Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light
Abstract
:Simple Summary
Abstract
1. Introduction
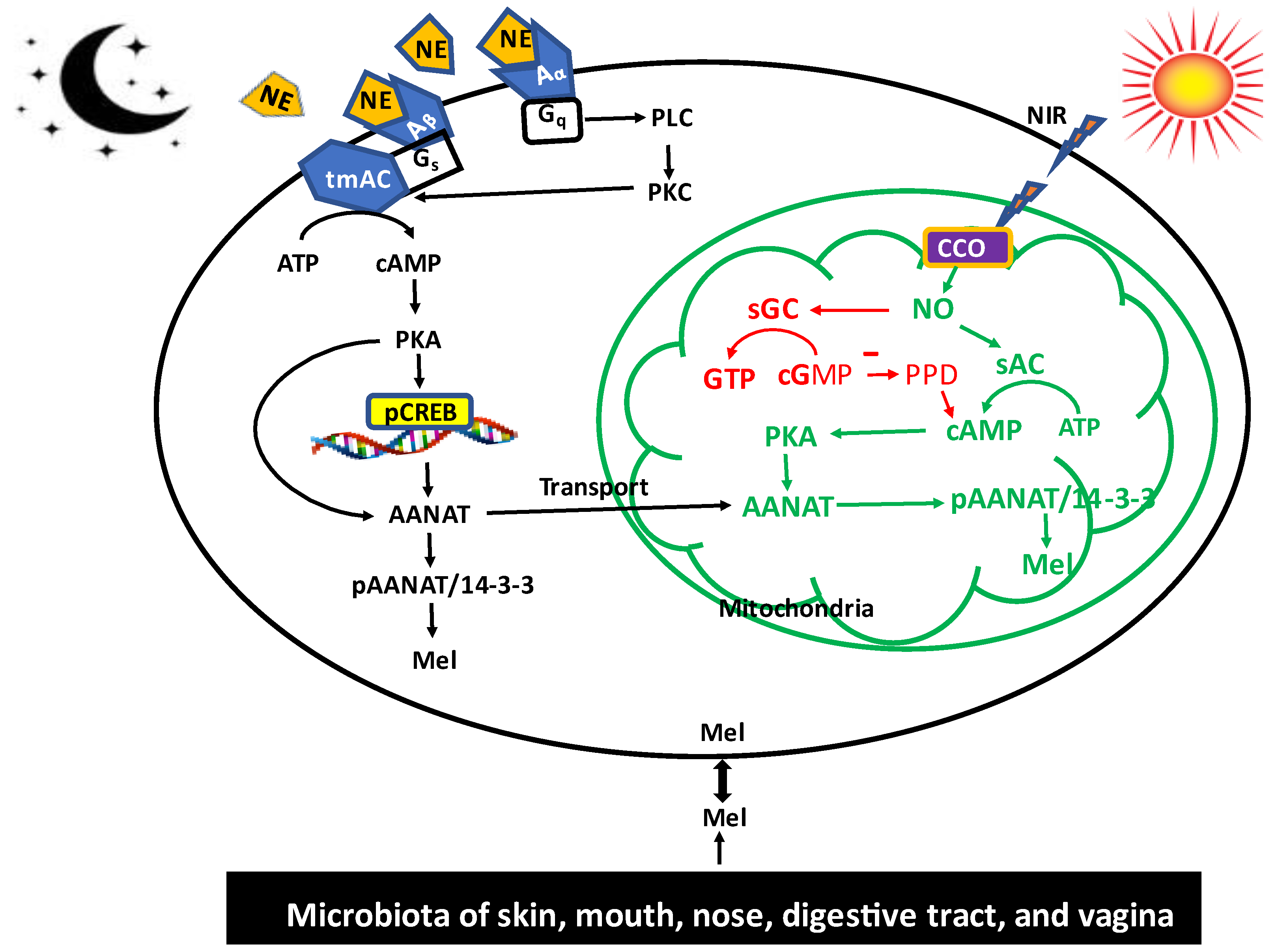
2. Melatonin Synthesis in Mitochondria and Its Biological Significance
- (1)
- Melatonin promotes the activity of pyruvate dehydrogenase (PDH) to enhance mitochondrial uptake of pyruvate, which increases the production of acetyl coenzyme A, a necessary co-factor for melatonin synthesis. Thus, melatonin has the capacity to switch the so-called Warburg’s effect to mitochondrial oxidative metabolism in cells [75,76,77].
- (2)
- (3)
- (4)
- Melatonin supports the transfer of functionally active mitochondria from healthy to injured cells, thereby rescuing the energy metabolism of the recipient cells [83]. The transfer of mitochondria is achieved either by tunneling nanotubes that develop between healthy and injured cells or via exosomes, whose cargo comprises diverse cellular materials including regulatory RNAs and organelles such as mitochondria [84].
- (5)
- Melatonin regulates mitochondrial dynamics. This includes melatonin’s promotion of mitochondrial biogenesis in both stem cells and postmitotic cells. Under most conditions, melatonin increases mitochondrial fusion, inhibits their fission, and enhances mitophagy [85,86,87,88], whereas the reverse only occurs in tumor cells [89]. In nontumor cells, it downregulates the genes involved in mitochondrial fission (DRP1, hFis1, MIEF2, MFF) and mitophagy (PINK, BNip3, NIX) to maintain mitochondrial homeostasis.
- (6)
- Melatonin inhibits the mitochondrial permeability transition pore (mtPTP) opening to preserve the mitochondrial membrane potential and maintain functionally intact mitochondria [90,91]. The activity of melatonin in influencing mitochondrial physiology may be in part receptor mediated, since the membrane melatonin receptor 1 (MT1) is not restricted to the plasma membrane, but also located on the mitochondrial membrane [41]. The signal transduction processes by which melatonin modulates mitochondrial biogenesis is via the MT1/SIRT1/PGC-1α/NRF2/PPAR-γ pathway [92].
