Capture Response and Long-Term Fate of White Sharks (Carcharodon carcharias) after Release from SMART Drumlines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
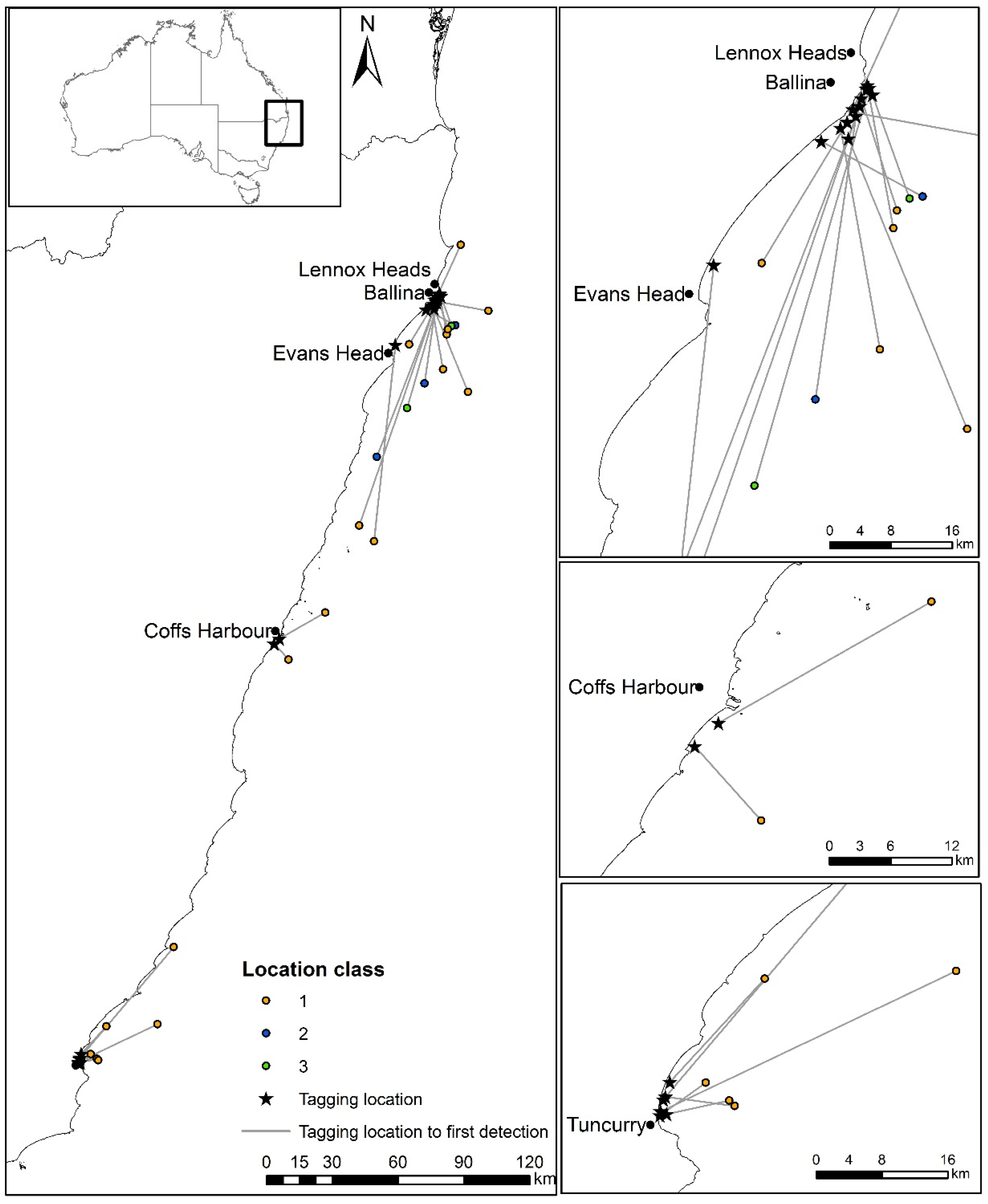
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- West, J.G. Changing patterns of shark attacks in Australian waters. Mar. Freshw. Res. 2011, 62, 744–754. [Google Scholar] [CrossRef]
- Curtis, T.; Bruce, B.; Cliff, G.; Dudley, S.; Klimley, A.; Kock, A.; Lea, R.; Lowe, C.; McCosker, J.; Skomal, G.; et al. Responding to the risk of white shark attack: Updated statistics, prevention, control methods, and recommendations. In Global Perspectives on the Biology and Life History of the Great White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 477–510. [Google Scholar]
- Ryan, L.A.; Lynch, S.K.; Harcourt, R.; Slip, D.J.; Peddemors, V.; Everett, J.D.; Harrison, L.M.; Hart, N.S. Environmental predictive models for shark attacks in Australian waters. Mar. Ecol. Prog. Ser. 2019, 631, 165–179. [Google Scholar] [CrossRef]
- Crossley, R.; Collins, C.M.; Sutton, S.G.; Huveneers, C. Public perception and understanding of shark attack mitigation measures in Australia. Hum. Dimens. Wildl. 2014, 19, 154–165. [Google Scholar] [CrossRef]
- Muter, B.A.; Gore, M.L.; Gledhill, K.S.; Lamont, C.; Huveneers, C. Australian and US news media portrayal of sharks and their conservation. Conser. Biol. 2013, 27, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; Fitzpatrick, R.; Bradley, M.; Miller, I.; Sheaves, M.; Chin, A.; Smith, B.; Diedrich, A.; Yick, J.L.; Lubitz, N.; et al. Scientific Response to a Cluster of Shark Bites. People Nat. 2022, 4, 963–982. [Google Scholar]
- Lagabrielle, E.; Allibert, A.; Kiszka, J.J.; Louiseau, N.; Kilfoil, J.P.; Lemahieu, A. Environmental and anthropogenic factors affecting the increasing occurrence of shark-human interactions around a fast-developing Indian Ocean island. Sci. Rep. 2018, 8, 3676. [Google Scholar] [CrossRef]
- Hazin, F.H.V.; Burgess, G.; Carvalho, F.C. A shark attack outbreak off Recife, Pernambuco, Brazil: 1992–2006. Bull. Mar. Sci. 2008, 82, 199–212. [Google Scholar]
