COI Haplotyping and Comparative Microbiomics of the Peach Fruit Fly, an Emerging Pest of Egyptian Olive Orchards
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Morphological Identification of Fruit Flies
- Thorax/Scutum: red brown scutum with lateral yellow vittae (stripes) down each side, in posterior part shorter (posterior 2/3)
- Abdomen: pair of dark marks on tergite III
- Wings: lack of a complete costal band, reduced to an isolated apical spot
- Thorax/Scutum: black scutum, lateral and medial vittae absent
- Abdomen: terga with dark anterolateral corners
- Wings: costal band absent and apical spot (small spot around the apex of R4 + 5)
- Thorax/Scutum: yellowish scutum with numerous black areas in a characteristic pattern
- Wings: wings are relatively broad in comparison to length, cloudy yellow, with three brown bands separated from each other and small dark irregular streaks in the proximal half of the wing
2.2. DNA Extraction and PCR Amplification
- the COI gene encoding cytochrome c oxidase I gene (primer pair LCO1490-mod/HCO2198-mod),
- the ef1a gene encoding translation elongation factor 1 alpha (M46-1/M4rc),
- the CAD1 region of the gene encoding the trifunctional carbamoyl-phosphate synthetase 2—aspartate transcarbamylase—dihydroorotase protein CAD (CAD-Bd-F/R),
- the per gene encoding the PERIOD protein involved in circadian rhythm regulation (Per2612F/Per3105R).
2.3. COI Haplotype Analysis
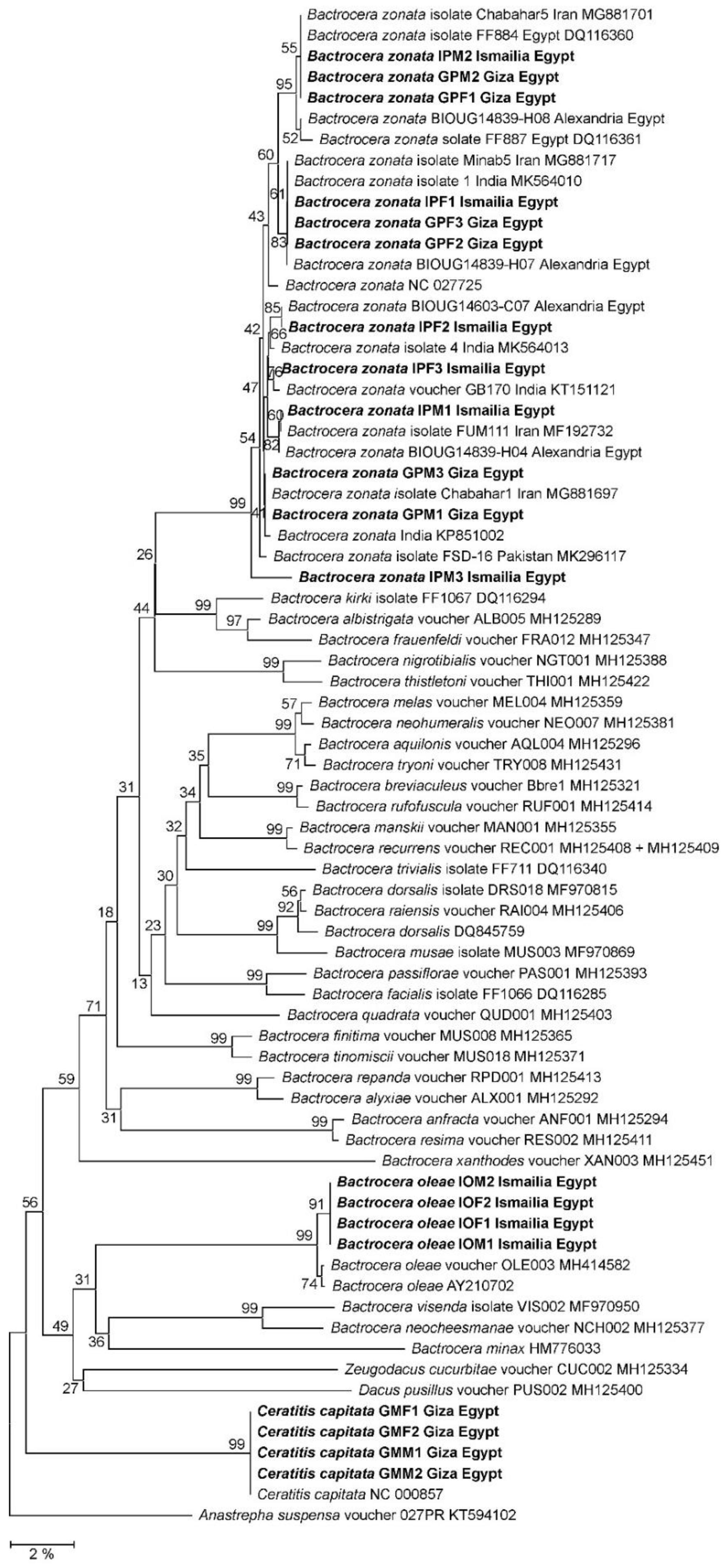
2.4. Phylogenetic Reconstruction
2.5. Microbiome Sequencing
3. Results
3.1. Sampling and Morphological Identification of Fruit Flies
3.2. Molecular Taxonomy of Fruit Flies
3.3. Diversity of Fruit Fly Populations
3.4. The Bacterial Microbiome of B. zonata from Different Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 November 2022).
