolation of insect chitin from black soldier fly exoskeleton. ACS Food Sci. Technol. 2021, 1, 698–706. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; von Seggern, N.; Falabella, P.; Salvia, R.; Thomä, J.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Stegbauer, L.; et al. Purification of chitin from pupal exuviae of the black soldier fly. Waste Biomass Valorization 2022, 13, 1993–2008. [Google Scholar] [CrossRef]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and chitosan based composites for energy and environmental applications: A review. Waste Biomass Valorization 2021, 12, 4777–4804. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in functional chitin materials: A review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect chitin-based nanomaterials for innovative cosmetics and cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Black soldier fly Hermetia illucens as a novel source of chitin and chitosan. Int. J. Sci. 2019, 8, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Hahn, T.; Roth, A.; Ji, R.; Schmitt, E.; Zibek, S. Chitosan production with larval exoskeletons derived from the insect protein production. J. Biotechnol. 2020, 310, 62–67. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, S.C.; Nam, K.D.; Kim, T.H.; Jung, B.O.; Park, Y.I.; Synytsya, A.; Park, J.K. Chitosan isolated from black soldier flies Hermetia illucens: Structure and enzymatic hydrolysis. Process Biochem. 2022, 118, 171–181. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A. Antibacterial action of insect chitosan/gum arabic nanocomposites encapsulating eugenol and selenium nanoparticles. J. King Saud Univ.—Sci. 2022, 34. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and dhitosan derivatives: A review of the structure-activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; a review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial – a review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Choi, W.H.; Yun, J.H.; Chu, J.P.; Chu, K.B. Antibacterial effect of extracts of Hermetia illucens (Diptera: Stratiomyidae) larvae against gram-negative bacteria. Entomol. Res. 2012, 42, 219–226. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrügg, C.; Lindström, A.; Vinnerås, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens) - from a hygiene aspect. Sci. Total Environ. 2013, 458–460, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2015, 35, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, K.; Kawasaki, T.; Hirayasu, H.; Matsumoto, Y.; Fujitani, Y. Evaluation of fertilizer value of residues obtained after processing household organic waste with black soldier fly larvae (Hermetia illucens). Sustainability 2020, 12, 4920. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Liu, T.; Awasthi, S.K.; Duan, Y.; Pandey, A.; Zhang, Z. Manure pretreatments with black soldier fly Hermetia illucens L. (Diptera: Stratiomyidae): A study to reduce pathogen content. Sci. Total Environ. 2020, 737. [Google Scholar] [CrossRef] [PubMed]
- Marusich, E.; Mohamed, H.; Afanasev, Y.; Leonov, S. Fatty acids from Hermetia illucens larvae fat inhibit the proliferation and growth of actual phytopathogens. Microorganisms 2020, 8, 1423. [Google Scholar] [CrossRef]
- Mohamed, H.; Marusich, E.; Afanasev, Y.; Leonov, S. Bacterial outer membrane permeability increase underlies the bactericidal effect of fatty acids from Hermetia illucens (black soldier fly) larvae fat against hypermucoviscous isolates of Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef]
- Choi, W.H.; Choi, H.J.; Goo, T.W.; Quan, F.S. Novel antibacterial peptides induced by probiotics in Hermetia illucens (Diptera: Stratiomyidae) larvae. Entomol. Res. 2018, 48, 237–247. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial peptides from black soldier fly (Hermetia illucens) as potential antimicrobial factors representing an alternative to antibiotics in livestock farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef]
- Lee, K.S.; Yun, E.Y.; Goo, T.W. Antimicrobial activity of an extract of Hermetia illucens larvae immunized with Lactobacillus casei against Salmonella species. Insects 2020, 11, 704. [Google Scholar] [CrossRef]
- Lee, K.; Yun, E.; Goo, T. Evaluation of the antimicrobial activity of an extract of Lactobacillus casei -infected Hermetia illucens. Animals 2020, 10, 2121. [Google Scholar] [CrossRef]