- Dudley, S.F.J. A comparison of the shark control programs of New South Wales and Queensland (Australia) and KwaZulu-Natal (South Africa). Ocean. Coast. Manag. 1997, 34, 1–27. [Google Scholar] [CrossRef]
- Reid, D.D.; Robbins, W.D.; Peddemors, V.M. Decadal trends in shark catches and effort from the New South Wales, Australia, Shark Meshing Program 1950–2010. Mar. Freshw. Res. 2011, 62, 676–693. [Google Scholar] [CrossRef]
- Cliff, G.; Dudley, S.F.J. Reducing the environmental impact of shark-control programs: A case study from KwaZulu-Natal, South Africa. Mar. Freshw. Res. 2011, 62, 700–709. [Google Scholar] [CrossRef]
- Cliff, G. Shark attacks on the South African coast between 1960 and 1990. S. Afr. J. Sci. 1991, 87, 513–518. [Google Scholar]
- Dudley, S.F.J.; Cliff, G. Sharks caught in the protective gill nets off Natal, South Africa. 7. The blacktip shark Carcharhinus limbatus (Valenciennes). S. Afr. J. Mar. Sci. 1993, 13, 237–254. [Google Scholar] [CrossRef]
- Krogh, M.; Reid, D. Bycatch in the protective shark meshing program off south-eastern New South Wales, Australia. Biol. Conser. 1996, 77, 219–226. [Google Scholar] [CrossRef]
- Sumpton, W.D.; Taylor, S.M.; Gribble, N.A.; McPherson, G.; Ham, T. Gear selectivity of large-mesh nets and drumlines used to catch sharks in the Queensland Shark Control Program. S. Afr. J. Mar. Sci. 2011, 33, 37–43. [Google Scholar] [CrossRef]
- Robbins, W.D.; Peddemors, V.M.; Kennelly, S.J.; Ives, M.C. Experimental evaluation of shark detection rates by aerial observers. PLoS ONE 2014, 9, e83456. [Google Scholar] [CrossRef] [PubMed]
- Colefax, A.P.; Butcher, P.A.; Kelaher, B.P. The potential for unmanned aerial vehicles (UAVs) to conduct marine fauna surveys in place of manned aircraft. ICES J. Mar. Sci. 2018, 75, 1–8. [Google Scholar] [CrossRef]
- Butcher, P.A.; Piddocke, T.P.; Colefax, A.P.; Hoade, B.; Peddemors, V.M.; Borg, L.; Cullis, B.R. Beach safety: Can drones provide a platform for sighting sharks? Wildli. Res. 2019, 46, 701–712. [Google Scholar] [CrossRef]