- Yacout, D.; Soliman, F.N.; Zhran, F.H. Potentials of a sustainable olive industry in Egypt. In Proceedings of the International Conference of Biotechnology and Environment, Alexandria, Egypt, 1–3 November 2016; p. 57. [Google Scholar]
- EPPO. Bactrocera oleae (DACUOL) Datasheet. EPPO Global Database. 2022. Available online: https://gd.eppo.int/taxon/DACUOL (accessed on 1 November 2022).
- EPPO. Ceratitis capitata (CERTCA) Datasheet. EPPO Global Database. 2022. Available online: https://gd.eppo.int/taxon/CERTCA (accessed on 1 November 2022).
- Davis, M.A. Invasion Biology; Oxford University Press: Oxford, UK, 2009; Volume 74–75, pp. 101–106. [Google Scholar]
- Zeng, Y.; Reddy, G.V.P.; Li, Z.; Qin, Y.; Wang, Y.; Pan, X.; Jiang, F.; Gao, F.; Zhao, Z.H. Global distribution and invasion pattern of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). J. Appl. Entomol. 2019, 143, 165–176. [Google Scholar] [CrossRef]
- Zingore, K.M.; Sithole, G.; Abdel-Rahman, E.M.; Mohamed, S.A.; Ekesi, S.; Tanga, C.M. Global risk of invasion by Bactrocera zonata: Implications on horticultural crop production under changing climatic conditions. PLoS ONE 2020, 15, e0243047. [Google Scholar] [CrossRef] [PubMed]
- EPPO. Bactrocera zonata (DACUZO) Datasheet. EPPO Global Database. 2022. Available online: https://gd.eppo.int/taxon/DACUZO (accessed on 1 November 2022).
- El-Samea, S.; Fetoh, B. New record of Bactrocera zonata (Saunders) (Diptera: Tephritidae) on potatoes in Egypt. Egypt. J. Agric. Res 2006, 84, 61–63. [Google Scholar]
- Delrio, G.; Cocco, A. The peach fruit fly, Bactrocera zonata: A major threat for Mediterranean fruit crops? In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on the 940, Lisbon, Portugal, 22–27 August 2010; pp. 557–566. [Google Scholar]
- Ni, W.L.; Li, Z.H.; Chen, H.J.; Wan, F.H.; Qu, W.W. Including climate change in pest risk assessment: The peach fruit fly, Bactrocera zonata (Diptera: Tephritidae). Bull. Entomol. Res 2012, 102, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayoumy, M.H.; Michaud, J.P.; Badr, F.A.A.; Ghanim, N.M. Validation of degree-day models for predicting the emergence of two fruit flies (Diptera: Tephritidae) in northeast Egypt. Insect Sci. 2021, 28, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Nugnes, F.; Russo, E.; Viggiani, G.; Bernardo, U. First Record of an Invasive Fruit Fly Belonging to Bactrocera dorsalis Complex (Diptera: Tephritidae) in Europe. Insects 2018, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- EPPO. European and Mediterranean Plant Protection Organization A1 and A2 Lists of Pests Recommended for Regulation as Quarantine Pests; EPPO: Paris, France, 2016. [Google Scholar]
- Taher, M. Bactrocera zonata (Saunders) in Egypt. Disease and pest outbreaks. Arab. Near East Plant Prot. Newsl. 1998, 27, 30. [Google Scholar]
- EPPO. EPPO Reporting Service no.04/1999. Available online: https://gd.eppo.int/reporting/article-3380 (accessed on 1 November 2022).