- Shin, H.S.; Park, S.I. Novel attacin from Hermetia illucens: cDNA cloning, characterization, and antibacterial properties. Prep. Biochem. Biotechnol. 2019, 49, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Moretta, A.; Cané, C.; Scieuzo, C.; Salvia, R.; Falabella, P.; Duilio, A. Structural and functional characterization of a novel recombinant antimicrobial peptide from Hermetia illucens. Cell. Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, X.; Fang, G.; Zhan, S.; Wu, J.; Wang, D.; Huang, Y. Transgenic expression of antimicrobial peptides from black soldier fly enhance resistance against entomopathogenic bacteria in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2020, 127. [Google Scholar] [CrossRef] [PubMed]
- Rabani, V.; Cheatsazan, H.; Davani, S. Proteomics and lipidomics of black soldier fly (Diptera: Stratiomyidae) and blow fly (Diptera: Calliphoridae) larvae. J. Insect Sci. 2019, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, D.; Wilkinson, K.A.; Treilhou, M.; Téné, N.; Castillo, D.; Sauvain, M. Prospecting peptides isolated from black soldier fly (Diptera: Stratiomyidae) with antimicrobial activity against Helicobacter pylori (Campylobacterales: Helicobacteraceae). J. Insect Sci. 2019, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Neshani, A.; Zare, H.; Akbari Eidgahi, M.R.; Hooshyar Chichaklu, A.; Movaqar, A.; Ghazvini, K. Review of antimicrobial peptides with anti-Helicobacter pylori activity. Helicobacter 2019, 24, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Matsue, M.; Mori, Y.; Nagase, S.; Sugiyama, Y.; Hirano, R.; Ogai, K.; Ogura, K.; Kurihara, S.; Okamoto, S. Measuring the antimicrobial activity of lauric acid against various bacteria in human gut microbiota using a new method. Cell Transplant. 2019, 28, 1528–1541. [Google Scholar] [CrossRef] [Green Version]
- Saviane, A.; Tassoni, L.; Naviglio, D.; Lupi, D.; Savoldelli, S.; Bianchi, G.; Cortellino, G.; Bondioli, P.; Folegatti, L.; Casartelli, M.; et al. Mechanical processing of Hermetia illucens larvae and Bombyx mori pupae produces oils with antimicrobial activity. Animals 2021, 11, 783. [Google Scholar] [CrossRef]
- Correa, Y.; Cabanillas, B.; Jullian, V.; Álvarez, D.; Castillo, D.; Dufloer, C.; Bustamante, B.; Roncal, E.; Neyra, E.; Sheen, P.; et al. Identification and characterization of compounds from Chrysosporium multifidum, a fungus with moderate antimicrobial activity isolated from Hermetia illucens gut microbiota. PLoS ONE 2019, 14, e0218837. [Google Scholar] [CrossRef] [Green Version]
- Dontsov, A.E.; Ushakova, N.A.; Sadykova, V.S.; Bastrakov, A.I. Ommochromes from Hermetia illucens: Isolation and study of antioxidant characteristics and antimicrobial activity. Appl. Biochem. Microbiol. 2020, 56, 91–95. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Luparelli, A.V.; Caligiani, A.; Macavei, L.I.; Maistrello, L.; Neviani, E.; Galaverna, G.; Sforza, S.; Lazzi, C. Antimicrobial biomasses from lactic acid fermentation of black soldier fly prepupae and related by-products. Microorganisms 2020, 8, 1785. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the black soldier fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Feng, W.; Xiong, H.; Wang, W.; Duan, X.; Yang, T.; Wu, C.; Yang, F.; Xiong, J.; Wang, T.; Wang, C. Energy consumption analysis of lipid extraction from black soldier fly biomass. Energy 2019, 185, 1076–1085. [Google Scholar] [CrossRef]
- Wong, C.Y.; Lim, J.W.; Chong, F.K.; Lam, M.K.; Uemura, Y.; Tan, W.N.; Bashir, M.J.K.; Lam, S.M.; Sin, J.C.; Lam, S.S. Valorization of exo-microbial fermented coconut endosperm waste by black soldier fly larvae for simultaneous biodiesel and protein productions. Environ. Res. 2020, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Rehman, K.U.; Yu, Y.; Liu, X.; Wang, H.; Tomberlin, J.K.; Sze, S.H.; Cai, M.; Zhang, J.; Yu, Z.; et al. De novo transcriptome sequencing and analysis revealed the molecular basis of rapid fat accumulation by black soldier fly (Hermetia illucens, L.) for development of insectival biodiesel. Biotechnol. Biofuels 2019, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Hasnol, S.; Lim, J.W.; Wong, C.Y.; Lam, M.K.; Ntwampe, S.K.O. Liminal presence of exo-microbes inoculating coconut endosperm waste to enhance black soldier fly larval protein and lipid. Environ. Sci. Pollut. Res. 2020, 27, 24574–24581. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Brodskii, E.S.; Kovalenko, A.A.; Bastrakov, A.I.; Kozlova, A.A.; Pavlov, D.S. Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl. Biochem. Biophys. 2016, 468, 209–212. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47, 84–90. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Surendra, K.C.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Elsayed, M.; Ran, Y.; Ai, P.; Azab, M.; Mansour, A.; Jin, K.; Zhang, Y.; Abomohra, A.E.F. Innovative integrated approach of biofuel production from agricultural wastes by anaerobic digestion and black soldier fly larvae. J. Clean. Prod. 2020, 263, 121495. [Google Scholar] [CrossRef]
