Simple Summary
Marine waters are the most susceptible to exposure to contaminants, specifically polyaromatic hydrocarbons (PAHs) and heavy metals that are poisonous and carcinogenic. On the other hand, a marine environment can provide various natural materials that can perform biodegradation and biosorption. This research seeks to investigate various species of symbiotic sponge bacteria and assess the feasibility of developing bacterial consortium formulations for the remediation of these hazardous contaminants. Several species of symbiotic sponge bacteria are capable of biodegrading polyaromatic compounds. According to the search and analysis results, several species of symbiotic sponge bacteria have the potential to biodegrade polyaromatic compounds, such as naphthalene, anthracene, and pyrene. Other sponge symbiont bacteria or bacteria isolated from the same sponge can also be used for the biosorption of heavy metals. The ability of the symbiotic sponge bacteria to be formulated as a consortium of bacteria for pollutant remediation applications, which we refer to as the metallohydrocarbonoclastic formula, is another feature that sets them apart. The proposed use is a bioremediation approach that the community can implement to the isolation processes, such as the remediation of contaminants in water used for fish farming and shrimp plotting methods, including in ponds, to provide aquaculture products free of toxic pollutants.
Abstract
Toxic materials in waste generally contain several components of the global trending pollutant category, especially PAHs and heavy metals. Bioremediation technology for waste management that utilizes microorganisms (bacteria) has not been fully capable of breaking down these toxic materials into simple and environmentally friendly chemical products. This review paper examines the potential application of a consortium of marine sponge symbionts with high performance and efficiency in removing PAHs and heavy metal contaminants. The method was carried out through a review of several related research articles by the author and published by other researchers. The results of the study conclude that the development of global trending pollutant (GTP) bioremediation technology could be carried out to increase the efficiency of remediation. Several types of marine sponge symbiont bacteria, hydrocarbonoclastic (R-1), metalloclastic (R-2), and metallo-hydro-carbonoclastic (R-3), have the potential to be applied to improve waste removal performance. A consortium of crystalline bacterial preparations is required to mobilize into GTP-exposed sites rapidly. Bacterial symbionts of marine sponges can be traced mainly to sea sponges, whose body surface is covered with mucus.
1. Introduction
Global trending pollutant (GTP) is a term applied to several types of pollutant materials (heavy metals, aromatic hydrocarbons, microplastics, medical waste, and pesticide residues) that pose many complex problems to the global environment [1,2,3,4,5,6,7]. The problem of environmental quality is especially felt by developing countries [3,8]. The issue of GTP in this decade has been very much discussed, not only by environmental observers and activists, as well as scientists in the field of environmental management, but also by several world leaders who have voiced the need for real action in reducing fossil fuel consumption [9,10,11]. The global policy of reducing carbon consumption is a tangible manifestation of the emergency of environmental quality [7,12]. It is very reasonable because the rate of increase in global trends of pollutants is increasing massively, far beyond the natural recovery ability of nature to reduce these pollutants [13,14,15]. Reducing carbon in the environment is not enough because various types of GTP and their adverse environmental effects are also different [7,16].
The aquatic environment is a giant container that is most vulnerable to being affected by GTP contaminants [17]. This is because the topography of the area is generally low. Almost all types of GTP contaminants are found in particulates and residues and can be dissolved in air, soil and water bodies [4,7]. These materials eventually empty into the aquatic environments of rivers, lakes, swamps and the sea [18,19]. In this environment, these toxic pollutants form parasitic accumulations in almost all marine organisms, especially fish, sponges, algae, plankton, phytoplankton and other types of biota [20,21].
At the same time, much research has been carried out related to the management of toxic pollutants in order to achieve the dream of creating a green environment [22,23]. The results of this research have yielded many findings, technologies, innovations and methods related to removing toxic contaminants [24,25]. Degradation, reduction, destruction, and absorption are recommended and can be applied to decompose carcinogenic pollutants in the environment [26,27,28]. This method can be called bioremediation technology if it involves the role of living organisms, including the contribution of microorganisms as biodegraders that can decompose or may be able to eliminate the toxic properties of GTP contaminants [29,30,31].
The principle of bioremediation technology involves the development of biological methods that can be applied in a gradual combination with physical, chemical, and biological methods, depending on the characteristics of the pollutant being degraded [32,33]. Bioremediation technology that uses microorganisms as a decomposer component is generally good and is more often applied to wastewater treatment [34,35]. Sources of microorganisms that can carry out bioremediation functions can be obtained in an aquatic environment contaminated with pollutants, for example, in port areas, offshore oil processing industries or marine areas around locations that have experienced oil spills [36,37,38]. Pollutant-degrading microorganisms can also be found in the soil, especially on land with a history of contamination due to oil spills and agricultural land exposed to pesticides [3,39]. Toxic pollutant-degrading microorganisms can also be found in other organisms that form a symbiotic relationship, for example, in sponges and mangroves [40,41].
Isolation and screening methods can be applied to obtain potential microorganisms as biomaterials for degrading pollutants [42,43]. In general, two microorganisms have potential as biomaterials for degrading pollutants, namely, bacteria and fungi [44,45,46]. These groups of microorganisms have different abilities and degradation mechanisms for pollutants [47]. The application of bacteria in bioremediation mainly removes hydrocarbon contaminants, especially polyaromatic hydrocarbons (PAHs) and heavy metal pollutants. At the same time, the use of fungi in pollutant bioremediation has also been carried out but is still limited or is not as popular as using bacteria [48,49,50].
The types of bacteria identified have bioremediation capabilities against PAHs, but the level of bioremediation achieved is still low, especially if only one type of bacteria is used [51,52]. This is because bioremediation bacteria are generally resistant to acidic environments. At the same time, one well-known hydrocarbon component of bioremediation products is the simple organic compounds resulting from oxidation reactions in the form of alcohols, aldehydes, ketones and possibly carboxylate group compounds [18,53,54]. Carboxylic compounds from bacterial bioremediation products can change the habitat conditions (media) to a more acidic state. Changes in the medium’s acidity is narrowly between 0.3 to 1.4 [26,50]. This condition is usually accompanied by an increase in temperature so that the degradation activity of bacteria decreases, or the resistance of bacteria becomes weak. If there is an increase in salinity in this condition, especially if accompanied by movement due to currents, it can result in significant bacterial cell death [16,26,51,55]. This is not a significant value, but the nature of bacteria makes them quite sensitive to environmental changes. Therefore, this may cause their performance to drop, or may even kill the bacteria [32,48]. This condition is the limiting factor for the performance of bacterial bioremediation [48,50,56].
In the aquatic environment, especially in marine ecosystems, there are several types of biota, such as sponges, which are known to be often used as objects for the biomonitoring of the pollution of hydrocarbon components and heavy metals; some types of sponges are even used as references or bioindicators in analyzing the levels of PAHs and heavy metal contaminants [54,55]. In addition to biomonitoring and acting as bioindicators of this type of GTP contamination, it is known that this sponge can perform the degradation of hydrocarbon components and the biosorption of heavy metals [7,56,57,58]. This is based on research results that show the ability of sponges to live and thrive in an environment contaminated with GTP pollutants [7,59]. The development of knowledge about the degradation and adsorption function of marine sponges against pollutants was revealed after discovering that these sponges can achieve a mutualistic symbiosis with microorganisms, especially bacteria [60,61,62].
The bacterial–sponge symbiosis model is intensified when the sponge habitat is exposed to hydrocarbon or heavy metal pollutants, or perhaps both, as the sponge tries to survive in the extreme conditions of its growing environment [63,64,65]. At the same time, symbiont bacteria also use these conditions to produce a mucus substance that behaves as an enzyme, which is then spread on the surface of the sponge’s body to avoid the toxic pollutant [66,67,68]. Internal sponges also independently stimulate immunity in themselves against all forms of predators and changes in their habitat by producing metabolic substances [53,69,70].
The types and populations of sponges are vast, so the right sponge selection must be made with symbiont bacteria that have the potential and ability to degrade and adsorb. This can be done by selecting sponges in their habitat, especially those with dark colors or smooth body surfaces because these are coated with mucus [20,21,71,72,73]. Bacterial symbionts from the selected sponge are then isolated to obtain a single isolate [74,75]. The phenotypic analysis of sponge symbiont bacteria through biochemical tests using standard reagents needs to be carried out to ensure that these symbiotic bacteria can perform the functions of PAH degradation and heavy metal adsorption [6,76]. Bacterial symbionts are potentially useful if they react positively with several biochemical reagents, especially Methyl Red, Voges Proskauer, citrates, lactose, catalase, nitrate reduction and indole reagents [77,78]. Genotypic analysis of bacterial symbionts is important to obtain complete information related to bacterial species and strain and the number of DNA base pairs, and it is also possible to carry out genotypic analyses of these symbiotic bacteria using 16S rRNA sequences [36,79,80,81].
The degradation function of sponge symbiont bacteria used for qualitative analysis can be ensured by the identification of several bacterial cells on media containing hydrocarbon components, such as pyrene [82,83,84]. Bacteria that can adapt to an environment containing PAHs are characterized by their activity after being incubated for ±24 h. This situation indicates that bacteria can carry out the biodegradation of polyaromatic hydrocarbon pollutants [85,86]. A preliminary qualitative test can determine the adsorption function of sponge symbiont bacteria by inserting ±1 mL of bacterial suspension into a medium containing heavy metal contaminants after being incubated for ±24 h and then measuring the optical density (OD600) of the interaction medium. If there is an increase in the turbidity or absorption, this indicates that the symbiont bacteria could perform the adsorption function on the heavy metals tested [87,88].
The mechanism of the bioremediation of hydrocarbon components is slightly different between aliphatic and aromatic hydrocarbons [89,90]. The mechanism of the degradation of hydrocarbon components generally proceeds via microorganisms, especially sponge symbiont bacteria, operating through oxidation reactions or biochemical reactions at the molecular level [69,91]. This involves the entry of metabolic substances or enzymes (dioxygenase) produced by the bacteria into the structure of hydrocarbon molecules that act as substrates to enable these molecules to undergo destruction, which causes the molecules to break down and produce organic compound products containing hydroxyl functional groups, and which then turn into keto-enol compounds [92,93,94].
After forming a keto-enol complex as the initial component of the remediation product, the oxidation reaction against PAH contaminants should continue. Further oxidation of these complex molecules produces organic compounds containing aldehyde, carboxylic, and perhaps ketone groups. [16,95,96]. Ideally, the oxidation reaction proceeds until compounds that can enter the metabolic cycle consist of simple organic molecules. [26,97]. The ability of bacterial cells to survive pressure fluctuations in pH, salinity and temperature significantly impacts the persistence of PAH bioremediation [42,43]. The oxidation reaction of hydrocarbon components can proceed if the ideal conditions required for the degradation of bacteria are met [32,48,98]. Generally, the degradation performance of bacteria decreases when the oxidation produces a carboxylic acid product [50,58,99]. Another factor that can inhibit the bacterial degradation of the substrate (reactant) of the hydrocarbon component is the low solubility of the hydrocarbon component, making it difficult for bacteria to penetrate [71,100,101].
The rate of the bacterial biodegradation of hydrocarbon contaminants varies in the range of 35–97%. Several factors cause this, such as the type of bacteria used; the type of hydrocarbon pollutant (aliphatic or PAHs) [26,50,102,103]; the interaction time; the degradation method; the concentration of hydrocarbon components used as reactants; the number of bacterial cells; the presence or absence of nutrition; oxygen injection (aeration); the scale of experiments carried out and other factors [32,48,104,105]. Variations in the level of bacterial degradation of hydrocarbon components indicate that multiple factors influence bioremediation [58,106]. Two things always occur in the biodegradation of hydrocarbon components (subtracts) by bacteria (degraders), namely: (1) The biodegradation of hydrocarbon pollutants by bacteria through an oxidation reaction pathway involving enzymes produced by bacteria in response to the presence of toxic substances in their growth habitat [71,107,108]. (2) A decrease in the performance of bacterial degradation when degradation products are formed as carboxylic compounds. These two things cause the degradation of hydrocarbon components, especially PAHs, to be incomplete, or may prevent the 100% degradation of the substrate [50,109]. Pyrene biodegradation generally stops at the stage at which the benzene component is formed [32,110,111]. Under these conditions, it is assumed that all bacterial cells will have died [110,112].
These data make it possible to modify the biodegradation of hydrocarbon components using a consortium of bacteria in diverse species or a consortium of microorganisms (a mix of bacteria and fungi) [51,92,113]. The first modification involves a consortium of certain species of bacteria (X) which have a high degradation activity, is expected to work at the beginning of contact, and is combined with bacteria (Y), which have a slow adaptation rate and continue the degradation process when the cells of the X-species bacteria have died [114]. The second modification, a consortium of X-species bacteria that perform degradation when combined with fungi of type (Z), is more tolerant of acids that can degrade hydrocarbons in acidic media [115,116]. This method is one of the potential alternatives for developing bioremediation technology. It is hoped that all hydrocarbon components will be wholly or 100% degraded and produce the final product of simple organic compounds in the form of salicylic acid and similar, which are environmentally friendly [117,118].
2. Polycyclic of Aromatic and Heavy Metals Bioremediation Analysis Instrument
Among the 16 other PAHs, pyrenes are placed in the middle based on their molecular size, their number of rings (four), their toxicity level, and their water solubility [26,71]. Pyrene is considered the most toxic pollutant since it can come from various sources, such as combustion activities and the petroleum processing industry [19]. So far, pyrene has been used as a substrate in bioremediation investigations of PAHs [58,113]. This is why pyrene was selected for this bioremediation study. The bioremediation of global pollutants necessitates applying new technologies and innovations to improve remediation efficiency, such that the natural balance, especially in marine ecosystems, can be maintained [119]. Bacterial consortia in bioremediation represent a new approach and are needed in the future. The analysis of the performance and efficiency of bacterial remediation against PAHs generally uses instruments such as gas chromatography–mass spectrometry (GC-MS) [3,57], Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS) and X-ray diffraction analysis (XRD). In contrast, the remediation of heavy metals by bacteria generally uses atomic absorption spectroscopy (AAS) analytical instruments; inductively coupled plasma (ICP) can be combined with optical emission spectroscopy or mass spectrometry (MS) [120,121,122,123,124]. The combination of analytical instruments in pollutant bioremediation can be useful, especially for the bioremediation of pollutants containing two or more types [122].