- Elnagar, S.; El-Sheikh, M.; Hashem, A.; Afia, Y. Recent invasion by Bactrocera zonata (Saunders) as a new pest competing with Ceratitis capitata (Wiedemann) in attacking fruits in Egypt. Asp. Appl. Biol. 2010, 104, 97–102. [Google Scholar]
- Hosni, M.E.; El-Husseini, M.M.; El-Heneidy, A.H.; Atallah, F.A. Biological aspects of the peach fruit fly, Bactrocera zonata (Saund.(Diptera: Tephritidae) and its parasitoid species, Aganaspis daci Weld. (Hymenoptera: Eucoilidae). Egypt. J. Biol. Pest Cont. 2011, 21, 137–142. [Google Scholar]
- San Jose, M.; Doorenweerd, C.; Leblanc, L.; Barr, N.; Geib, S.; Rubinoff, D. Incongruence between molecules and morphology: A seven-gene phylogeny of Dacini fruit flies paves the way for reclassification (Diptera: Tephritidae). Mol. Phylogenet. Evol. 2018, 121, 139–149. [Google Scholar] [CrossRef]
- Krosch, M.N.; Schutze, M.K.; Armstrong, K.F.; Graham, G.C.; Yeates, D.K.; Clarke, A.R. A molecular phylogeny for the Tribe Dacini (Diptera: Tephritidae): Systematic and biogeographic implications. Mol. Phylogenet. Evol. 2012, 64, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krosch, M.N.; Strutt, F.; Blacket, M.J.; Batovska, J.; Starkie, M.; Clarke, A.R.; Cameron, S.L.; Schutze, M.K. Development of internal COI primers to improve and extend barcoding of fruit flies (Diptera: Tephritidae: Dacini). Insect. Sci. 2020, 27, 143–158. [Google Scholar] [CrossRef]
- San Jose, M.; Doorenweerd, C.; Leblanc, L.; Barr, N.; Geib, S.; Rubinoff, D. Tracking the Origins of Fly Invasions; Using Mitochondrial Haplotype Diversity to Identify Potential Source Populations in Two Genetically Intertwined Fruit Fly Species (Bactrocera carambolae and Bactrocera dorsalis [Diptera: Tephritidae]). J. Econ. Entomol. 2018, 111, 2914–2926. [Google Scholar] [CrossRef] [PubMed]
- Doorenweerd, C.; San Jose, M.; Barr, N.; Leblanc, L.; Rubinoff, D. Highly variable COI haplotype diversity between three species of invasive pest fruit fly reflects remarkably incongruent demographic histories. Sci. Rep. 2020, 10, 6887. [Google Scholar] [CrossRef]
- Lauzon, C.R. Symbiotic relationships of tephritids. In Insect Symbiosis, 2nd ed.; Bourtzis, K., Miller, T.A., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 115–130. [Google Scholar]
- Behar, A.; Jurkevitch, E.; Yuval, B. Bringing back the fruit into fruit fly-bacteria interactions. Mol. Ecol. 2008, 17, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. Open Sci. 2015, 2, 150170. [Google Scholar] [CrossRef] [Green Version]
- De Cock, M.; Virgilio, M.; Vandamme, P.; Bourtzis, K.; De Meyer, M.; Willems, A. Comparative Microbiomics of Tephritid Frugivorous Pests (Diptera: Tephritidae) From the Field: A Tale of High Variability Across and Within Species. Front. Microbiol. 2020, 11, 1890. [Google Scholar] [CrossRef]
- Bigiotti, G.; Sacchetti, P.; Pastorelli, R.; Lauzon, C.R.; Belcari, A. Bacterial symbiosis in Bactrocera oleae, an Achilles’ heel for its pest control. Insect Sci. 2021, 28, 874–884. [Google Scholar] [CrossRef]
- Capuzzo, C.; Firrao, G.; Mazzon, L.; Squartini, A.; Girolami, V. ‘Candidatus Erwinia dacicola’, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin). Int. J. Syst. Evol. Microbiol. 2005, 55, 1641–1647. [Google Scholar] [CrossRef]
