M.tb-Rv2462c of Mycobacterium tuberculosis Shows Chaperone-like Activity and Plays a Role in Stress Adaptation and Immunomodulation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cell Lines
2.2. Cloning and Purification of M.tb-Rv2462c by Ni-NTA Column
2.3. Enzyme Activity
2.4. Chaperone-like Activity of M.tb Rv2462c
2.5. Expression of M.tb-Rv2462c in Mycobacterium Smegmatis
2.6. Examining In Vitro Growth and Effect of H2O2
2.7. Intracellular Replication of M. Smegmatis Strains in THP-1 Cells
2.8. Biofilm Estimation
2.9. Prediction of Antigenicity
2.10. Immune Assay
3. Results
3.1. rTig of M.tb Is Enzymatically Active
3.2. rTig Displays Properties of a Chaperone Protein
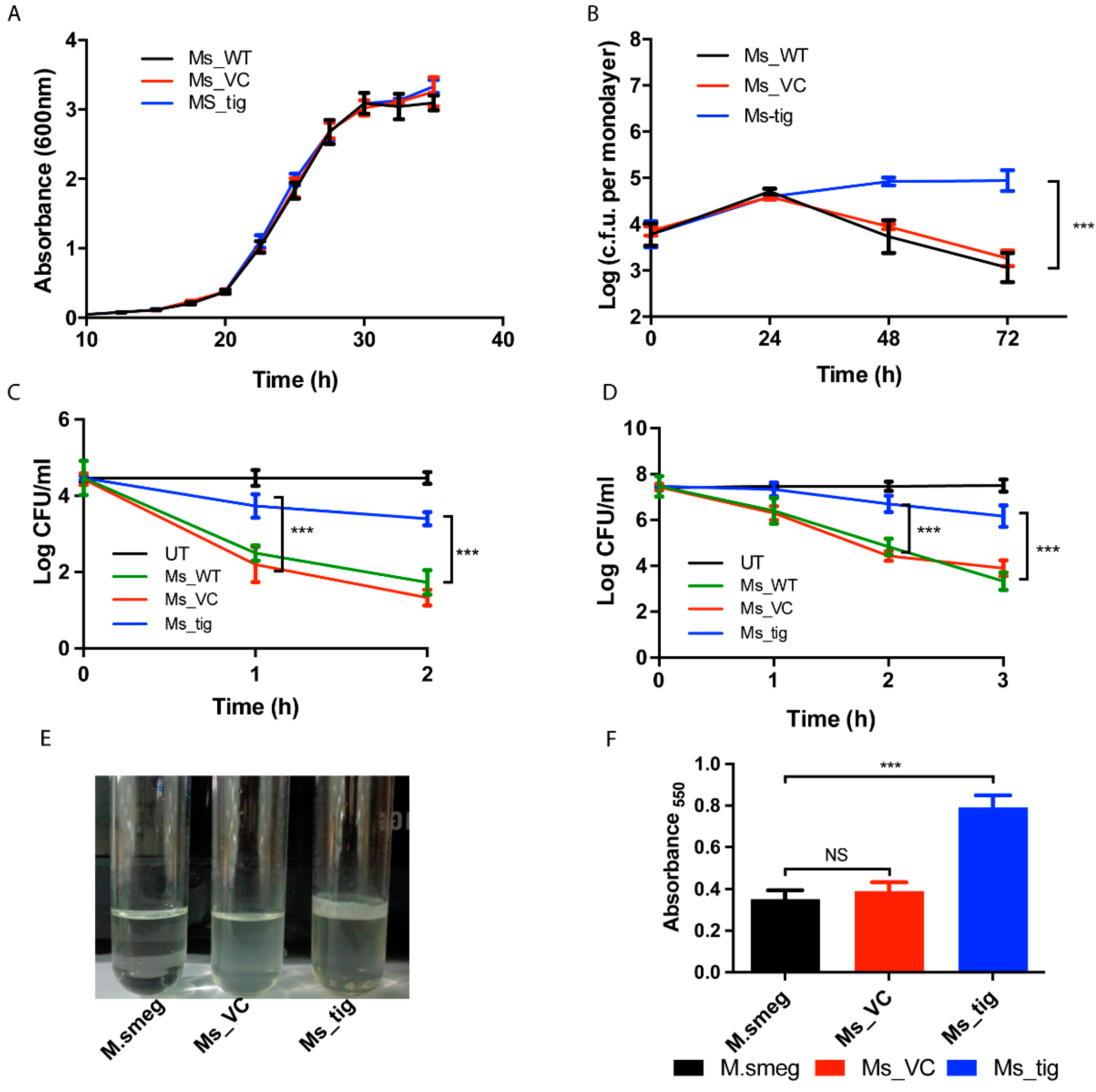
3.3. M.tb Tig Helps Mycobacterial Survival within THP-1 Macrophages
3.4. Exposure of THP-1 Cell with M.tb Tig Elicited Pro-Inflammatory Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrt, S.; Schnappinger, D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell. Microbiol. 2009, 11, 1170–1178. [Google Scholar] [CrossRef]
- Glaziou, P.; Floyd, K.; Raviglione, M.C. Global Epidemiology of Tuberculosis. Semin. Respir. Crit. Care Med. 2018, 39, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet. Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.G.; Amici, C.; Rossi, A. Role of Heat Shock Proteins in Viral Infection. In Prokaryotic and Eukaryotic Heat Shock Proteins in Infectious Disease; Springer: Berlin/Heidelberg, Germany, 2009; Volume 4, pp. 51–84. [Google Scholar] [CrossRef]
- Kramer, G.; Boehringer, D.; Ban, N.; Bukau, B. The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat. Struct. Mol. Biol. 2009, 16, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Stoller, G.; Rücknagel, K.P.; Nierhaus, K.H.; Schmid, F.X.; Fischer, G.; Rahfeld, J.U. A ribosome-associated peptidyl-prolyl cis/trans isomerase identified as the trigger factor. EMBO J. 1995, 14, 4939–4948. [Google Scholar] [CrossRef]
- Ferbitz, L.; Maier, T.; Patzelt, H.; Bukau, B.; Deuerling, E.; Ban, N. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature 2004, 431, 590–596. [Google Scholar] [CrossRef]
- Merz, F.; Hoffmann, A.; Rutkowska, A.; Zachmann-Brand, B.; Bukau, B.; Deuerling, E. The C-terminal domain of Escherichia coli trigger factor represents the central module of its chaperone activity. J. Biol. Chem. 2006, 281, 31963–31971. [Google Scholar] [CrossRef]
- Huang, G.C.; Li, Z.Y.; Zhou, J.M.; Fischer, G. Assisted folding of D-glyceraldehyde-3-phosphate dehydrogenase by trigger factor. Protein Sci. A Publ. Protein Soc. 2000, 9, 1254–1261. [Google Scholar] [CrossRef]
