Climate Change Dependence in Ex Situ Conservation of Wild Medicinal Plants in Crete, Greece
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Medicinal Plants and Study Area
2.2. Data Collection
2.3. Ecological Niche Modeling (ENM)
2.4. Future Climate Scenarios
2.5. Data Processing and Visualization
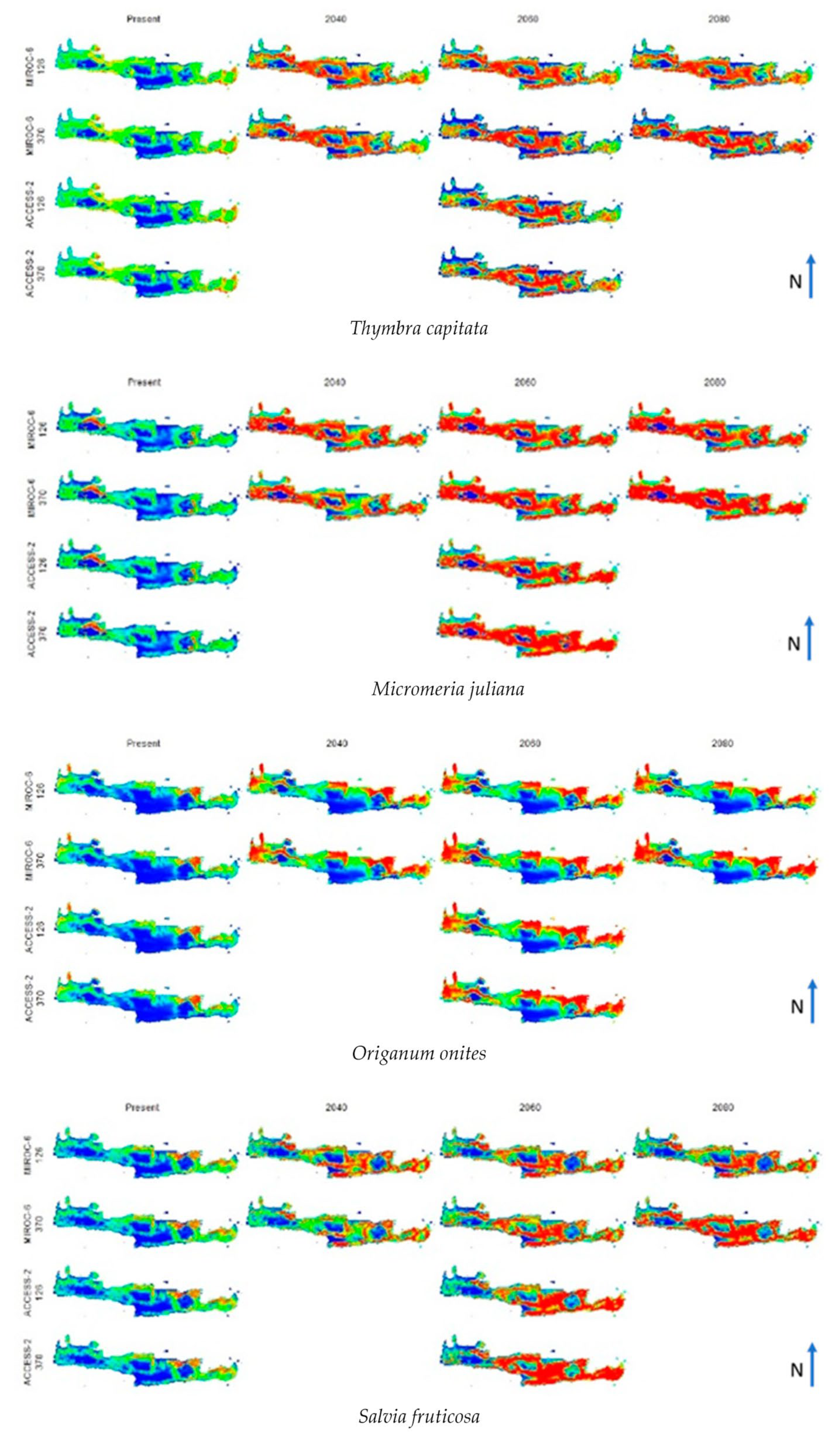
