Caffeine Delays Ethanol-Induced Sedation in Drosophila
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Stocks and Maintenance
2.2. Food Preparation
2.3. Caffeine Supplementation
2.4. Caffeine-Induced Mortality
2.5. Ethanol-Induced Sedation Assay
2.6. Statistical Analysis
3. Results
3.1. Caffeine Delays Onset of Ethanol-Induced Sedation in Wild Type Flies
3.2. Exposure to Caffeine for One Day Is Sufficient to Increase Ethanol-Induced Sedation Time
3.3. The Effect of Caffeine on Ethanol-Induced Sedation Reverses after Caffeine Withdrawal
3.4. Adenosine Receptor Mutation Delays Onset of Ethanol-Induced Sedation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2004, 9, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Osz, B.E.; Jitca, G.; Stefanescu, R.E.; Puscas, A.; Tero-Vescan, A.; Vari, C.E. Caffeine and its antioxidant properties-it is all about dose and source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Agarwal, S.; Fulgoni, V.L., 3rd. Daily patterns of caffeine intake and the association of intake with multiple sociodemographic and lifestyle factors in us adults based on the nhanes 2007–2012 surveys. J. Acad. Nutr. Diet. 2019, 119, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, M.; Calvello, R.; Porro, C.; Messina, G.; Cianciulli, A.; Panaro, M.A. Neurodegenerative diseases: Can caffeine be a powerful ally to weaken neuroinflammation? Int. J. Mol. Sci. 2022, 23, 12958. [Google Scholar] [CrossRef]
- Nehlig, A.; Daval, J.L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Battig, K.; Holmen, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Ribeiro, J.A.; Sebastiao, A.M. Caffeine and adenosine. J. Alzheimer’s Dis. 2010, 20 (Suppl. 1), S3–S15. [Google Scholar] [CrossRef] [Green Version]