- Li, Z.; Wu, D.; Zhan, B.; Hu, X.; Gan, J.; Ji, C.; Li, J. Structural insights into the complex of trigger factor chaperone and ribosomal protein S7 from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 2019, 512, 838–844. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., 3rd; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, B.; Lata, M.; Joshi, B.; Venkatesan, K.; Shukla, S.; Bisht, D. Comparative Proteomic Analysis of Aminoglycosides Resistant and Susceptible Mycobacterium tuberculosis Clinical Isolates for Exploring Potential Drug Targets. PLoS ONE 2015, 10, e0139414. [Google Scholar] [CrossRef]
- Hampshire, T.; Soneji, S.; Bacon, J.; James, B.W.; Hinds, J.; Laing, K.; Stabler, R.A.; Marsh, P.D.; Butcher, P.D. Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: A model for persistent organisms? Tuberculosis 2004, 84, 228–238. [Google Scholar] [CrossRef]
- Rifat, D.; Bishai, W.R.; Karakousis, P.C. Phosphate depletion: A novel trigger for Mycobacterium tuberculosis persistence. J. Infect. Dis. 2009, 200, 1126–1135. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, A.; Tripathi, D.; Kumar, A.; Khubaib, M.; Bhuwan, M.; Chaudhuri, T.K.; Hasnain, S.E.; Ehtesham, N.Z. Mycobacterium tuberculosis peptidyl-prolyl isomerases also exhibit chaperone like activity in-vitro and in-vivo. PLoS ONE 2016, 11, e0150288. [Google Scholar] [CrossRef]
- Perez, C.; Maier, T. Expression, Purification, and Structural Biology of Membrane Proteins; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Suragani, M.; Aadinarayana, V.D.; Pinjari, A.B.; Tanneeru, K.; Guruprasad, L.; Banerjee, S.; Pandey, S.; Chaudhuri, T.K.; Ehtesham, N.Z. Human resistin, a proinflammatory cytokine, shows chaperone-like activity. Proc. Natl. Acad. Sci. USA 2013, 110, 20467–20472. [Google Scholar] [CrossRef]
- Fischer, G.; Bang, H.; Mech, C. Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed. Biochim. Acta 1984, 43, 1101–1111. [Google Scholar]
- Wu, T.; Zhao, Z.; Zhang, L.; Ma, H.; Lu, K.; Ren, W.; Liu, Z.; Chang, H.; Bei, W.; Qiu, Y.; et al. Trigger Factor of Streptococcus Suis Is Involved in Stress Tolerance and Virulence. Microb. Pathog. 2011, 51, 69–76. [Google Scholar] [CrossRef]
- Pandey, S.; Tripathi, D.; Khubaib, M.; Kumar, A.; Sheikh, J.A.; Sumanlatha, G.; Ehtesham, N.Z.; Hasnain, S.E. Mycobacterium tuberculosis Peptidyl-Prolyl Isomerases Are Immunogenic, Alter Cytokine Profile and Aid in Intracellular Survival. Front. Cell. Infect. Microbiol. 2017, 7, 38. [Google Scholar] [CrossRef]