Waste generated in the petroleum processing industry in the form of sludge, which generally contains hydrocarbon component pollutants, both aliphatic and aromatic, also contains heavy metal toxic materials, so a combination of analytical instruments is often used to obtain data related to performance, efficiency, mechanism and remediation products, including models of and changes to the pollutant material during remediation [125,126]. Researchers often use a combination of analytical instruments in the bioremediation of petroleum sludge waste with a single bacterium/bacterial consortium, such as GC-MS, FTIR and AAS [20,57,121]. SEM, EDS and XRD instruments usually observe changes in the surface shape or structure of bacteria-heavy metal complexes through extracellular bonding [120,121,122,123].
3. Bacterial Performance in Pollutant Bioremediation
Bioremediation innovation using a consortium of bacteria in pollutant remediation is a global trend, especially for PAHs and heavy metal pollutants. It aims to improve remediation efficiency and performance such that the final product of remediation is given in the form of simple organic compounds which are environmentally friendly and no longer cause health effects on living things in their environment [51,91,110].
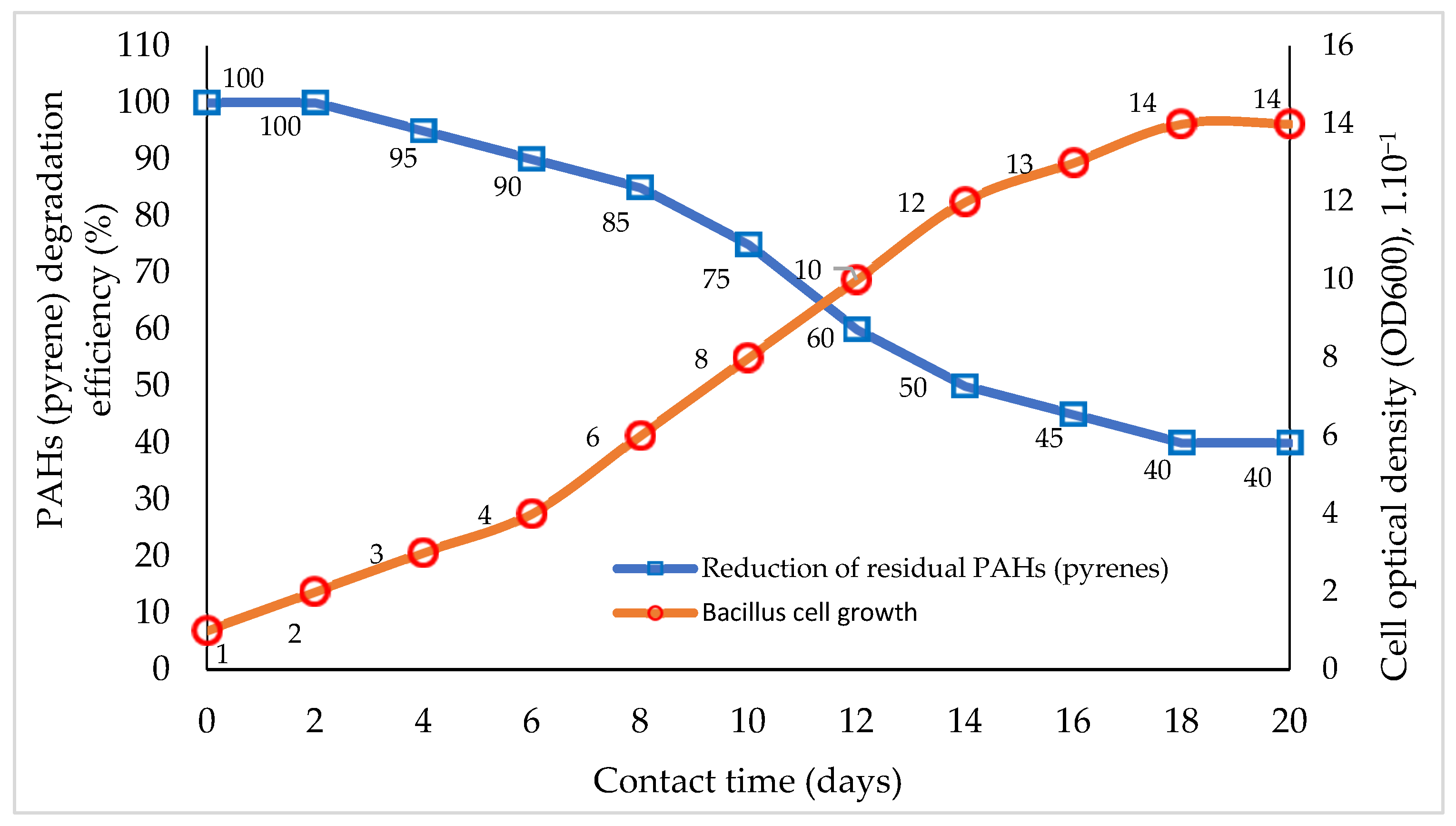
The relationship between single-species biodegrader remediation performance on PAH components and bacterial cell growth based on interaction time is presented in Figure 1.
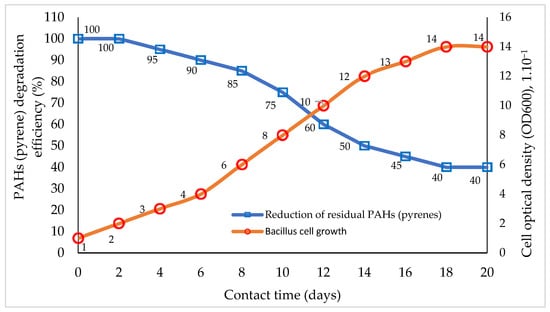
Figure 1.
Comparison of the percentage of PAH (pyrene) bioremediation with bacterial cell growth rates based on interaction time. Graphically modified figure [13,16,110,113,116].
The bioremediation of PAHs utilizing a single type of microbe (bacteria) is frequently inefficient. These bacteria perform poorly in the breakdown of PAH substrates, both when conducted on a laboratory scale with a single substrate pyrene and when used in natural conditions that are difficult to manage. This is due to the fluctuating influence of the external environment, as well as the salinity and pH levels [48,106,107]. This occurs because the biodegradation of PAHs by bacteria takes place in several stages, generally starting with an oxidation reaction and then destroying the substrate’s molecular structure. The substrate degradation process, via both biostimulation and bioaugmentation methods [16,127,128], using bacteria, continues until transition products are obtained in the form of acidic compounds (carboxylic) [8,9,10,11,26,33,56]. At this stage, the performance of bacteria is often reduced significantly due to their inability to tolerate an acidic medium [28,71,77,113]. As a result of this process, bacterial cells cannot continue the process of cell division, so there is no more prolonged regeneration of bacterial cells that grow to continue the degradation process [58,116,124]. The degradation step involves the formation of carboxylic acid compounds, but most bacteria cannot tolerate acidic conditions, so this step in the bioremediation method is often called the rate-limiting step of degradation [11,23,41,50,116].
The reduction in PAH components (pyrene) by bacillus bacteria (Figure 1) increases with increasing interaction time, followed by an increase in the growth of bacillus cells [38,51]. The growth of bacillus cells appears to cease on the 18th to 20th days of interaction (Figure 1). Under this condition, the growth activity of bacillus cells was considered non-existent, so the biodegradation process of the pyrene substrate also stopped, while the pyrene residue remained at ±40% of the initial amount [30,129].
Figure 1 above illustrates the weaknesses or limitations of using one type of microorganism species (bacteria) in the biodegradation process. This illustration shows the performance of bacillus used in pyrene degradation for 18 days [6,109]. Bacteria is used this time to carry out the degradation of pyrene through the adaptation of the interaction environment, cell growth and multiplication, and stationary and cell–cell death phases [111,113]. Bacterial degradation results in the gradual destruction of the pyrene molecular structure following the growth and development of bacterial cells, resulting in the production of transitional organic compound products until the bacteria reach the degradation stage of the production of carboxylic acid components [44,129]. The concentration of ±40% pyrene remaining at the end of the biodegradation process is still high, and if this enters the environment, the safety of living creatures in the area is not guaranteed [4,98].
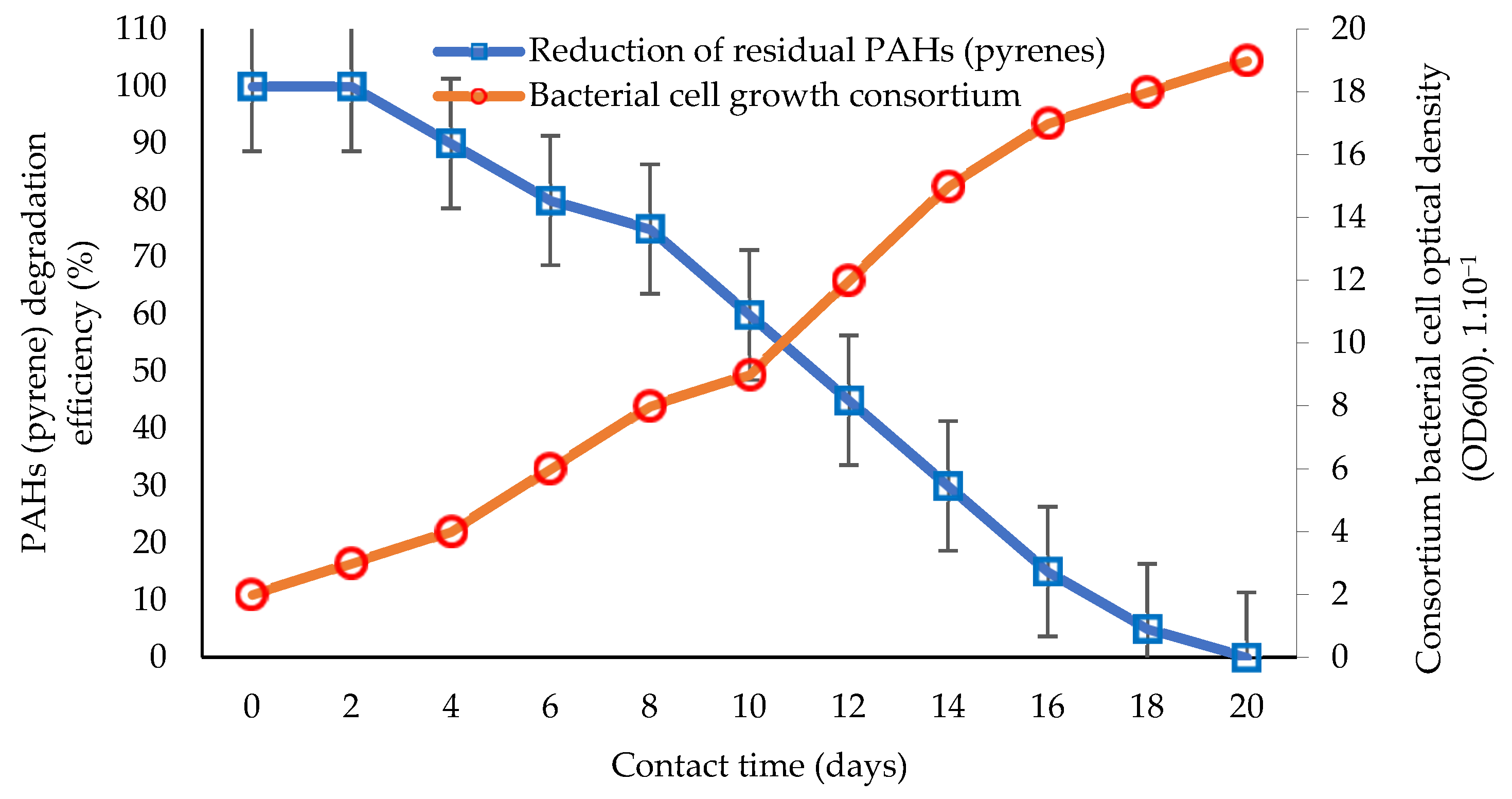
Bioremediation technology continues to develop today. One of the developments in bioremediation technology is the innovation of using a consortium of bacteria to remediate toxic and carcinogenic PAHs [22,105,124]. The study of the performance and efficiency of biodegradation via the application of consortia or groups of bacteria tolerant to the toxicity of PAHs (hydrocarbonoclastic) [68,113] is presented in Figure 2.
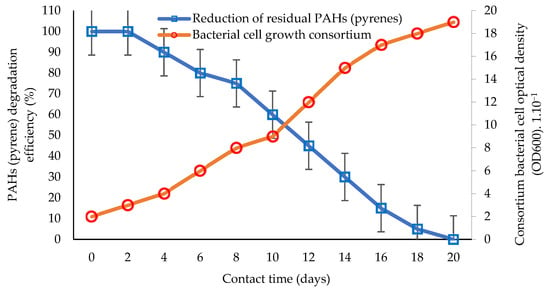
Figure 2.
Comparison of the percentage of PAH (pyrene) bioremediation with the bacterial cell growth rate of the consortium based on interaction time. Graphically modified figure [51,64,71,113,116].
An illustration of biodegradation in aquatic ecosystems by utilizing pyrene as the primary substrate for degradation is shown in Figure 1, and the biodegradation performance using a bacterial consortium is shown in Figure 2. Studies using hydrocarbonoclastic biodegraders in the bioremediation of PAHs can give better results than those using single species of bacteria [81,106]. The application of consortium bacteria in the bioremediation of PAHs is considered to achieve a higher performance and substrate degradation power, and is more efficient, as it is estimated that the bioremediation performance of PAHs increases to 100% (Figure 2) [27,60,126]. The time required by the consortium bacteria to degrade potential PAHs is less than 20 days, which can be inferred from the consortium bacterial cells that still show growth [51,92]. This condition indicates that bacterial cells can still carry out cell division and the degradation of hydrocarbon components for use as an energy source [5,31].