- Dolezelova, E.; Nothacker, H.P.; Civelli, O.; Bryant, P.J.; Zurovec, M. A drosophila adenosine receptor activates camp and calcium signaling. Insect Biochem. Mol. Biol. 2007, 37, 318–329. [Google Scholar] [CrossRef]
- Sperlagh, B.; Vizi, E.S. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and basal ganglia: Pharmacological and clinical aspects. Curr. Top. Med. Chem. 2011, 11, 1034–1046. [Google Scholar] [CrossRef] [Green Version]
- Ruby, C.L.; Adams, C.A.; Knight, E.J.; Nam, H.W.; Choi, D.S. An essential role for adenosine signaling in alcohol abuse. Curr. Drug Abus. Rev. 2010, 3, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.F.; Deboer, T.; Landolt, H.P. Adenosine, caffeine, and sleep-wake regulation: State of the science and perspectives. J. Sleep Res. 2022, 31, e13597. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.N.; Ho, K.; Crocker, A.; Yue, Z.; Koh, K.; Sehgal, A. The effects of caffeine on sleep in drosophila require pka activity, but not the adenosine receptor. J. Neurosci. 2009, 29, 11029–11037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.N.; Kumar, S.; Rai, S.; Methippara, M.; Szymusiak, R.; McGinty, D. Role of adenosine a(1) receptor in the perifornical-lateral hypothalamic area in sleep-wake regulation in rats. Brain Res. 2009, 1304, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Parikh, M.; Mishra, V.; Zuniga, A.; Sahota, P.; Thakkar, M. Sleep, sleep homeostasis and arousal disturbances in alcoholism. Brain Res. Bull. 2022, 182, 30–43. [Google Scholar] [CrossRef]
- Ferre, S.; O’Brien, M.C. Alcohol and caffeine: The perfect storm. J. Caffeine Res. 2011, 1, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Erickson, E.K.; DaCosta, A.J.; Mason, S.C.; Blednov, Y.A.; Mayfield, R.D.; Harris, R.A. Cortical astrocytes regulate ethanol consumption and intoxication in mice. Neuropsychopharmacology 2021, 46, 500–508. [Google Scholar] [CrossRef]