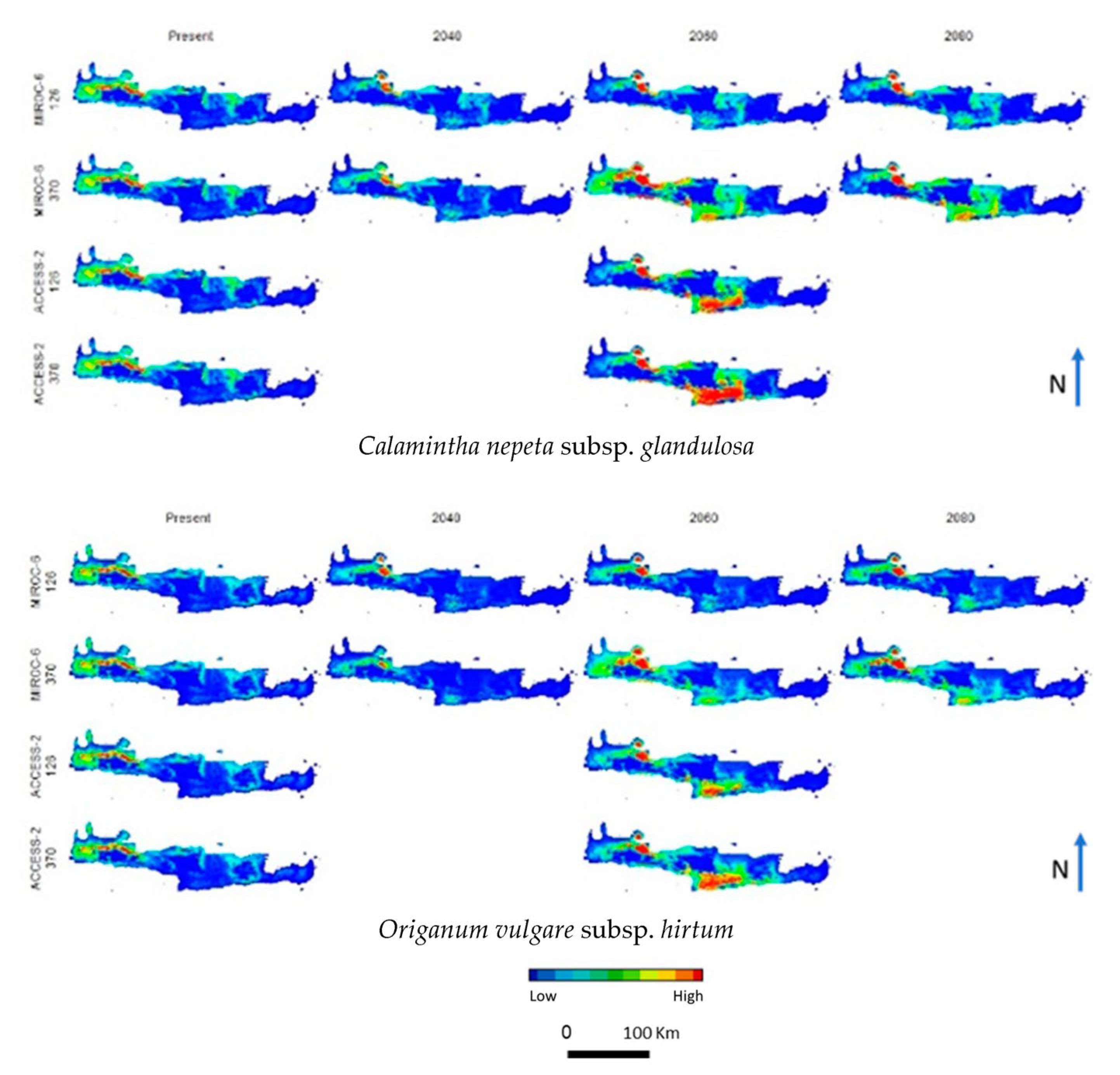
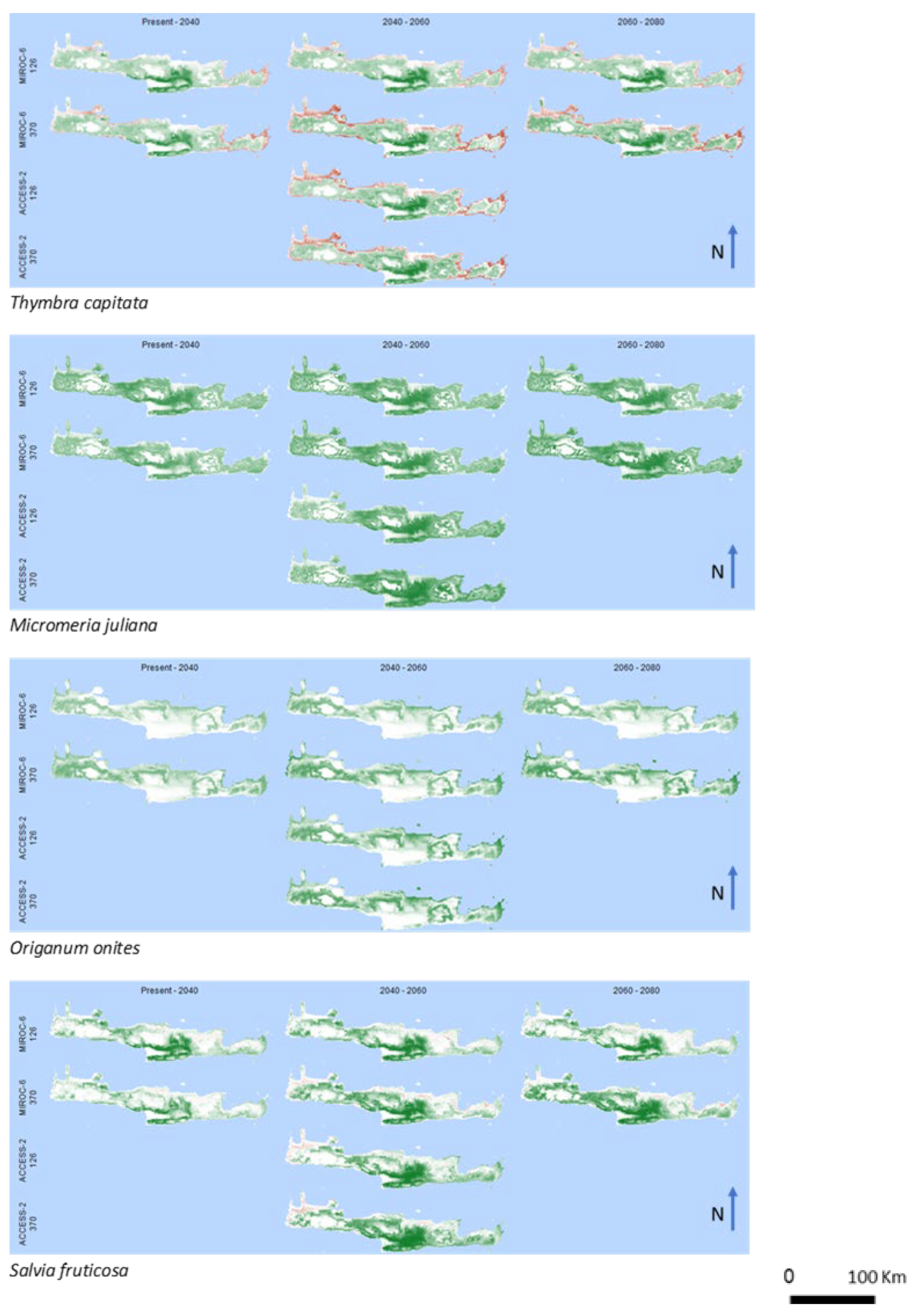
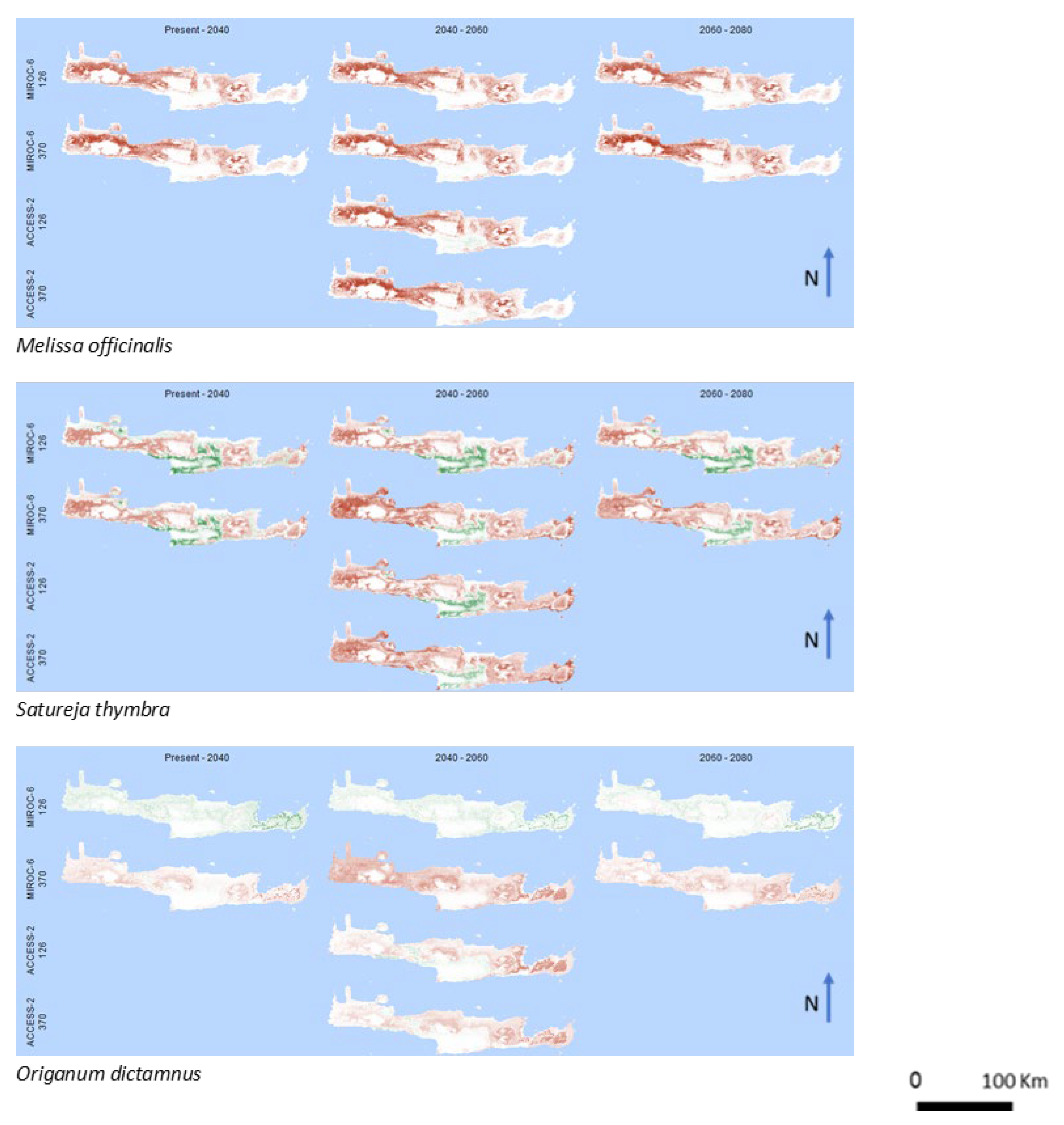
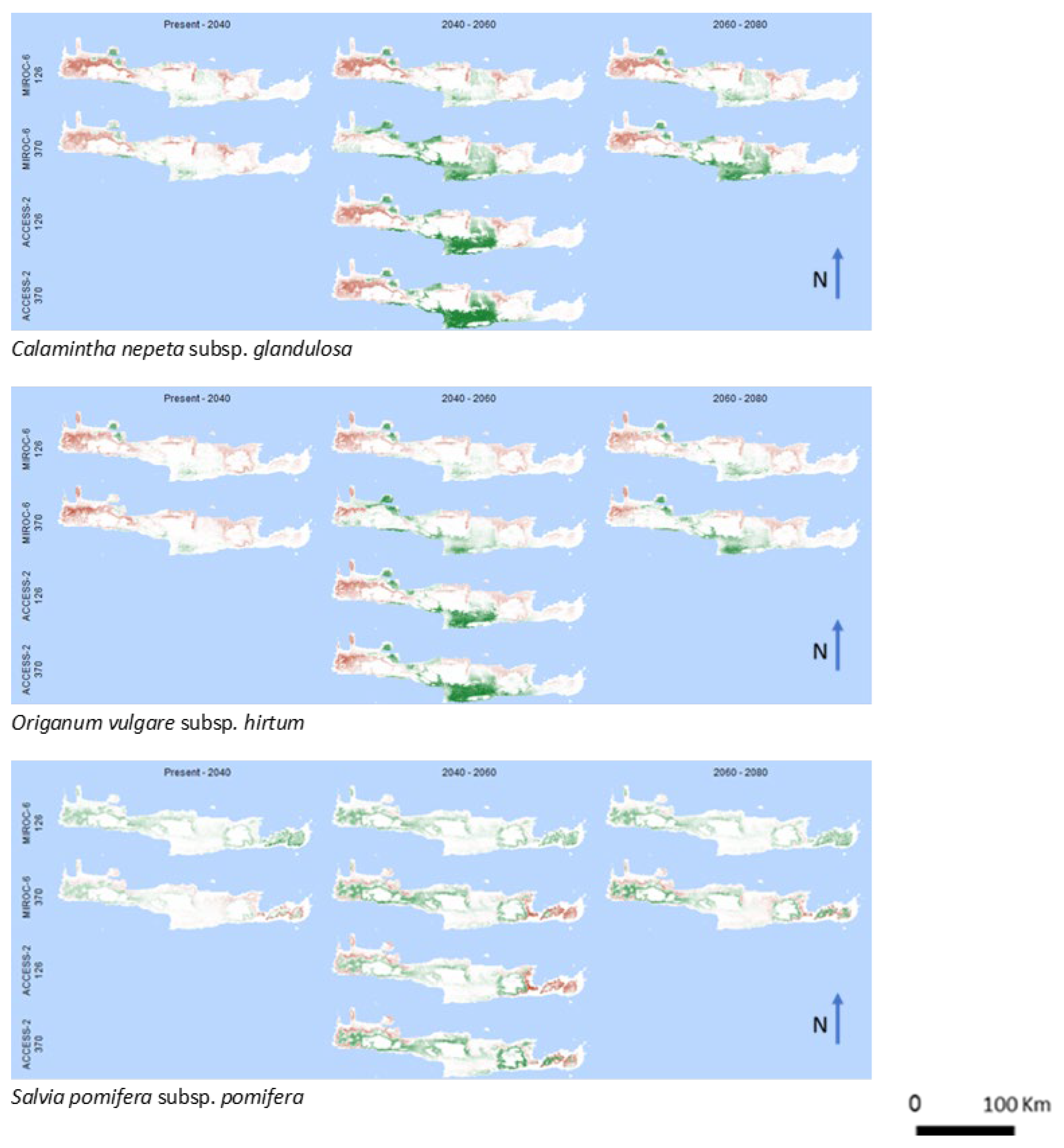
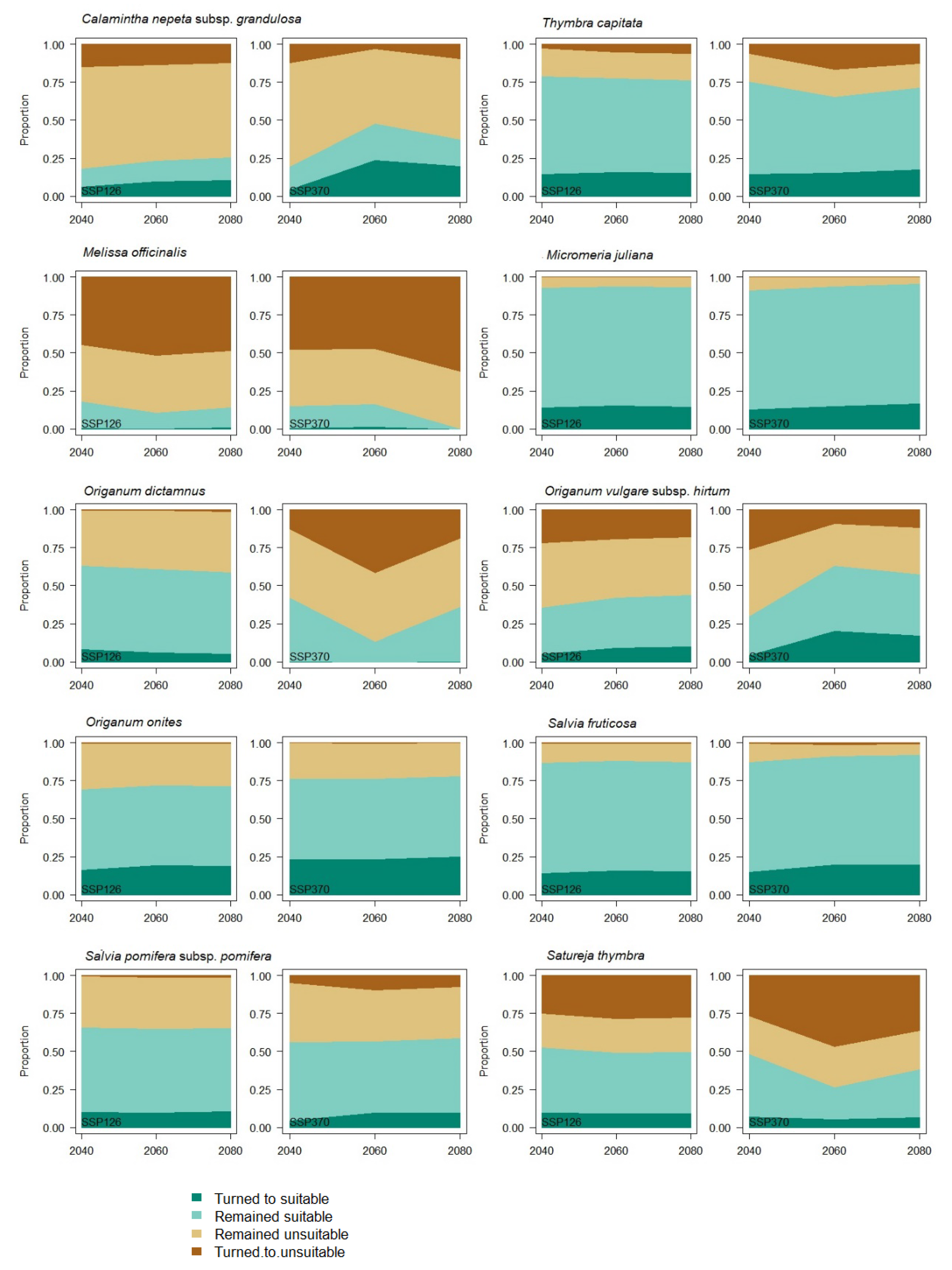