3. Regulation of the Visible Light (Blue Wavelength) on Cerebrospinal Fluid (CSF) and Serum Melatonin Circadian Rhythms: Melatonin Serving as the Chemical Expression of Darkness
4. Role of Non-Visible Near Infrared (NIR) Radiation on Melatonin Synthesis: Melatonin as a Participant of Sunlight Exposure
5. Melatonin Synthetic System of the Gut Microbiota
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, Y.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Zheng, X.; Kong, J.; Manchester, L.; Hardeland, R.; Kim, S.; Xu, X.; Reiter, R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int. J. Mol. Sci. 2014, 15, 15858–15890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Choi, G.-H.; Back, K. Functional characterization of serotonin n-acetyltransferase in archaeon Thermoplasma volcanium. Antioxidants 2022, 11, 596. [Google Scholar] [CrossRef]
- Rosen, J.; Than, N.N.; Koch, D.; Poeggeler, B.; Laatsch, H.; Hardeland, R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006, 41, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Fuhrberg, B.; Hardeland, R.; Poeggeler, B.; Behrmann, C. Dramatic rises of melatonin and 5-methoxytryptamine in gonyaulax exposed to decreased temperature. Biol. Rhythm Res. 1997, 28, 144–150. [Google Scholar] [CrossRef]
- Lee, K.; Back, K. Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J. Pineal Res. 2017, 62, e12392. [Google Scholar] [CrossRef]
- Jand, Y.; Ghahremani, M.H.; Ghanbari, A.; Ejtemaei-Mehr, S.; Guillemin, G.J.; Ghazi-Khansari, M. Melatonin ameliorates disease severity in a mouse model of multiple sclerosis by modulating the kynurenine pathway. Sci. Rep. 2022, 12, 15963. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, R.; Wang, Z.; Chen, Z.; Wang, G.; Guan, S.; Lu, J. Melatonin prevents against ethanol-induced liver injury by mitigating ferroptosis via targeting brain and muscle ARNT-like 1 in mice liver and HepG2 cells. J. Agric. Food Chem. 2022, 70, 12953–12967. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: The chemical expression of darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and reproduction revisited. Biol. Reprod. 2009, 81, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenshen, Y.; Minshu, W.; Qing, Y.; Yang, L.; Suodi, Z.; Wei, W. The effect of cataract surgery on salivary melatonin and sleep quality in aging people. Chronobiol. Int. 2016, 33, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Heydari, K.; Mehravaran, H.; Saeedi, M.; Alizadeh-Navaei, R.; Hedayatizadeh-Omran, A.; Shamshirian, A. Melatonin effects on sleep quality and outcomes of COVID-19 patients: An open-label, randomized, controlled trial. J. Med. Virol. 2022, 94, 263–271. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127–128, 46–63. [Google Scholar] [CrossRef] [Green Version]
- Mota, A.D.L.; Jardim-Perassi, B.V.; De Castro, T.B.; Colombo, J.; Sonehara, N.M.; Nishiyama, V.K.G.; Pierri, V.A.G.; de Campos Zuccari, D.A.P. Melatonin modifies tumor hypoxia and metabolism by inhibiting HIF-1α and energy metabolic pathway in the in vitro and in vivo models of breast cancer. Melatonin Res. 2019, 2, 83–98. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Ojike, N.I.; Akinseye, O.A.; Kendzerska, T.; Buttoo, K.; Dhandapany, P.S.; Brown, G.M.; Cardinali, D.P. Melatonin and human cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 122–132. [Google Scholar] [CrossRef]
- Spinedi, E.; Cardinali, D.P. Neuroendocrine-metabolic dysfunction and sleep disturbances in neurodegenerative disorders: Focus on alzheimer’s disease and melatonin. Neuroendocrinology 2019, 108, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Prodhan, A.H.M.S.U.; Cavestro, C.; Kamal, M.A.; Islam, M.A. Melatonin and sleep disturbances in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2021, 20, 736–754. [Google Scholar] [CrossRef]
- Cheng, Z.; Xiang, Q.; Wang, J.; Zhang, Y. The potential role of melatonin in retarding intervertebral disc ageing and degeneration: A systematic review. Ageing Res. Rev. 2021, 70, 101394. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory. Int J Mol Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinali, D.P. Melatonin and healthy aging. Vitam. Horm. 2021, 115, 67–88. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Ma, Q.; Sharma, R. Melatonin in mitochondria: Mitigating clear and present dangers. Physiology 2020, 35, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Huether, G.; Poeggeler, B.; Reimer, A.; George, A. Effect of tryptophan administration on circulating melatonin levels in chicks and rats: Evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992, 51, 945–953. [Google Scholar] [CrossRef]
- Hajak, G.; Huether, G.; Blanke, J.; Blomer, M.; Freyer, C.; Poeggeler, B.; Reimer, A.; Rodenbeck, A.; Schulz-Varszegi, M.; Ruther, E. The influence of intravenous L-tryptophan on plasma melatonin and sleep in men. Pharmacopsychiatry 1991, 24, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Germann, S.M.; Baallal Jacobsen, S.A.; Schneider, K.; Harrison, S.J.; Jensen, N.B.; Chen, X.; Stahlhut, S.G.; Borodina, I.; Luo, H.; Zhu, J.; et al. Glucose-based microbial production of the hormone melatonin in yeast Saccharomyces cerevisiae. Biotechnol. J. 2016, 11, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo-Fernández, M.; Valls-Fonayet, J.; Valero, E.; Hornedo-Ortega, R.; Richard, T.; Troncoso, A.M.; Garcia-Parrilla, M.C. Isotopic labelling-based analysis elucidates biosynthesis pathways in Saccharomyces cerevisiae for Melatonin, Serotonin and Hydroxytyrosol formation. Food Chem. 2022, 374, 131742. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Saxena, P.K. A melatonin-rich germplasm line of St John’s wort (Hypericum perforatum L.). J. Pineal Res. 2006, 41, 284–287. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef] [PubMed]