Studies conducted regarding the bioremediation of hydrocarbon components using microorganisms have applied different methods, such as biodegradation, biostimulation, bioaugmentation or a combination of these methods (Table 1) and have shown that none of the experiments succeed in degrading hydrocarbon pollutants with an efficiency reaching 100% [94,128]. The study of heavy metal bioremediation by microorganisms also showed that no single type of bacteria could absorb heavy metal contaminants in waste with 100% efficiency [52,116,130].

Table 1.
Results of recent studies on the application, performance and efficiency of bacteria in the remediation of polycyclic aromatic hydrocarbons (PAHs) and heavy metal pollutants.
The results of this research indicate the need to develop a bioremediation technology for hydrocarbon pollutants (PAHs) and heavy metals. One of the innovations in remediation engineering is the use of several types of bacteria that can bioremediate PAHs and heavy metals to achieve 100% remediation efficiency [37,60,66].
Sea sponges generally undergo a mutualistic symbiosis with microorganisms, especially bacteria [31,113]. Research on the bioremediation of waste containing hydrocarbon components (aliphatic, aromatic) using several types of marine sponge symbiont bacteria shows the ability of these bacteria to degrade hydrocarbon components (Table 2) [12,100]. The search results above show that there are three groups of symbiotic sponge bacteria (Bacillus, Pseudomonas and Acinetobacter) [38,52,127]. These bacteria showed good biodegradation performance against hydrocarbon components [116]. The research findings related to tracking the remediation performance of sponge symbiont bacteria against pollutants containing hydrocarbon components show that these bacteria are generally isolated from sponges whose body surface is covered with mucus, or dark-colored sponges [73,130]. This has to do with the dynamics shown by sponges suspected of being exposed to pollutants in their habitat, which stimulate themselves to survive in that environment by producing mucus substances [13,20,131].

Table 2.
Various studies on the biodegradation performance of sponge symbiont bacteria on hydrocarbon components.
Similar research has also been conducted to evaluate the capability and efficacy of symbiotic sponge bacteria in removing heavy metal pollution (Table 3). Numerous investigations indicate that certain varieties of bacteria isolated from sea sponges with mucus-coated body surfaces can also adsorb multiple types of heavy metal pollution [32,58,77,132]. In general, symbiotic sponge bacteria are effective and show a great performance against heavy metal adsorption [6,25,133].

Table 3.
Various studies on the bio-adsorption performance of sponge symbiont bacteria against heavy metal contaminants.
The adsorption patterns of sponge symbionts on several types of heavy metal pollutants are different from those of the biodegradation of PAHs, which tends to be directly proportional to the duration of the interaction [6,28,109]. The adsorption of heavy metal pollutants by bacteria occurs extracellularly through the binding of heavy metal ions by extracellular polymers produced by bacterial cells, which act as negatively charged biosorbents on the cell surface that bind and form complexes with positively charged heavy metal ions [59,127].
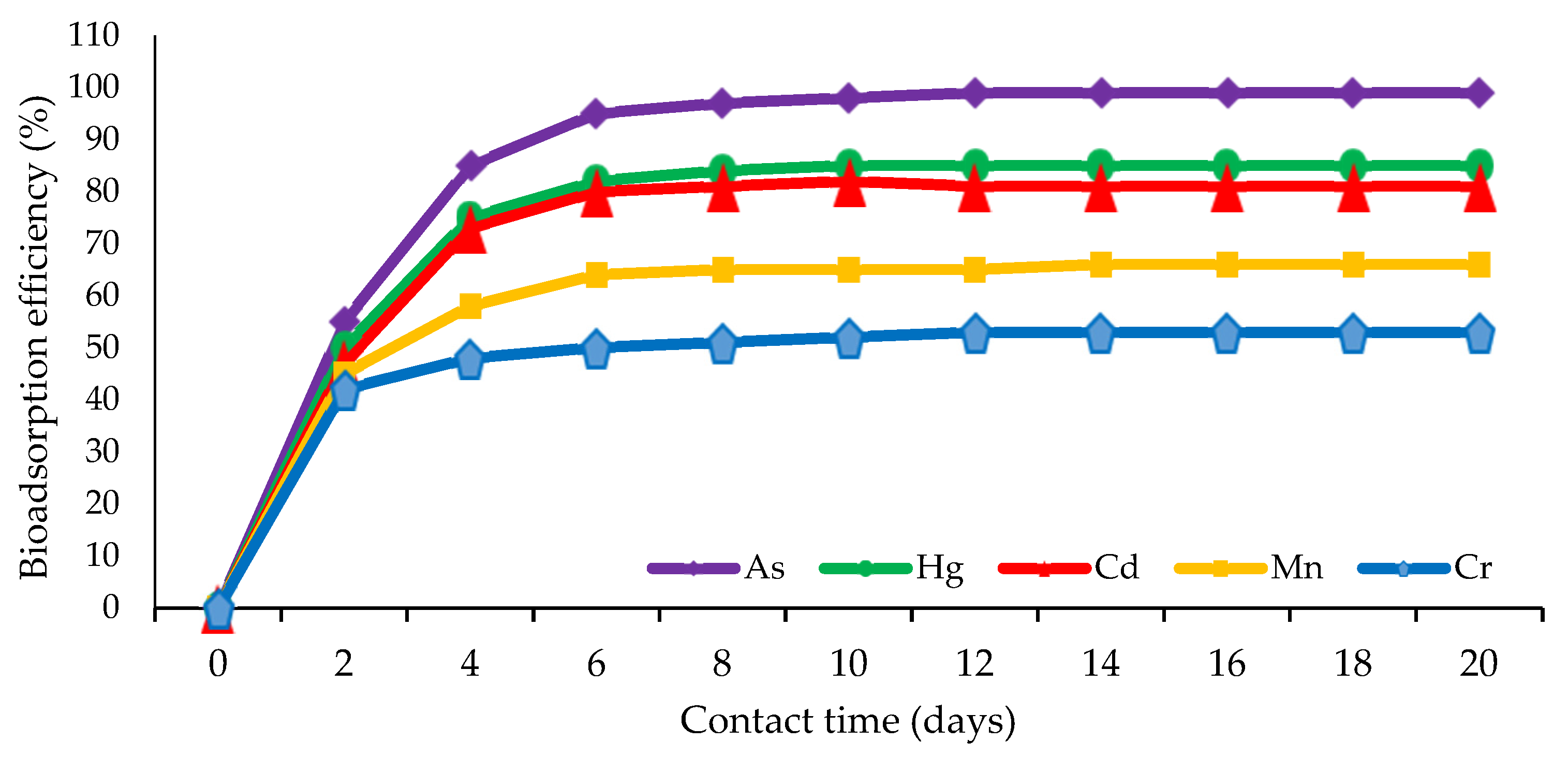
The analysis of the bioremediation pattern of marine sponge symbiont bacteria against several kinds of heavy metals showed that the optimal adsorption of heavy metal pollutants by bacteria generally occurred at a contact duration of 2–6 days or when the bacteria had passed the adaptation phase in a new environment exposed to heavy metal toxins [20,130]. Bacterial remediation activity decreased until it reached a contact time of 20 days. This biosorption pattern is illustrated in Figure 3.
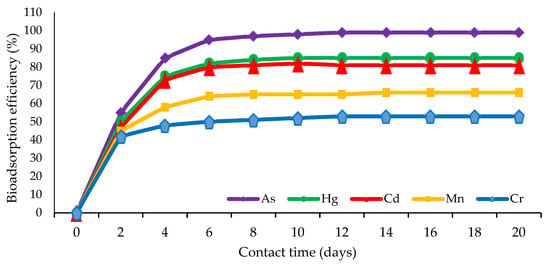
Figure 3.
The pattern of adsorption of sponge symbiont bacteria on some heavy metal pollutants.
This phenomenon indicates that the adsorption model involves an extracellular ionic bond between the positive pole of the heavy metals and the negative pole of the bacteria on the surface, such that adsorption can occur very quickly when the negative surface of the bacteria is active [60,65,132,134]. The contact duration of 6 days is considered the general period required for the fishery to reach the saturation stage, whereat most of the negative poles of the bacterial surface will have formed ionic bonds with heavy metal ions [35,124]. Under these conditions, the bacterial cells can no longer continue their adsorption activity, and the process towards the division phase and cell growth is declared to have stopped [28,107].
4. Process and Mechanism of Pollutant Bioremediation by Marine Sponge Symbiont Bacteria
The degradation process of PAH components by bacteria differs from the heavy metal adsorption process [65,131]. This difference also pertains between the degradation mechanism of PAHs and the mechanism of heavy metal adsorption by bacteria as a bioremediator, despite using the same bacterial species to remove different types of pollutants (PAHs and heavy metals) [16,24,25]. The research on bioremediation using bacteria raises several assumptions that can be scientifically justified [17,42,43].
4.1. Processes and Mechanisms of Biodegradation of PAHs
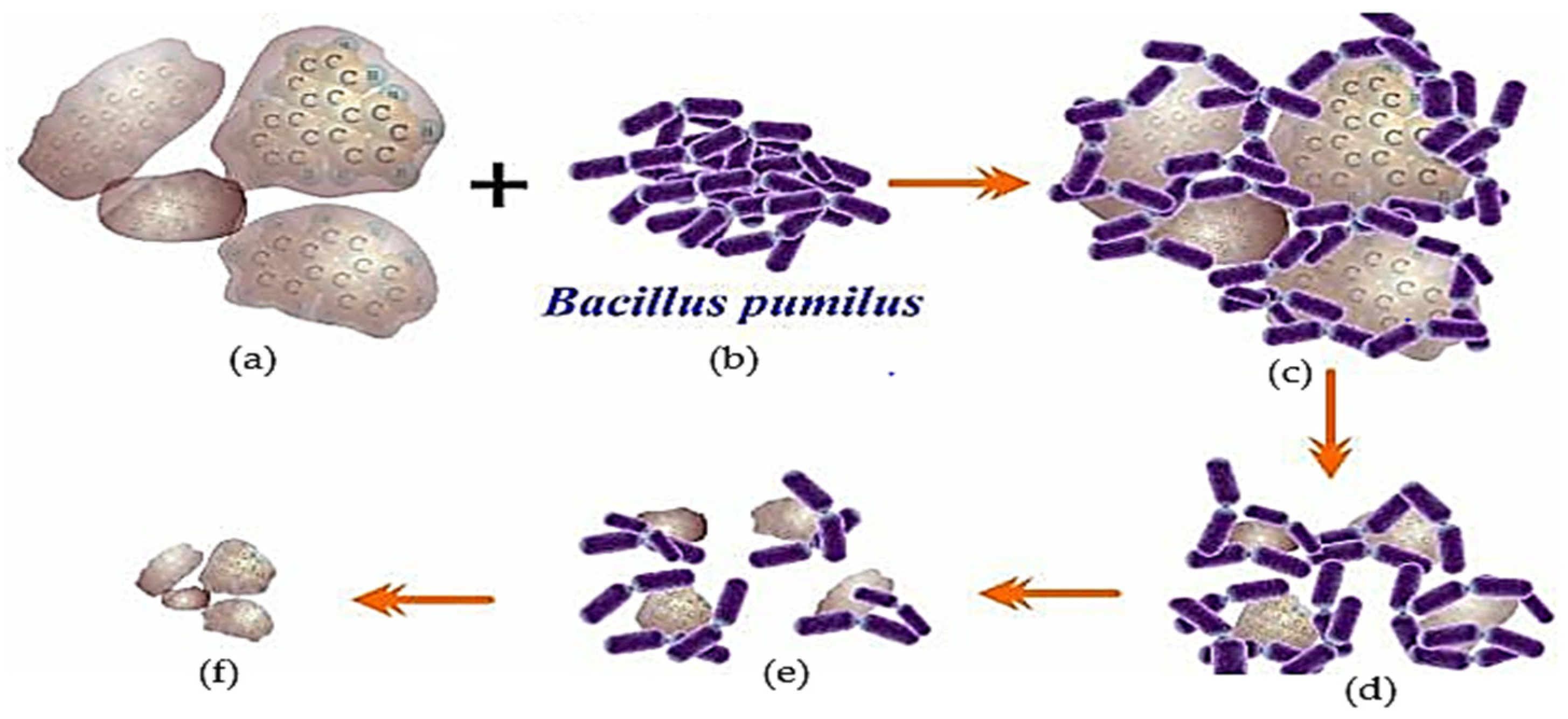
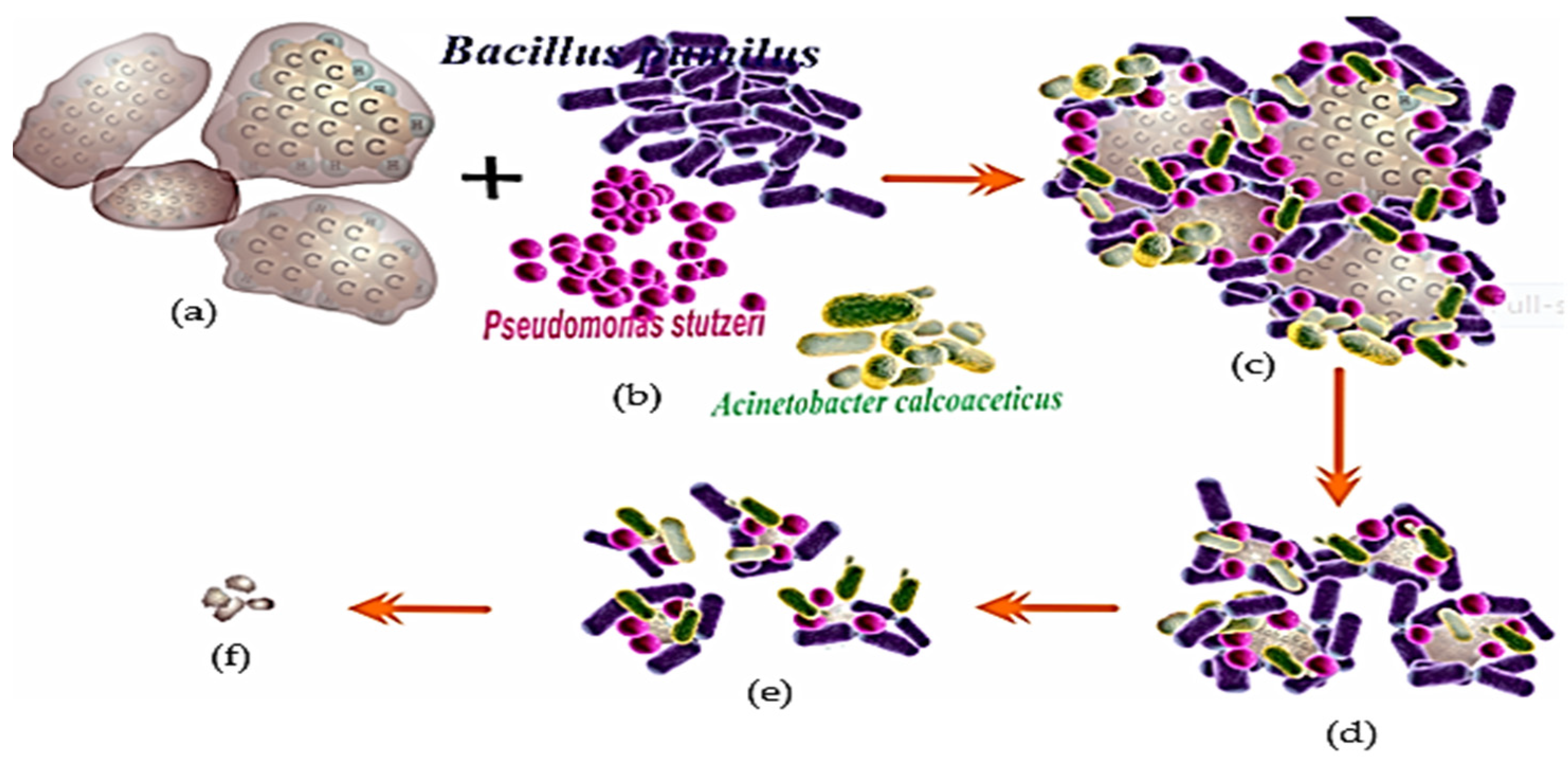
The pyrene biodegradation process using the Bacillus pumilus strain GLB197 isolated from marine sponge Niphates sp. (Table 2) [124] is illustrated in Figure 4. The illustration shows that the degradation performance when using one type of bacteria is not significant, or the degradation will be incomplete [9,11,31,135].