- Kaun, K.R.; Devineni, A.V.; Heberlein, U. Drosophila melanogaster as a model to study drug addiction. Hum. Genet. 2012, 131, 959–975. [Google Scholar] [CrossRef] [Green Version]
- Philyaw, T.J.; Rothenfluh, A.; Titos, I. The use of drosophila to understand psychostimulant responses. Biomedicines 2022, 10, 119. [Google Scholar] [CrossRef]
- Devineni, A.V.; Heberlein, U. The evolution of drosophila melanogaster as a model for alcohol research. Annu. Rev. Neurosci. 2013, 36, 121–138. [Google Scholar] [CrossRef]
- Rodan, A.R.; Rothenfluh, A. The genetics of behavioral alcohol responses in drosophila. Int. Rev. Neurobiol. 2010, 91, 25–51. [Google Scholar] [PubMed]
- Liao, J.; Seggio, J.A.; Ahmad, S.T. Mutations in the circadian gene period alter behavioral and biochemical responses to ethanol in drosophila. Behav. Brain Res. 2016, 302, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maples, T.; Rothenfluh, A. A simple way to measure ethanol sensitivity in flies. J. Vis. Exp. 2011, 48, e2541. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.; Shen, H.Y.; Cherasse, Y.; Qu, W.M.; Huang, Z.L.; Bass, C.E.; Winsky-Sommerer, R.; Semba, K.; Fredholm, B.B.; Boison, D.; et al. Arousal effect of caffeine depends on adenosine a2a receptors in the shell of the nucleus accumbens. J. Neurosci. 2011, 31, 10067–10075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustard, J.A. The buzz on caffeine in invertebrates: Effects on behavior and molecular mechanisms. Cell Mol. Life Sci. 2014, 71, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Devineni, A.V.; Heberlein, U. Acute ethanol responses in drosophila are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2012, 109, 21087–21092. [Google Scholar] [CrossRef] [Green Version]