- Kappers, J.A. Localization of indoleamine and protein synthesis in the mammalian pineal gland. J. Neural Transm. Suppl. 1978, 12, 13–24. [Google Scholar]
- Kerényi, N.A.; Sótonyi, P.; Somogyi, E. Localizing acethyl-serotonin transferase by electron microscopy. Histochemistry 1975, 46, 77–80. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Park, O.K.; Yoo, K.Y.; Lee, C.H.; Choi, J.H.; Hwang, I.K.; Park, J.H.; Kwon, Y.G.; Kim, Y.M.; Won, M.H. Arylalkylamine N-acetyltransferase (AANAT) is expressed in astrocytes and melatonin treatment maintains AANAT in the gerbil hippocampus induced by transient cerebral ischemia. J. Neurol. Sci. 2010, 294, 7–17. [Google Scholar] [CrossRef]
- Uz, T.; Qu, T.; Sugaya, K.; Manev, H. Neuronal expression of arylalkylamine N-acetyltransferase (AANAT) mRNA in the rat brain. Neurosci. Res. 2002, 42, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hidalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwas, D.K.; Haldar, C. MT1 receptor expression and AA-NAT activity in lymphatic tissue following melatonin administration in male golden hamster. Int. Immunopharmacol. 2014, 22, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002, 16, 896–898. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, F.; Sutradhar, S.; Das, P.; Mukherjee, S. Gut melatonin: A potent candidate in the diversified journey of melatonin research. Gen. Comp. Endocrinol. 2021, 303, 113693. [Google Scholar] [CrossRef]
- Gonzalez-Arto, M.; Hamilton, T.R.D.S.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Bozenna, O.; Paweł, M.; Bogdan, L.; Urszula, S. Melatonin and its synthesizing enzymes (arylalkylamine N-acetyltransferase-like and hydroxyindole-O-methyltransferase) in avian eggs and early embryos. J. Pineal Res. 2007, 42, 310–318. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R. The Reserve/Maximum Capacity of Melatonin’s synthetic function for the potential dimorphism of melatonin production and its biological significance in mammals. Molecules 2021, 26, 7302. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Rodriguez, C.; Martin, V.; Rosales-Corral, S.; de Campos Zuccari, D.A.; de Almeida Chuffa, L.G. Part-time cancers and role of melatonin in determining their metabolic phenotype. Life Sci. 2021, 278, 119597. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, A.; Do Kim, H.; Chang, Y.C.; Kim, C.H. Revisited metabolic control and reprogramming cancers by means of the warburg effect in tumor Cells. Int. J. Mol. Sci. 2022, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Gaiotte, L.B.; Carvalho Cesário, R.; Silveira, H.S.; Augusto De Morais Oliveira, D.; Cucielo, M.S.; Gorete Romagnoli, G.; Kaneno, R.; Aparecida, D.; De Campos Zuccari, P.; Reiter, R.J.; et al. Combination of melatonin with paclitaxel reduces the TLR4-mediated inflammatory pathway, PD-L1 levels, and survival of ovarian carcinoma cells. Melatonin Res. 2022, 5, 34–51. [Google Scholar] [CrossRef]
- Cucielo, M.S.; Cesário, R.C.; Silveira, H.S.; Gaiotte, L.B.; Dos Santos, S.A.A.; de Campos Zuccari, D.A.P.; Seiva, F.R.F.; Reiter, R.J.; de Almeida Chuffa, L.G. Melatonin reverses the warburg-type metabolism and reduces mitochondrial membrane potential of ovarian cancer cells independent of mt1 receptor activation. Molecules 2022, 27, 4350. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Claes, V.; Vallée, A.; Hébert, J. Thermodynamics in cancers: Opposing interactions between PPAR gamma and the canonical WNT/beta-catenin pathway. Clin. Transl. Med. 2017, 6, 5879090. [Google Scholar] [CrossRef] [Green Version]
- Maestroni, G.J.M.; Conti, A. Melatonin in human breast cancer tissue: Association with nuclear grade and estrogen receptor status. Lab. Investig. 1996, 75, 557–561. [Google Scholar]
- Azama, T.; Yano, M.; Oishi, K.; Kadota, K.; Hyun, K.; Tokura, H.; Nishimura, S.; Matsunaga, T.; Iwanaga, H.; Miki, H.; et al. Altered expression profiles of clock genes hPer1 and hPer2 in peripheral blood mononuclear cells of cancer patients undergoing surgery. Life Sci. 2007, 80, 1100–1108. [Google Scholar] [CrossRef]
- Elorza, A.A.; Soffia, J.P. mtDNA heteroplasmy at the core of aging-associated heart failure. An integrative view of OXPHOS and mitochondrial life cycle in cardiac mitochondrial physiology. Front. Cell Dev. Biol. 2021, 9, 625020. [Google Scholar] [CrossRef]
- Wei, Y.H. Mitochondrial DNA mutations and oxidative damage in aging and diseases: An emerging paradigm of gerontology and medicine. Proc. Natl. Sci. Counc. Repub. China B 1998, 22, 55–67. [Google Scholar]
- Gemmell, N.J.; Metcalf, V.J.; Allendorf, F.W. Mother’s curse: The effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004, 19, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, P.W. Reversing mother’s curse revisited. Evolution 2012, 66, 612–616. [Google Scholar] [CrossRef]
- Wehbe, Z.; Hammoud, S.H.; Yassine, H.M.; Fardoun, M.; El-Yazbi, A.F.; Eid, A.H. Molecular and biological mechanisms underlying gender differences in COVID-19 severity and mortality. Front. Immunol. 2021, 1603. [Google Scholar] [CrossRef]