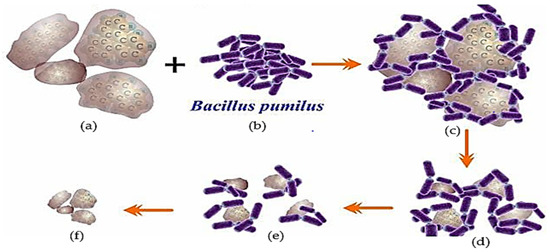
Figure 4.
Description of the biodegradation process of PAH (pyrene) contaminants using one type of bacteria, Bacillus pumilus type. (a) Pyrene as a substrate; (b) Bacillus pumilus (Bp); (c) pyrene-Bp complex; (d) Degraded pyrene; (e) The population of Bp was significantly reduced and the pyrene concentration was not significantly reduced and (f) the remaining pyrene was not degraded, an illustration [16,71,99,116].
One of the PAHs (Figure 4a) interacts with the Bacillus pumilus (Bp) bacterium (Figure 4b); this contact leads to the Bp bacterial population decomposing PAHs (Figure 4c). In addition, the deterioration that occurs continues to weaken when the Bp population decreases as a result of the increasing acidity of the remediation media. The success of bio-remediation decreases as this technique is implemented in an unregulated, unrestricted environment. For instance, in marine ecosystems, the elements that suppress the performance of deterioration, such as salinity, currents, and temperature, are becoming increasingly variable (Figure 4d). The population of Bp decreases because the remaining bacteria are unable to divide; as a result, the process of degradation barely progresses (Figure 4e), until the point at which all Bp communities have died, and the PAHs remain undigested (Figure 4f) [16,26,110,113,116].
The process of pyrene degradation by bacillus begins with adaptation in the medium, which usually lasts 1–3 days [129]. Then the bacteria enter the enlargement phase, cells divide to form colonies, crowding around the pyrene pieces until the cells enter the pyrene body and cut the pyrene component until it decomposes into small particles (Figure 4) [46,136]. Suppose the bacteria are still able to carry out the remediation activity. In that case, each of these bacterial communities takes pieces to continue the degradation process until the bacterial cell activity stops, perhaps because it has been killed by the changing of the medium to become more acidic [15,34,137].
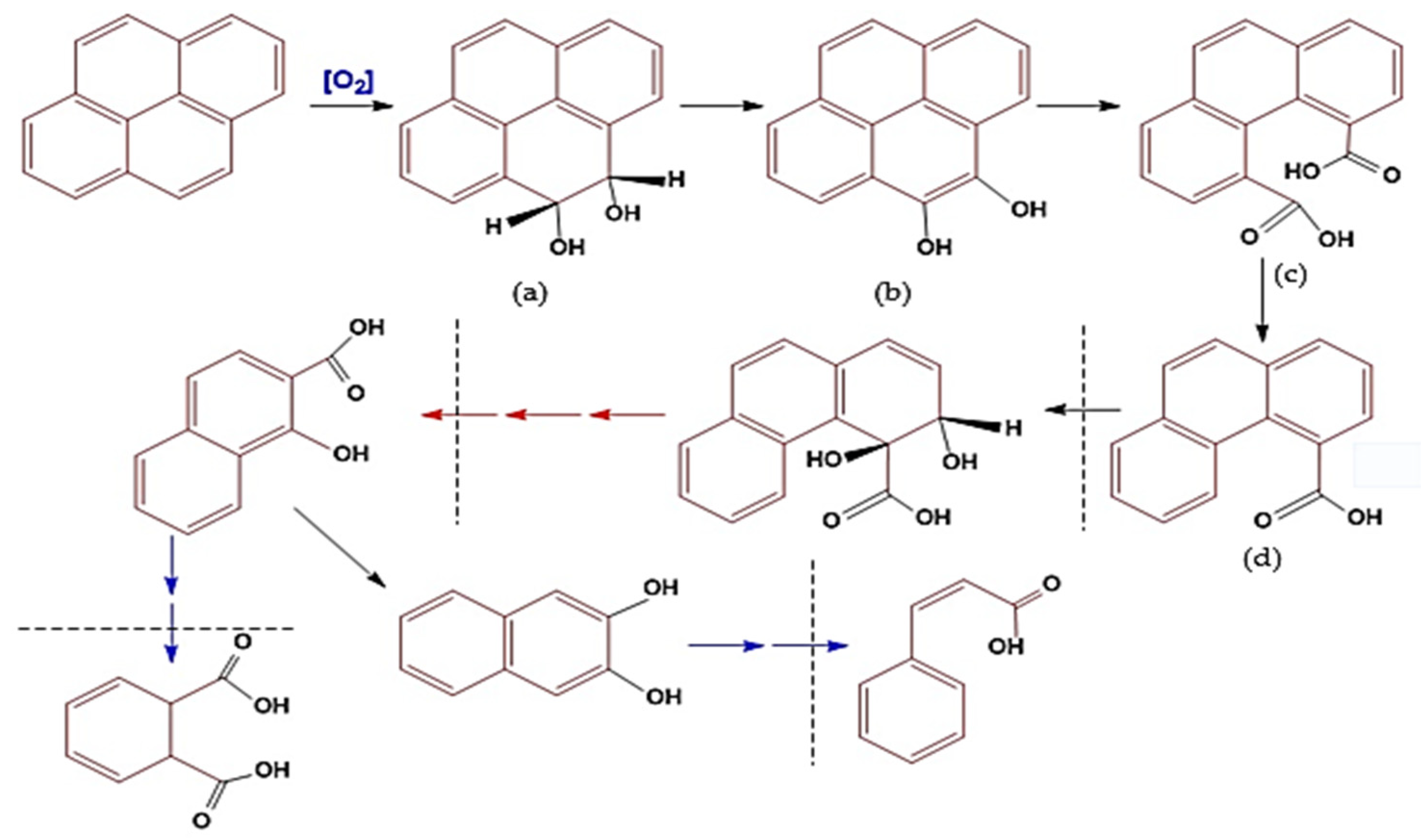
The process of pyrene degradation by bacillus is similar to the bioremediation mechanism that occurs, and the difference is that the bioremediation mechanism takes place at the molecular level or in metabolic substances (micromolecules). In contrast, the remediation process occurs at the component or macromolecular level [11,34,118]. The degradation mechanism of PAHs (pyrene) by bacteria is known as a cycle. Namely, one cycle involves a series of changes in the molecular structure of PAHs from pyrene (four aromatic benzene rings) to phenanthrene (three aromatic rings). The following cycle changes phenanthrene to naphthalene (two aromatic rings; see the imaginary line (Figure 5)), and so on until the conversion of benzene (one aromatic ring) into simple non-aromatic organic compounds that are environmentally friendly [83,117].
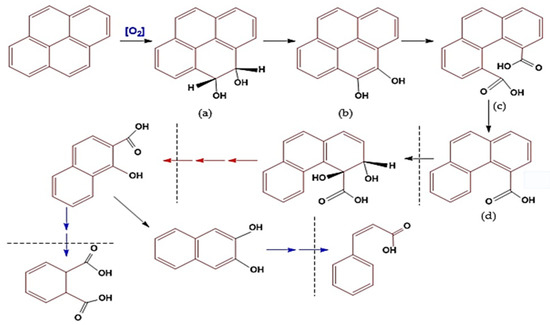
Figure 5.
Illustration of the mechanism of biodegradation of pyrene components using Bacillus sp. bacteria, through the following metabolic reactions: (a) Pyrene molecules undergo oxidation; (b) a keto-enol molecule is formed; (c) One benzene ring is broken to form a carboxylic acid compound; (d) a simple carboxylate molecule separates to form penanthroic acid. The next degradation process is through an oxidation reaction which leaves benzoate compounds. Figure has been modified based on some references [99,110,113,116].
The mechanism of pyrene degradation by Bacillus pumilus strain GLB197 [113] is similar to the degradation pathway of PAHs by Cycloclasticus sp. [110,112,116] and has been adopted into the mechanism of pyrene degradation by Mycobacterium sp. [50,91,99,132], combined with genomic analysis and experimental analysis (Figure 5). The mechanism of pyrene degradation is described through an oxidation reaction in four stages of change or one cycle, i.e., starting with the change that produces the cis/trans transition product 4,5-dihydrodiol-pyrene (Figure 5a), which is then converted to 4,5-dihydroxy pyrene (Figure 5b), then to 4,5-dicarboxylic acid phenanthrene (Figure 5c) and finally to the product phenanthrene-4-carboxylate transition (Figure 5d) [17,46,58,64,99].
The fourth stage of the first cycle is the first point of vulnerability for bacteria because, at that stage, a carboxylic acid transition product is formed, which causes the acidity of the media to increase [110,112,113]. Under this condition, the bacterial cells are susceptible, so the potential cell activity decreases drastically, and they can even undergo mass death [91,99,138]. The mechanism of the conversion of pyrene to the transition product of phenanthrene can be called destruction, namely, the destruction of the pyrene molecular structure or the open aromatic ring [117,136].
4.2. Process and Mechanism of Heavy Metal Bioabsorption
The process of heavy metal adsorption using bacteria is similar to the process of using ethylene diamine tetra acetate (EDTA) and a heavy metal (X) to form an X-EDTA complex [55,136]. The mechanism of heavy metal adsorption involves forming extracellular ionic bonds on the surfaces of bacterial cells that are negatively charged with positively charged heavy metals [16,118]. The process of the adsorption of heavy metal ions lasts for a shorter duration than the degradation of PAHs [25,128]. The adsorption process continues until the saturation point is reached [65,96]. The mechanism and changes in heavy metal adsorption by bacterial cells can be observed using analytical instruments such as SEM, EDS and XRD, while determining the adsorption efficiency can be done using AAS or ICP [125,139,140].
5. Parameters of Pollutant Bioremediation
Several changes can be observed either directly by observation or by using analytical instruments to assess the performance of microorganisms (bacteria) in the bioremediation process of PAHs and heavy metal pollutants [141]. These changes are indicators and parameters that can be used to infer the occurrence of pollutant bioremediation activities by bacteria [27,95].
