Obstructive Sleep Apnea: A Look towards Micro-RNAs as Biomarkers of the Future
Abstract
:Simple Summary
Abstract
1. Obstructive Sleep Apnea
- (1)
- (2)
- (3)
- Sleeping in the supine position facilitates the onset of apnea;
- (4)
- The presence of craniofacial anomalies (retrognathia and micrognathia, angulation of the skull base), nasal obstructions, tonsillar and/or adenoid hypertrophy, ogival palate, prolapse of the uvula, macroglossia, or edema of the larynx [14];
- (5)
- The use of alcohol or other substances such as muscle relaxants or sedatives [16].
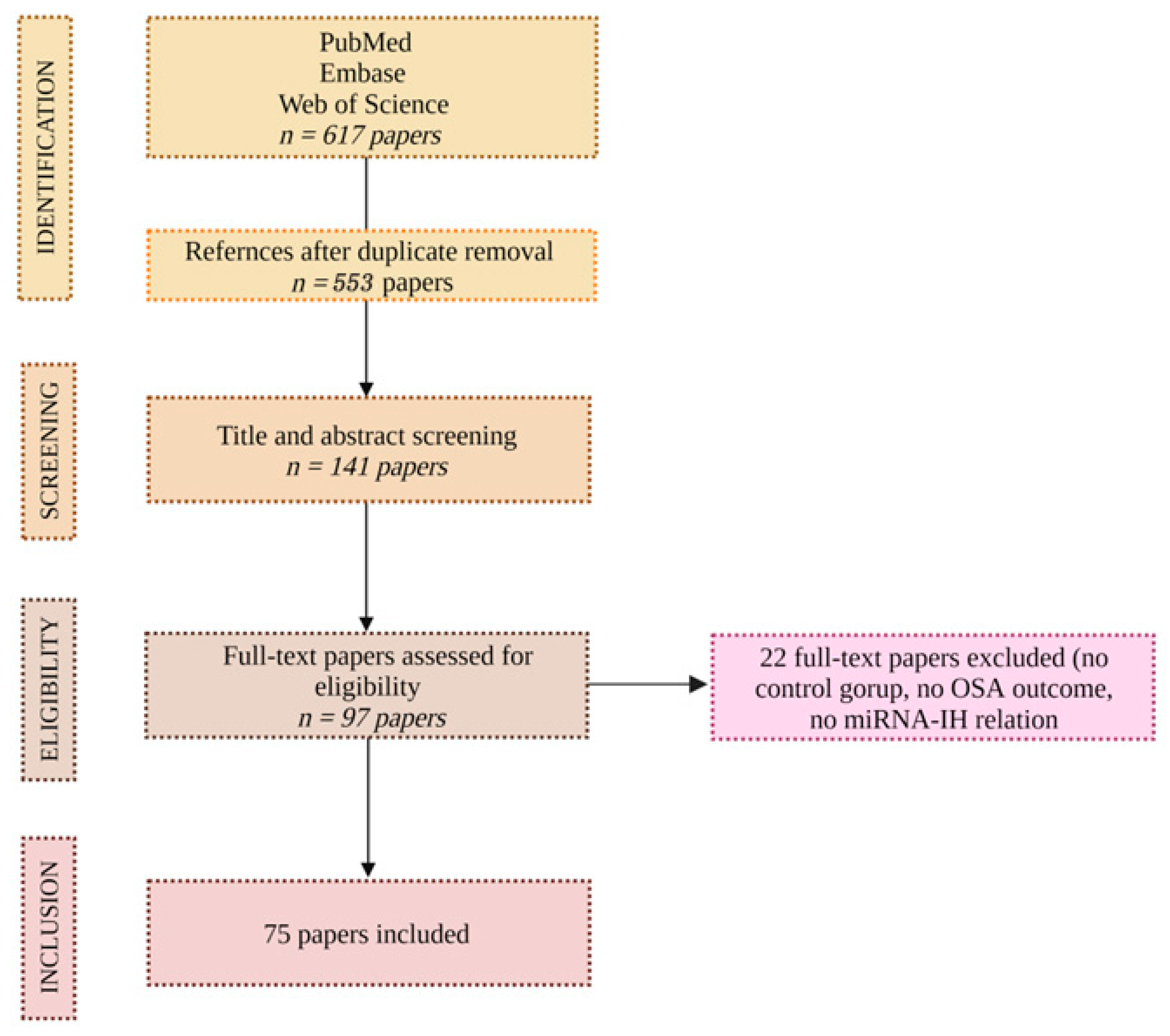
2. Methods
3. OSA and Intermittent Hypoxia
4. OSA and Cancer
5. Promising New Biomarkers in OSA: Micro-RNAs
6. Micro-RNAs and OSA
| Reference | Title | Publication Year | Experimental design | Samples | Samples size | Methods | Main Findings |
|---|---|---|---|---|---|---|---|
| Sánchez-de-la-Torre M et al. [62] | Precision Medicine in Patients with Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. | 2015 | Patients with OSA and CPAP treatment | PLASMA | 38 | Microarray + RT-qPCR | MiR-100-5p, miR-378a-3p, and miR-486-5p predict responses to CPAP treatment in patients with OSA. |
| Santamaria-Martos F et al. [54] | Circulating microRNA profile as a potential biomarker for obstructive sleep apnea diagnosis. | 2019 | Differences between OSA and non-OSA | PLASMA | 230 | TLDA + RT-qPCR | Lower levels of miR-133a, miR-181a, miR-199b, miR-340, miR-345, and miR-486-3p in OSA patients compared with non-OSA. |
| Targa A et al. [72] | Circulating MicroRNA Profile Associated with Obstructive Sleep Apnea in Alzheimer’s Disease. | 2020 | Circulating microRNA profile associated with OSA in Alzheimer’s disease. | PLASMA | 29 | RT-qPCR | 15 miRNAs are differentially expressed between OSA and non-OSA patients with AD. |
| Khalyfa A et al. [67] | Circulating microRNAs as Potential Biomarkers of Endothelial Dysfunction in Obese Children. | 2016 | Obese or non-obese children with OSA and with endothelial dysfunction or normal endothelial function. | EXOSOMES IN PLASMA | 128 | Microarray + RT-qPCR | MiR-125a-5p, miR-342-3p, and miR-365b-3p were identified as potential biomarkers of children with endothelial dysfunction |
| Khalyfa A et al. [66] | Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults | 2016 | Human model of intermittent hypoxia. | EXOSOMES IN PLASMA | 10 | Microarray + RT-qPCR | Plasma exosomal micro RNAs (miRNAs) profile. |
| Khalyfa A et al. [68] | Circulating plasma exosomes in obstructive sleep apnoea and reverse dipping blood pressure | 2020 | Exosomal microRNA in untreated OSA patients with normal immersion blood pressure, reverse immersion blood pressure, and an extreme form of non-immersion. | EXOSOMES IN PLASMA | 46 | Microarray + RT-qPCR | Exosomes from reverse immersion blood pressure patients increased the permeability of endothelial cell tight junctions and adhesion molecule expression. |
| Li K et al. [63] | MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. | 2017 | OSA patients and healthy subjects | SERUM | 6 | Sequencing + RT-qPCR | Different expression of miR-107, miR-199-3p, miR-485-5p, and miR-574-5 in patients with OSA and healthy controls. |