- Larouche, J.; Deschamps, M.-H.; Saucier, L.; Lebeuf, Y.; Doyen, A.; Vandenberg, G.W. Effects of killing methods on lipid oxidation, colour and microbial load of black soldier fly (Hermetia illucens) larvae. Animals 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Jing Lim, J.; Seng Liew, C.; Raksasat, R.; Merican, Z.M.A.; Kiatkittipong, K.; Alaaeldin Abdelfattah, E.; Mohamad, M.; Bashir, M.J.K.; Karabo Obed Ntwampe, S.; Lim, J.W. Cellulase pretreated palm decanter cake for feeding of black soldier fly larvae in triggering bioaccumulation of protein and lipid into biodiesel productions. Sustain. Energy Technol. Assess. 2022, 53, 102485. [Google Scholar] [CrossRef]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2019, 238. [Google Scholar] [CrossRef]
- Feng, W.; Xiong, H.; Wang, W.; Duan, X.; Yang, T.; Wu, C.; Yang, F.; Wang, T.; Wang, C. A facile and mild one-pot process for direct extraction of lipids from wet energy insects of black soldier fly larvae. Renew. Energy 2020, 147, 584–593. [Google Scholar] [CrossRef]
- Ishak, S.; Kamari, A. A review of optimum conditions of transesterification process for biodiesel production from various feedstocks. Int. J. Environ. Sci. Technol. 2019, 16, 2481–2502. [Google Scholar] [CrossRef]
- He, S.; Lian, W.; Liu, X.; Xu, W.; Wang, W.; Qi, S. Transesterification synthesis of high-yield biodiesel from black soldier fly larvae using the combination of lipase eversa transform 2.0 and lipase SMG1. Food Sci. Technol. 2022, 42, e103221. [Google Scholar] [CrossRef]
- Jung, S.; Jung, J.M.; Tsang, Y.F.; Bhatnagar, A.; Chen, W.H.; Lin, K.Y.A.; Kwon, E.E. Biodiesel production from black soldier fly larvae derived from food waste by non-catalytic transesterification. Energy 2022, 238, 121700. [Google Scholar] [CrossRef]
- Kamarulzaman, M.K.; Hafiz, M.; Abdullah, A.; Chen, A.F.; Awad, O.I. Combustion, performances and emissions characteristics of black soldier fly larvae oil and diesel blends in compression ignition engine. Renew. Energy 2019, 142, 569–580. [Google Scholar] [CrossRef]
- ur Rehman, K.; Liu, X.; Wang, H.; Zheng, L.; ur Rehman, R.; Cheng, X.; Li, Q.; Li, W.; Cai, M.; Zhang, J.; et al. Effects of black soldier fly biodiesel blended with diesel fuel on combustion, performance and emission characteristics of diesel engine. Energy Convers. Manag. 2018, 173, 489–498. [Google Scholar] [CrossRef]
- Bulak, P.; Proc, K.; Pawłowska, M.; Kasprzycka, A.; Berus, W.; Bieganowski, A. Biogas generation from insects breeding post production wastes. J. Clean. Prod. 2020, 244, 118777. [Google Scholar] [CrossRef]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens-a promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforsch.-Sect. C J. Biosci. 2017, 72, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Nordberg, Å.; Vinnerås, B. A comparison in product-value potential in four treatment strategies for food waste and faeces–assessing composting, fly larvae composting and anaerobic digestion. GCB Bioenergy 2018, 10, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities—A review. Front. Environ. Sci. Eng. 2018, 12, 4. [Google Scholar] [CrossRef]
- Win, S.S.; Ebner, J.H.; Brownell, S.A.; Pagano, S.S.; Cruz-Diloné, P.; Trabold, T.A. Anaerobic digestion of black solider fly larvae (BSFL) biomass as part of an integrated biorefinery. Renew. Energy 2018, 127, 705–712. [Google Scholar] [CrossRef]
- Czekała, W.; Janczak, D.; Cieślik, M.; Mazurkiewicz, J.; Pulka, J. Food waste management using the Hermetia illucens insect. J. Ecol. Eng. 2020, 21, 214–216. [Google Scholar] [CrossRef]
- Ewuim, S. Entomoremediation-a novel in-situ bioremediation approach. Anim. Res. Int. 2013, 10, 1681–1684. [Google Scholar]
- Bulak, P.; Polakowski, C.; Nowak, K.; Waśko, A.; Wiącek, D.; Bieganowski, A. Hermetia illucens as a new and promising species for use in entomoremediation. Sci. Total Environ. 2018, 633, 912–919. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Bioaccumulation of heavy metals in the black soldier fly, Hermetia illucens and effects on its life cycle. J. Insects Food Feed 2015, 1, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Van Der Fels-Klerx, H.J.; Camenzuli, L.; Van Der Lee, M.K.; Oonincx, D.G.A.B. Uptake of cadmium, lead and arsenic by Tenebrio molitor and Hermetia illucens from contaminated substrates. PLoS ONE 2016, 11, e0166186. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, X.; Wang, W.; Lei, C.; Zhu, F. Influences of chromium and cadmium on the development of black soldier fly larvae. Environ. Sci. Pollut. Res. 2017, 24, 8637–8644. [Google Scholar] [CrossRef] [PubMed]