- Oyeyinka, A.; Kansal, M.; O’Sullivan, S.M.; Gualtieri, C.; Smith, Z.M.; Vonhoff, F.J. Corazonin neurons contribute to dimorphic ethanol sedation sensitivity in drosophila melanogaster. Front. Neural Circuits 2022, 16, 702901. [Google Scholar] [CrossRef]
- Francikowski, J.; Baran, B.; Płachetka-Bożek, A.; Krzyżowski, M.; Augustyniak, M. Caffeine effects on ador mrna expression in drosophila melanogaster. Open Life Sci. 2016, 11, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Itoyama, M.M.; Bicudo, H.E.M.d.C. Effects of caffeine on fecundity, egg laying capacity, development time and longevity in drosophila prosaltans. Rev. Bras. Genét. 1992, 15, 303–321. [Google Scholar]
- Ebbs, M.L.; Amrein, H. Taste and pheromone perception in the fruit fly drosophila melanogaster. Pflug. Arch. 2007, 454, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Moon, S.J.; Montell, C. Multiple gustatory receptors required for the caffeine response in drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 4495–4500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, H.J.; Shin, B.; Han, S.H.; Woo, M.J.; Hong, K.B. Behavioral changes and survival in drosophila melanogaster: Effects of ascorbic acid, taurine, and caffeine. Biol. Pharm. Bull. 2017, 40, 1873–1882. [Google Scholar] [CrossRef]
- Coelho, A.; Fraichard, S.; Le Goff, G.; Faure, P.; Artur, Y.; Ferveur, J.F.; Heydel, J.M. Cytochrome p450-dependent metabolism of caffeine in drosophila melanogaster. PLoS ONE 2015, 10, e0117328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- El