| Yang X et al. [70] | MiRNA expression profiles in healthy OSAHS and OSAHS with arterial hypertension: potential diagnostic and early warning markers. | 2018 | 3 patient groups: non-OSA, non-hypertensive OSA patients, and hypertensive OSA patients. | SERUM | 60 | Microarray + RT-qPCR | Let-7d-5p and miR-145-5p allow the identification of non-hypertensive patients with OSA. The miR-26a-5p, miR-107, and miR-126-3p identify hypertensive patients with OSA. |
| Li K et al. [73] | MiR-664a-3p expression in patients with obstructive sleep apnea. | 2018 | Patients divided into four groups based on the presence of OSA and carotid intima-media thickness test. | SERUM | 116 | Sequencing + RT-qPCR | MiR-664a-3p was downregulated in patients with OSA, and non-OSA with CIMT increased compared to controls. |
| Shao H et al. [65] | Expression Profile Analysis and Image Observation of miRNA in Serum of Patients with Obstructive Sleep Apnea-Hypopnea Syndrome. | 2021 | Differential miRNAs of OSAHS-related hypertension. | SERUM | - | Bioinformatics methods | MiR-22-3p, miR-595, and miR-6856-are involved in the pathogenesis of OSAHS-related hypertension. |
| Freitas LS et al. [64] | Severe obstructive sleep apnea is associated with circulating microRNAs related to heart failure, myocardial ischemia, and cancer proliferation. | 2020 | Four groups: non-OSA, mild OSA, moderate OSA, and severe OSA | BLOOD | 48 | Microarray + RT-qPCR | MiR-320e and miR-1254 are associated with severe OSA. |
| Slouka D et al. [71] | The potential of miR-499 plasmatic level as a biomarker of obstructive sleep apnea syndrome | 2021 | Study of miR-1-3p, miR-133a-3p, and miR-499a-5p plasmatic levels in OSA | BLOOD | - | Reverse transcription-PCR | MiR-499 influences gene expression and could be a putative biomarker for OSA. |
| Chen YC et al. [74] | miR-21-5p Under-Expression in Patients with Obstructive Sleep Apnea Modulates Intermittent Hypoxia with Re-Oxygenation-Induced-Cell Apoptosis and Cytotoxicity by Targeting Pro-Inflammatory TNF-α-TLR4 Signaling. | 2020 | Levels of miR-21, miR-23a, and their target genes are assessed in PBMC from patients with severe OSA and 20 subjects with primary snoring (PS). | PBMC | 60 | RT-qPCR | Lower levels of miR-21-5p and miR-23-3p and higher levels of TNF-α both in OSA patients and in IHR-induced apoptotic monocytes. |
| He L et al. [69] | miR-126a-3p targets HIF-1α and alleviates obstructive sleep apnea syndrome with hypertension. | 2020 | Role of miR-126a-3p in OSA-hypertension. | Sprague–Dawley rats and rat aortic smooth muscle cells (A7r5) | 24 rats | RT-qPCR | MiR-126a-3p is a novel potential therapeutic target for the treatment of OSA-hypertension. |
7. Strengths and Limitations
8. Conclusions
- (i)
- The extreme variability of the clinical presentation of the patient with obstructive sleep apnea and must reflect the anthropometric characteristics, the associated comorbidities, and the environmental factors involved, such as cigarette smoking and sedentary habits;
- (ii)