- Fidecicchi, T.; Fruzzetti, F.; Lete Lasa, L.I.; Calaf, J. COVID-19, gender and estroprogestins, what do we know? Eur. J. Contracept. Reprod. Health Care 2022, 27, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tao, J.; Wu, H.; Guan, S.; Liu, L.; Zhang, L.; Deng, S.; He, C.; Ji, P.; Liu, J.; et al. Aanat knockdown and melatonin supplementation in embryo development: Involvement of mitochondrial function and DNA methylation. Antioxid. Redox Signal. 2018, 30, 2050–2065. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef] [PubMed]
- Jauhari, A.; Baranov, S.V.; Suofu, Y.; Kim, J.; Singh, T.; Yablonska, S.; Li, F.; Wang, X.; Oberly, P.; Minnigh, M.B.; et al. Melatonin inhibits cytosolic mitochondrial DNA-induced neuroinflammatory signaling in accelerated aging and neurodegeneration. J. Clin. Investig. 2020, 130, 3124–3136. [Google Scholar] [CrossRef] [Green Version]
- Mai, F.; Del Pinto, R.; Ferri, C. COVID-19 and cardiovascular diseases. J. Cardiol. 2020, 141, 1648–1655. [Google Scholar] [CrossRef]
- Yu, L.; Di, W.; Dong, X.; Li, Z.; Zhang, Y.; Xue, X.; Xu, Y.; Zhang, J.; Xiao, X.; Han, J.; et al. Melatonin protects diabetic heart against ischemia-reperfusion injury, role of membrane receptor-dependent cGMP-PKG activation. Biochim. Biophys. Acta—Mol. Basis Dis. 2018, 1864, 563–578. [Google Scholar] [CrossRef]
- Rudnitskaya, E.A.; Muraleva, N.A.; Maksimova, K.Y.; Kiseleva, E.; Kolosova, N.G.; Stefanova, N.A. Melatonin attenuates memory impairment, amyloid-β accumulation, and neurodegeneration in a rat model of sporadic Alzheimer’s disease. J. Alzheimers. Dis. 2015, 47, 103–116. [Google Scholar] [CrossRef]
- Brusco, L.I.; Márquez, M.; Cardinali, D.P. Monozygotic twins with Alzheimer’s disease treated with melatonin: Case report. J. Pineal Res. 1998, 25, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Reiter, R.J.; Alipoor, R.; Dadgostar, E.; Kouchaki, E.; Asemi, Z. Melatonin and Parkinson disease: Current status and future perspectives for molecular mechanisms. Cell. Mol. Neurobiol. 2020, 40, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wongprayoon, P.; Govitrapong, P. Melatonin as a mitochondrial protector in neurodegenerative diseases. Cell. Mol. Life Sci. 2017, 74, 3999–4014. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; El-Missiry, Z.M.A.; Othman, A.I. Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of Covid-19. Eur. J. Pharmacol. 2020, 882, 173329. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rorsales-Corral, S.; de Almeida Chuffa, L.G. Melatonin inhibits Warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: A mechanistic hypothesis. Cell. Mol. Life Sci. 2020, 77, 2527–2542. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hao, B.; Li, D.; Reiter, R.J.; Bai, Y.; Abay, B.; Chen, G.; Lin, S.; Zheng, T.; Ren, Y.; et al. Melatonin inhibits lung cancer development by reversing the Warburg effect via stimulating the SIRT3/PDH axis. J. Pineal Res. 2021, 71, e12755. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rosales-Corral, S.; Acuna-Castroviejo, D.; Escames, G. Inhibition of mitochondrial pyruvate dehydrogenase kinase: A proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2019, 2, 105–119. [Google Scholar] [CrossRef]
- Martín, M.; Macías, M.; Escames, G.; Reiter, R.J.; Agapito, M.T.; Ortiz, G.G.; Acuña-Castroviejo, D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 2000, 28, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Solís-Muñoz, P.; Solís-Herruzo, J.A.; Fernández-Moreira, D.; Gómez-Izquierdo, E.; García-Consuegra, I.; Muñoz-Yagüe, T.; García Ruiz, I. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J. Pineal Res. 2011, 51, 113–123. [Google Scholar] [CrossRef]
- Jiménez-Aranda, A.; Fernández-Vázquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.X.; Reiter, R.J.; Agil, A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, S.; Wen, H.; Liu, T.; Cai, J.; Du, D.; Zhu, D.; Chen, F.; Xia, C. Melatonin decreases M1 polarization via attenuating mitochondrial oxidative damage depending on UCP2 pathway in prorenin-treated microglia. PLoS ONE 2019, 14, e0212138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslan, G.; Gül, H.F.; Tektemur, A.; Sahna, E. Ischemic postconditioning reduced myocardial ischemia-reperfusion injury: The roles of melatonin and uncoupling protein 3. Anatol. J. Cardiol. 2020, 23, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S. Melatonin, tunneling nanotubes and anastasis: Cheating cell death. Melatonin Res. 2021, 4, 566–580. [Google Scholar] [CrossRef]
- Novais, A.A.; de Chuffa, L.G.A.; de Zuccari, D.A.P.C.; Reiter, R.J. Exosomes and melatonin: Where their destinies intersect. Front. Immunol. 2021, 12, 692022. [Google Scholar] [CrossRef]
- Xue, R.; Li, S.; Wei, Z.; Zhang, Z.; Cao, Y. Melatonin attenuates di-(2-ethylhexyl) phthalate-induced apoptosis of human granulosa cells by inhibiting mitochondrial fission. Reprod. Toxicol. 2022, 113, 18–29. [Google Scholar] [CrossRef]
- Agil, A.; Chayah, M.; Visiedo, L.; Navarro-Alarcon, M.; Ferrer, J.M.R.; Tassi, M.; Reiter, R.J.; Fernández-Vázquez, G. Melatonin improves mitochondrial dynamics and function in the kidney of zücker diabetic fatty rats. J. Clin. Med. 2020, 9, 2916. [Google Scholar] [CrossRef]
- Chen, W.R.; Zhou, Y.J.; Sha, Y.; Wu, X.P.; Yang, J.Q.; Liu, F. Melatonin attenuates vascular calcification by inhibiting mitochondria fission via an AMPK/Drp1 signalling pathway. J. Cell. Mol. Med. 2020, 24, 6043–6054. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.A.; Yu, F.; Shi, L.; Ko, M.L.; Ko, G.Y.P. Melatonin affects mitochondrial fission/fusion dynamics in the diabetic retina. J. Diabetes Res. 2019, 2019, 8463125. [Google Scholar] [CrossRef] [Green Version]
- Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; Martinez-Ruiz, L.; Rodríguez-Santana, C.; Shen, Y.Q.; García-Verdugo, J.M.; López-Rodríguez, A.; Rusanova, I.; Quiñones-Hinojosa, A.; et al. Melatonin targets metabolism in head and neck cancer cells by regulating mitochondrial structure and function. Antioxidants 2021, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhao, C.; Xiang, H.; Jia, G.X.; Zhong, R. Melatonin improves cryopreservation of ram sperm by inhibiting mitochondrial permeability transition pore opening. Reprod. Domest. Anim. 2020, 55, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Meng, J.; Zhu, Y.; Ding, M.; Zhang, Y.; Zhou, J. Melatonin enhances SIRT1 to ameliorate mitochondrial membrane damage by activating PDK1/Akt in granulosa cells of PCOS. J. Ovarian Res. 2021, 14, 152. [Google Scholar] [CrossRef]
- Guo, P.; Pi, H.; Xu, S.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; Li, R.; et al. Melatonin Improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol. Sci. 2014, 142, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Rosengarten, H.; Meller, E.; Friedhoff, A.J. In vitro enzymatic formation of melatonin by human erythrocytes. Res. Commun. Chem. Pathol. Pharmacol. 1972, 4, 457–465. [Google Scholar] [PubMed]
- Ramakrishna, A.; Dayananda, C.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G.A. Photoperiod influences endogenous indoleamines in cultured green alga Dunaliella bardawil. Indian J. Exp. Biol. 2011, 49, 234–240. [Google Scholar]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar] [PubMed]
- Hardeland, R.; Poeggeler, B.; Balzer, I.; Behrmann, G. A hypothesis on the evolutionary origins of photoperiodism based on circadian rhythmicity of melatonin in phylogenetically distant organisms. Chronobiol. Chronomedicine 1993, 1, 113–120. [Google Scholar]
- Menaker, M.; Moreira, L.F.; Tosini, G. Evolution of circadian organization in vertebrates. Braz. J. Med. Biol. Res. 1997, 30, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Goldman, H.; Wurtman, R.J. Flow of Blood to the Pineal Body of the Rat. Nature 1964, 203, 87–88. [Google Scholar] [CrossRef]
- Tan, D.X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H.C. Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X. Role of CSF in the transport of melatonin. J. Pineal Res. 2002, 33, 61. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Interaction of neurons and astrocytes underlies the mechanism of Aβ-induced neurotoxicity. Biochem. Soc. Trans. 2014, 42, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Zhuang, J.; Zhu, H.-Y.; Shen, Y.-X.; Tan, Z.-L.; Zhou, J.-N. Cultured rat cortical astrocytes synthesize melatonin: Absence of a diurnal rhythm. J. Pineal Res. 2007, 43, 232–238. [Google Scholar] [CrossRef]
- Ibañez Rodriguez, M.P.; Noctor, S.C.; Muñoz, E.M. Cellular basis of pineal gland development: Emerging role of microglia as phenotype regulator. PLoS ONE 2016, 11, e0167063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upson, R.H.; Benson, B.; Satterfield, V. Quantitation of ultrastructural changes in the mouse pineal in response to continuous illumination. Anat. Rec. 1976, 184, 311–323. [Google Scholar] [CrossRef]
- Quay, W.B. The demonstration of a secretory material and cycle in the parenchymal cells of the mammalian pineal organ. Exp. Cell Res. 1956, 10, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; de Mange, J.; Phillips, W.T.; Tan, D.X.; Bitar, R.D. Melatonin in ventricular and subarachnoid cerebrospinal fluid: Its function in the neural glymphatic network and biological significance for neurocognitive health. Biochem. Biophys. Res. Commun. 2022, 605, 70–81. [Google Scholar] [CrossRef]
- Hedlund, L.; Lischko, M.M.; Rollag, M.D.; Niswender, G.D. Melatonin: Daily cycle in plasma and cerebrospinal fluid of calves. Science 1977, 195, 686–687. [Google Scholar] [CrossRef]
- Tricoire, H.; Møller, M.; Chemineau, P.; Malpaux, B. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod. Suppl. 2003, 61, 311–321. [Google Scholar] [CrossRef]
- Tricoire, H.; Locatelli, A.; Chemineau, P.; Malpaux, B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology 2002, 143, 84–90. [Google Scholar] [CrossRef]
- Klein, D.C.; Ganguly, S.; Coon, S.; Weller, J.L.; Obsil, T.; Hickman, A.; Dyda, F. 14-3-3 Proteins and photoneuroendocrine transduction: Role in controlling the daily rhythm in melatonin. Biochem. Soc. Trans. 2002, 30, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Schomerus, C.; Korf, H.-W. Mechanisms regulating melatonin synthesis in the mammalian pineal organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–383. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, F.H.; Hull, J.T.; Peirson, S.N.N.; Wulff, K.; Aeschbach, D.; Gooley, J.J.; Brainard, G.C.C.; Gregory-Evans, K.; Rizzo, J.F.F.; Czeisler, C.A.; et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr. Biol. 2007, 17, 2122–2128. [Google Scholar] [CrossRef] [Green Version]
- Souman, J.L.; Borra, T.; de Goijer, I.; Schlangen, L.J.M.; Vlaskamp, B.N.S.; Lucassen, M.P. Spectral tuning of white light allows for strong reduction in melatonin suppression without changing illumination level or color temperature. J. Biol. Rhythms 2018, 33, 420–431. [Google Scholar] [CrossRef]