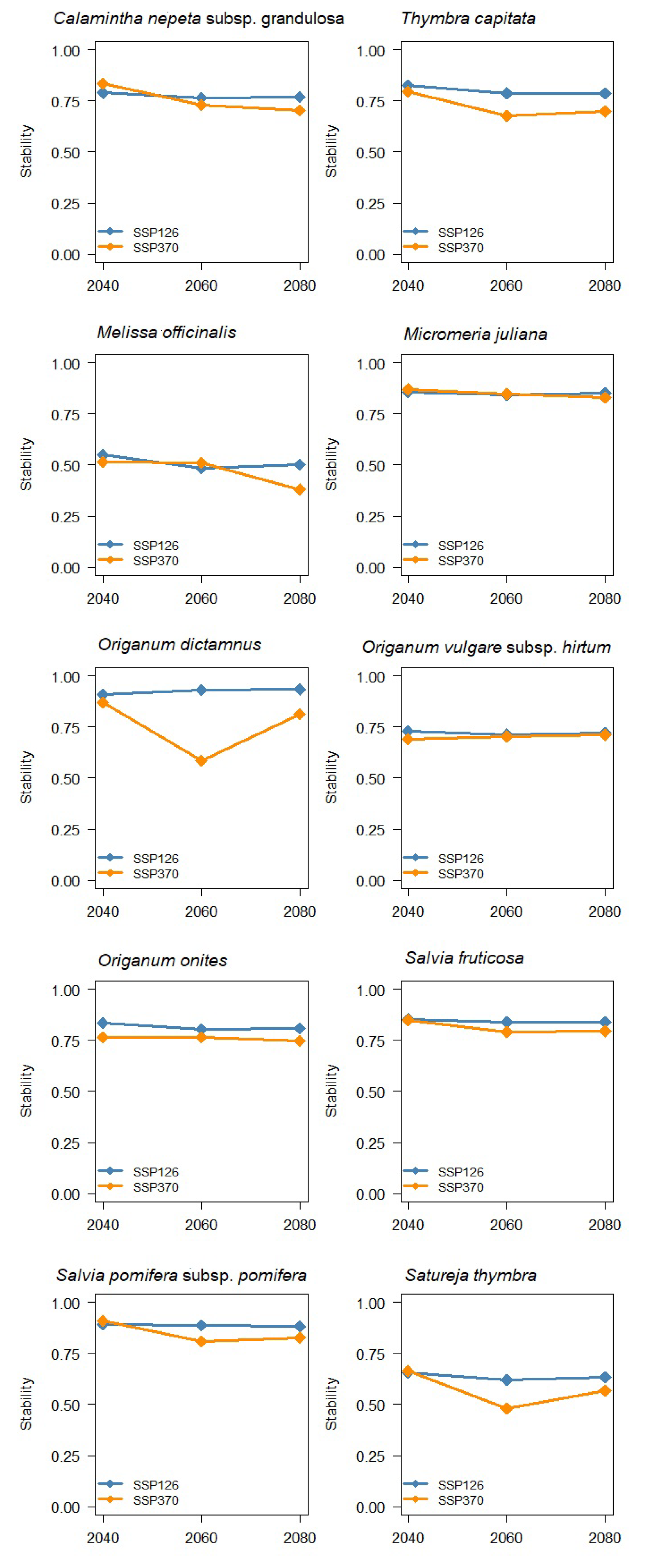
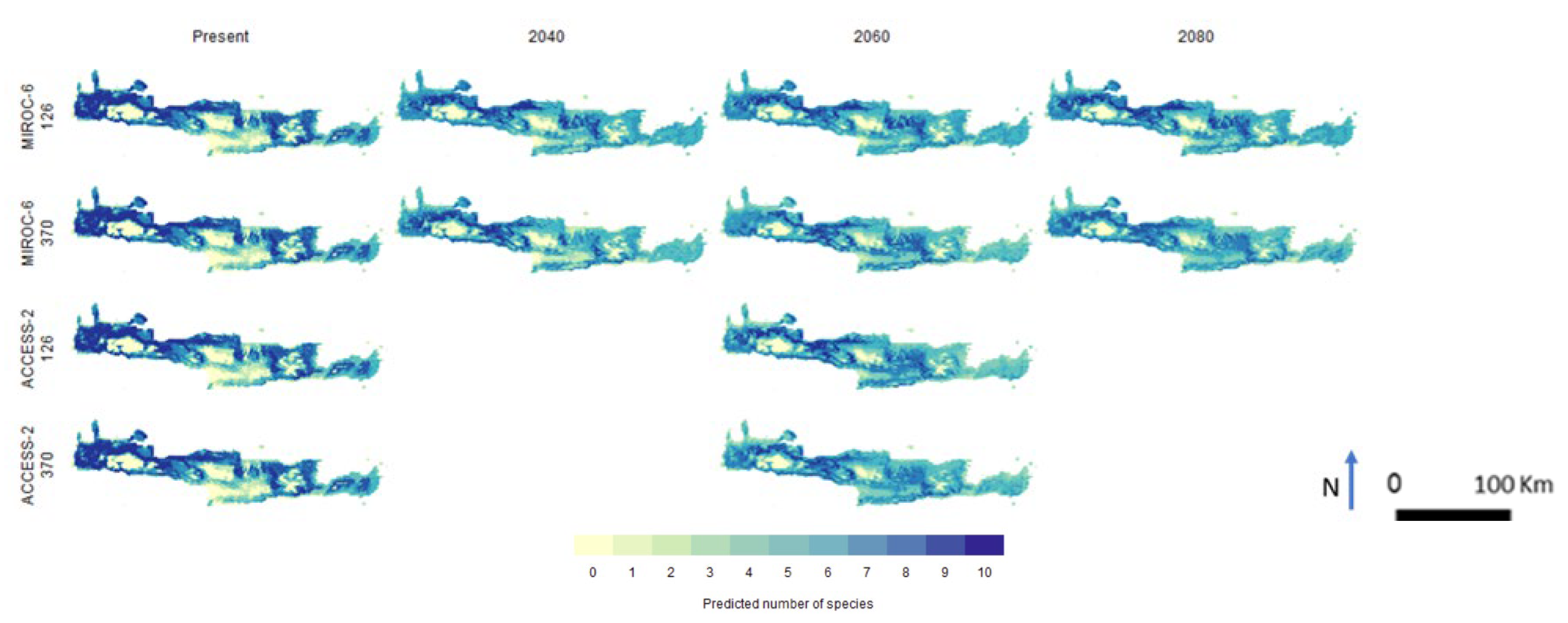
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Regional Office for South-East Asia. The Use of Herbal Medicines in Primary Health Care. WHO Regional Office for South-East Asia. 2009. Available online: https://iris.who.int/handle/10665/206476 (accessed on 30 August 2023).