- Purschke, B.; Scheibelberger, R.; Axmann, S.; Adler, A.; Jäger, H. Impact of substrate contamination with mycotoxins, heavy metals and pesticides on growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit. Contam. Part A 2017, 34, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Proc, K.; Bulak, P.; Wiącek, D.; Bieganowski, A. Hermetia illucens exhibits bioaccumulative potential for 15 different elements–implications for feed and food production. Sci. Total Environ. 2020, 723, 138125. [Google Scholar] [CrossRef]
- Proc, K.; Bulak, P.; Kaczor, M.; Bieganowski, A. A new approach to quantifying bioaccumulation of elements in biological processes. Biology 2021, 10, 345. [Google Scholar] [CrossRef]
- Bulak, P.; Walkiewicz, A.; Brzezińska, M. Plant growth regulators-assisted phytoextraction. Biol. Plant. 2014, 58, 1–8. [Google Scholar] [CrossRef]
- Mohanty, M. Post-harvest management of phytoremediation technology. J. Environ. Anal. Toxicol. 2016, 6, 5. [Google Scholar] [CrossRef]
- Schmitt, E.; Belghit, I.; Johansen, J.; Leushuis, R.; Lock, E.-J.; Melsen, D.; Kathirampatti Ramasamy Shanmugam, R.; Van Loon, J.; Paul, A. Growth and safety assessment of feed streams for aquaculture sludge. Animals 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutiérrez-Ginés, M.J. Black Soldier Fly-based bioconversion of biosolids creates high-value products with low heavy metal concentrations. Resour. Conserv. Recycl. 2022, 180, 106149. [Google Scholar] [CrossRef]
- Jiang, D.; Jiang, K.; Li, R.; Zhao, L.; Liu, Z.; Xiong, B.; Jin, D.; Hao, X.; Zhu, L.; Kang, B.; et al. Influence of different inoculation densities of black soldier fly larvae (Hermetia illucens) on heavy metal immobilization in swine manure. Environ. Sci. Pollut. Res. 2022, 29, 54378–54390. [Google Scholar] [CrossRef]
- Leni, G.; Cirlini, M.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Sforza, S.; Dall’Asta, C. Impact of naturally contaminated substrates on alphitobius diaperinus and Hermetia illucens: Uptake and excretion of mycotoxins. Toxins 2019, 11, 476. [Google Scholar] [CrossRef] [Green Version]
- Meijer, N.; Stoopen, G.; van Der Fels-Klerx, H.J.; van Loon, J.J.A.; Carney, J.; Bosch, G. Aflatoxin B1 conversion by black soldier fly (Hermetia illucens) larval enzyme extracts. Toxins 2019, 11, 532. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Liu, N.; Wu, X.; Zhang, J.; Cai, M. Tolerance and removal of four polycyclic aromatic hydrocarbon compounds (PAHs) by black soldier fly (Diptera: Stratiomyidae). Environ. Entomol. 2020, 49, 667–672. [Google Scholar] [CrossRef]
- Meijer, N.; de Rijk, T.; van Loon, J.J.A.; Zoet, L.; van der Fels-Klerx, H.J. Effects of insecticides on mortality, growth and bioaccumulation in black soldier fly (Hermetia illucens) larvae. PLoS ONE 2021, 16, e0249362. [Google Scholar] [CrossRef]
- Liu, C.; Yao, H.; Chapman, S.J.; Su, J.; Wang, C. Changes in gut bacterial communities and the incidence of antibiotic resistance genes during degradation of antibiotics by black soldier fly larvae. Environ. Int. 2020, 142, 105834. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Q.; Lin, Y.; Tang, Z.; Tomberlin, J.K.; Liu, W.; Huang, Y. Black soldier fly larvae effectively degrade lincomycin from pharmaceutical industry wastes. J. Environ. Manag. 2022, 307, 114539. [Google Scholar] [CrossRef]
- Guyomard, H.; Bouamra-Mechemache, Z.; Chatellier, V.; Delaby, L.; Détang-Dessendre, C.; Peyraud, J.L.; Réquillart, V. Review: Why and how to regulate animal production and consumption: The case of the European Union. Animals 2021, 15, 100283. [Google Scholar] [CrossRef]
- Bosch, G.; van Zanten, H.H.E.; Zamprogna, A.; Veenenbos, M.; Meijer, N.P.; van der Fels-Klerx, H.J.; van Loon, J.J.A. Conversion of organic resources by black soldier fly larvae: Legislation, efficiency and environmental impact. J. Clean. Prod. 2019, 222, 355–363. [Google Scholar] [CrossRef]
- EU Commission Regulation 2021/1925 of 5 November 2021 amending certain Annexes to Regulation (EU) No 142/2011 as regards the requirements for placing on the market of certain insect products and the adaptation of a containment method. Official Journal of the European Union, L 393, 8.11.2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32021R1925&from=EN (accessed on 22 December 2022).