- New biomarkers useful for disease stratification and treatment response.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tondo, P.; Fanfulla, F.; Sabato, R.; Scioscia, G.; Barbaro, M.P.F.; Lacedonia, D. Obstructive Sleep Apnoea-Hypopnoea Syndrome (OSAHS): State of the art. Minerva Medica 2022. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- E Mirrakhimov, A.; Sooronbaev, T.; Mirrakhimov, E.M. Prevalence of obstructive sleep apnea in Asian adults: A systematic review of the literature. BMC Pulm. Med. 2013, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzer, R.; Marti-Soler, H.; Marques-Vidal, P.; Tobback, N.; Andries, D.; Waeber, G.; Preisig, M.; Vollenweider, P.; Haba-Rubio, J. Impact of sex and menopausal status on the prevalence, clinical presentation, and comorbidities of sleep-disordered breathing. Sleep Med. 2018, 51, 29–36. [Google Scholar] [CrossRef]
- Pillar, G.; Lavie, P. Obstructive sleep apnea: Diagnosis, risk factors, and pathophysiology. Handb. Clin. Neurology 2011, 98, 383–399. [Google Scholar] [CrossRef]
- Savini, S.; Ciorba, A.; Bianchini, C.; Stomeo, F.; Corazzi, V.; Vicini, C.; Pelucchi, S. Assessment of obstructive sleep apnoea (OSA) in children: An update. Acta Otorhinolaryngol. Ital. 2019, 39, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Huon, L.-K.A.; Guilleminault, C. A Succinct History of Sleep Medicine. Sleep-Relat. Breath. Disord. 2017, 80, 1–6. [Google Scholar] [CrossRef]
- Park, J.G.; Ramar, K.; Olson, E.J. Updates on Definition, Consequences, and Management of Obstructive Sleep Apnea. Mayo Clin. Proc. 2011, 86, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Pien, G.W.; Ratcliffe, S.; Björnsdottir, E.; Arnardottir, E.S.; Pack, A.; Benediktsdottir, B.; Gislason, T. The different clinical faces of obstructive sleep apnoea: A cluster analysis. Eur. Respir. J. 2014, 44, 1600–1607. [Google Scholar] [CrossRef]
- McNicholas, W.T. Diagnosis of Obstructive Sleep Apnea in Adults. Proc. Am. Thorac. Soc. 2008, 5, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Narang, I.; Al-Saleh, S.; Amin, R.; Propst, E.J.; Bin-Hasan, S.; Campisi, P.; Ryan, C.; Kendzerska, T. Utility of Neck, Height, and Tonsillar Size to Screen for Obstructive Sleep Apnea among Obese Youth. Otolaryngol. Neck Surg. 2017, 158, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Pirklbauer, K.; Russmueller, G.; Stiebellehner, L.; Nell, C.; Sinko, K.; Millesi, G.; Klug, C. Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea Syndrome: A Systematic Review. J. Oral Maxillofac. Surg. 2011, 69, e165–e176. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Dixon-Williams, S.; Thornton, J.D. Where There Is Smoke…There Is Sleep Apnea. Chest 2014, 146, 1673–1680. [Google Scholar] [CrossRef] [Green Version]
- Varol, Y.; Anar, C.; Tuzel, O.E.; Guclu, S.Z.; Ucar, Z.Z. The impact of active and former smoking on the severity of obstructive sleep apnea. Sleep Breath. 2015, 19, 1279–1284. [Google Scholar] [CrossRef]
- Kolla, B.P.; Foroughi, M.; Saeidifard, F.; Chakravorty, S.; Wang, Z.; Mansukhani, M.P. The impact of alcohol on breathing parameters during sleep: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 42, 59–67. [Google Scholar] [CrossRef]
- Berry, R.B.; Quan, S.F.; Abreu, A.R.; Bibbs, M.L.; DelRosso, L.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6. Darien, Illinois: American Academy of Sleep Medicine. 2020. Available online: http://www.aasmnet.org/scoringmanual/ (accessed on 21 December 2022).