5.1. Biodegradation of PAHs
The biodegradation parameters of PAHs, which indicate the presence of degradation activity performed by the bacteria, include the following: (1) The growth of bacterial cells can be seen from the increase in the optical density of the interaction medium (OD600) [5,53]. The increase in OD600 medium is an indicator that bacterial cells have passed the adaptation phase and are heading to the phase of enlargement and division [16,33]. At this stage, the degradation activity of PAHs has taken place. (2) The increase in the acidic properties of the media is a manifestation of the work of bacterial cell degradation, which has succeeded in destroying the molecular structure of PAHs, forming several new components, among which are carboxylic compounds, which results in increased media acidity [110,112]. This stage is a vulnerable period for bacteria, which can result in cells not being able to enlarge and divide, and the bacterial cells may even be threatened with mass death [103,134]. (3) The increase in the temperature of the interaction media due to the formation of new compounds resulting from degradation. This parameter only increases by a few points, generally in the 0.4–1.2 °C range [23,37]. (4) The emergence of gas bubbles indicates that these bacteria are part of an aerobic group that requires oxygen in carrying out PAH remediation. The oxygen demand of the interaction media is met by aeration using a shaker, or it can be injected directly (5). The smell of fermentation is a characteristic feature of the enzymatic reaction that occurs at the stage of the destruction of the molecular structure of PAHs [17,46,68]. The existing enzymes are produced by bacteria as a response of the cells in order to defend themselves in extreme environments exposed to PAHs [66,67]. (6) New peaks are identified on the GC-MS chromatogram [120,121]. The peaks recorded with varying abundance are valid evidence of all the previously described events (points 1–5) [8,126]. The detection of functional groups of organic compounds via the FTIR chromatogram strengthens the GC-MS data showing that the degradation products produce organic compounds, one of which is a carboxylic component containing carbonyl and hydroxyl groups [122,142,143].
5.2. Heavy Metal Bioadsorption
The parameters of heavy metal adsorption by bacteria that can be observed and measured include increased media turbidity, a limited change in the pH ranging 0.2–0.4, temperature increases in the narrow range of 0.3–0.8 °C, gas bubbles appearing, and the smell of fermentation being very weak [3,50]. Changes during the adsorption process are not strong enough to be described in detail, as in the biodegradation of PAHs [87,106]. This is because the remediation occurs in the form of adsorption, with the extracellular ionic bonding of the negative part of the bacterial surface to the positive charge of the heavy metals [7,37,61]. Therefore, at a certain duration of contact, a saturation point can be reached in the media, but this saturation point cannot be observed directly. The saturation point is inferred if adsorption efficiency is determined by bidaily contacts using the AAS instrument [20,60]. In this case, the saturation point is assumed to arise in the contact period of 6–20 days (Figure 3).
6. Development and Formulation of Remediator Bacteria Consortium
The performance and efficiency of PAH pollutant biodegradation can be improved by making experimental modifications, especially using consortium bacteria. Similarly, the capacity and level of bacterial adsorption to heavy metal contaminants can be improved with the modification of a consortium of bacterial biodegraders [35,92,130].
6.1. Hydrocarbonoclastic Bacteria
The application of hydrocarbonoclastic bacteria (R-1) in the biodegradation of PAHs is one of the modifications that is considered to improve the performance and efficiency of bioremediation [34,95]. Several types of bacteria can be used, for example, Bacillus pumilus, Pseudomonas stutzeri and Acinetobacter calcoaceticus [9,127], all of which can biodegrade PAHs (hydrocarbonoclastic bacteria) [99,144]. These bacteria were formulated as a consortium bacterial suspension and interacted with pollutant PAHs, e.g., pyrene (Figure 6) [112,145].
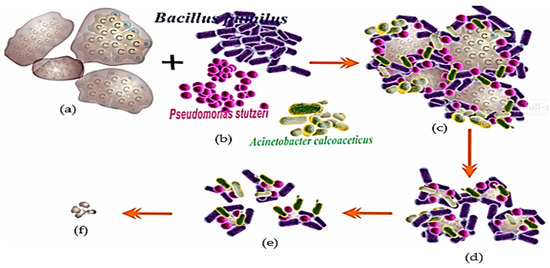
Figure 6.
The process of the biodegradation of PAH contaminants uses a consortium of hydrocarbonoclastic bacteria. An illustration, bacterial consortium biodegradation of pyrene: (a) Pyrene substrate; (b) consortium of bacterial degraders (hydrocarbonoclastic); (c) Complex of consortium bacterial-pyrene; (d) Pyrene begins to degraded; (e) Pyrene is significantly degraded and (f) A small portion of pyrene is not degraded and the bacterial consortium population has died [58,99,110,113,116].
This modification is believed to increase the degradability of PAHs because each type of bacteria can enact degradation in parallel, enabling them to complete one cycle of the conversion of pyrene to phenanthrene in a shorter time [71,90]. The study of the potential of the R-1 consortium bacteria was intended to help remediate waste containing hydrocarbon components, especially PAHs, with high performance and efficiency [38,145].
The bioremediation of PAHs (pyrene) utilizes a consortium of bacteria (hydrocarbonoclastic), namely, Bacillus pumilus (Bp), Pseudomonas stutzeri (Ps), and Acinetobacter calcoacceticus (Ac) (Figure 6a,b). The bioremediation process appears to proceed more rapidly because the bacterial population is large, and the three types of bacteria (Figure 6c) undergo distinct remediation procedures based on their features (Figure 6d). This also indicates that bioremediation is more effective than the use of a single bacterium (Figure 6e) (Figure 4). This causes the pyrene to be significantly destroyed (Figure 6e). However, it is expected that consortium bacteria cannot completely digest pyrene since the state of the bacterial cells is very poor due to the increasing acidity of the medium caused by the creation of degradation products of aldehyde and carboxylate components. Other hypotheses attribute the inability of bacterial cells to divide to a lack of energy and nutrients or the toxicity of PAHs on bacterial cells. [24,26,116].
The degradability of bacteria was significantly increased, resulting in a high-efficiency degradation [50,110]. Thus, all pyrene could be degraded to produce the final product in the form of simple non-aromatic organic compounds that are environmentally friendly (Figure 6) [44,117]. The problem of the limiting factors in the PAHs degradation process in relation to the formation of transition products of carboxylic acid compounds is also assumed to be minimized, so that bacterial cells can work continuously to convert carbon elements into energy [82,110].
6.2. Metalloclastic Bacteria
We have considered the performance, capacity and efficiency of heavy metal adsorption using several types of bacteria in the form of a consortium, or bacteria of the metalloclastic category (R-2) [9,131]. An increase in the capacity and efficiency of heavy metal pollutant adsorption occurs due to the abundance of negative poles on the bacterial cells’ surface, allowing the formation of ionic bonds that occur at the surface at almost the same time [28,77].
In general, bioremediation of PAH and heavy metal-polluted soil is simpler than in aquatic ecosystems [32]. This is due to the relatively static soil media, which enables superior bacterial bioremediation performance since it suits the features of bacteria as a decomposer of the PAH molecular structure and turns it into energy to be used for subsequent bacterial growth and regeneration. Due to the adhesive nature of bacteria, heavy metal pollution can become entrapped in their bodies.
The effectiveness of bacterial bioremediation in aquatic ecosystems against PAH and heavy metal pollution appears to be more dynamic and weak. This assumption is brought about by external influences (salinity, pH, temperature, currents or waves, and mineral availability) [26]. Another aspect is internal bacteria (tolerance level, metabolic processes, cell resistance) [106] Salinity, pH, and temperature are thought to be the most influential factors impacting the performance of bacterial biodegradation of PAH and heavy metal contaminants, whilst all other factors are assumed to be constant. The condition of bacteria as bioremediators in extreme environments contaminated with PAHs and heavy metals has been under strain due to salinity and the toxicity of pollutants since the beginning of the interaction. In contrast, these bacteria are still able to perform remediation duties. When a new material in the form of a carboxylic acid component is created as a result of remediation, the acidity of the remediation medium and the temperature increase within a restricted range [13,90,107]. This weakens the resilience of bacterial cells and can be lethal if there is an increase in salinity and the presence of currents [48,116].
It is proposed to utilize a consortium of bacteria with diverse metabolic methods to boost cells’ tolerance and resistance, hence improving the performance of pollution bioremediation. However, salinity pressure, pH variations, temperature rises, and mass movement related to tides remain the factors that influence and might diminish bioremediation performance.
The differences in adsorption efficiency due to differences in the reactivity and affinity of each heavy metal, as well as the presence of several types of bacterial cells in the consortium formulation, allow the barriers that suppress the abundance of ionic bond formation to be overcome, because each type of bacteria has a specific adsorption model [6,20]. The positive charge of each heavy metal also affects the bonds formed. The adaptability factor of bacterial cells to an environment containing heavy metals greatly determines the adsorption process. In contrast, the external influence of adsorption, such as the provision of nutrients, and the presence of aeration, do not positively impact the number of ionic bonds formed [35,113]. The consortium of bacteria coded R-2 has high adsorption power and efficiency, and they are intended to be applied in the remediation of waste exposed to heavy metals [77,131].
The bioremediation of heavy metals by bacteria resembles a chelate formation reaction; it involves the adsorption of positive ions from heavy metal contaminants on the surface of bacteria [9,10]. Suppose, according to the ionic size, that a bond is formed that eventually forms a heavy metal complex with the active side of the bacteria. In that case, the ionic size determines the formation of this complex. Heavy metal bioremediation is based on ion trapping. In contrast, PAH bioremediation is based on the breakdown of molecular structures [15,58].
6.3. Metallo-Hydrocarbonoclastic Bacteria
In addition to containing aliphatic and aromatic hydrocarbon components, sludge waste originates from petroleum processing and contains several types of heavy metals [65,96]. This condition requires using bacteria that have a biodegradation function and an adsorption function [60]. Several studies have shown that several types of sponge symbiont bacteria can degrade PAH components and adsorb heavy metals, although these ability tests were carried out separately [6,45,146]. Groups of bacteria with multiple abilities are called metallo-hydrocarbonoclastic (R-3) bacteria.
Several types of research have been conducted; it was found that several sponge symbiont bacteria can be included in the group of bacteria coded R-3, these representing a collection of bacteria that can biodegrade PAHs and also have the ability to adsorb heavy metals [34,109].
This study concludes with recommendations and suggestions for developing bioremediation technology for GTP, especially with PAHs and heavy metals that are important to producing and improving remediation efficiency [19,110]. The bacterial consortium of marine sponge symbionts, both hydrocarbonoclastic (R-1) and metalloclastic (R-2), or metallo-hydrocarbonoclastic (R-3), bacteria, have the potential to increase remediation efficiency in various types of waste [51,81,92]. Screening is important to find and categorize bacteria (R-1; R-2; R-3) with different abilities in terms of GTP remediation [1,7,19]. The bacterial formulation codes R-1, R-2, and R-3 can also be developed in the future into crystalline preparations so that these bacteria will be more easily mobilized for rapid culturing at sites exposed to GTP [2,7,147]. The search for marine sponge symbiont bacteria for bioremediation with high performance and efficiency can be traced only to marine sponges whose body surface is covered with mucus, or which is dark in color [59,88].
Author Contributions
Conceptualization, I.M., R.R., A.M. and B.B.; study design, I.M., R.R., A.M., S.S., I.S.S., E.S. (Early Septiningsih); software, R.R., K.K., E.A.H., I.S.S.; validation, I.M., R.R., A.M., A.A. and T.T.; data analysis, I.M., A.M., T.T. and S.H.; literature search, I.M., R.R., A.S., A.M., R.A.; resources, R.R., S.S., M.M., E.S. (Endang Susianingsih); data collection, I.M., R.R., E.R., A.M. and K.K.; writing—original draft preparation, I.M., A.M., B.H.I., B.B., K.N.; writing—review and editing, I.M., R.R., A.M., E.S. (Early Septiningsih), A.A., R.A. and K.N.; figure and visualization, E.A.H., A.S., B.H.I., E.S. (Endang Susianingsih); supervision, I.M., S.G., E.S.K., B.B.; project administration, A.A., E.R., M.M., S.H., S.G.; funding acquisition, I.M., R.R., A.M., R.A. and E.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This article is an additional output funded by the authors, because it is not included in the financing of the RI Ministry of Research, Technology and Higher Education, even though this article is part of the research assignment of the Ministry of Higher Education of the Republic of Indonesia based on work contract number: “315/E4.1/AK.04.PT/2021”, and “Number: 1868/E4/AK.04/2021”, dated 7 June 2021.
Data Availability Statement
No specific statement regarding data availability.