- Borkent, S.; Hodge, S. Glasshouse evaluation of the black soldier fly waste product hexafrassTM as an organic fertilizer. Insects 2021, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Van Looveren, N.; Vandeweyer, D.; Van Campenhout, L. Impact of heat treatment on the microbiological quality of frass originating from black soldier fly larvae (Hermetia illucens). Insects 2022, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Gebremikael, M.T.; Ranasinghe, A.; Hosseini, P.S.; Laboan, B.; Sonneveld, E.; Pipan, M.; Oni, F.E.; Montemurro, F.; Höfte, M.; Sleutel, S.; et al. How do novel and conventional agri-food wastes, co-products and by-products improve soil functions and soil quality? Waste Manag. 2020, 113, 132–144. [Google Scholar] [CrossRef]
- Arabzadeh, G.; Delisle-Houde, M.; Tweddell, R.J.; Deschamps, M.H.; Dorais, M.; Lebeuf, Y.; Derome, N.; Vandenberg, G. Diet composition influences growth performance, bioconversion of black soldier fly larvae: Agronomic value and in vitro biofungicidal activity of derived frass. Agronomy 2022, 12, 1765. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Turan, V.; Juárez, M.F.D.; Oberegger, S.; Insam, H. Suitability of black soldier fly frass as soil amendment and implication for organic waste hygienization. Agronomy 2020, 10, 578. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N. Fruit, vegetable, and starch mixtures on the nutritional quality of black soldier fly (Hermetia illucens) larvae and resulting frass. J. Insects Food Feed. 2021, 7, 319–327. [Google Scholar] [CrossRef]
- Sarpong, D.; Oduro-Kwarteng, S.; Gyasi, S.F.; Buamah, R.; Donkor, E.; Awuah, E.; Baah, M.K. Biodegradation by composting of municipal organic solid waste into organic fertilizer using the black soldier fly (Hermetia illucens) (Diptera: Stratiomyidae) larvae. Int. J. Recycl. Org. Waste Agric. 2019, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Mazza, L.; Yu, Y.; Cai, M.; Zheng, L.; Tomberlin, J.K.; Yu, J.; van Huis, A.; Yu, Z.; Fasulo, S.; et al. Efficient co-conversion process of chicken manure into protein feed and organic fertilizer by Hermetia illucens L. (Diptera: Stratiomyidae) larvae and functional bacteria. J. Environ. Manag. 2018, 217, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Dubois, T.; Subramanian, S.; Wangu, M.M.; Ekesi, S.; et al. Biochar and gypsum amendment of agro-industrial waste for enhanced black soldier fly larval biomass and quality frass fertilizer. PLoS ONE 2020, 15, e0238154. [Google Scholar] [CrossRef] [PubMed]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Wangu, M.M.; Dubois, T.; Ekesi, S.; et al. Low-cost technology for recycling agro-industrial waste into nutrient-rich organic fertilizer using black soldier fly. Waste Manag. 2021, 119, 183–194. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.; Musyoka, M.W.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Ekesi, S.; et al. Nitrogen Fertilizer Equivalence of Black Soldier Fly Frass Fertilizer and Synchrony of Nitrogen Mineralization for Maize Production. Agronomy 2020, 10, 1395. [Google Scholar] [CrossRef]
- Wu, X.; Cai, R.; Wang, X.; Wu, N.; Xu, X. Study on effects of black soldier fly feces on rice growth. IOP Conf. Ser. Earth Environ. Sci. 2020, 450, 012099. [Google Scholar] [CrossRef]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae processing residue in peat-based growing media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef]
- Romano, N.; Powell, A.; Islam, S.; Fischer, H.; Renukdas, N.; Sinha, A.K.; Francis, S. Supplementing aquaponics with black soldier fly (Hermetia illucens) larvae frass tea: Effects on the production and composition of sweetpotato slips and sweet banana peppers. Aquaculture 2022, 555. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World; FAO: Rome, Italy, 2017; ISBN 978-92-5-109888-2. [Google Scholar]