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Tondo, P.; Drigo, R.; Scioscia, G.; Ballarin, A.; Rossi, E.; Floriani, A.F.; Pauletti, A.; Barbaro, M.P.F.; Lacedonia, D. Usefulness of sleep events detection using a wrist worn peripheral arterial tone signal device (WatchPAT™) in a population at low risk of obstructive sleep apnea. J. Sleep Res. 2021, 30, e13352. [Google Scholar] [CrossRef]
- Faber, J.; Faber, C.; Faber, A.P. Obstructive sleep apnea in adults. Dent. Press J. Orthod. 2019, 24, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Tingting, X.; Danming, Y.; Xin, C. Non-surgical treatment of obstructive sleep apnea syndrome. Eur. Arch. Otorhinolaryngol. 2017, 275, 335–346. [Google Scholar] [CrossRef]
- Lombardi, C.; Mattaliano, P.; Parati, G. Sleep and Cardiac Disorders. In Medlink Neurology; Gilman, S., Ed.; MedLink Corporation: San Diego, CA, USA, 2013. [Google Scholar]
- Kendzerska, T.; Povitz, M.; Leung, R.S.; Boulos, M.I.; McIsaac, D.I.; Murray, B.J.; Bryson, G.L.; Talarico, R.; Hilton, J.F.; Malhotra, A.; et al. Obstructive Sleep Apnea and Incident Cancer: A Large Retrospective Multicenter Clinical Cohort Study. Cancer Epidemiology Biomarkers Prev. 2021, 30, 295–304. [Google Scholar] [CrossRef]
- Tondo, P.; Fanfulla, F.; Scioscia, G.; Sabato, R.; Salvemini, M.; De Pace, C.C.; Barbaro, M.P.F.; Lacedonia, D. The Burden of Respiratory Alterations during Sleep on Comorbidities in Obstructive Sleep Apnoea (OSA). Brain Sci. 2022, 12, 1359. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Lombardi, C.; Narkiewicz, K. Sleep apnea: Epidemiology, pathophysiology, and relation to cardiovascular risk. Am. J. Physiol. Integr. Comp. Physiol. 2007, 293, R1671–R1683. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, X.; Lu, Y. Obstructive Sleep Apnea Syndrome and Metabolic Diseases. Endocrinology 2018, 159, 2670–2675. [Google Scholar] [CrossRef] [Green Version]
- Saunamäki, T.; Jehkonen, M. Depression and anxiety in obstructive sleep apnea syndrome: A review. Acta Neurol. Scand. 2007, 116, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Hunyor, I.; Cook, K.M. Models of intermittent hypoxia and obstructive sleep apnea: Molecular pathways and their contribution to cancer. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R669–R687. [Google Scholar] [CrossRef] [Green Version]
- Lacedonia, D.; Landriscina, M.; Scioscia, G.; Tondo, P.; Caccavo, I.; Bruno, G.; Giordano, G.; Piscazzi, A.; Barbaro, M.P.F. Obstructive Sleep Apnea Worsens Progression-Free and Overall Survival in Human Metastatic Colorectal Carcinoma. J. Oncol. 2021, 2021, 1–5. [Google Scholar] [CrossRef]
- Lacedonia, D.; E Carpagnano, G.; Crisetti, E.; Cotugno, G.; Palladino, G.P.; Patricelli, G.; Sabato, R.; Barbaro, M.P.F. Mitochondrial DNA alteration in obstructive sleep apnea. Respir. Res. 2015, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Brahimi-Horn, M.C.; Pouysségur, J. HIF at a glance. J. Cell Sci. 2009, 122, 1055–1057. [Google Scholar] [CrossRef]