- Nair, S.; Ramaswamy, P.A.; Ghosh, S.; Joshi, D.C.; Pathak, N.; Siddiqui, I.; Sharma, P.; Hasnain, S.E.; Mande, S.C.; Mukhopadhyay, S. The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J. Immunol. 2009, 183, 6269–6281. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Nandyala, A.; Podili, R.; Katoch, V.; Murthy, K.; Hasnain, S.E. Mycobacterium tuberculosis (Mtb) isocitrate dehydrogenases show strong B cell response and distinguish vaccinated controls from TB patients. Proc. Natl. Acad. Sci. USA 2004, 101, 12652–12657. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Tripathi, D.; Kant, S.; Chandra, H.; Bhatnagar, R.; Banerjee, N. The conserved hypothetical protein Rv0574c is required for cell wall integrity, stress tolerance, and virulence of Mycobacterium tuberculosis. Infect. Immun. 2015, 83, 120–129. [Google Scholar] [CrossRef]
- Schulz, C.; Lai, X.; Bertrams, W.; Jung, A.L.; Sittka-Stark, A.; Herkt, C.E.; Janga, H.; Zscheppang, K.; Stielow, C.; Schulte, L.; et al. Thp-1-Derived Macrophages Render Lung Epithelial Cells Hypo-Responsive to Legionella Pneumophila—A Systems Biology Study. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gasymov, O.K.; Glasgow, B.J. Ans Fluorescence: Potential to Augment the Identification of the External Binding Sites of Proteins. Biochim. Biophys. Acta 2007, 1774, 403–411. [Google Scholar] [CrossRef]
- Vanghele, M.; Ganea, E. The role of bacterial molecular chaperones in pathogen survival within the host. Rom. J. Biochem. 2010, 47, 100. [Google Scholar]
- Buchmeier, N.A.; Heffron, F. Induction of Salmonella stress proteins upon infection of macrophages. Science 1990, 248, 730–732. [Google Scholar] [CrossRef]
- Henderson, B.; Allan, E.; Coates, A.R. Stress wars: The direct role of host and bacterial molecular chaperones in bacterial infection. Infect. Immun. 2006, 74, 3693–3706. [Google Scholar] [CrossRef]
- Fossati, G.; Izzo, G.; Rizzi, E.; Gancia, E.; Modena, D.; Moras, M.L.; Niccolai, N.; Giannozzi, E.; Spiga, O.; Bono, L.; et al. Mycobacterium tuberculosis chaperonin 10 is secreted in the macrophage phagosome: Is secretion due to dissociation and adoption of a partially helical structure at the membrane? J. Bacteriol. 2003, 185, 4256–4267. [Google Scholar] [CrossRef]
- Crooke, E.; Wickner, W. Trigger factor: A soluble protein that folds pro-OmpA into a membrane-assembly-competent form. Proc. Natl. Acad. Sci. USA 1987, 84, 5216–5220. [Google Scholar] [CrossRef]
- Kandror, O.; Goldberg, A.L. Trigger factor is induced upon cold shock and enhances viability of Escherichia coli at low temperatures. Proc. Natl. Acad. Sci. USA 1997, 94, 4978–4981. [Google Scholar] [CrossRef]