- Reiter, R.J. Influence of pinealectomy on the breeding capability of hamsters maintained under natural photoperiodic and temperature conditions. Neuroendocrinology 1974, 13, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Malek, I.; Haim, A. Bright artificial light at night is associated with increased body mass, poor reproductive success and compromised disease tolerance in Australian budgerigars (Melopsittacus undulatus). Integr. Zool. 2019, 14, 589–603. [Google Scholar] [CrossRef]
- Zeman, M.; Józsa, R.; Cornélissen, G.; Stebelova, K.; Bubenik, G.; Olah, A.; Poeggeler, B.; Huether, G.; Hardeland, R.; Nagy, G.; et al. Chronomics: Circadian lead of extrapineal vs. pineal melatonin rhythms with an infradian hypothalamic exploration. Biomed. Pharmacother. 2005, 59, S213–S219. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Cornélissen, G.; Huether, G.; Hardeland, R.; Józsa, R.; Zeman, M.; Stebelova, K.; Oláh, A.; Bubenik, G.; Pan, W.; et al. Chronomics affirm extending scope of lead in phase of duodenal vs. pineal circadian melatonin rhythms. Biomed. Pharmacother. 2005, 59, S220–S224. [Google Scholar] [CrossRef] [Green Version]
- Stebelova, K.; Zeman, M.; Cornélissen, G.; Bubenik, G.; Jozsa, R.; Hardeland, R.; Poeggeler, B.; Huether, G.; Olah, A.; Nagy, G.; et al. Chronomics reveal and quantify circadian rhythmic melatonin in duodenum of rats. Biomed. Pharmacother. 2005, 59, S209–S212. [Google Scholar] [CrossRef] [Green Version]
- Ondrusova, K.; Fatehi, M.; Barr, A.; Czarnecka, Z.; Long, W.; Suzuki, K.; Campbell, S.; Philippaert, K.; Hubert, M.; Tredget, E.; et al. Subcutaneous white adipocytes express a light sensitive signaling pathway mediated via a melanopsin/TRPC channel axis. Sci. Rep. 2017, 7, 16332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, S.; Reiter, R.J. Melatonin and the optics of the human body. Melatonin Res. 2019, 2, 138–160. [Google Scholar] [CrossRef]
- Holick, M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar] [PubMed]
- Sebastian, P.; Cherbuin, N.; Barcellos, L.F.; Roalstad, S.; Casper, C.; Hart, J.; Aaen, G.S.; Krupp, L.; Benson, L.; Gorman, M.; et al. Association between time spent outdoors and risk of multiple sclerosis. Neurology 2022, 98, E267–E278. [Google Scholar] [CrossRef] [PubMed]
- Philipp, D.; Vogel, M.; Brandt, M.; Rauscher, F.G.; Hiemisch, A.; Wahl, S.; Kiess, W.; Poulain, T. The relationship between myopia and near work, time outdoors and socioeconomic status in children and adolescents. BMC Public Health 2022, 22, 2058. [Google Scholar] [CrossRef] [PubMed]
- Tedford, C.E.; Delapp, S.; Jacques, S.; Anders, J.J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg. Med. 2015, 47, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Brunke, M.W.; Sakovitch, K. Transcranial photobiomodulation (laser) therapy for cognitive impairment: A review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). Open Vet. J. 2022, 12, 256–263. [Google Scholar] [CrossRef]
- Stepanov, Y.V.; Golovynska, I.; Zhang, R.; Golovynskyi, S.; Stepanova, L.I.; Gorbach, O.; Dovbynchuk, T.; Garmanchuk, L.V.; Ohulchanskyy, T.Y.; Qu, J. Near-infrared light reduces β-amyloid-stimulated microglial toxicity and enhances survival of neurons: Mechanisms of light therapy for Alzheimer’s disease. Alzheimers. Res. Ther. 2022, 14. [Google Scholar] [CrossRef]
- Eells, J.T.; Gopalakrishnan, S.; Valter, K. Near-infrared photobiomodulation in retinal injury and disease. Adv. Exp. Med. Biol. 2016, 854, 437–441. [Google Scholar] [CrossRef]
- Chan, A.S.; Lee, T.L.; Hamblin, M.R.; Cheung, M.C. Photobiomodulation enhances memory processing in older adults with mild cognitive impairment: A functional near-infrared spectroscopy study. J. Alzheimers. Dis. 2021, 83, 1471–1480. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, S.; Liu, M. The review of the light parameters and mechanisms of photobiomodulation on melanoma cells. Photodermatol. Photoimmunol. Photomed. 2022, 38, 3–11. [Google Scholar] [CrossRef]
- Lipko, N.B. Photobiomodulation: Evolution and adaptation. Photobiomodulation Photomed. Laser Surg. 2022, 40, 213–233. [Google Scholar] [CrossRef]
- Beirne, K.; Rozanowska, M.; Votruba, M. Photostimulation of mitochondria as a treatment for retinal neurodegeneration. Mitochondrion 2017, 36, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odinokov, D.; Hamblin, M.R. Aging of lymphoid organs: Can photobiomodulation reverse age-associated thymic involution via stimulation of extrapineal melatonin synthesis and bone marrow stem cells? J. Biophotonics 2018, 11, e201700282. [Google Scholar] [CrossRef]
- Agil, A.; Navarro-Alarcon, M.; Ali, F.A.Z.; Albrakati, A.; Salagre, D.; Campoy, C.; Elmahallawy, E.K. Melatonin enhances the mitochondrial functionality of brown adipose tissue in obese-diabetic rats. Antioxidants 2021, 10, 1482. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, F.; Mostafavinia, A.; Sharifi, Z.; Mohaghegh Shalmani, L.; Amini, A.; Ahmadi, H.; Omidi, H.; Hajihosseintehrani, M.; Bayat, S.; Hamblin, M.R.; et al. Effects of photobiomodulation on mitochondrial function in diabetic adipose-derived stem cells in vitro. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 285, 121835. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Mehrvar, S.; Maleki, S.; Schmitt, H.; Summerfelt, P.; Dubis, A.M.; Abroe, B.; Connor, T.B.; Carroll, J.; Huddleston, W.; et al. Photobiomodulation preserves mitochondrial redox state and is retinoprotective in a rodent model of retinitis pigmentosa. Sci. Rep. 2020, 10, 20382. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A. Therapeutic photobiomodulation: Nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov. Med. 2011, 11, 154–159. [Google Scholar]