- Rocha, S. Gene regulation under low oxygen: Holding your breath for transcription. Trends Biochem. Sci. 2007, 32, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, N.S.; Rocha, S. Regulation of gene expression by hypoxia. Biochem. J. 2008, 414, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pialoux, V.; Mounier, R.; Brown, A.D.; Steinback, C.D.; Rawling, J.M.; Poulin, M.J. Relationship between oxidative stress and HIF-1α mRNA during sustained hypoxia in humans. Free Radic. Biol. Med. 2009, 46, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Garvey, J.F.; Taylor, C.T.; McNicholas, W.T. Cardiovascular disease in obstructive sleep apnoea syndrome: The role of intermittent hypoxia and inflammation. Eur. Respir. J. 2009, 33, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Almendros, I.; Montserrat, J.M.; Torres, M.; Dalmases, M.; Cabañas, M.L.; Campos-Rodríguez, F.; Navajas, D.; Farré, R. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir. Physiol. Neurobiol. 2013, 186, 303–307. [Google Scholar] [CrossRef]
- Almendros, I.; Montserrat, J.M.; Ramirez, J.; Torres, M.; Durán-Cantolla, J.; Navajas, D.; Farre, R. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur. Respir. J. 2011, 39, 215–217. [Google Scholar] [CrossRef]
- Nieto, F.; Peppard, P.; Young, T.; Finn, L.; Hla, K.; Farré, R. Sleep-disordered breathing and cancer mortality: Results from the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2012, 186, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Campos-Rodriguez, F.; Martinez-Garcia, M.; Martinez, M.; Duran-Cantolla, J.; Peña, M.L.; Masdeu, M.; Gonzalez, M.; Campo, F.; Gallego, I.; Marin, J.; et al. Association between Obstructive Sleep Apnea and Cancer Incidence in a Large Multicenter Spanish Cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Kendzerska, T.; Leung, R.S.; Hawker, G.; Tomlinson, G.; Gershon, A.S. Obstructive sleep apnea and the prevalence and incidence of cancer. Can. Med. Assoc. J. 2014, 186, 985–992. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakim, F.; Wang, Y.; Zhang, S.X.; Zheng, J.; Yolcu, E.S.; Carreras, A.; Khalyfa, A.; Shirwan, H.; Almendros, I.; Gozal, D. Fragmented Sleep Accelerates Tumor Growth and Progression through Recruitment of Tumor-Associated Macrophages and TLR4 Signaling. Cancer Res 2014, 74, 1329–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.; Campillo, N.; Nonaka, P.N.; Montserrat, J.M.; Gozal, D.; Martínez-García, M.A.; Campos-Rodriguez, F.; Navajas, D.; Farré, R.; Almendros, I. Aging Reduces Intermittent Hypoxia–induced Lung Carcinoma Growth in a Mouse Model of Sleep Apnea. Am. J. Respir. Crit. Care Med. 2018, 198, 1234–1236. [Google Scholar] [CrossRef]
- Pataka, A.; Bonsignore, M.R.; Ryan, S.; Riha, R.L.; Pépin, J.L.; Schiza, S.; Basoglu, O.K.; Sliwinski, P.; Ludka, O.; Steiropoulos, P.; et al. Cancer prevalence is increased in females with sleep apnoea: Data from the ESADA study. Eur. Respir. J. 2019, 53, 1900091. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Bhadra, M.P.; Girschick, H.J.; Bhadra, U. MicroRNAs - micro in size but macro in function. FEBS J. 2008, 275, 4929–4944. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Calin, G.A.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Med. 2009, 60, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Castanotto, D.; Rossi, J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009, 457, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Roberts, T.C. The MicroRNA Biology of the Mammalian Nucleus. Mol. Ther.-Nucleic Acids 2014, 3, e188. [Google Scholar] [CrossRef]