Acknowledgments
I would like to thank the provider of this research fund, in this case, the Indonesian Ministry of Higher Education. Thanks also to the research collaboration team at the Research Center for Fishery National Research and Innovation Agency and the Research Center for Conservation of Marine and Inland Water Resources, National Research and Innovation Agency (BRIN) West Java, Indonesia for their cooperation in conducting research, especially in completing the task of reviewing various related journals and also in assisting in particular in the provision of research facilities and infrastructure. Analysts at the Hasanuddin University Biochemistry Laboratory are also thanked.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gad, A.K.; Midway, S.R. Relationship of Microplastics to Body Size for Two Estuarine Fishes. Microplastics 2022, 1, 211–220. [Google Scholar] [CrossRef]
- Akoto, O.; Azuure, A.A.; Adotey, K.D. Pesticide residues in water, sediment and fish from Tono Reservoir and their health risk implications. SpringerPlus 2016, 5, 1849. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, N.; Kratofil Krehula, L.; Ptiček Siročić, A.; Hrnjak-Murgić, Z. Analysis of recycled PET bottles products by pyrolysis-gas chromatography. Polym. Degrad. Stab. 2013, 98, 972–979. [Google Scholar] [CrossRef]
- Ye, J.; Song, Y.; Liu, Y.; Zhong, Y. Assessment of medical waste generation, associated environmental impact, and management issues after the outbreak of COVID-19: A case study of the Hubei Province in China. PLoS ONE 2022, 17, e0259207. [Google Scholar] [CrossRef]
- Abraham, N.A.; Offiong, N.A.O.; Ukafia, O.P.; Akpan, P.E. Source Apportionment of Polycyclic Aromatic Hydrocarbons (PAHs) in a Tropical Estuarine Epipelic Sediment and Its Associated Bacterial Degrading Potentials. Curr. J. of Appl. Sci. Technol. 2018, 32, 1–11. [Google Scholar] [CrossRef]
- Gran, S.A.; Ramos, Z.J.; Fuentes, E.; Bravo, D.; Pérez, D.J.M. Effect of co-contamination by PAHs and heavy metals on bacterial communities of diesel contaminated soils of south shetland islands, antarctica. Microorganisms 2020, 8, 1749. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, I.; Septiningsih, E.; Kaseng, E.S.; Herlinah, H.; Sahrijanna, A.; Sahabuddin, S.; Asaf, R.; Athirah, A.; Isnawan, B.H.; Samidjo, G.S.; et al. Investigation of Global Trends of Pollutants in Marine Ecosystems around Barrang Caddi Island, Spermonde Archipelago Cluster: An Ecological Approach. Toxics 2022, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Asgari, A.; Nabizadeh, R.; Mahvi, A.H.; Nasseri, S.; Dehghani, M.H.; Nazmara, S.; Yaghmaeian, K. Biodegradation of total petroleum hydrocarbons from acidic sludge produced by rerefinery industries of waste oil using invessel composting. J. Env. Health Sci. Eng. 2017, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Medic, A.; Lješević, M.; Inui, H.; Stojanovi, K.; Karadzi, I.; Beskoski, V.; Koji, I. Efficient biodegradation of petroleum: N -alkanes and polycyclic aromatic hydrocarbons by polyextremophilic Pseudomonas aeruginosa sanai with multidegradative capacity. RSC Adv. 2020, 10, 14060–14070. [Google Scholar] [CrossRef]
- Al-Dhabaan, F.A. Morphological, biochemical and molecular identification of petroleum hydrocarbons biodegradation bacteria isolated from oil polluted soil in Dhahran, Saud Arabia. Saudi. J. Biol. Sci. 2019, 26, 1247–1252. [Google Scholar] [CrossRef]
- Akinde, S.B.; Iwuozor, C.C. Alkane Degradative Potentials of Bacteria Isolated From the Deep Atlantic Ocean of the Gulf of Guinea. J. Bioremediat. Biodegrad. 2012, 3, 135. [Google Scholar] [CrossRef]
- Khabouchi, I.; Khadhar, S.; Chaouachi, D.; Chekirbene, A.; Doumenq, P. Study of organic pollution in superficial sediments of Meliane river catchment area: Aliphatic and polycyclic aromatic hydrocarbons. Enviroment Monit. Assess 2020, 192, 283. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, I.; Noor, A.; La-Nafie, N.; Djide, M.N. Sponge Role In Alleviating Oil Pollution Through Sludge Reduction, A Preliminary Approach. Int. J. Appl. Chem. 2015, 11, 427–441. [Google Scholar]
- Marzuki, I.; Pratama, I.; Heryani, H.I.; Paserangi, I.; Kamaruddin, M.; Chaerul, M.; Ahmad, R. The Identification and Distribution Components of Polycyclic Aromatic Hydrocarbon Contaminants at the Port of Paotere, Makassar, South Sulawesi. In Proceedings of the The 1st International Conference on Biotechnology and Food Sciences, Surabaya, Indonesia, 11 September 2020; Volume 679. [Google Scholar]
- Zhang, B.; Zhang, L.; Zhang, X. Bioremediation of petroleum hydrocarbon-contaminated soil by petroleum degrading bacteria immobilized on biochar. RSC Adv. 2019, 9, 35304–35311. [Google Scholar] [CrossRef]
- Košnář, Z.; Částková, T.; Wiesnerová, L.; Praus, L.; Jablonský, I.; Koudela, M.; Tlustoš, P. Comparing the removal of polycyclic aromatic hydrocarbons in soil after different bioremediation approaches in relation to the extracellular enzyme activities. J. Env. Sci. 2019, 76, 249–258. [Google Scholar] [CrossRef]
- Sibero, M.T.; Igarashi, Y.; Radjasa, O.K.; Sabdono, A.; Trianto, A.; Zilda, D.S.; Wijaya, Y.J. Sponge-associated fungi from a mangrove habitat in Indonesia: Species composition, antimicrobial activity, enzyme screening and bioactive profiling. Int. Aquat. Res. 2019, 11, 173–186. [Google Scholar] [CrossRef]
- Bendouz, M.; Dionne, D.; Tran, L.H.; Coudert, L.; Mercier, G.; Blais, J.F. Polycyclic Aromatic Hydrocarbon Oxidation from Concentrates Issued from an Attrition Process of Polluted Soil Using the Fenton Reagent and Permanganate. Water Air Soil Pollut. 2017, 228, 114–127. [Google Scholar] [CrossRef]
- Bojes, H.K.; Pope, P.G. Characterization of EPA’s 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) in tank bottom solids and associated contaminated soils at oil exploration and production sites in Texas. Regul. Toxicol. Pharmacol. 2007, 47, 288–295. [Google Scholar] [CrossRef]
- Abdel-monem, N.M.; Abdel-azeem, A.M.; Ghareeb, D.A.; Nabil-adam, A. Pretreatment Hepatoprotective Effect of the Marine Fungus Derived from Sponge on Hepatic Toxicity Induced by Heavy Metals in Rats. Biomed. Res. Int. 2013, 2013, 510879. [Google Scholar] [CrossRef]
- Baquiran, J.I.P.; Nada, M.A.L.; Posadas, N.; Manogan, D.P.; Cabaitan, P.C.; Conaco, C. Population structure and microbial community diversity of two common tetillid sponges in a tropical reef lagoon. Peer J. 2020, 4, e9017. [Google Scholar] [CrossRef]
- Al-Hawash, A.B.; Dragh, M.A.; Li, S.; Alhujaily, A.; Abbood, H.A.; Zhang, X.; Ma, F. Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt. J. Aquat. Res. 2018, 44, 71–76. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lyu, C.; Li, Y. Analysis of Factors Influencing Plant–Microbe Combined Remediation of Soil Contaminated by Polycyclic Aromatic Hydrocarbons. Sustainability 2021, 13, 10695. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy metals removal from water by efficient adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Bisht, S.; Pandey, P.; Bhargava, B.; Sharma, S.; Kumar, V.; Krishan, D. Bioremediation of polyaromatic hydrocarbons (PAHs) using rhizosphere technology. Braz. J. Microbiol. 2015, 46, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Shityakov, S. New Trends in Bioremediation Technologies Toward Environment-Friendly Society: A Mini-Review. Front. Bioeng. Biotechnol. 2021, 9, 666858. [Google Scholar] [CrossRef]
- Khan, M.N.; Ullah, H.; Naeem, S.; Uddin, J.; Hamid, Y.; Ahmad, W.; Ding, J. Remediation of Emerging Heavy Metals from Water Using Natural Adsorbent: Adsorption Performance and Mechanistic Insights. Sustainability 2021, 13, 8817. [Google Scholar] [CrossRef]
- Duran, R.; Cravo, L.C. Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol. Rev. 2016, 40, 814–830. [Google Scholar] [CrossRef]
- Hou, L.; Majumder, E.L.W. Potential for and distribution of enzymatic biodegradation of polystyrene by environmental microorganisms. Materials 2021, 14, 503. [Google Scholar] [CrossRef]
- Marzuki, I.; Chaerul, M.; Erniati, E.; Asmeati, A.; Paserangi, I. Biodegradation of aliphatic waste components of oil sludge used micro symbiont of Sponge Niphates sp. In Proceedings of the 3rd International Conference on Marine Science, Towards Sustainable Marine Resources and Environment, Bogor City, Indonesia, 4 September 2019. [Google Scholar]
- Marzuki, I.; Daris, L.; Nisaa, K.; Emelda, A. The power of biodegradation and bio-adsorption of bacteria symbiont sponges sea on waste contaminated of polycyclic aromatic hydrocarbons and heavy metals. In Proceedings of the International Conference on Fisheries and Marine Research, North Maluku, Indonesia, 13–14 July 2020. [Google Scholar]
- Nikel, P.I.; Silva-Rocha, R.; Benedetti, I.; De-Lorenzo, V. The private life of environmental bacteria: Pollutant biodegradation at the single cell level. Environ. Microbiol. 2014, 16, 628–642. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil Fisseha. Chemosphere 2016, 144, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Kui-Ma, X.; Ling-Wu, L.; Fam, H. Heavy metal ions affecting the removal of polycyclic aromatic hydrocarbons by fungi with heavy-metal resistance. Appl. Microbiol. Biotechnol. 2014, 98, 9817–9827. [Google Scholar]
- Yu, Y.; Zhang, Y.; Zhao, N.; Guo, J.; Xu, W.; Ma, M.; Li, X. Remediation of crude oil-polluted soil by the bacterial rhizosphere community of suaeda salsa revealed by 16S rRNA genes. Int. J. Environ. Res. Public Health 2020, 17, 1471. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Zeng, G.-M.; Niu, Q.-Y.; Liu, Y.; Zhou, L.; Jiang, L.-H.; Tang, Z.-F.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Elenga-Wilson, P.S.; Kayath, C.A.; Mokemiabeka, N.S.; Nzaou, S.A.E.; Nguimbi, E.; Ahombo, G. Profiling of Indigenous Biosurfactant-Producing Bacillus Isolates in the Bioremediation of Soil Contaminated by Petroleum Products and Olive Oil. Int. J. Microbiol. Hindawi. 2021, 221, 9565930. [Google Scholar] [CrossRef] [PubMed]
- Essumang, D.K.; Togoh, G.K.; Chokky, L. Pesticide residues in the water and Fish (lagoon tilapia) samples from lagoons in Ghana. Bull. Chem. Soc. Ethiop. 2009, 23, 19–27. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Gadd, G.M. Fungal bioremediation of soil co-contaminated with petroleum hydrocarbons and toxic metals. Appl. Microbiol. Biotechnol. 2020, 104, 8999–9008. [Google Scholar] [CrossRef]
- Medaura, M.C.; Guivernau, M.; Moreno-Ventas, X.; Prenafeta-Boldú, F.X.; Viñas, M. Bioaugmentation of Native Fungi, an Efficient Strategy for the Bioremediation of an Aged Industrially Polluted Soil With Heavy Hydrocarbons. Front. Microbiol. 2021, 12, 626436. [Google Scholar] [CrossRef]
- Yetti, E.; Thontowi, A.; Yopi, Y.; Lisdiyanti, P. Screening of Marine bacteria capable of degrading various polyaromatic hydrocarbons. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2015, 10, 121–127. [Google Scholar] [CrossRef][Green Version]
- Kamaruddin, M.; Marzuki, I.; Burhan, A.; Ahmad, R. Screening acetylcholinesterase inhibitors from marine-derived actinomycetes by simple chromatography. In Proceedings of the 1st International Conference on Biotechnology and Food Sciences, Surabaya, Indonesia, 11 September 2020. [Google Scholar]
- Agrawal, N.; Verma, P.; Shahi, S.K. Degradation of polycyclic aromatic hydrocarbons (phenanthrene and pyrene) by the ligninolytic fungi Ganoderma lucidum isolated from the hardwood stump. Bioresour. Bioprocess. 2018, 5, 11. [Google Scholar] [CrossRef]
- Atagana, H.I. Biodegradation of PAHs by fungi in contaminated-soil containing cadmium and nickel ions. Afr. J. Biotechnol. 2009, 8, 5780–5789. [Google Scholar]
- Cao, H.; Wang, C.; Liu, H.; Jia, W.; Sun, H. Enzyme activities during Benzo[a]pyrene degradation by the fungus Lasiodiplodia theobromae isolated from a polluted soil. Sci. Rep. 2020, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Armus, R.; Selry, C.; Marzuki, I.; Hasan, H.; Syamsia; Sapar, A. Investigation of Potential Marine Bacterial Isolates in Biodegradation Methods on Hydrocarbon Contamination. In Proceedings of the 2nd Workshop on Engineering, Education, Applied Sciences and Technology (WEAST), Makassar, Indonesia, 5 October 2020. [Google Scholar]
- Obire, O.; Aleruchi, O.; Wemedo, S. Fungi in Biodegradation of Polycyclic Aromatic Hydrocarbons in Oilfield Wastewater. Acta Sci Microbiol. 2020, 3, 220–224. [Google Scholar]