- Smit, C.F.; Peddemors, V. Estimating the probability of a shark attack when using an electrical repellent. S. Afr. Stat. J. 2003, 37, 59–78. [Google Scholar]
- Huveneers, C.; Rogers, P.J.; Semmens, J.; Beckmann, C.; Kock, A.A.; Page, B.; Goldsworthy, S.D. Effects of an electric field on white sharks: In situ testing of an electric deterrent. PLoS ONE 2013, 8, e62730. [Google Scholar] [CrossRef]
- Huveneers, C.; Whitmarsh, S.; Thiele, M.; Meyer, L.; Fox, A.; Bradshaw, C.J.A. Effectiveness of five personal shark-bite deterrents for surfers. PeerJ 2018, 6, e5554. [Google Scholar] [CrossRef]
- Hart, N.S.; Collin, S.P. Shark senses and shark repellents. Int. Zool. 2015, 10, 38–64. [Google Scholar] [CrossRef] [PubMed]
- Blount, C.; Pygas, D.; Lincoln Smith, M.P.; McPhee, D.P.; Bignell, C.; Ramsey, O. Effectiveness Against White Sharks of the Rpela Personal Shark Deterrent Device Designed for Surfers. J. Mar. Sci. Technol. 2021, 29, 13. [Google Scholar] [CrossRef]
- Parsons, M.; Parnum, I.; Allen, K.; McCauley, R.; Erbe, C. Detection of sharks with the Gemini imaging sonar. Acoust. Aust. 2014, 42, 185–189. [Google Scholar]
- O’Connell, C.P.; Andreotti, S.; Rutzen, M.; Meӱer, M.; Matthee, C.A. Testing the exclusion capabilities and durability of the Sharksafe Barrier to determine its viability as an eco-friendly alternative to current shark culling methodologies. Aqu. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 252–258. [Google Scholar] [CrossRef]
- Stroud, E.M.; O’Connell, C.P.; Rice, P.H.; Snow, N.; Barnes, B.B.; Hanson, J.E. Existence of a shark necromone derived from putrefied shark tissue. Oce. Coa. Manag. 2014, 97, 50–57. [Google Scholar]
- Broadhurst, M.K.; Tolhurst, D.J. Null effects of decomposing shark issue on baited-hook catches of elasmobranchs. Reg. Stud. Mar. 2021, 46, 10898. [Google Scholar]
- Davison, A.; Kock, A. Fish Hoek Exclusion Net Evaluation Report; Shark Spotter Report; Shark Spotters: Cape Town, South Africa, 2014; Available online: https://sharkspotters.org.za/wp-content/uploads/2016/10/FINAL-Exclusion-net-report-24-06-14.pdf (accessed on 1 April 2023).