- Weinmann, L.; Höck, J.; Ivacevic, T.; Ohrt, T.; Mütze, J.; Schwille, P.; Kremmer, E.; Benes, V.; Urlaub, H.; Meister, G. Importin 8 Is a Gene Silencing Factor that Targets Argonaute Proteins to Distinct mRNAs. Cell 2009, 136, 496–507. [Google Scholar] [CrossRef]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef]
- Santamaria-Martos, F.; Benítez, I.; Ortega, F.; Zapater, A.; Giron, C.; Pinilla, L.; Pascual, L.; Cortijo, A.; Dalmases, M.; Fernandez-Real, J.M.; et al. Circulating microRNA profile as a potential biomarker for obstructive sleep apnea diagnosis. Sci. Rep. 2019, 9, 13456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soccio, P.; Moriondo, G.; Lacedonia, D.; Tondo, P.; Quarato, C.M.I.; Barbaro, M.P.F.; Scioscia, G. EVs-miRNA: The New Molecular Markers for Chronic Respiratory Diseases. Life 2022, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Sakaguchi, Y.; Miyauchi, K.; Suzuki, T.; Kashiwabara, S.-I.; Baba, T.; Suzuki, T. Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev. 2009, 23, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of MicroRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; Jiang, Z.; Zhang, Z.; Yang, R.; Chen, J.; et al. MicroRNA Expression Signatures of Bladder Cancer Revealed by Deep Sequencing. PLoS ONE 2011, 6, e18286. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-De-La-Torre, M.; Khalyfa, A.; Sánchez-De-La-Torre, A.; Martinez-Alonso, M.; Martinez-García, M.; Barceló, A.; Lloberes, P.; Campos-Rodriguez, F.; Capote, F.; Diaz-De-Atauri, M.J.; et al. Precision Medicine in Patients with Resistant Hypertension and Obstructive Sleep Apnea. J. Am. Coll. Cardiol. 2015, 66, 1023–1032. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine 2017, 96, e7917. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.S.; Silveira, A.C.; Martins, F.C.; Costa-Hong, V.; Lebkuchen, A.; Cardozo, K.H.M.; Bernardes, F.M.; Bortolotto, L.A.; Lorenzi-Filho, G.; Oliveira, E.M.; et al. Severe obstructive sleep apnea is associated with circulating microRNAs related to heart failure, myocardial ischemia, and cancer proliferation. Sleep Breath. 2020, 24, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Shen, P.; Chen, J. Expression Profile Analysis and Image Observation of miRNA in Serum of Patients with Obstructive Sleep Apnea-Hypopnea Syndrome. Contrast Media Mol. Imaging 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Khalyfa, A.; Zhang, C.; Khalyfa, A.A.; Foster, G.E.; Beaudin, A.E.; Andrade, J.; Hanly, P.J.; Poulin, M.J.; Gozal, D. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep 2016, 39, 2077–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalyfa, A.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Khalyfa, A.A.; Gozal, D. Circulating microRNAs as Potential Biomarkers of Endothelial Dysfunction in Obese Children. Chest 2016, 149, 786–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalyfa, A.; Gozal, D.; Chan, W.-C.; Andrade, J.; Prasad, B. Circulating plasma exosomes in obstructive sleep apnoea and reverse dipping blood pressure. Eur. Respir. J. 2019, 55, 1901072. [Google Scholar] [CrossRef]