- Newman, G.W.; Gan, H.X.; McCarthy, P.L., Jr.; Remold, H.G. Survival of human macrophages infected with Mycobacterium avium intracellulare correlates with increased production of tumor necrosis factor-alpha and IL-6. J. Immunol. 1991, 147, 3942–3948. [Google Scholar]
- Tsao, T.C.; Hong, J.; Huang, C.; Yang, P.; Liao, S.K.; Chang, K.S. Increased TNF-alpha, IL-1 beta and IL-6 levels in the bronchoalveolar lavage fluid with the upregulation of their mRNA in macrophages lavaged from patients with active pulmonary tuberculosis. Tuber. Lung Dis. 1999, 79, 279–285. [Google Scholar] [CrossRef]
- Ilonidis, G.; Parapanisiou, E.; Anogeianaki, A.; Giavazis, I.; Theofilogiannakos, E.K.; Tsekoura, P.; Kidonopoulou, K.; Trakatelli, C.; Polimenidis, Z.; Conti, P.; et al. Interleukin-1beta (IL-1 beta), interleukin 6 (IL-6) and tumor necrosis factor (TNF) in plasma and pleural fluid of pneumonia, lung cancer and tuberculous pleuritis. J. Biol. Regul. Homeost. Agents 2006, 20, 41–46. [Google Scholar]
- van der Poll, T. Pro-Inflammatory Cytokines: Double-Edged Swords in the Pathogenesis of Bacterial Infection. In Mechanisms of Organ Dysfunction in Critical Illness; Evans, T.W., Fink, M.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 146–158. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Kandror, O.; Sherman, M.; Goldberg, A. Rapid degradation of an abnormal protein in Escherichia coli proceeds through repeated cycles of association with GroEL. J. Biol. Chem. 1999, 274, 37743–37749. [Google Scholar] [CrossRef]
- Kandror, O.; Sherman, M.; Rhode, M.; Goldberg, A.L. Trigger factor is involved in GroEL-dependent protein degradation in Escherichia coli and promotes binding of GroEL to unfolded proteins. EMBO J. 1995, 14, 6021–6027. [Google Scholar] [CrossRef]
- Lyon, W.R.; Caparon, M.G. Trigger factor-mediated prolyl isomerization influences maturation of the Streptococcus pyogenes cysteine protease. J. Bacteriol. 2003, 185, 3661–3667. [Google Scholar] [CrossRef]
- Lyon, W.R.; Gibson, C.M.; Caparon, M.G. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 1998, 17, 6263–6275. [Google Scholar] [CrossRef]
- Wen, Z.T.; Burne, R.A. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 2004, 186, 2682–2691. [Google Scholar] [CrossRef]
- Lenaerts, A.J.; Hoff, D.; Aly, S.; Ehlers, S.; Andries, K.; Cantarero, L.; Orme, I.M.; Basaraba, R.J. Location of Persisting Mycobacteria in a Guinea Pig Model of Tuberculosis Revealed by R207910. Antimicrob. Agents Chemother. 2007, 51, 3338–3345. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrands, I.; Weldingh, K.; Jacobsen, S.; Hansen, C.V.; Florio, W.; Gianetri, I.; Andersen, P. Mapping and Identification of Mycobacterium Tuberculosis Proteins by Two-Dimensional Gel Electrophoresis, Microsequencing and Immunodetection. Electrophoresis 2000, 21, 935–948. [Google Scholar] [CrossRef]