- Estes, A.M.; Hearn, D.J.; Burrack, H.J.; Rempoulakis, P.; Pierson, E.A. Prevalence of Candidatus Erwinia dacicola in wild and laboratory olive fruit fly populations and across developmental stages. Environ. Entomol. 2012, 41, 265–274. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. J. Evol. Biol. 2014, 27, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.M.; Hearn, D.J.; Agrawal, S.; Pierson, E.A.; Dunning Hotopp, J.C. Comparative genomics of the Erwinia and Enterobacter olive fly endosymbionts. Sci. Rep. 2018, 8, 15936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blow, F.; Gioti, A.; Goodhead, I.B.; Kalyva, M.; Kampouraki, A.; Vontas, J.; Darby, A.C. Functional Genomics of a Symbiotic Community: Shared Traits in the Olive Fruit Fly Gut Microbiota. Genome Biol. Evol. 2020, 12, 3778–3791. [Google Scholar] [CrossRef] [Green Version]
- White, I.M.; Hancock, D.L. CABIKEY to the Dacini (Diptera, Tephritidae) of the Asia Pacific Australasian Regions; CAB International: Wallingford, UK, 1997. [Google Scholar]
- White, I.M. Morphological features of the tribe Dacini (Dacinae): Their significance to behavior and classification. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 505–546. [Google Scholar]
- The Australian Handbook for the Identification of Fruit Flies, Version 3.1.; Plant Health Australia: Canberra, ACT, Australia, 2018.
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D. The Barcode of Life Data System. Mol. Ecol. Note 2007, 7, 355–364. [Google Scholar] [CrossRef] [Green Version]
- EPPO. Bactrocera zonata (DACUZO), Host plant. EPPO Global Database. 2022. Available online: https://gd.eppo.int/taxon/DACUZO/hosts (accessed on 1 November 2022).
- Drew, R.A.I.; Hancock, D.L. Phylogeny of the Tribe Dacini (Dacinae) based on morphological, distributional, and biological data. In Phylogeny and Evolution of Behavior, 2nd ed.; Aluja, M., Norrbom, A.L., Eds.; Fruit Flies (Tephritidae); CRC Press: Boca Raton, FL, USA, 2000; pp. 491–504. [Google Scholar]
- Choudhary, J.S.; Naaz, N.; Lemtur, M.; Das, B.; Singh, A.K.; Bhatt, B.P.; Prabhakar, C.S. Genetic analysis of Bactrocera zonata (Diptera: Tephritidae) populations from India based on cox1 and nad1 gene sequences. Mitochondrial DNA Part A 2018, 29, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Samie, E.M.; El Fiky, Z.A. Molecular phylogeny and identification of the peach fruit fly, Bactrocera zonata, established in Egypt. J. Insect Sci. 2011, 11, 177. [Google Scholar] [CrossRef] [Green Version]
- Abd-El-Samie, E.M.; El Fiky, Z.A. Retraction of: Abd-El-Samie, E.M.; El Fiky, Z.A. Molecular phylogeny and identification of the peach fruit fly, Bactrocera zonata, established in Egypt. J. Insect Sci. 2015, 15, 118. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.; Sharma, K.; Singh, S. Attractancy potential of culturable bacteria from the gut of peach fruit fy, Bactrocera zonata (Saunders). Phytoparasitica 2014, 42, 691–698. [Google Scholar] [CrossRef]