- Jimoh, M.A.; Jimoh, M.O.; Saheed, S.A.; Bamigboye, S.O.; Laubscher, C.P.; Kambizi, L. Commercialization of Medicinal Plants: Opportunities for Trade and Concerns for Biodiversity Conservation. Sustain. Uses Prospect. Med. Plants 2023, 309–332. [Google Scholar]
- Barata, A.M.; Rocha, F.; Lopes, V.; Carvalho, A.M. Conservation and sustainable uses of medicinal and aromatic plants genetic resources on the worldwide for human welfare. Ind. Crop. Prod. 2016, 88, 8–11. [Google Scholar] [CrossRef]
- Lange, D. The role of east and southeast Europe in the medicinal and aromatic plants’ trade. Med. Plant Conserv. 2002, 8, 14–18. [Google Scholar]
- Qazi, M.; Molvi, K. Herbal medicine: A comprehensive review. Int. J. Pharm. Res. 2016, 8, 1–5. [Google Scholar]
- Vasisht, K.; Sharma, N.; Karan, M. Current perspective in the international trade of medicinal plants material: An update. Curr. Pharm. Des. 2016, 22, 4288–4336. [Google Scholar] [CrossRef]
- Saeed, S.T.; Samad, A. Emerging threats of begomoviruses to the cultivation of medicinal and aromatic crops and their management strategies. Virus Dis. 2017, 28, 1–17. [Google Scholar] [CrossRef]
- Ticktin, T. The ecological implications of harvesting non-timber forest products. J. Appl. Ecol. 2004, 41, 11–21. [Google Scholar] [CrossRef]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Thompson, N.M.; Bir, C.; Widmar, D.A.; Mintert, J.R. Farmer perceptions of precision agriculture technology benefits. J. Agric. Appl. Econ. 2019, 51, 142–163. [Google Scholar] [CrossRef]
- Shafi, U.; Mumtaz, R.; García-Nieto, J.; Hassan, S.A.; Zaidi, S.A.R.; Iqbal, N. Precision agriculture techniques and practices: From considerations to applications. Sensors 2019, 19, 3796. [Google Scholar] [CrossRef]
- Karim, F.; Karim, F. Monitoring system using web of things in precision agriculture. Procedia Comput. Sci. 2017, 110, 402–409. [Google Scholar] [CrossRef]
- Bariotakis, M.; Georgescu, L.; Laina, D.; Oikonomou, I.; Ntagounakis, G.; Koufaki, M.-I.; Souma, M.; Choreftakis, M.; Zormpa, O.G.; Smykal, P. From wild harvest towards precision agriculture: Use of Ecological Niche Modelling to direct potential cultivation of wild medicinal plants in Crete. Sci. Total Environ. 2019, 694, 133681. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.C.; Valin, H.; Sands, R.D.; Havlík, P.; Ahammad, H.; Deryng, D.; Elliott, J.; Fujimori, S.; Hasegawa, T.; Heyhoe, E. Climate change effects on agriculture: Economic responses to biophysical shocks. Proc. Natl. Acad. Sci. USA 2014, 111, 3274–3279. [Google Scholar] [CrossRef]
- Dono, G.; Cortignani, R.; Dell’Unto, D.; Deligios, P.; Doro, L.; Lacetera, N.; Mula, L.; Pasqui, M.; Quaresima, S.; Vitali, A. Winners and losers from climate change in agriculture: Insights from a case study in the Mediterranean basin. Agric. Syst. 2016, 147, 65–75. [Google Scholar] [CrossRef]
- Duijker, G.; Bertsias, A.; Symvoulakis, E.; Moschandreas, J.; Malliaraki, N.; Derdas, S.; Tsikalas, G.; Katerinopoulos, H.; Pirintsos, S.; Sourvinos, G. Reporting effectiveness of an extract of three traditional Cretan herbs on upper respiratory tract infection: Results from a double-blind randomized controlled trial. J. Ethnopharmacol. 2015, 163, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Giweli, A.; Džamić, A.M.; Soković, M.; Ristić, M.S.; Marin, P.D. Antimicrobial and antioxidant activities of essential oils of Satureja thymbra growing wild in Libya. Molecules 2012, 17, 4836–4850. [Google Scholar] [CrossRef] [PubMed]
- Sarac, N.; Ugur, A. Antimicrobial activities of the essential oils of Origanum onites L. Origanum vulgare L. subspecies hirtum (Link) Ietswaart, Satureja thymbra L. and Thymus cilicicus Boiss. & Bal. growing wild in Turkey. J. Med. Food 2008, 11, 568–573. [Google Scholar]
- Pirintsos, S.; Bariotakis, M.; Kampa, M.; Sourvinos, G.; Lionis, C.; Castanas, E. The therapeutic potential of the essential oil of Thymbra capitata (L.) Cav. Origanum dictamnus L. and Salvia fruticosa Mill. and a case of plant-based pharmaceutical development. Front. Pharmacol. 2020, 11, 522213. [Google Scholar] [CrossRef]
- Pirintsos, S.; Panagiotopoulos, A.; Bariotakis, M.; Daskalakis, V.; Lionis, C.; Sourvinos, G.; Karakasiliotis, I.; Kampa, M.; Castanas, E. From traditional ethnopharmacology to modern natural drug discovery: A methodology discussion and specific examples. Molecules 2022, 27, 4060. [Google Scholar] [CrossRef]
- Pitarokili, D.; Tzakou, O.; Couladis, M.; Verykokidou, E. Composition and antifungal activity of the essential oil of Salvia pomifera subsp. calycina growing wild in Greece. J. Essent. Oil Res. 1999, 11, 655–659. [Google Scholar] [CrossRef]
- Stojanović, G.; Palić, I.; Ursić-Janković, J. Composition and antimicrobial activity of the essential oil of Micromeria cristata and Micromeria juliana. Flavour Fragr. J. 2006, 21, 77–79. [Google Scholar] [CrossRef]