Yacoubi, M.; Ledent, C.; Parmentier, M.; Costentin, J.; Vaugeois, J.M. Caffeine reduces hypnotic effects of alcohol through adenosine a2a receptor blockade. Neuropharmacology 2003, 45, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, E.; Li, Q.; Rao, Y.; Kitamoto, T. The unique dopamine/ecdysteroid receptor modulates ethanol-induced sedation in drosophila. J. Neurosci. 2016, 36, 4647–4657. [Google Scholar] [CrossRef] [Green Version]
- Andretic, R.; Kim, Y.C.; Jones, F.S.; Han, K.A.; Greenspan, R.J. Drosophila d1 dopamine receptor mediates caffeine-induced arousal. Proc. Natl. Acad. Sci. USA 2008, 105, 20392–20397. [Google Scholar] [CrossRef] [Green Version]
- Nall, A.H.; Shakhmantsir, I.; Cichewicz, K.; Birman, S.; Hirsh, J.; Sehgal, A. Caffeine promotes wakefulness via dopamine signaling in drosophila. Sci. Rep. 2016, 6, 20938. [Google Scholar] [CrossRef] [Green Version]
- Kucerova, L.; Broz, V.; Fleischmannova, J.; Santruckova, E.; Sidorov, R.; Dolezal, V.; Zurovec, M. Characterization of the drosophila adenosine receptor: The effect of adenosine analogs on camp signaling in drosophila cells and their utility for in vivo experiments. J. Neurochem. 2012, 121, 383–395. [Google Scholar] [CrossRef]
- Mailliard, W.S.; Diamond, I. Recent advances in the neurobiology of alcoholism: The role of adenosine. Pharmacol. Ther. 2004, 101, 39–46. [Google Scholar] [CrossRef]
- Patrick, M.E.; Maggs, J.L. Energy drinks and alcohol: Links to alcohol behaviors and consequences across 56 days. J. Adolesc. Health 2014, 54, 454–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Mana, C.; Mateus, J.A.; Diaz-Pellicer, P.; Diaz-Baggerman, A.; Perez, M.; Pujadas, M.; Fonseca, F.; Papaseit, E.; Pujol, J.; Langohr, K.; et al. Effects of mixing energy drinks with alcohol on driving-related skills. Int. J. Neuropsychopharmacol. 