- O’Connell, C.; Crews, J.; King, A.; Gressle, J. Evaluating the shark deterrent effects of the novel exclusion barrier in comparison to the rigorously tested sharksafe barrier technology. J. Mar. Sci. Eng. 2022, 10, 634. [Google Scholar] [CrossRef]
- Engelbrecht, T.; Kock, A.; Waries, S.; O’Riain, M.J. Shark Spotters: Successfully reducing spatial overlap between white sharks (Charcharodon carcharias) and recreational water users in False Bay, South Africa. PLoS ONE 2017, 12, e0185335. [Google Scholar] [CrossRef]
- Chapuis, L.; Collin, S.P.; Yopak, K.E.; McCauley, R.D.; Kempster, R.M.; Ryan, L.A.; Schmidt, C.; Kerr, C.C.; Gennari, E.; Egeberg, C.A.; et al. The effect of underwater sounds on shark behaviour. Sci. Rep. 2019, 9, 6924. [Google Scholar] [CrossRef]
- Ryan, L.A.; Chapuis, L.; Hemmi, J.M.; Collin, S.P.; McCauley, R.D.; Yopak, K.E.; Gennari, E.; Huveneers, C.; Kempster, R.M.; Kerr, C.C.; et al. Effects of auditory and visual stimuli on shark feeding behaviour: The disco effect. Mar. Biol. 2018, 165, 11. [Google Scholar] [CrossRef]
- Bradford, R.W.; Bruce, B.D.; McAuley, R.B.; Robinson, G. An evaluation of passive acoustic monitoring using satellite communication technology for near real-time detection of tagged animals in a marine setting. Open Fish Sci. J. 2011, 4, 10–20. [Google Scholar] [CrossRef]
- Lee, K.A.; Smoothey, A.F.; Harcourt, R.G.; Roughan, M.; Butcher, P.A.; Peddemors, V.M. Environmental drivers of abundance and residency of a large migratory shark, Carcharhinus leucas, inshore of a dynamic western boundary current. Mar. Ecol. Prog. Ser. 2019, 622, 121–137. [Google Scholar] [CrossRef]
- Smoothey, A.F.; Lee, K.A.; Peddemors, V.M. Long-term patterns of abundance, residency and movements of bull sharks (Carcharhinus leucas) in Sydney Harbour, Australia. Sci. Rep. 2019, 9, 18864. [Google Scholar] [CrossRef] [PubMed]
- Niella, Y.; Butcher, P.; Holmes, B.; Barnett, A.; Harcourt, R. Forecasting intraspecific changes in distribution of a wide-ranging marine predator under climate change. Oecologia 2022, 198, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Bruce, B.D.; Harasti, D.; Lee, K.; Gallen, C.; Bradford, R. Broad-scale movements of juvenile white sharks Carcharodon carcharias in eastern Australia from acoustic and satellite telemetry. Mar. Ecol. Prog. Ser. 2019, 619, 1–15. [Google Scholar] [CrossRef]
- Spaet, J.; Patterson, T.; Bradford, R.; Butcher, P. Spatiotemporal distribution patterns of immature Australasian white sharks (Carcharodon carcharias). Sci. Rep. 2020, 10, 10169. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L.; Curley, B.; Wolfenden, K.; Green, M.; Moltschaniwskyj, N.A. The Social Dimension to the New South Wales Shark Management Strategy, 2015–2020, Australia: Lessons learned. Mar. Pol. 2022, 141, 105079. [Google Scholar] [CrossRef]
- McPhee, D. Unprovoked shark bites: Are they becoming more prevalent? Coast. Manage. 2014, 42, 478–492. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species in Date; FAO: Rome, Italy, 2001; pp. 1–269. [Google Scholar]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia; CSIRO: Collingwood, Australia, 2009; p. 656. [Google Scholar]
- Bruce, B.D.; Stevens, J.D.; Malcolm, H. Movements and swimming behaviour of white Sharks (Carcharodon carcharias) in Australian waters. Mar. Biol. 2006, 150, 161–172. [Google Scholar] [CrossRef]
- Bruce, B.D.; Bradford, R.W. Habitat use and spatial dynamics of juvenile white sharks, Carcharodon carcharias, in eastern Australia. In Global Perspectives on the Biology and Life History of the White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 225–254. [Google Scholar]