- Schirmer, I.; Bualeong, T.; Budde, H.; Cimiotti, D.; Appukuttan, A.; Klein, N.; Steinwascher, P.; Reusch, P.; Mügge, A.; Meyer, R.; et al. Soluble adenylyl cyclase: A novel player in cardiac hypertrophy induced by isoprenaline or pressure overload. PLoS ONE 2018, 13, e0192322. [Google Scholar] [CrossRef] [Green Version]
- Szanda, G.; Wisniewski, É.; Rajki, A.; Spät, A. Mitochondrial cAMP exerts positive feedback on mitochondrial Ca2+ uptake via the recruitment of Epac1. J. Cell Sci. 2018, 131, jcs215178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, T.; Jackvony, S.; Buck, J.; Levin, L.R. Bicarbonate, carbon dioxide and pH sensing via mammalian bicarbonate-regulated soluble adenylyl cyclase. Interface Focus 2021, 11, 20200034. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Espiritu, L.; Kleinboelting, S.; Navarrete, F.A.; Alvau, A.; Visconti, P.E.; Valsecchi, F.; Starkov, A.; Manfredi, G.; Buck, H.; Adura, C.; et al. Discovery of LRE1 as a specific and allosteric inhibitor of soluble adenylyl cyclase. Nat. Chem. Biol. 2016, 12, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Sisson, J.H.; Pavlik, J.A.; Wyatt, T.A. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol. Clin. Exp. Res. 2009, 33, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valsecchi, F.; Ramos-Espiritu, L.S.; Buck, J.; Levin, L.R.; Manfredi, G. cAMP and mitochondria. Physiology 2013, 28, 199–209. [Google Scholar] [CrossRef]
- Jakobsen, E.; Lange, S.C.; Bak, L.K. Soluble adenylyl cyclase-mediated cAMP signaling and the putative role of PKA and EPAC in cerebral mitochondrial function. J. Neurosci. Res. 2019, 97, 1018–1038. [Google Scholar] [CrossRef]
- Valsecchi, F.; Konrad, C.; Manfredi, G. Role of soluble adenylyl cyclase in mitochondria. Biochim. Biophys. Acta 2014, 1842, 2555–2560. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Ye, J.; Bai, Y.; Hu, S.; Tan, C.; Bazer, F.W.; Johnson, G.A.; Jiang, Z.; Wu, G. Arginine promotes the expression of aquaporin-3 and water transport in porcine trophectoderm cells through NO- and cAMP-dependent mechanisms. Front. Biosci. 2022, 27, 83. [Google Scholar] [CrossRef]
- Bassil, M.; Anand-Srivastava, M.B. Cyclic GMP modulates the expression of Gi protein and adenylyl cyclase signaling in vascular smooth muscle cells. Cell Biochem. Biophys. 2007, 47, 99–108. [Google Scholar] [CrossRef]
- Kalyanaraman, H.; Schall, N.; Pilz, R.B. Nitric oxide and cyclic GMP functions in bone. Nitric Oxide Biol. Chem. 2018, 76, 62–70. [Google Scholar] [CrossRef]
- Friebe, A.; Englert, N. NO-sensitive guanylyl cyclase in the lung. Br. J. Pharmacol. 2022, 179, 2328–2343. [Google Scholar] [CrossRef]
- Algara-Suárez, P.; Espinosa-Tanguma, R. 8Br-cGMP mediates relaxation of tracheal smooth muscle through PKA. Biochem. Biophys. Res. Commun. 2004, 314, 597–601. [Google Scholar] [CrossRef]
- Roh, S.; Choi, S.; Lim, I. Involvement of protein kinase A in nitric oxide stimulating effect on a BK(Ca) channel of human dermal fibroblasts. J. Investig. Dermatol. 2007, 127, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- Baumann, R.; Blass, C.; Götz, R.; Dragon, S. Ontogeny of catecholamine and adenosine receptor-mediated cAMP signaling of embryonic red blood cells: Role of cGMP-inhibited phosphodiesterase 3 and hemoglobin. Blood 1999, 94, 4314–4320. [Google Scholar] [CrossRef]
- Jaworek, J.; Leja-Szpak, A.; Bonior, J.; Nawrot, K.; Tomaszewska, R.; Stachura, J.; Sendur, R.; Pawlik, W.; Brzozowski, T.; Konturek, S.J. Protective effect of melatonin and its precursor L-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J. Pineal Res. 2003, 34, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Shi, K.; Shan, D.; Zhu, Y.; Wang, C.; Bai, Y.; Yan, T.; Zheng, X.; Kong, J. Apple tree flowering is mediated by low level of melatonin under the regulation of seasonal light signal. J. Pineal Res. 2019, 66, e12551. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, L.; Tai, M.; Ding, Q.; Yao, W.; Shen, M. Light from heat lamps affects sow behaviour and piglet salivary melatonin levels. Animal 2022, 16, 100534. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, Y.; Nie, J.; Xu, J.; Liu, D. Red light and the sleep quality and endurance performance of Chinese female basketball players. J. Athl. Train. 2012, 47, 673–678. [Google Scholar] [CrossRef] [Green Version]
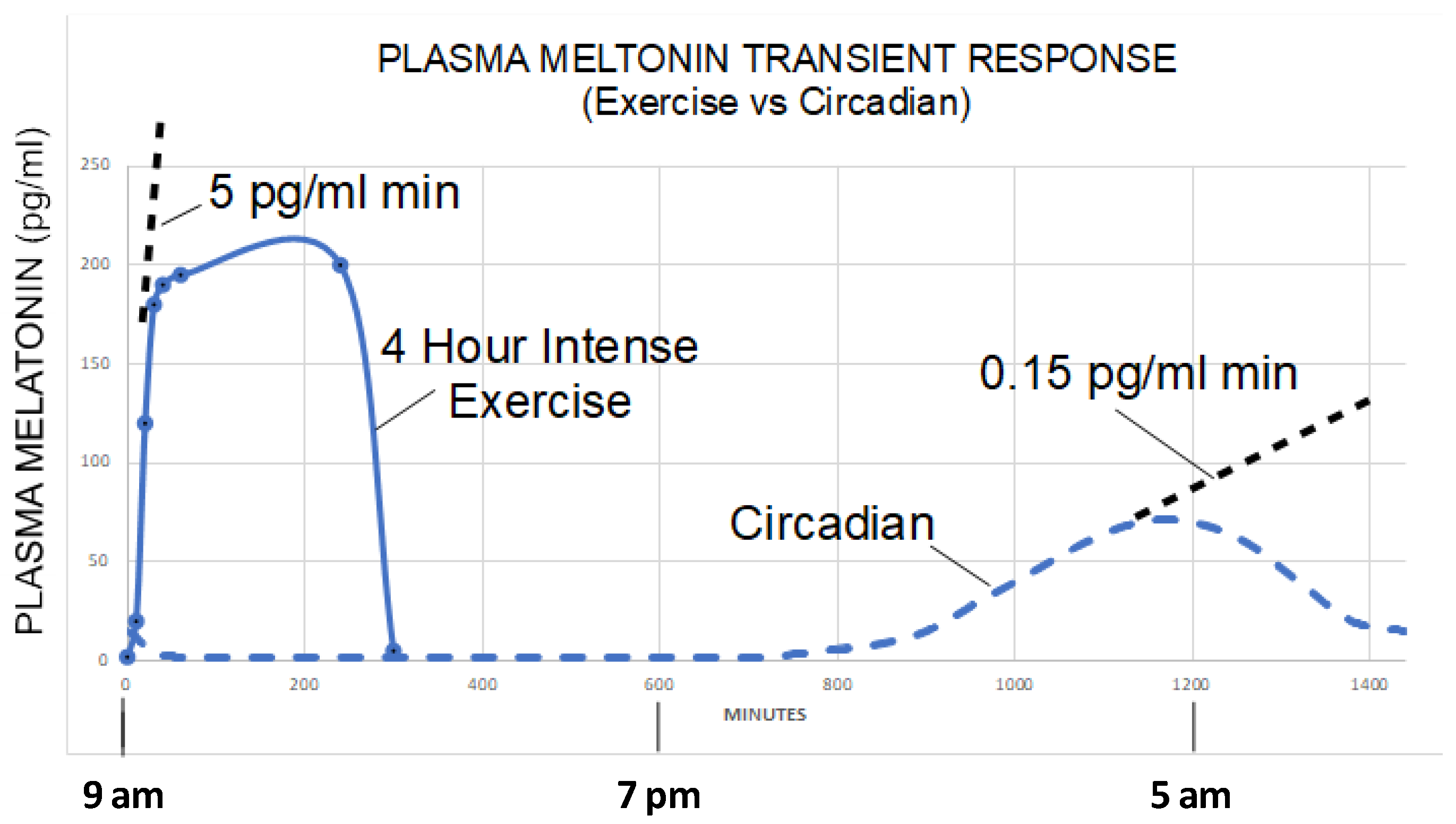
- Zimmerman, S.; Reiter, R.J. Transient responses of melatonin to stress. Melatonin Res. 2022, 5, 295–303. [Google Scholar] [CrossRef]
- Theron, J.J.; Oosthuizen, J.M.; Rautenbach, M.M. Effect of physical exercise on plasma melatonin levels in normal volunteers. Afr. Med. J. 1984, 66, 838–841. [Google Scholar]
- Zhu, M.; Anderson, K.; Gronowski, A. Investigating Eccrine Sweat as a Noninvasive Biomarker Resource. Ph.D. Thesis, Arizona state university, Lake Havasu, AZ, USA, 2018. [Google Scholar]
- Burcelin, R.; Luche, E.; Serino, M.; Amar, J. The gut microbiota ecology: A new opportunity for the treatment of metabolic diseases? Front. Biosci. 2009, 14, 5107–5117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. The gut microbiota: A treasure for human health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Fernández, M.; Porras, D.; García-Mediavilla, M.V.; Román-Sagüillo, S.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Aging, gut microbiota and metabolic diseases: Management through physical exercise and nutritional interventions. Nutrients 2020, 13, 16. [Google Scholar] [CrossRef]