- Omoni, V.T.; Lagb, A.J.; Ibeto, C.N.; Semple, K.T. Effects of biological pre-treatment of lignocellulosic waste with white-rot fungi on the stimulation of 14C-phenanthrene catabolism in soils. Int. Biodeterior Biodegrad. 2021, 165, 105324. [Google Scholar] [CrossRef]
- Saraswath, A.; Hallberg, R. Degradation of pyrene by indigenous fungi from a former gasworks site. FEMS Microbiol. Lett. 2002, 210, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Nedoroda, V.; Trokhymenko, G.; Khrapko, T.; Koliehova, A. Analysis of Petroleum Biodegradation by a Bacterial Consortium of Bacillus amyloliquefaciens ssp. Plantarum and Bacillus subtilis. J. Ecol. Eng. 2021, 22, 36–42. [Google Scholar] [CrossRef]
- Bello-, A.M.; Adeleke, R.; Swanevelder, D.; Thantsha, M. Draft Genome Sequence of Pseudomonas sp. Strain 10-1B, a Polycyclic Aromatic Hydrocarbon Degrader in Contaminated Soil. Genome Announc. 2015, 3, e00325-15. [Google Scholar]
- Brzeszcz, J.; Kaszycki, P. Aerobic bacteria degrading both n-alkanes and aromatic hydrocarbons: An undervalued strategy for metabolic diversity and flexibility. Biodegradation 2018, 29, 359–407. [Google Scholar] [CrossRef]
- Crampon, M.; Cébron, A.; Portet-Koltalo, F.; Uroz, S.; Le Derf, F.; Bodilis, J. Low effect of phenanthrene bioaccessibility on its biodegradation in di_usely contaminated soil. Environ. Pollut. 2017, 225, 663–673. [Google Scholar] [CrossRef]
- Dadrasnia, A.; Usman, M.M.; Lim, K.T.; Farahiyah, F.H.; Rodzhan, N.S.M.; Karim, S.H.A.; Ismail, S. Bio-Enhancement of Petroleum Hydrocarbon Polluted Soil Using Newly Isolated Bacteria. Polycycl. Aromat. Compd. 2020, 40, 484–493. [Google Scholar] [CrossRef]
- Guo, J.; Wen, X. Performance and kinetics of benzo(a)pyrene biodegradation in contaminated water and soil and improvement of soil properties by biosurfactant amendment. Ecotoxicol. Environ. Saf. 2021, 207, 111292. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Himber, C.; Boricaud, B.; Kazour, M.; Amara, R.; Cassone, A.-L.; Laurentie, M.; Paul-Pont, I.; Soudant, P.; Dehaut, A.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, I.; Sinardi, S.; Pratama, I.; Chaerul, M.; Paserangi, I.; Kamaruddin, M. Performance of sea sponges micro symbionts as a biomaterial in biodegradation naphthalene waste of modified. In Proceedings of the 5th International Seminar on Sustainable Urban Development, Jakarta, Indonesia, 5 August 2020. [Google Scholar]
- Freeman, C.J.; Easson, C.G.; Fiore, C.L.; Thacker, R.W. Sponge–Microbe Interactions on Coral Reefs: Multiple Evolutionary Solutions to a Complex Environment. Front. Mar. Sci. 2021, 8, 705053. [Google Scholar] [CrossRef]
- Zahirnejad, M.; Ziarati, P.; Asgarpanah, J. The Efficiency of Bio-adsorption of Heavy Metals from Pharmaceutical Effluent by Rumex crispus L. Seed. J. Pharm. Health Sci. 2017, 5, 231–243. [Google Scholar]
- Abass, O.K.; Zhuo, M.; Zhang, K. Concomitant degradation of complex organics and metals recovery from fracking wastewater: Roles of nano zerovalent iron. Chem. Eng. J. 2017, 328, 159–171. [Google Scholar] [CrossRef]
- Lavy, A.; Keren, R.; Haber, M.; Schwartz, I.; Ilan, M. Implementing sponge physiological and genomic information to enhance the diversity of its culturable associated bacteria. FEMS Microbiol. Ecol. 2014, 87, 486–502. [Google Scholar] [CrossRef]
- Maldonado, M.; López, A.M.; Busch, K.; Slaby, B.M.; Bayer, K.; Beazley, L.; Hentschel, U.; Kenchington, E.; Rapp, H.T. A Microbial Nitrogen Engine Modulated by Bacteriosyncytia in Hexactinellid Sponges: Ecological Implications for Deep-Sea Communities. Front. Mar. Sci. 2021, 8, 638505. [Google Scholar] [CrossRef]
- Marzuki, I.; Enryani, H.I.; Nafie, N.-L.; Dali, S. Study Biodegradation of Aromatics Pyrene Using Bacterial Isolates from the Sea and micro symbionts Sponges. Int. J. Appl. Chem. 2017, 13, 707–720. [Google Scholar]
- Marzuki, I. The Bio-adsorption Pattern Bacteria Symbiont Sponge Marine Against Contaminants Chromium and Manganese In The Waste Modification of Laboratory Scale. Indo Chim. Acta. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Cajthaml, T.; Erbanová, P.; Kollmann, A.; Novotný, C.; Sasek, V.; Mougin, C. Degradation of PAHs by ligninolytic enzymes of Irpex lacteus. Folia. Microbiol. 2008, 53, 289–294. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Brar, S.K.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Env. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Huang, Y.; Zhou, F.; Zhao, Q.; Zhang, W.; Chen, G.; Gao, Y. Isolation of Petroleum Degraders and Petroleum-Degradation Characteristics of Crude Enzymes from Providencia rettgeri L1. Polish J. Envirin. Stud. 2022, 31, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Ohowa, B.; Kiteresi, L.I.; Wanjeri, V.W.; Mwamburi, S.M.; Tunje, S.L. Sponges as simple biomonitoring tools for trace element pollution in marine environments: Insights from a Kenyan study focused on the leaf sponge Phyllospongia foliascens. Afr. J. Mar. Sci. 2021, 43, 533–538. [Google Scholar] [CrossRef]
- Marzuki, I.; Gusty, S.; Armus, R.; Sapar, A.; Asaf, R.; Athirah, A.; Jaya, J. Secondary Metabolite Analysis and Anti-Bacteria and Fungal Activities of Marine Sponge Methanol Extract Based on Coral Cover. In Proceedings of the 6th International Conference on Basic Sciences, Ambon City, Indonesia, 4–5 November 2020. [Google Scholar]
- Marzuki, I.; Nisaa, K.; Asaf, R.; Paena, M.; Athirah, A.; Susianingsih, E.; Nurhidayah, N.; Kadriah, I.A.K.; Kamaruddin, K.; Sahabuddin, S.; et al. Comparison of Pyrene Biodegradation Using Two Types of Marine Bacterial Isolates. Sustainability 2022, 14, 9890. [Google Scholar] [CrossRef]
- Rua, C.P.J.; Oliveira, L.S.; De-Froes, A.; Tschoeke, D.A.; Soares, A.C.; Leomil, L.; Gregoracci, G.B.; Coutinho, R.; Hajdu, E.; Thompson, C.C.; et al. Microbial and Functional Biodiversity Patterns in Sponges that Accumulate Bromopyrrole Alkaloids Suggest Horizontal Gene Transfer of Halogenase Genes. Microb. Ecol. J. 2018, 76, 825–838. [Google Scholar] [CrossRef]
- Marzuki, I.; Daris, L.; Yunus, S.; Riana, A.D. Selection and characterization of potential bacteria for polycyclic aromatic biodegradation of hydrocarbons in sea sponges from Spermonde Islands, Indonesia. AACL Bioflux. 2020, 13, 3493–3506. [Google Scholar]
- Kim, H.-W.; Jo, J.H.; Kim, Y.-B.; Le, T.-K.; Cho, C.-W.; Yun, C.-H.; Chi, W.S.; Yeom, S.-J. Biodegradation of polystyrene by bacteria from the soil in common environments. J. Hazard. Mater. 2021, 416, 126239. [Google Scholar] [CrossRef]
- Fu, X.; Wang, H.; Bai, Y.; Xue, J.; Gao, Y.; Hu, S.; Wu, T.; Sum, J. Systematic degradation mechanism and pathways analysis of the immobilized bacteria: Permeability and biodegradation, kinetic and molecular simulation. Env. Sci. Ecotechnol. 2020, 2, 100028. [Google Scholar] [CrossRef]
- Marzuki, I.; Kamaruddin, M.; Ahmad, R. Identification of marine sponges-symbiotic bacteria and their application in degrading polycyclic aromatic hydrocarbons. Biodiversitas 2021, 22, 1481–1488. [Google Scholar] [CrossRef]
- Marzuki, I.; Ahmad, R.; Kamaruddin, M.; Asaf, R.; Armus, R.; Siswanty, I. Performance of cultured marine sponges-symbiotic bacteria as a heavy metal bio-adsorption. Biodiversitas 2021, 22, 5536–5543. [Google Scholar] [CrossRef]
- Marzuki, I.; Ali, M.Y.; Syarif, H.U.; Erniati, E.; Gusty, S.; Ritnawati, R.; Daris, L.; Nisaa, K. Investigation of Biodegradable Bacteria as Bio indicators of the Presence of PAHs Contaminants in Marine Waters in the Marine Tourism Area of Makassar City. In Proceedings of the 6th International Conference on Tropical Coastal Region Eco-Development, Semarang, Indonesia, 27–28 October 2020. [Google Scholar]
- Liu, Y.F.; Mbadinga, S.M.; Gu, J.D.; Mu, B.Z. Type II chaperonin gene as a complementary barcode for 16S rRNA gene in study of Archaea diversity of petroleum reservoirs. Int. Biodeterior Biodegrad. 2017, 123, 113–120. [Google Scholar] [CrossRef]
- Su, X.M.; Bamba, A.M.; Zhang, S.; Zhang, Y.G.; Hashmi, M.Z.; Lin, H.J.; Ding, L.X. Revealing potential functions of VBNC bacteria in polycyclic aromatic hydrocarbons biodegradation. Lett. Appl. Microbiol. 2018, 66, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Syakti, A.D.; Yani, M.; Hidayati, N.V.; Siregar, A.S.; Doumenq, P.; Sudiana, I.M. The bioremediation potential of hydrocarbonoclastic bacteria isolated from a mangrove contaminated by petroleum hydrocarbons on the cilacap coast, Indonesia. Bioremediat. J. 2013, 17, 11–20. [Google Scholar] [CrossRef]
- Ahmad, M.; Wang, P.; Li, J.L.; Wang, R.; Duan, L.; Luo, X.; Irfan, M.; Peng, Z.; Yin, L.; Li, W. Impacts of bio-stimulants on pyrene degradation, prokaryotic community compositions, and functions. Environ. Pollut. 2021, 289, 117863. [Google Scholar] [CrossRef]
- Fang, H.; Shi, Y.; Zhou, M.; Niu, Q. Influence of n-Hexadecane and Naphthalene on Anaerobic Digestion: Kinetic Simulation, DOM Variation and Microbial Community Assessment. In Proceedings of the 2020 International Conference on Green Energy, Environment and Sustainable Development, Wuhan, China, 24–25 April 2020. [Google Scholar]
- Galitskaya, P.; Biktasheva, L.; Blagodatsky, S.; Selivanovskaya, S. Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci. Rep. 2021, 11, 164. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Peng, C. Shift of Soil Polycyclic Aromatic Hydrocarbons (PAHs) dissipation pattern and microbial community composition due to rhamnolipid supplementation. Water Air Soil Pollut. 2019, 230, 107. [Google Scholar] [CrossRef]
- Mao, J.; Guan, W. Fungal degradation of polycyclic aromatic hydrocarbons (PAHs) by Scopulariopsis brevicaulis and its application in bioremediation of PAH-contaminated soil. Acta Agric. Scand Sect. B Soil Plant Sci. 2016, 66, 399–405. [Google Scholar]
- Sandhu, M.; Paul, A.T. Metagenomic Analysis for Taxonomic and Functional Potential of Polyaromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyl ( PCB ) Degrading Bacterial Communities in Steel Industrial Soil. PLoS ONE 2022, 17, e0266808. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the marine sponges Axinella cannabina and Suberites carnosus: Isolation and morphological, biochemical, and biophysical characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef]
- Marzuki, I.; Nisaa, K.; Asaf, R.; Armus, R.; Kamaruddin, M.; Sapar, A.; Emelda, A. Biodegradation mechanism of naphthalene using marine sponge symbiotic bacteria. In Proceedings of the 2nd International Conference on Fisheries and Marine, Ternate, Indonesia, 15 July 2021. [Google Scholar]
- Chulalaksananukul, S.; Gadd, G.M.; Sangvanich, P.; Sihanonth, P.; Piapukiew, J.; Vangnai, A.S. Biodegradation of benzo(a)pyrene by a newly isolated Fusarium sp. FEMS Microbiol. Lett. 2006, 262, 99–106. [Google Scholar] [CrossRef]
- Govarthanan, M.; Fuzisawa, S.; Hosogai, T.; Chang, Y.C. Biodegradation of aliphatic and aromatic hydrocarbons using the filamentous fungus Penicillium sp. CHY-2 and characterization of its manganese peroxidase activity. RSC Adv. 2017, 7, 20716–20723. [Google Scholar] [CrossRef]
- Laothamteep, N.; Kawano, H.; Vejarano, F.; Minakuchi, C.S.; Shintani, M.; Nojiri, H.; Pinyakong, O. Effects of environmental factors and coexisting substrates on PAH degradation and transcriptomic responses of the defined bacterial consortium OPK. Environ. Pollut. 2021, 277, 116769. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Xu, M.; Sun, K.; Cao, L.H.; Dai, C.; Jia, Y. Biodegradation of phenanthrene by endophytic fungus Phomopsis liquidambari in vitro and in vivo. Chemosphere 2018, 203, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Haleyur, N.; Shahsavari, E.; Jain, S.S.; Koshlaf, E.; Ravindran, V.B.; Morrison, P.D.; Osborn, A.M.; Ball, A.S. Influence of bioaugmentation and biostimulation on PAH degradation in aged contaminated soils: Response and dynamics of the bacterial community. J. Environ. Manag. 2019, 238, 49–58. [Google Scholar] [CrossRef]
- Guerra, A.B.; Oliveira, J.S.; Silva-Portela, R.C.B.; Araújo, W.; Carlos, A.C.; Vasconcelos, A.T.R.; Freitas, A.T.; Domingos, Y.S.; de Farias, M.F.; Fernandes, G.J.T.; et al. Metagenome enrichment approach used for selection of oil-degrading bacteria consortia for drill cutting residue bioremediation. Environ. Pollut. 2018, 235, 869–880. [Google Scholar] [CrossRef]
- Janczuk, B.; Szymczyk, K.; Zdziennicka, A. Adsorption Properties of Hydrocarbon and Fluorocarbon Surfactants Ternary Mixture at the Water-Air Interface. Molecules 2021, 26, 4313. [Google Scholar] [CrossRef]