2022, 25, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Powers, G.; Berger, L. Alcohol mixed with energy drinks: Expectancies of use and alcohol-related negative consequences among a young adult sample. Addict. Behav. Rep. 2020, 12, 100292. [Google Scholar] [CrossRef] [PubMed]
- Sampasa-Kanyinga, H.; Masengo, L.; Hamilton, H.A.; Chaput, J.P. Energy drink consumption and substance use among middle and high school students. Int. J. Environ. Res. Public Health 2020, 17, 3110. [Google Scholar] [CrossRef] [PubMed]

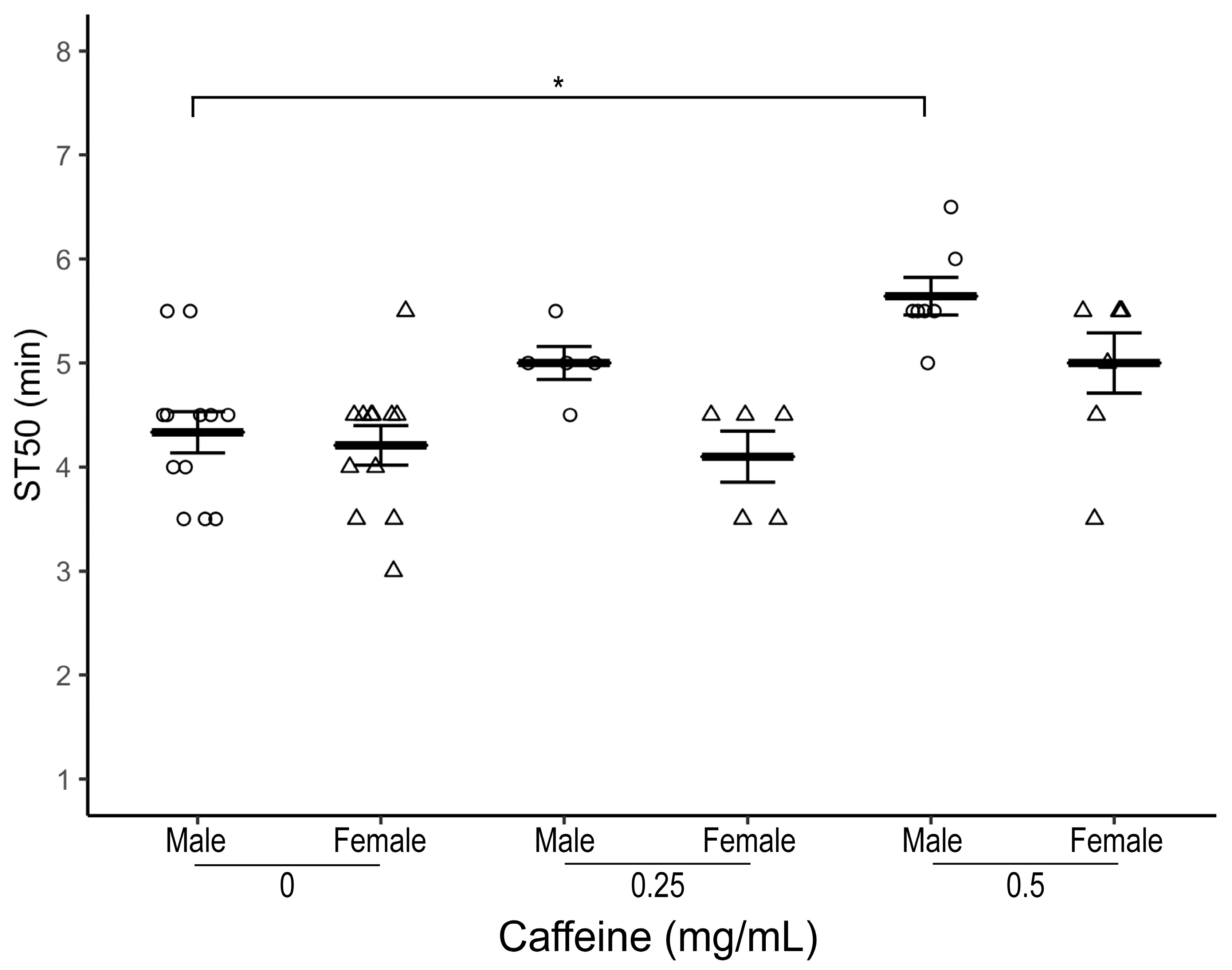

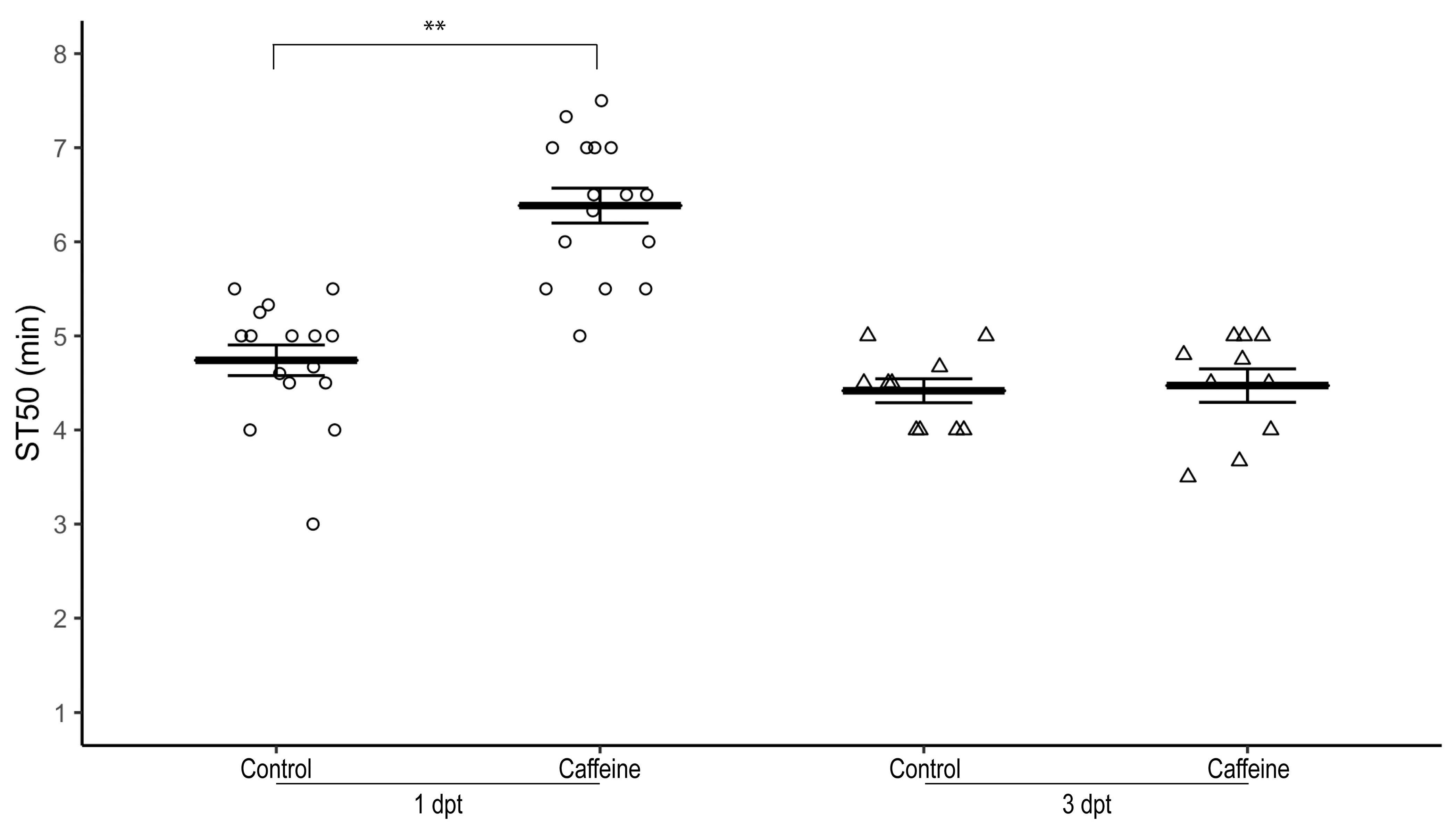
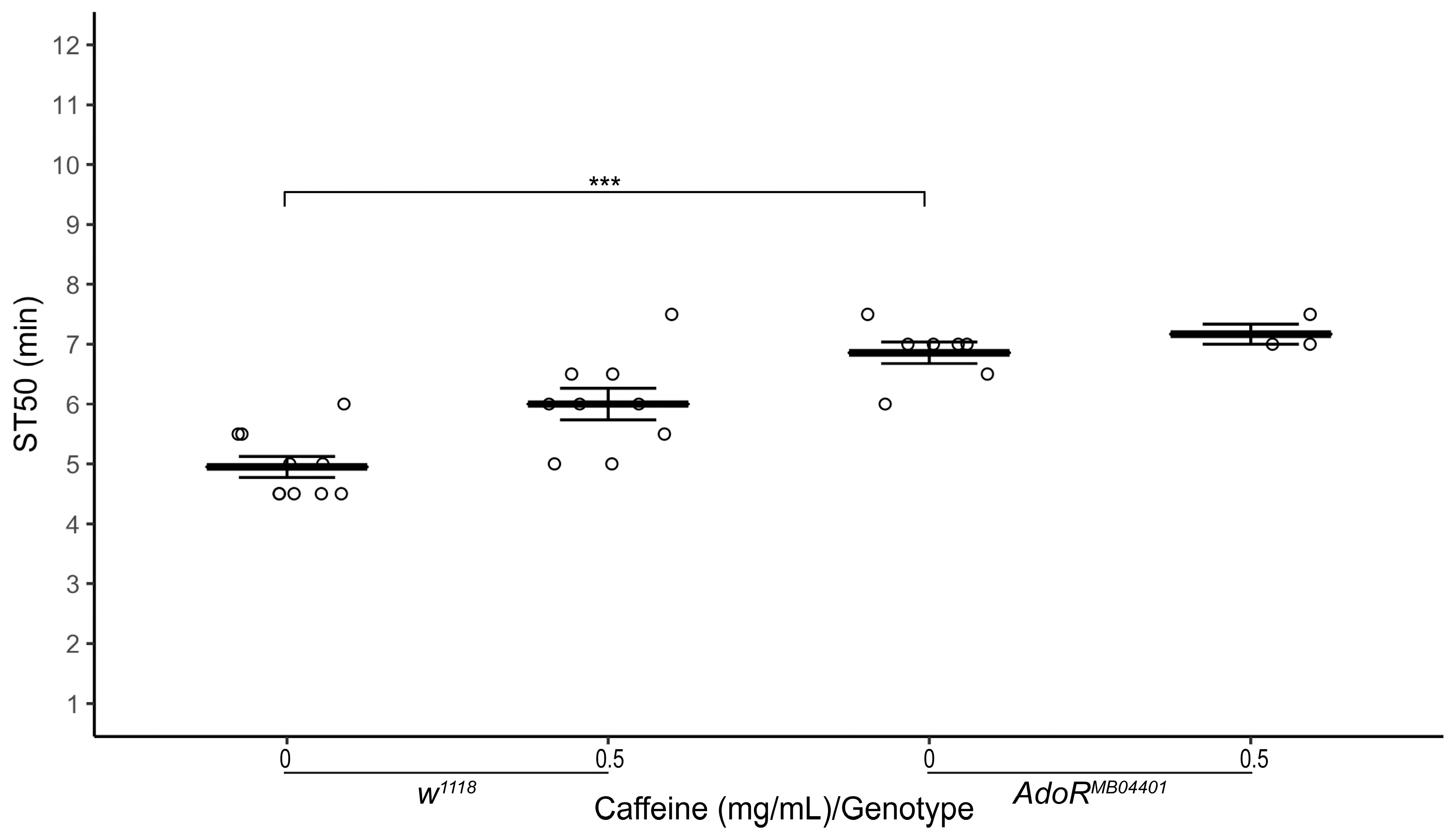
| Median Sedation Time ST50 (min) | Change in ST50 (%) Caffeine vs. Control | ||
|---|---|---|---|
| Ethanol (%)/Caffeine (mg/mL) | 0 | 0.5 | |
| 50 | 12.12 ± 1.5 a,b | 20.03 ± 1.7 i,ii | 65.26 ** |
| 75 | 6.97 ± 0.5 a | 11.37 ± 0.9 i,iii | 63.13 ** |
| 100 | 4.66 ± 0.3 b | 6.23 ± 0.3 ii,iii | 33.70 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremblay, S.; Zeng, Y.; Yue, A.; Chabot, K.; Mynahan, A.; Desrochers, S.; Bridges, S.; Ahmad, S.T. Caffeine Delays Ethanol-Induced Sedation in Drosophila. Biology 2023, 12, 63. https://doi.org/10.3390/biology12010063
Tremblay S, Zeng Y, Yue A, Chabot K, Mynahan A, Desrochers S, Bridges S, Ahmad ST. Caffeine Delays Ethanol-Induced Sedation in Drosophila. Biology. 2023; 12(1):63. https://doi.org/10.3390/biology12010063
Chicago/Turabian StyleTremblay, Sonia, Yanqiqi Zeng, Aixin Yue, Kiana Chabot, Abigail Mynahan, Stephanie Desrochers, Sarra Bridges, and S. Tariq Ahmad. 2023. "Caffeine Delays Ethanol-Induced Sedation in Drosophila" Biology 12, no. 1: 63. https://doi.org/10.3390/biology12010063