- Naaz, N.; Choudhary, J.S.; Choudhary, A.; Dutta, A. Morphological and Biochemical Characterization of Bacteria Associated with the Developmental Stage of the Peach Fruit Fly, Bactrocera zonata (Diptera: Tephritidae). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1349–1360. [Google Scholar] [CrossRef]
- Naaz, N.; Choudhary, J.S.; Choudhary, A.; Dutta, A. Das, B. Developmental stage-associated microbiota profile of the peach fruit fly, Bactrocera zonata (Diptera: Tephritidae) and their functional prediction using 16S rRNA gene metabarcoding sequencing. 3 Biotech 2020, 10, 390. [Google Scholar] [CrossRef]
- Kounatidis, I.; Crotti, E.; Sapountzis, P.; Sacchi, L.; Rizzi, A.; Chouaia, B.; Bandi, C.; Alma, A.; Daffonchio, D.; Mavragani-Tsipidou, P.; et al. Acetobacter tropicalis is a major symbiont of the olive fruit fly (Bactrocera oleae). Appl. Environ. Microbiol. 2009, 75, 3281–3288. [Google Scholar] [CrossRef] [Green Version]
- Chou, J.H.; Chen, W.M.; Arun, A.B.; Young, C.C. Trabulsiella odontotermitis sp. nov., isolated from the gut of the termite Odontotermes formosanus Shiraki. Int. J. Syst. Evol. Microbiol. 2007, 57, 696–700. [Google Scholar] [CrossRef] [Green Version]
- Tainchum, K.; Dupont, C.; Chareonviriyaphap, T.; Jumas-Bilak, E.; Bangs, M.J.; Manguin, S. Bacterial Microbiome in Wild-Caught Anopheles Mosquitoes in Western Thailand. Front. Microbiol. 2020, 11, 965. [Google Scholar] [CrossRef]
- Juneja, P.; Lazzaro, B.P. Providencia sneebia sp. nov. and Providencia burhodogranariea sp. nov., isolated from wild Drosophila melanogaster. Int. J. Syst. Evol. Microbiol. 2009, 59, 1108–1111. [Google Scholar] [CrossRef] [Green Version]
- Galac, M.R.; Lazzaro, B.P. Comparative pathology of bacteria in the genus Providencia to a natural host, Drosophila melanogaster. Microbes Infect 2011, 13, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Kuzina, L.V.; Peloquin, J.J.; Vacek, D.C.; Miller, T.A. Isolation and identification of bacteria associated with adult laboratory Mexican fruit flies, Anastrepha ludens (Diptera: Tephritidae). Curr. Microbiol. 2001, 42, 290–294. [Google Scholar] [CrossRef]
- Msaad Guerfali, M.; Djobbi, W.; Charaabi, K.; Hamden, H.; Fadhl, S.; Marzouki, W.; Dhaouedi, F.; Chevrier, C. Evaluation of Providencia rettgeri pathogenicity against laboratory Mediterranean fruit fly strain (Ceratitis capitata). PLoS ONE 2018, 13, e0196343. [Google Scholar] [CrossRef] [Green Version]
- Ksentini, I.; Gharsallah, H.; Sahnoun, M.; Schuster, C.; Hamli Amri, S.; Gargouri, R.; Triki, M.A.; Ksantini, M.; Leclerque, A. Providencia entomophila sp. nov., a new bacterial species associated with major olive pests in Tunisia. PLoS ONE 2019, 14, e0223943. [Google Scholar] [CrossRef]
- Tsiropoulos, G.J. Microflora associated with wild and laboratory reared adult olive fruit flies, Dacus oleae (Gmel.). Z. für Angew. Entomol. 1983, 96, 337–340. [Google Scholar] [CrossRef]
- Ercolani, G.L. Pseudomonas savastanoi and other bacteria colonizing the surface of olive leaves in the field. J. Gen. Microbiol. 1978, 109, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Drew, R.A.I.; Lloyd, A.C. Relationship of fruit flies (Diptera: Tephritidae) and their bacteria to host plants. Ann. Entomol. Soc. Am. 1987, 80, 629–636. [Google Scholar] [CrossRef]