- Mshayisa, V.V.; Van Wyk, J.; Zozo, B. Nutritional, techno-functional and structural properties of black soldier fly (Hermetia illucens) larvae flours and protein concentrates. Foods 2022, 11, 724. [Google Scholar] [CrossRef]
- Anankware, J.P.; Roberts, B.J.; Cheseto, X.; Osuga, I.; Savolainen, V.; Collins, C.M. The nutritional profiles of five important edible insect species from west Africa—An analytical and literature synthesis. Front. Nutr. 2021, 8, 792941. [Google Scholar] [CrossRef]
- Raes, K.; Balcaen, A.; Dirinck, P.; De Winne, A.; Claeys, E.; Demeyer, D.; De Smet, S. Meat quality, fatty acid composition and flavour analysis in belgian retail beef. Meat Sci. 2003, 65, 1237–1246. [Google Scholar] [CrossRef]
- Zozo, B.; Wicht, M.M.; Mshayisa, V.V.; van Wyk, J. The nutritional quality and structural analysis of black soldier fly larvae flour before and after defatting. Insects 2022, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for feed and not for human consumption? The black soldier fly larvae. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Dhanani, K.; Hoffman, L.C. Food safety of consuming black soldier fly (Hermetia illucens) larvae: Microbial, heavy metal and cross-reactive allergen risks. Foods 2021, 10, 1934. [Google Scholar] [CrossRef]
- Bertola, M.; Mutinelli, F. A systematic review on viruses in mass-reared edible insect species. Viruses 2021, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Rijo, P.; Rosado, C. Bioactive compounds from Hermetia illucens larvae as natural ingredients for cosmetic application. Biomolecules 2020, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an innovative and sustainable source. Sustainability 2021, 13, 198. [Google Scholar] [CrossRef]
- Xu, W.; Xu, L.; Liu, X.; He, S.; Ji, Y.; Wang, W.; Wang, F. An Effective strategy for the production of lauric acid–enriched monoacylglycerol via enzymatic glycerolysis from black soldier fly (Hermetia illucens) larvae (BSFL) oil. Appl. Biochem. Biotechnol. 2021, 193, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Nugroho, D.S.; Cheng, Y.S.; Chang, J.Y. Development and characterization of nano-emulsions based on oil extracted from black soldier fly larvae. Appl. Biochem. Biotechnol. 2020, 191, 331–345. [Google Scholar] [CrossRef]
- Almeida, C.; Murta, D.; Nunes, R.; Baby, A.R.; Fernandes, Â.; Barros, L.; Rijo, P.; Rosado, C. Characterization of lipid extracts from the Hermetia illucens larvae and their bioactivities for potential use as pharmaceutical and cosmetic ingredients. Heliyon 2022, 8, e09455. [Google Scholar] [CrossRef]
- Verheyen, G.R.; Ooms, T.; Vogels, L.; Vreysen, S.; Bovy, A.; Van Miert, S.; Meersman, F. Insects as an alternative source for the production of fats for cosmetics. J. Cosmet. Sci. 2018, 69, 187–202. [Google Scholar]
- Barbi, S.; Messori, M.; Manfredini, T.; Pini, M.; Montorsi, M. Rational design and characterization of bioplastics from Hermetia illucens prepupae proteins. Biopolymers 2019, 110, e23250. [Google Scholar] [CrossRef] [PubMed]
- Nuvoli, D.; Montevecchi, G.; Lovato, F.; Masino, F.; Van Der Borght, M.; Messori, M.; Antonelli, A. Protein films from black soldier fly (Hermetia illucens, Diptera: Stratiomyidae) prepupae: Effect of protein solubility and mild crosslinking. J. Sci. Food Agric. 2021, 101, 4506–4513. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Francia, E.; Pulvirenti, A.; De Leo, R.; Martinelli, S.; Maistrello, L.; MacAvei, L.I.; Montorsi, M.; Barbi, S.; Ronga, D. Bioplastic film from black soldier fly prepupae proteins used as mulch: Preliminary results. Agronomy 2020, 10, 933. [Google Scholar] [CrossRef]
- Józefiak, D.; Józefiak, A.; Kierończyk, B.; Rawski, M.; Świątkiewicz, S.; Długosz, J.; Engberg, R.M. Insects—A natural nutrient source for poultry-a review. Ann. Anim. Sci. 2016, 16, 297–313. [Google Scholar] [CrossRef] [Green Version]