- Tseliou, M.; Pirintsos, S.A.; Lionis, C.; Castanas, E.; Sourvinos, G. Antiviral effect of an essential oil combination derived from three aromatic plants (Coridothymus capitatus (L.) Rchb. f. Origanum dictamnus L. and Salvia fruticosa Mill.) against viruses causing infections of the upper respiratory tract. J. Herb. Med. 2019, 17, 100288. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanischer Garten und Botanisches Museum Berlin-Dahlem: Berlin, Germany; Hellenic Botanical Society: Athens, Greece, 2013. [Google Scholar]
- Bosque, M.; Adamogianni, M.-I.; Bariotakis, M.; Fazan, L.; Stoffel, M.; Garfi, G.; Gratzfeld, J.; Kozlowski, G.; Pirintsos, S. Fine-scale spatial patterns of the Tertiary relict Zelkova abelicea (Ulmaceae) indicate possible processes contributing to its persistence to climate changes. Reg. Environ. Chang. 2014, 14, 835–849. [Google Scholar] [CrossRef]
- Vrochidou, A.-E.; Tsanis, I. Assessing precipitation distribution impacts on droughts on the island of Crete. Nat. Hazards Earth Syst. Sci. 2012, 12, 1159–1171. [Google Scholar] [CrossRef]
- Kourgialas, N.N.; Anyfanti, I.; Karatzas, G.P.; Dokou, Z. An integrated method for assessing drought prone areas-Water efficiency practices for a climate resilient Mediterranean agriculture. Sci. Total Environ. 2018, 625, 1290–1300. [Google Scholar] [CrossRef]
- Tsakiris, G. Drought risk assessment and management. Water Resour. Manag. 2017, 31, 3083–3095. [Google Scholar] [CrossRef]
- Turland, N.J.; Chilton, L.; Press, J.R. Flora of the Cretan Area: Annotated Checklist and Atlas; HMSO: London, UK, 1993. [Google Scholar]
- Fick, S.E.; Hijmans, R. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Bariotakis, M.; Koutroumpa, K.; Karousou, R.; Pirintsos, S.A. Environmental (in) dependence of a hybrid zone: Insights from molecular markers and ecological niche modeling in a hybrid zone of Origanum (Lamiaceae) on the island of Crete. Ecol. Evol. 2016, 6, 8727–8739. [Google Scholar] [CrossRef]
- Bariotakis, M.; Pirintsos, S.A. Mapping absences within the BAM concept: Towards a new generation of ecological and environmental indicators. Ecol. Indic. 2018, 90, 564–568. [Google Scholar] [CrossRef]
- Smýkal, P.; Chaloupská, M.; Bariotakis, M.; Marečková, L.; Sinjushin, A.; Gabrielyan, I.; Akopian, J.; Toker, C.; Kenicer, G.; Kitner, M. Spatial patterns and intraspecific diversity of the glacial relict legume species Vavilovia formosa (Stev.) Fed. in Eurasia. Plant Syst. Evol. 2017, 303, 267–282. [Google Scholar] [CrossRef]
- Smýkal, P.; Hradilová, I.; Trněný, O.; Brus, J.; Rathore, A.; Bariotakis, M.; Das, R.R.; Bhattacharyya, D.; Richards, C.; Coyne, C. Genomic diversity and macroecology of the crop wild relatives of domesticated pea. Sci. Rep. 2017, 7, 17384. [Google Scholar] [CrossRef] [PubMed]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Shabani, F.; Kumar, L.; Taylor, S. Suitable regions for date palm cultivation in Iran are predicted to increase substantially under future climate change scenarios. J. Agric. Sci. 2014, 152, 543–557. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions, version 3.4.1. 2017. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/(accessed on 30 August 2023).
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Hajima, T.; Watanabe, M.; Yamamoto, A.; Tatebe, H.; Noguchi, M.A.; Abe, M.; Ohgaito, R.; Ito, A.; Yamazaki, D.; Okajima, H. Development of the MIROC-ES2L Earth system model and the evaluation of biogeochemical processes and feedbacks. Geosci. Model Dev. 2020, 13, 2197–2244. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Bi, D.; Dix, M.; Marsland, S.; O’farrell, S.; Sullivan, A.; Bodman, R.; Law, R.; Harman, I.; Srbinovsky, J.; Rashid, H.A. Configuration and spin-up of ACCESS-CM2, the new generation Australian community climate and earth system simulator coupled model. J. South. Hemisphere Earth Syst. Sci. 2020, 70, 225–251. [Google Scholar] [CrossRef]
- Rashid, H.; Dix, M.; Sullivan, A.; Bodman, R.; Zhu, H. ACCESS Climate Model Simulations for the Coupled Model Intercomparison Project (CMIP6); Earth Systems and Climate Change Hub Report No. 14; Earth Systems and Climate Change Hub: Canberra, Australia, 2020. [Google Scholar]
- Rashid, H. Delving Deeper into Australia’s National Climate Model: The Australian Community Climate and Earth System Simulator (ACCESS); Earth Systems and Climate Change Hub Report No. 12; Earth Systems and Climate Change Hub: Canberra, Australia, 2020. [Google Scholar]
- Team, R.C.R. A Language and Environment for Statistical Computing, version 4.2.0; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Bivand, R.; Keitt, T.; Rowlingson, B.; Pebesma, E.; Sumner, M.; Hijmans, R.; Rouault, E.; Bivand, M.R. Package ‘rgdal’. Bind. Geospat. Data Abstr. Libr. 2015, 172. Available online: https://cran.r-project.org/web/packages/rgdal/index.html (accessed on 30 August 2023).
- Pebesma, E.; Bivand, R.S. S classes and methods for spatial data: The sp package. R News 2005, 5, 9–13. [Google Scholar]
- Hijmans, R.; van Etten, J. R package, version 3.4–5. Raster: Geographic Data Analysis and Modeling. 2020. Available online: https://cran.r-project.org/package=raster(accessed on 30 August 2023).