- Rosenkrands, I.; King, A.; Weldingh, K.; Moniatte, M.; Moertz, E.; Andersen, P. Towards the Proteome of Mycobacterium Tuberculosis. Electrophoresis 2000, 21, 3740–3756. [Google Scholar] [CrossRef] [PubMed]
- Jungblut, P.R.; Schaible, U.E.; Mollenkopf, H.J.; Zimny-Arndt, U.; Raupach, B.; Mattow, J.; Halada, P.; Lamer, S.; Hagens, K.; Kaufmann, S.H. Comparative proteome analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG strains: Towards functional genomics of microbial pathogens. Mol. Microbiol. 1999, 33, 1103–1117. [Google Scholar] [CrossRef]
- Boyd, C.D.; Smith, T.J.; El-Kirat-Chatel, S.; Newell, P.D.; Dufrene, Y.F.; O’Toole, G.A. Structural features of the Pseudomonas fluorescens biofilm adhesin LapA required for LapG-dependent cleavage, biofilm formation, and cell surface localization. J. Bacteriol. 2014, 196, 2775–2788. [Google Scholar] [CrossRef]
- Zhou, G.; Yuan, J.; Gao, H. Regulation of biofilm formation by BpfA, BpfD, and BpfG in Shewanella oneidensis. Front. Microbiol. 2015, 6, 790. [Google Scholar] [CrossRef]
- Keogh, R.A.; Zapf, R.L.; Frey, A.; Marino, E.C.; Null, G.G.; Wiemels, R.E.; Holzschu, D.L.; Shaw, L.N.; Carroll, R.K. Staphylococcus aureus Trigger Factor Is Involved in Biofilm Formation and Cooperates with the Chaperone PpiB. J. Bacteriol. 2021, 203, e00681-20. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khawary, M.; Rakshit, R.; Bahl, A.; Juneja, P.; Kant, S.; Pandey, S.; Tripathi, D. M.tb-Rv2462c of Mycobacterium tuberculosis Shows Chaperone-like Activity and Plays a Role in Stress Adaptation and Immunomodulation. Biology 2023, 12, 69. https://doi.org/10.3390/biology12010069
Khawary M, Rakshit R, Bahl A, Juneja P, Kant S, Pandey S, Tripathi D. M.tb-Rv2462c of Mycobacterium tuberculosis Shows Chaperone-like Activity and Plays a Role in Stress Adaptation and Immunomodulation. Biology. 2023; 12(1):69. https://doi.org/10.3390/biology12010069
Chicago/Turabian StyleKhawary, Masuma, Roopshali Rakshit, Aayush Bahl, Pallavi Juneja, Sashi Kant, Saurabh Pandey, and Deeksha Tripathi. 2023. "M.tb-Rv2462c of Mycobacterium tuberculosis Shows Chaperone-like Activity and Plays a Role in Stress Adaptation and Immunomodulation" Biology 12, no. 1: 69. https://doi.org/10.3390/biology12010069
APA StyleKhawary, M., Rakshit, R., Bahl, A., Juneja, P., Kant, S., Pandey, S., & Tripathi, D. (2023). M.tb-Rv2462c of Mycobacterium tuberculosis Shows Chaperone-like Activity and Plays a Role in Stress Adaptation and Immunomodulation. Biology, 12(1), 69. https://doi.org/10.3390/biology12010069