- Majewski, P.; Zapotoczny, P.; Lampa, P.; Burduk, R.; Reiner, J. Multipurpose monitoring system for edible insect breeding based on machine learning. Sci. Rep. 2022, 12, 7892. [Google Scholar] [CrossRef]
- Łochyńska, M.; Frankowski, J. The biogas production potential from silkworm waste. Waste Manag. 2018, 79, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, M.; Krzeminska, I. Enhancement of biogas production by co-digestion of maize silage with common goldenrod rich in biologically active compounds. BioResources 2017, 12, 704–714. [Google Scholar] [CrossRef] [Green Version]
- Brooks, R.R.; Lee, J.; Jaffré, T. Some New Zealand and New Caledonian plant accumulators of nickel. J. Ecol. 1974, 62, 493–499. [Google Scholar] [CrossRef]
- Mogren, C.L.; Trumble, J.T. The impacts of metals and metalloids on insect behavior. Entomol. Exp. Appl. 2010, 135, 1–17. [Google Scholar] [CrossRef]
- Boyd, R.S. High-nickel insects and nickel hyperaccumulator plants: A review. Insect Sci. 2009, 16, 19–31. [Google Scholar] [CrossRef]
- Thompson, E.D.; Hogstrand, C.; Glover, C.N. From sea squirts to squirrelfish: Facultative trace element hyperaccumulation in animals. Metallomics 2018, 10, 777–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Microbial producers of plant growth stimulators and their practical use: A review. Appl. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- Dutta, T.; Nandy, S.; Singh, J.; Pandey, D.K.; Dey, A. Chitin and chitosan as elicitors in sustainable production of medicinal crops. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 413–426. [Google Scholar] [CrossRef]
| Animal | Form of H. illucens | Effects | References |
|---|---|---|---|
| Positive influence | |||
| Laying hens | Soybean meal and soybean oil + H. illucens pre-pupae | Increased egg weight by 1.1 times, increased SCFA concentration by 1.3 times | [32] |
| Laying hens | Corn-soybean meal + 25% of replaced protein by partially defatted H. illucens | Despite the reduced length of the intestinal villi, increased the amount of volatile fatty acids by 1.1 times and the amount of butyrate by 1.2 times in intestines | [33] |
| Hen broilers | Chicken feed + 5% or 10% of live H. illucens larvae | Decreased timidity of hens, increased activity of hens | [34] |
| Atlantic salmon (Salmo salar) | Corn protein, soybean meal + (200 g·kg−1) H. illucens meal | No significant differences to control | [36] |
| European seabass (Dicentrarchus labrax) | Fishmeal + 50% dried H. illucens larvae meal | No significant differences to control | [38] |
| Finishing pigs | Corn, wheat bran and soybean meal + 4% dried and crushed H. illucens prepupea | Decreased expression of pro-inflammatory cytokines and concentrations of total amines and phenol, increased expression of anti-inflammatory cytokines, intestinal barrier genes and concentrations of short-chain fatty acids (SCFA) and butyrate (prebiotic effect) | [39] |
| Weanling piglets | Fishmeal + 2% full-fat H. illucens larvae meal | Increased lactate in illeum by 1.6 times, in cecum by 2.2 times, and SCFA by 1.2 times in illeum and by 1.1 in cecum (probiotic effect), increased anti-inflammatory protein IL-10 by 1.3 times, decreased pro-inflammatory protein TNF-α by 1.3 times | [40] |
| Beagle dogs | Grain-based diet + 2% defatted H. illucens larvae meal | Improved dry matter digestibility by 1.1 times, decreased TNF-α levels by 1.8 times (anti-inflammatory effect), increased glutathione peroxidase levels by 1.23 times (antioxidant effect) | [41] |
| Rabbits | Rabbits feed + 1.5% H. illucens fat | Inhibition of the growth of the pathogens Pasteurella multocida by 3.2 times, Yersinia enterocolitica by 2.5 times, Listeria monocytogenes by 2.1 times | [43] |
| Muscovy ducklings (Cairina moschata domestica) | 9% partially defatted H. illucens meal | Decrease in uric acid by 1.2 times and creatinine by 1.2 times (improved kidney function), increase in serum iron Fe by 1.3 times | [45] |