- He, L.; Liao, X.; Zhu, G.; Kuang, J. miR-126a-3p targets HIF-1α and alleviates obstructive sleep apnea syndrome with hypertension. Hum. Cell 2020, 33, 1036–1045. [Google Scholar] [CrossRef]
- Yang, X.; Niu, X.; Xiao, Y.; Lin, K.; Chen, X. MiRNA expression profiles in healthy OSAHS and OSAHS with arterial hypertension: Potential diagnostic and early warning markers. Respir. Res. 2018, 19, 194. [Google Scholar] [CrossRef]
- Slouka, D.; Windrichova, J.; Rezackova, H.; Houfkova, K.; Kucera, R.; Cerna, V.; Kostlivy, T.; Topolcan, O.; Pesta, M. The potential of miR-499 plasmatic level as a biomarker of obstructive sleep apnea syndrome. Biomarkers Med. 2021, 15, 1011–1019. [Google Scholar] [CrossRef]
- Targa, A.; Dakterzada, F.; Benítez, I.D.; De Gonzalo-Calvo, D.; Moncusí-Moix, A.; López, R.; Pujol, M.; Arias, A.; De Batlle, J.; Sánchez-De-La-Torre, M.; et al. Circulating MicroRNA Profile Associated with Obstructive Sleep Apnea in Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 4363–4372. [Google Scholar] [CrossRef]
- Li, K.; Chen, Z.; Qin, Y.; Wei, Y. MiR-664a-3p expression in patients with obstructive sleep apnea. Medicine 2018, 97, e9813. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Hsu, P.-Y.; Su, M.-C.; Chin, C.-H.; Liou, C.-W.; Wang, T.-Y.; Lin, Y.-Y.; Lee, C.P.; Lin, M.-C.; Hsiao, C.-C. miR-21-5p Under-Expression in Patients with Obstructive Sleep Apnea Modulates Intermittent Hypoxia with Re-Oxygenation-Induced-Cell Apoptosis and Cytotoxicity by Targeting Pro-Inflammatory TNF-α-TLR4 Signaling. Int. J. Mol. Sci. 2020, 21, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriondo, G.; Scioscia, G.; Soccio, P.; Tondo, P.; De Pace, C.C.; Sabato, R.; Barbaro, M.P.F.; Lacedonia, D. Effect of Hypoxia-Induced Micro-RNAs Expression on Oncogenesis. Int. J. Mol. Sci. 2022, 23, 6294. [Google Scholar] [CrossRef] [PubMed]

| SYMPTOMS | |
|---|---|
| TYPES |
|
| FREQUENT |
|
| LESS COMMON |
|
| SIGNS | |
| BODY MASS INDEX—BMI | >29 |
| NECK CIRCOMFERENCE | >43 cm (men) >41 cm (women) |
| CRANIAL-FACIAL DYSMORPHISMS | Cause a reduction in the caliber of the upper airways |
| PHARYNGEAL ANOMALIES | Cause a reduction in the caliber of the upper airways |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriondo, G.; Soccio, P.; Tondo, P.; Scioscia, G.; Sabato, R.; Foschino Barbaro, M.P.; Lacedonia, D. Obstructive Sleep Apnea: A Look towards Micro-RNAs as Biomarkers of the Future. Biology 2023, 12, 66. https://doi.org/10.3390/biology12010066
Moriondo G, Soccio P, Tondo P, Scioscia G, Sabato R, Foschino Barbaro MP, Lacedonia D. Obstructive Sleep Apnea: A Look towards Micro-RNAs as Biomarkers of the Future. Biology. 2023; 12(1):66. https://doi.org/10.3390/biology12010066
Chicago/Turabian StyleMoriondo, Giorgia, Piera Soccio, Pasquale Tondo, Giulia Scioscia, Roberto Sabato, Maria Pia Foschino Barbaro, and Donato Lacedonia. 2023. "Obstructive Sleep Apnea: A Look towards Micro-RNAs as Biomarkers of the Future" Biology 12, no. 1: 66. https://doi.org/10.3390/biology12010066