- Ercolani, G.L. Distribution of epiphytic bacteria on olive leaves and the influence of leaf age and sampling time. Microb. Ecol. 1991, 21, 35–48. [Google Scholar] [CrossRef]
- Liscia, A.; Angioni, P.; Sacchetti, P.; Poddighe, S.; Granchietti, A.; Setzu, M.D.; Belcari, A. Characterization of olfactory sensilla of the olive fly: Behavioral and electrophysiological responses to volatile organic compounds from the host plant and bacterial filtrate. J. Insect Physiol. 2013, 59, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.M.; Hearn, D.J.; Bronstein, J.L.; Pierson, E.A. The olive fly endosymbiont, “Candidatus Erwinia dacicola”, switches from an intracellular existence to an extracellular existence during host insect development. Appl. Environ. Microbiol. 2009, 75, 7097–7106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlidi, N.; Gioti, A.; Wybouw, N.; Dermauw, W.; Ben-Yosef, M.; Yuval, B.; Jurkevich, E.; Kampouraki, A.; Van Leeuwen, T.; Vontas, J. Transcriptomic responses of the olive fruit fly Bactrocera oleae and its symbiont Candidatus Erwinia dacicola to olive feeding. Sci. Rep. 2017, 7, 42633. [Google Scholar] [CrossRef] [Green Version]
- Jurkevitch, E. Riding the Trojan horse: Combating pest insects with their own symbionts. Microb. Biotechnol. 2011, 4, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Nobre, T. Symbiosis in sustainable agriculture: Can olive fruit fly bacterial microbiome be useful in pest management? Microorganisms 2019, 7, 238. [Google Scholar] [CrossRef] [Green Version]
- Bigiotti, G.; Pastorelli, R.; Belcari, A.; Sacchetti, P. Symbiosis interruption in the olive fly: Effect of copper and propolis on Candidatus Erwinia dacicola. J. Appl. Entomol. 2019, 143, 357–364. [Google Scholar] [CrossRef]
- Sinno, M.; Bezier, A.; Vinale, F.; Giron, D.; Laudonia, S.; Garonna, A.P.; Pennacchio, F. Symbiosis disruption in the olive fruit fly, Bactrocera oleae (Rossi), as a potential tool for sustainable control. Pest Manag. Sci. 2020, 76, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.S.; Liu, L.; Bai, Z.; Li, Z. Tephritidae bacterial symbionts: Potentials for pest management. Bull. Entomol. Res. 2020, 110, 1–14. [Google Scholar] [CrossRef]



| Acronym | Location | GPS Coordinates |
|---|---|---|
| Giza-1 | Agricultural Research Center, Giza | 30°01′23.8″ N 31°12′26.0″ E |
| Giza-2 | Cairo–Alexandria Road, Wadi Food Farm, Giza | 30°14′17.6″ N 30°47′14.6″ E |
| Ismailia-1 | El Ferdan, Ismailia | 30.655947° N, 32.325767° E |
| Ismailia-2 | Suez Canal University Farm, Ismailia | 30.6205° N, 32.2697° E |
| Primer Designation | Primer Sequence | Reference |
|---|---|---|
| LCO1490-mod | 5′-TYTCAACAAATCATAAAGATATTGG-3′ | [21] |
| HC02198-mod | 5′-TAAACTTCAGGGTGWCCAAARAATCA-3′ | |
| Dac-COI-f | 5′-GCHTTCCCHCGAATAAATAATA-3′ | |
| Dac-COI-r | 5′-GTTCAACCTGTACCVGCYCCGTTTTC-3′ | |
| M46-1 | 5′-CAGGAAACGCTATGACCGAGGAAATYAARAAGGAAG-3′ | [19] |
| M4rc | 5′-TGTAAAACGACGGCCAGTACAGCVACKGTYTGYCTCATRTC-3′ | |
| CAD-Bd-F | 5′-CCGGTAAATTTTGAATGGTTC-3′ | |
| CAD-Bd-R | 5′-GCRGTKGCGAGCARYTGATG-3′ | |
| Per2612F | 5′-ATTCATGGGAAGGAGATGCC-3′ | |
| Per3105R | 5′-AABGACATGGGTTGGTACATC-3′ |