- Lasota, J.; Łyszczarz, Y.; Kempf, P.; Kempf, M.; Błońska, E. Effect of Species Composition on Polycyclic Aromatic Hydrocarbon (PAH) Accumulation in Urban Forest Soils of Krakow. Water. Air. Soil Pollut. 2021, 232, 74. [Google Scholar] [CrossRef]
- Lu, C.; Hong, Y.; Liu, J.; Gao, Y.; Ma, Z.; Yang, B.; Ling, W.; Waigi, M.G. A PAH-degrading bacterial community enriched with contaminated agricultural soil and its utility for microbial bioremediation. Environ. Pollut. 2019, 251, 773–782. [Google Scholar] [CrossRef]
- Lundstedt, S. Analysis of PAHs and Their Transformation Products in Contaminated Soil and Remedial Processes; Department of Environment and Toxicological Chemistry, University of Amsterdam: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Nzila, A. Current status of the degradation of aliphatic and aromatic petroleum hydrocarbons by thermophilic microbes and future perspectives. Int. J. Environ. Res. Public Health 2018, 15, 2782. [Google Scholar] [CrossRef]
- Pandey, P.; Kapley, A.; Brar, S.K. Editorial: Biodegradation of High Molecular Weight Polyaromatic Hydrocarbons in Different Environments. Front. Microbiol. 2021, 12, 2020–2022. [Google Scholar] [CrossRef] [PubMed]
- Sawulski, P.; Boots, B.; Clipson, N.; Doyle, E. Differential degradation of polycyclic aromatic hydrocarbon mixtures by indigenous microbial assemblages in soil. Lett. Appl. Microbiol. 2015, 61, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Wang, H.; Li, Y.; Duan, X. Impact of co-contamination by PAHs and heavy metals on micro-ecosystem in bioretention systems with soil, sand, and water treatment residuals. J. Clean. Prod. 2023, 383, 135417. [Google Scholar] [CrossRef]
- Shehu, U.; Ahmad, F.A.; Yusuf, F.; Muhammad, F.; Yakasai, H.M. Isolation and Identification of Anthracene Utilizing Proteus vulgaris from Oil Spill Contaminated Soil at NNPC Depot Kano State Nigeria. J. Adv. Biol. Biotechnol. 2021, 24, 46–53. [Google Scholar] [CrossRef]
- Smułek, W.; Sydow, M.; Matejuk, Z.J.; Kaczorek, E. Bacteria involved in biodegradation of creosote PAH–A case study of longterm contaminated industrial area. Ecotoxicol. Environ. Saf. 2020, 187, 109843. [Google Scholar] [CrossRef] [PubMed]
- Souza, H.M.d.L.; Barreto, L.R.; da Mota, A.J.; de Oliveira, L.A.; Barroso, H.d.S.; Zanotto, S.P. Tolerance to polycyclic aromatic hydrocarbons (PAHs) by filamentous fungi isolated from contaminated sediment in the Amazon region. Acta Sci. Biol. Sci. 2017, 39, 481–488. [Google Scholar] [CrossRef]
- Ray, M.; Kumar, V.; Banerjee, C.; Gupta, P.; Singh, S.; Singh, A. Investigation of biosurfactants produced by three indigenous bacterial strains, their growth kinetics and their anthracene and fluorene tolerance. Ecotoxicol. Environ. Saf. 2021, 208, 111621. [Google Scholar] [CrossRef]
- Sahu, L. Presence of Hydrocarbon Degrading Bacteria in Contaminated Soil Collected From Various Fuel Station in Bhilai, Chhattisgarh. Int. J. Res. Appl. Sci. Eng. Technol. 2021, 9, 1802–1804. [Google Scholar] [CrossRef]
- Vaezzadeh, V.; Zakaria, M.P.; Bong, C.W.; Masood, N.; Magam, M.S.; Alkhadher, S. Mangrove Oyster (Crassostrea belcheri) as a Biomonitor Species for Bioavailability of Polycyclic Aromatic Hydrocarbons (PAHs) from Sediment of the West Coast of Peninsular Malaysia. Polycycl. Aromat. Compd. 2019, 39, 470–485. [Google Scholar] [CrossRef]
- Wang, B.; Lai, Q.; Cui, Z.; Tan, T.; Shao, Z. A pyrene-degrading consortium from deep-sea sediment of the West Pacific and its key member Cycloclasticus sp. P1. Environ. Microbiol. J. 2008, 10, 1948–1963. [Google Scholar] [CrossRef]
- Wolf, D.C.; Gan, J. Influence of rhamnolipid biosurfactant and Brij-35 synthetic surfactant on 14C-pyrene mineralization in soil. Environ. Pollut. 2018, 243, 1846–1853. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Shao, Z. Polycyclic Aromatic Hydrocarbon (PAH) Degradation Pathways of the Obligate Marine PAH Degrader Cycloclasticus sp. Strain P1. App. Env. Microbiol. 2018, 84, e1261-18. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, I.; Asaf, R.; Paena, M.; Athirah, A.; Nisaa, K.; Ahmad, R.; Kamaruddin, M. Anthracene and Pyrene Biodegradation Performance of Marine Sponge Symbiont Bacteria Consortium. Molecules 2021, 26, 6851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, F.; Qiao, J.; Li, G.; Shen, C.; Huang, T.; Hu, Z. Draft Genome Sequence of Rhodococcus sp. Strain P14, a Biodegrader of High-Molecular-Weight Polycyclic Aromatic Hydrocarbons. J. Bacteriol. 2012, 194, 3546. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Tauler, M.; Grifoll, M. Bacterial PAH degradation in marine and terrestrial habitats. Curr. Opin. Biotechnol. 2015, 33, 95–102. [Google Scholar] [CrossRef]
- Tawniczak, T.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, T. Microbial degradation of hydrocarbons-basic principles for bioremediation: A review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef]
- Miao, L.L.; Qu, J.; Liu, Z.P. Hydroxylation at Multiple Positions Initiated the Biodegradation of Indeno[1,2,3-cd]Pyrene in Rhodococcus aetherivorans IcdP1. Front. Microbiol. 2020, 11, 568381. [Google Scholar] [CrossRef]
- Bodor, A.; Bounedjouma, N.; Feigl, G.; Duzs, A.; Laczi, K.; Szilágyi, A.; Rákhely, G.; Perei, K. Exploitation of extracellular organic matter from Micrococcus luteus to enhance ex situ bioremediation of soils polluted with used lubricants. J. Hazard. Mater. 2021, 417, 125996. [Google Scholar] [CrossRef]
- Zakaria, H.Y.; Hassan, A.K.M.; El-Naggar, H.A.; Abo-Senna, F.M. Biomass determination based on the individual volume of the dominant copepod species in the Western Egyptian Mediterranean Coast. Egypt J. Aquat. Res. 2018, 44, 89–99. [Google Scholar] [CrossRef]
- Çoban-Yıldız, Y.; Chiavari, G.; Fabbri, D.; Gaines, A.F.; Galletti, G.; Tuğrul, S. The chemical composition of Black Sea suspended particulate organic matter: Pyrolysis-GC/MS as a complementary tool to traditional oceanographic analyses. Mar. Chem. 2000, 69, 55–67. [Google Scholar] [CrossRef]
- Gomiero, A.; Øysæd, K.B.; Palmas, L.; Skogerbø, G. Application of GC/MS-pyrolysis to estimate the levels of microplastics in a drinking water supply system. J. Hazard. Mater. 2021, 416, 125708. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, M.; Scholz-Böttcher, B.M.; Oberbeckmann, S.; Labrenz, M.; Fischer, D.; Eichhorn, K.-J.; Voit, B. Comparison of μ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal. Bioanal. Chem. 2018, 410, 5313–5327. [Google Scholar] [CrossRef] [PubMed]
- Spini, G.; Spina, F.; Poli, A.; Blieux, A.L.; Regnier, T.; Gramellini, C.; Varese, G.C.; Puglisi, E. Molecular and Microbiological Insights on the Enrichment Procedures for the Isolation of Petroleum Degrading Bacteria and Fungi. Front. Microbiol. 2018, 9, 2543. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, I.; Asdar, A.M.; Hardimas, H. Performance Analysis of bio-sorption of Heavy Metal and Biodegradation PAH of Isolates Marine Sponges Symbiont Bacteria. Indo Chim. Acta. 2021, 13, 18333. [Google Scholar]
- Tsaboula, A.; Papadakis, E.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Papadopoulou, M.E. Assessment and Management of Pesticide Pollution at a River Basin Level Part II: Optimization of Pesticide Monitoring Networks on Surface Aquatic Ecosystems by Data Analysis Methods. Sci. Total Environ. 2019, 653, 1597–1611. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, B.K.; Paudel, K.P. Assessing the efficiency of alternative best management practices to reduce nonpoint source pollution in a rural watershed located in Louisiana, USA. Water 2019, 11, 1714. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Dick, R.P.; Li, H.; Shen, D.; Gao, Y.; Waigi, M.G.; Ling, W. Rhamnolipid influences biosorption and biodegradation of phenanthrene by phenanthrene-degrading strain Pseudomonas sp. Ph6. Environ. Pollut. 2018, 240, 359–367. [Google Scholar] [CrossRef]
- Roy, A.; Dutta, A.; Pal, S.; Gupta, A.; Sarkar, J.; Chatterjee, A.; Saha, A.; Sarkar, P.; Sar, P.; Kazy, S.K. Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour. Technol. 2018, 253, 22–32. [Google Scholar] [CrossRef]
- Liu, X.; Hu, X.; Cao, Y.; Jing, W.P.; Yu, H.J.; Guo, P.; Huang, L. Biodegradation of Phenanthrene and Heavy Metal Removal by Acid-Tolerant Burkholderia fungorum FM-2. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Selvin, J.; Shanmugha Priya, S.; Seghal-Kiran, G.; Thangavelu, T.; Sapna-Bai, N. Sponge-associated marine bacteria as indicators of heavy metal pollution. Microbiol. Res. 2009, 164, 352–363. [Google Scholar] [CrossRef]
- Anggela, A.; Marzuki, I. Bioadsorption capacity of marine sponge symbiotic bacteria against heavy metal Contaminants. Kovalen J. Ris. Kim. 2021, 7, 12–22. (In Indonesia) [Google Scholar]
- Knobloch, S.; Jóhannsson, R.; Marteinsson, V. Bacterial diversity in the marine sponge Halichondria panicea from Icelandic waters and host-specificity of its dominant symbiont candidatus Halichondribacter symbioticus. FEMS Microbiol. Ecol. 2018, 95, 1–13. [Google Scholar]
- Ziss, E.; Friesl, H.W.; Noller, C.; Watzinger, A.; Hood, N.R. Heavy Metal City-Zen. Exploring the potential risk of heavy metal contamination of food crop plants in urban gardening contexts using a citizen science approach. EGU Gen. Assem. Conf. Abstract 2020, 13, 8626. [Google Scholar]
- White, J.R.; Patel, J.; Ottesen, A.; Arce, G.; Blackwelder, P.; Lopez, J.V. Pyrosequencing of Bacterial Symbionts within Axinella corrugata Sponges: Diversity and Seasonal Variability. PLoS ONE 2012, 7, e38204. [Google Scholar] [CrossRef]
- Parhamfar, M.; Abtahia, H.; Godinib, K.; Saeedi, R.; Sartaje, M.; Villaseñorf, J.; Coulong, F.; Kumarg, V.; Soltanighiash, T.; Radi, E.G.; et al. Biodegradation of heavy oily sludge by a two-step inoculation composting process using synergistic effect of indigenous isolated bacteria. Process Biochem. 2020, 91, 223–230. [Google Scholar] [CrossRef]
- Arroyo, A.; Provoste, F.; Rodríguez, M.; Prieto, A.L. A mechanistic model to assess the fate of naphthalene and Benzo(A)etilene in a chilean wwtp. Processes 2021, 9, 1313. [Google Scholar] [CrossRef]
- Okoro, C.C. Biosurfactant-enhanced remediation of hydrocarbon contaminated mangrove swamp. Nat. Sci. 2010, 8, 152–162. [Google Scholar] [CrossRef]
- Liu, W.J.; Duan, X.D.; Wu, L.P.; Masakorala, K. Biosurfactant Production by Pseudomonas aeruginosa SNP0614 and its Effect on Biodegradation of Petroleum. Appl. Biochem. Microbiol. 2018, 54, 155–162. [Google Scholar] [CrossRef]
- Tenea, A.G.; Vasile, G.G.; Dinu, C.; Gheorgh, S.; Pascu, L.F.; Mureseanu, M.; Ene, C. Behavior of Cd accumulation in sinapis alba L. In the presence of essential elements (Ca, Mg, Fe, Zn, Mn, Cu, Ni). Rev. Chim. 2020, 71, 378–389. [Google Scholar] [CrossRef]
- Alaboudi, A.A.; Ahmed, B.; Brodie, G. Annals of Agricultural Sciences Phytoremediation of Pb and Cd contaminated soils by using sun fl ower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Isikhuemhen, O.S.; Mikiashvili, N.A.; Senwo, Z.N.; Ohimain, E.I. Biodegradation and Sugar Release from Canola Plant Biomass by Selected White Rot Fungi. Adv. Biol. Chem. 2014, 04, 395–406. [Google Scholar] [CrossRef]
- Al-Nasrawi, H. Role of Fungi in Bioremediation. Adv. Biotechnol. Microbiol. 2019, 12, 77–81. [Google Scholar]
- Fomina, M.; Charnock, J.M.; Hillier, S.; Alvarez, R.; Livens, F.; Gadd, G.M. Role of fungi in the biogeochemical fate of depleted uranium. Curr. Biol. 2008, 18, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Morganti, T.M.; Ribes, M.; Yahel, G.; Coma, R. Size Is the Major Determinant of Pumping Rates in Marine Sponges. Front. Physiol. 2019, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Keller-Costa, T.; Jousset, A.; Van-Overbeek, L.; Van-Elsas, J.D.; Costa, R. The freshwater sponge Ephydatia fluviatilis harbours diverse Pseudomonas species (Gammaproteobacteria, Pseudomonadales) with broad-spectrum antimicrobial activity. PLoS ONE 2014, 9, e88429. [Google Scholar] [CrossRef]
- Shimoda, T.; Suryati, E.; Ahmad, T. Evaluation in a Shrimp Aquaculture System Using Mangroves, Oysters, and Seaweed as Biofilters Based on the Concentrations of Nutrients and Chlorophyll. JARQ 2006, 40, 189–193. [Google Scholar] [CrossRef][Green Version]
- Cecotti, M.; Coppotelli, B.M.; Mora, V.C.; Viera, M.; Morelli, I.S. Efficiency of surfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbon-contaminated soil: Link with bioavailability and the dynamics of the bacterial community. Sci. Total Environ. 2018, 634, 224–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).