- Wu, H.; Chen, X.; Zhang, S.; Li, J. Gut microbiota, the potential biological medicine for prevention, intervention and drug sensitization to fight diseases. Nutrients 2022, 14, 4220. [Google Scholar] [CrossRef] [PubMed]
- Saint-Georges-Chaumet, Y.; Attaf, D.; Pelletier, E.; Edeas, M. Targeting microbiota-mitochondria inter-talk: Microbiota control mitochondria metabolism. Cell. Mol. Biol. 2015, 61, 121–124. [Google Scholar] [CrossRef]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin attenuates microbiota dysbiosis of jejunum in short-term sleep deprived mice. J. Microbiol. 2020, 58, 588–597. [Google Scholar] [CrossRef]
- Tilden, A.R.; Becker, M.A.; Amma, L.L.; Arciniega, J.; McGaw, A.K. Melatonin production in an aerobic photosynthetic bacterium: An evolutionarily early association with darkness. J. Pineal Res. 1997, 22, 102–106. [Google Scholar] [CrossRef]
- Balzer, I.; Höcker, B.; Kapp, H.; Bartolomaeus, B. Occurrence and comparative physiology of melatoninin evolutionary diverse organisms. In The Redox State and Circadian Rhythms; Vanden Driessche, T., Guisset, J.-L., Petiau-de Vries, G.M., Eds.; Kluver: Dordrecht, The Netherlands, 2000; pp. 95–119. [Google Scholar]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and 5-methoxytryptamine in non-metazoans. Reprod. Nutr. Dev. 1999, 39, 399–408. [Google Scholar] [CrossRef]
- Morcillo-Parra, M.Á.; Valera, M.J.; Beltran, G.; Mas, A.; Torija, M.J. Glycolytic proteins interact with intracellular melatonin in saccharomyces cerevisiae. Front. Microbiol. 2019, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, E.; Carrasco-Galán, F.; Cerezo-López, A.B.; Valero, E.; Morcillo-Parra, M.Á.; Beltran, G.; Torija, M.J.; Troncoso, A.M.; García-Parrilla, M.C. Occurrence of melatonin and indolic compounds derived from l-tryptophan yeast metabolism in fermented wort and commercial beers. Food Chem. 2020, 331, 127192. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; He, C.; Han, L. Heterologous expression of ZjOMT from Zoysia japonica in Escherichia coli confers aluminum resistance through melatonin production. PLoS ONE 2018, 13, e0196952. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Hanes, M.A.; Farley, N.J. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999, 65, 2523–2529. [Google Scholar] [CrossRef]
- Reiter, R.; Rosales-Corral, S.; Manchester, L.; Liu, X.; Tan, D.-X. Melatonin in the biliary tract and liver: Health implications. Curr. Pharm. Des. 2014, 20, 4788–4801. [Google Scholar] [CrossRef]
- Luo, H.; Schneider, K.; Christensen, U.; Lei, Y.; Herrgard, M.; Palsson, B.; Palsson, B.; Palsson, B. Microbial synthesis of human-hormone melatonin at gram scales. ACS Synth. Biol. 2020, 9, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Wang, M.; Bu, D.; Ma, L.; Liu, F.; Xue, C.; Du, C.; Aboragah, A.; Loor, J.J. Ruminal microbes exhibit a robust circadian rhythm and are sensitive to melatonin. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Z.; Cao, J.; Gao, T.; Dong, Y.; Chen, Y. Role of melatonin in murine “restraint stress”-induced dysfunction of colonic microbiota. J. Microbiol. 2021, 59, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; de Campos Zuccari, D.A.P.; de Almeida Chuffa, L.G. Melatonin: A mitochondrial resident with a diverse skill set. Life Sci. 2022, 301, 120612. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Stokkan, K.A.; Reiter, R.J.; Nonaka, K.O.; Lerchl, A.; Yu, B.P.; Vaughan, M.K. Food restriction retards aging of the pineal gland. Brain Res. 1991, 545, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Lane, M.A.; Roth, G.S.; Ingram, D.K. Calorie restriction in rhesus monkeys. Exp. Gerontol. 2003, 38, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, C.H.; Hsieh, C.W.; Hsieh, C.H.; Chen, D.Y.; Wang, C.H.; Chen, J.H.; Lee, S.C. Detection of nighttime melatonin level in Chinese Original Quiet Sitting. J. Formos. Med. Assoc. 2010, 109, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Tse, A.C.Y.; Lee, P.H.; Zhang, J.; Chan, R.C.Y.; Ho, A.W.Y.; Lai, E.W.H. Effects of exercise on sleep, melatonin level, and behavioral functioning in children with autism. Autism 2022, 26, 1712–1722. [Google Scholar] [CrossRef]
- Kim, T.K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell. Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mézes, M.; Surai, P.; Sályi, G.; Speake, B.K.; Gaál, T.; Maldjian, A. Nutritional metabolic diseases of poultry and disorders of the biological antioxidant defence system. Acta Vet. Hung. 1997, 45, 349–360. [Google Scholar] [PubMed]
- Hardeland, R. Melatonin and microglia. Int. J. Mol. Sci. 2021, 22, 8296. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, D.-X.; Reiter, R.J.; Zimmerman, S.; Hardeland, R. Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light. Biology 2023, 12, 89. https://doi.org/10.3390/biology12010089
Tan D-X, Reiter RJ, Zimmerman S, Hardeland R. Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light. Biology. 2023; 12(1):89. https://doi.org/10.3390/biology12010089
Chicago/Turabian StyleTan, Dun-Xian, Russel J. Reiter, Scott Zimmerman, and Ruediger Hardeland. 2023. "Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light" Biology 12, no. 1: 89. https://doi.org/10.3390/biology12010089