| Geographic Origin | Sampling Date | Host Plant | Male Adults | Female Adults | Identification |
|---|---|---|---|---|---|
| Giza-1 | <09/2019 | Guava | 10 | 10 (5) | Bactrocera zonata |
| Giza-1 | <09/2019 | Apple | 10 | 10 (5) | Bactrocera zonata |
| Giza-1 | <09/2019 | Pear | 5 | 3 (3) | Bactrocera zonata |
| Giza-1 | 10–11/2019 | Olive | 8 | 8 (5) | Bactrocera zonata |
| Giza-2 | 10–11/2019 | Olive | 2 | 3 | Ceratitis capitata |
| Ismailia-1 | <09/2019 | Mango | 0 | 1 (1) | Bactrocera zonata |
| Ismailia-1 | <09/2019 | Pear | 0 | 1 (1) | Bactrocera zonata |
| Ismailia-2 | 10–11/2019 | Olive | 6 | 8 | Bactrocera zonata |
| Ismailia-2 | 10–11/2019 | Olive | 4 | 6 | Bactrocera oleae |
| Ismailia-2 | 12/2019 | Olive | 3 | 6 (5) | Bactrocera zonata |
| Sample-ID (Haplotype) | Best Hit—ID | Maximal Similarity Percentage | Minimal Similarity Percentage | Best Hit (Accession Number, Geographic Origin) |
|---|---|---|---|---|
| IPF3 (A) | Bactrocera zonata | 100 | 99.13 | MK564024, India |
| GPM3 (B) | Bactrocera zonata | 100 | 98.95 | MG881761, Iran |
| IPF2 (C) | Bactrocera zonata | 100 | 98.78 | BIOUG14603-C07, Egypt |
| GPF1 (D) | Bactrocera zonata | 100 | 98.43 | MG881762, Iran |
| GPF2 (E) | Bactrocera zonata | 100 | 98.78 | MK564010, India |
| IPM3 (F) | Bactrocera zonata | 98.34 | 97.24 | MK296117, Pakistan |
| IPM1 (G) | Bactrocera zonata | 100 | 98.78 | MF192732, Iran |
| GMF1 | Ceratitis capitata | 100 | 99.65 | MT474895, Australia |
| IOF1 | Bactrocera oleae | 100 | 99.3 | KY111512, Turkey |
| Location | Giza-1 | Ismailia-1 | Ismailia-2 | ||||
|---|---|---|---|---|---|---|---|
| Fruit | Apple | Guava | Pear | Olive | Mango | Pear | Olive |
| Enterobacter | 69.3 | 34.8 | 73.9 | 54.1 | 66.1 | 32.5 | 34.7 |
| Klebsiella | 7.7 | 26.8 | 7.7 | 15.8 | 14.6 | 49.0 | 3.6 |
| Acetobacter | 6.5 | 2.5 | 1.5 | 0 | 1.1 | 4.3 | 0 |
| Gluconobacter | 1.3 | 0.7 | 0.4 | 0 | 0.3 | 2.0 | 0 |
| Trabulsiella | 2.9 | 2.7 | 2.1 | 5.5 | 3.9 | 3.8 | 1.2 |
| Pseudomonas | 0.7 | 0.8 | 0.3 | 4.6 | 0 | 0 | 8.1 |
| Erwinia | 0.5 | 1.5 | 0.4 | 1.5 | 5.1 | 0.7 | 13.2 |
| Providencia | 0 | 0.1 | 0 | 0 | 0 | 0 | 30.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.; Ben Gharsa, H.; ElKraly, O.A.; Leclerque, A.; Elnagdy, S.M. COI Haplotyping and Comparative Microbiomics of the Peach Fruit Fly, an Emerging Pest of Egyptian Olive Orchards. Biology 2023, 12, 27. https://doi.org/10.3390/biology12010027
Awad M, Ben Gharsa H, ElKraly OA, Leclerque A, Elnagdy SM. COI Haplotyping and Comparative Microbiomics of the Peach Fruit Fly, an Emerging Pest of Egyptian Olive Orchards. Biology. 2023; 12(1):27. https://doi.org/10.3390/biology12010027
Chicago/Turabian StyleAwad, Mona, Haifa Ben Gharsa, Omnia Abdullah ElKraly, Andreas Leclerque, and Sherif M. Elnagdy. 2023. "COI Haplotyping and Comparative Microbiomics of the Peach Fruit Fly, an Emerging Pest of Egyptian Olive Orchards" Biology 12, no. 1: 27. https://doi.org/10.3390/biology12010027
APA StyleAwad, M., Ben Gharsa, H., ElKraly, O. A., Leclerque, A., & Elnagdy, S. M. (2023). COI Haplotyping and Comparative Microbiomics of the Peach Fruit Fly, an Emerging Pest of Egyptian Olive Orchards. Biology, 12(1), 27. https://doi.org/10.3390/biology12010027