| African catfish (Clarias gariepinus) | Fishmeal + 50% partially defatted H. illucens larvae meal | Increase in body weight by 1.5 times | [47] |
| Rainbow trout (Oncorhynchus mykiss) | Control diet (wheat gluten, soybean meal and hemoglobin) + 15% H. illucens larvae meal | Increase in the number of beneficial Lactobacillus and Bacillus bacteria, reduction in Aeromonas pathogens in fish gut | [48] |
| Female turkeys | Soybean-maize enriched with 50 g/kg H. illucens larvae fat (50% and 100%) | Improved intestinal digestibility of the ether extract Increase in lipase activity Reduction of Bacteroides-Prevotella clusters | [50] |
| Negative influence | |||
| Meagre (Argyrosomus regius) | Partially defatted H. illucens + fishmeal | Weight loss, decrease in protein efficiency | [37] |
| Atlantic salmon (Salmo salar) | Control diet with full-fat H. illucens larvae meal, substituting 12.5% content of protein and control diet with full-fat H. illucens larvae paste, substituting 6.7% of protein | Decrease in protein and lipid efficiency and protein efficiency index, decrease in phosphorus retention | [49] |
| Extraction Methods | Insect Material | Chitin Content (%) | Crystalline Index (%) | References |
|---|---|---|---|---|
| Demineralization: 1 M HCl (1 h), deproteinization: 1 M NaOH (80 °C, 24 h), depigmentation: 1% KMnO4 | puparium | n.d. | 35.0 | [64] |
| adults | n.d. | 24.9 | ||
| Demineralization: 2 M HCl (55 °C, 1 h, 200 rpm·min−1), deproteinization: 2 M NaOH (50 °C, 18 h, 200 rpm·min−1), depigmentation: 3.6% HCl (0.5 h), 10-fold diluted NaClO (80 °C, 4 h, 200 rpm·min−1) | larvae | 3.6 | 33.1 | [65] |
| prepupae | 3.1 | 35.1 | ||
| puparium | 14.1 | 68.4 | ||
| adults | 2.9 | 87.92 | ||
| Demineralization: 1 M HCl (1:10 (m:v), room temp., 1 h), deproteinization: 1 M NaOH (1:25, 80 °C, 1 h), depigmentation: 12-fold repetition of the deproteinization process | larvae | 9.5 | ~88 | [67] |
| prepupae | 9.1 | ~95 | ||
| pupae | 10.3 | ~93 | ||
| larvae shedding | 31.1 | ~90 | ||
| puparium | 23.8 | ~94 | ||
| adults | 5.6 | ~89 | ||
| Demineralization: 1 M HCl (100 °C, 0.5 h), deproteinization: 1 M NaOH (24 h) | puparium | 25.4 | 74.1 | [68] |
| flakes after oil extraction | 20.7 | 61.1 | ||
| adults | 7.8 | 77.8 | ||
| Acid detergent fiber—acid detergent lignin | puparium | 21.2 | 70.8 | |
| flakes after oil extraction | 26.8 | 50.0 | ||
| adults | 7.9 | 39.0 | ||
| Demineralization: 1 M HCl, 22 °C, 1 h, deproteinization: 1 M NaOH, 80 °C, 24 h, depigmentation: 9% H2O2, 80 °C, 2.5 h | puparium | 7.0 | 60.0 | [69] |
| Demineralization: 0.5 M formic acid (1:10 (m:v)), 1 h, room temperature, deproteinization: 2 M NaOH (1:10 (m:v)), 2 h, 80 °C | larvae | 13.0 | 74.0 | [70] |
| puparium | 31.0 | 78.0 | ||
| adults | 9.0 | 79.0 | ||
| Demineralization: 0.5 M formic acid (1:10 (m:v)), 1 h, room temperature, deproteinization: 2 M NaOH (1:10 (m:v)), 2 h, 80 °C, depigmentation: 5% H2O2, (1:20–30), 30–60 min, 90 °C | larvae | 10.0 | 77.0 | |
| puparium | 23.0 | 80.0 | ||
| adults | 6.0 | 86.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczor, M.; Bulak, P.; Proc-Pietrycha, K.; Kirichenko-Babko, M.; Bieganowski, A. The Variety of Applications of Hermetia illucens in Industrial and Agricultural Areas—Review. Biology 2023, 12, 25. https://doi.org/10.3390/biology12010025
Kaczor M, Bulak P, Proc-Pietrycha K, Kirichenko-Babko M, Bieganowski A. The Variety of Applications of Hermetia illucens in Industrial and Agricultural Areas—Review. Biology. 2023; 12(1):25. https://doi.org/10.3390/biology12010025
Chicago/Turabian StyleKaczor, Monika, Piotr Bulak, Kinga Proc-Pietrycha, Marina Kirichenko-Babko, and Andrzej Bieganowski. 2023. "The Variety of Applications of Hermetia illucens in Industrial and Agricultural Areas—Review" Biology 12, no. 1: 25. https://doi.org/10.3390/biology12010025