- Pearce, J.; Ferrier, S. An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecol. Model. 2000, 128, 127–147. [Google Scholar] [CrossRef]
- de Moraes Weber, M.; Stevens, R.D.; Lorini, M.L.; Grelle, C.E.V. Have old species reached most environmentally suitable areas? A case study with S outh A merican phyllostomid bats. Glob. Ecol. Biogeogr. 2014, 23, 1177–1185. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for climate change: Forestry and assisted migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Vincent, H.; Amri, A.; Castañeda-Álvarez, N.P.; Dempewolf, H.; Dulloo, E.; Guarino, L.; Hole, D.; Mba, C.; Toledo, A.; Maxted, N. Modeling of crop wild relative species identifies areas globally for in situ conservation. Commun. Biol. 2019, 2, 136. [Google Scholar] [CrossRef]
- Shahzad, Z.; Rouached, H. Protecting plant nutrition from the effects of climate change. Curr. Biol. 2022, 32, R725–R727. [Google Scholar] [CrossRef]
- Cahyaningsih, R.; Phillips, J.; Brehm, J.M.; Gaisberger, H.; Maxted, N. Climate change impact on medicinal plants in Indonesia. Glob. Ecol. Conserv. 2021, 30, e01752. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Thapa-Magar, K.B.; Subedi, S.C.; Kutal, D.H.; Baral, B.; Joshi, N.R.; Adhikari, B.; Upadhyaya, K.S.; Thapa-Magar, S.; Ansari, A.S. Distribution of important medicinal plant species in Nepal under past, present, and future climatic conditions. Ecol. Indic. 2023, 146, 109879. [Google Scholar] [CrossRef]
- Tshabalala, T.; Mutanga, O.; Abdel-Rahman, E.M. Predicting the Geographical Distribution Shift of Medicinal Plants in South Africa Due to Climate Change. Conservation 2022, 2, 694–708. [Google Scholar] [CrossRef]
- Kaky, E.; Gilbert, F. Predicting the distributions of Egypt’s medicinal plants and their potential shifts under future climate change. PLoS ONE 2017, 12, e0187714. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Qin, X.; Ranjitkar, S.; Lougheed, S.C.; Wang, M.; Zhou, W.; Ouyang, D.; Zhou, Y.; Xu, J.; Zhang, W. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Sci. Rep. 2018, 8, 5879. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Abdukeyum, N.; Ling, Y. Potential Geographical Distribution of Medicinal Plant Ephedra sinica Stapf under Climate Change. Forests 2022, 13, 2149. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, H.; Lu, C.; Gao, B.; Gu, W. Predictions of potential geographical distribution and quality of Schisandra sphenanthera under climate change. PeerJ 2016, 4, e2554. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cantino, P.D.; Olmstead, R.G.; Bramley, G.L.; Xiang, C.-L.; Ma, Z.-H.; Tan, Y.-H.; Zhang, D.-X. A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. Sci. Rep. 2016, 6, 34343. [Google Scholar] [CrossRef]
- Saccone, P.; Hoikka, K.; Virtanen, R. What if plant functional types conceal species-specific responses to environment? Study on arctic shrub communities. Ecology 2017, 98, 1600–1612. [Google Scholar] [CrossRef]
- Thomas, H.J.; Myers-Smith, I.H.; Bjorkman, A.D.; Elmendorf, S.C.; Blok, D.; Cornelissen, J.H.; Forbes, B.C.; Hollister, R.D.; Normand, S.; Prevéy, J.S. Traditional plant functional groups explain variation in economic but not size-related traits across the tundra biome. Glob. Ecol. Biogeogr. 2019, 28, 78–95. [Google Scholar] [CrossRef]
- Petersm, R.; Darling, J. The greenhouse effect and nature reserves: Global warming would diminish biological diversity by causing extinctions among reserve species. BioScience-Am. Inst. Biol. Sci. 1985. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts–A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Gairola, S.; Shariff, N.M.; Bhatt, A.; Kala, C.P. Influence of climate change on production of secondary chemicals in high altitude medicinal plants: Issues needs immediate attention. J. Med. Plants Res. 2010, 4, 1825–1829. [Google Scholar]
- Georgescu, L.; Stefanakis, M.K.; Kokkini, S.; Katerinopoulos, H.E.; Pirintsos, S.A. Chemical and genetic characterization of Phlomis species and wild hybrids in Crete. Phytochemistry 2016, 122, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant responses to climate change: Metabolic changes under combined abiotic stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Sun, Y.; Alseekh, S.; Fernie, A.R. Plant secondary metabolic responses to global climate change: A meta-analysis in medicinal and aromatic plants. Glob. Chang. Biol. 2023, 29, 477–504. [Google Scholar] [CrossRef]
- Chen, S.; Gong, B. Response and adaptation of agriculture to climate change: Evidence from China. J. Dev. Econ. 2021, 148, 102557. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bariotakis, M.; Georgescu, L.; Laina, D.; Koufaki, M.; Souma, M.; Douklias, S.; Giannakakis, K.A.; Chouli, K.N.; Paoli, L.; Loppi, S.; et al. Climate Change Dependence in Ex Situ Conservation of Wild Medicinal Plants in Crete, Greece. Biology 2023, 12, 1327. https://doi.org/10.3390/biology12101327
Bariotakis M, Georgescu L, Laina D, Koufaki M, Souma M, Douklias S, Giannakakis KA, Chouli KN, Paoli L, Loppi S, et al. Climate Change Dependence in Ex Situ Conservation of Wild Medicinal Plants in Crete, Greece. Biology. 2023; 12(10):1327. https://doi.org/10.3390/biology12101327
Chicago/Turabian StyleBariotakis, Michael, Luciana Georgescu, Danae Laina, Margianna Koufaki, Maria Souma, Sotirios Douklias, Konstantinos A. Giannakakis, Kyriaki N. Chouli, Luca Paoli, Stefano Loppi, and et al. 2023. "Climate Change Dependence in Ex Situ Conservation of Wild Medicinal Plants in Crete, Greece" Biology 12, no. 10: 1327. https://doi.org/10.3390/biology12101327