- Spaet, J.L.Y.; Butcher, P.A.; Manica, A.; Lam, C.H. Spatial dynamics and fine-scale vertical behaviour of immature eastern Australian white sharks. Biology 2022, 11, 1689. [Google Scholar] [CrossRef]
- Coxon, J.L.; Butcher, P.A.; Spaet, J.L.Y.; Rizzari, J.R. Preliminary data about habitat use of subadult and adult white sharks (Carcharodon carcharias) in eastern Australian waters. Biology 2022, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.; Booth, D. Seasonal, sexual and size segregation of White sharks, Carcharodon carcharias, at the Neptune Islands, South Australia. In Global Perspectives on the Biology and Life History of the White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 225–254. [Google Scholar]
- Bruce, B.D.; Bradford, R.W. The effects of shark cage-diving operations on the behaviour and movements of white sharks, Carcharodon carcharias, at the Neptune Islands, South Australia. Mar. Biol. 2013, 160, 889–907. [Google Scholar] [CrossRef]
- Bruce, B.D.; Bradford, R. Segregation or aggregation? Sex-specific patterns in the seasonal occurrence of white sharks Carcharodon carcharias at the Neptune Islands, South Australia. J. Fish Biol. 2015, 87, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- Spaet, J.L.Y.; Manica, A.; Brand, C.P.; Gallen, C.; Butcher, P.A. Environmental conditions are poor predictors of immature white shark Carcharodon carcharias occurrences on coastal beaches of eastern Australia. Mar. Ecol. Prog. Ser. 2020, 653, 167–179. [Google Scholar] [CrossRef]
- Guyomard, D.; Perry, C.; Tournoux, P.U.; Cliff, G.; Peddemors, V.; Jaquemet, S.J.F.R. An innovative fishing gear to enhance the release of non-target species in coastal shark-control programs: The SMART (shark management alert in real-time) drumline. Fish. Res. 2019, 216, 6–17. [Google Scholar] [CrossRef]
- Tate, R.D.; Cullis, B.R.; Smith, S.D.A.; Kelaher, B.P.; Brand, C.P.; Gallen, C.R.; Mandelman, J.W.; Butcher, P.A. The acute physiological status of white sharks (Carcharodon carcharias) exhibits minimal variation after capture on SMART drumlines. Conserv. Physiol. 2019, 7, 1–9. [Google Scholar]
- Lipscombe, R.S.; Scott, A.; Morris, S.; Peddemors, V.M.; Smoothey, A.F.; Butcher, P.A. The influence of bait position on the catch of target and non-target sharks in a SMART drumline bather protection program. Fish. Res. 2023, 257, 106501. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Meyer, L.; Pethybridge, H.R.; Huveneers, C.; Butcher, P.A. Effects of short-term capture on the physiology of white sharks Carcharodon carcharias: Amino acids and fatty acids. Endanger. Species Res. 2019, 40, 297–308. [Google Scholar] [CrossRef]
- CLS. 2011. Argos User’s Manual. Available online: http://www.argos-system.org (accessed on 1 May 2023).
- McAuley, R.B.; Bruce, B.D.; Keay, I.S.; Mountford, S.; Pinnell, T.; Whoriskey, F.G. Broad-scale coastal movements of white sharks off Western Australia described by passive acoustic telemetry data. Mar. Freshw. Res. 2017, 68, 1518–1531. [Google Scholar] [CrossRef]
- McAuley, R.; Bruce, B.; Keay, I.; Mountford, S.; Pinnell, T. Evaluation of Passive Acoustic Telemetry Approaches for Monitoring and Mitigating Shark Hazards Off the Coast of Western Australia; Department of Fisheries, Government of Western Australia: Joondalup, Australia, 2016.
- Wood, S. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 May 2023).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Meth. Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bartoń, K. Mumin: Multi-Model Inference. R Package Version 1.15.6. Available online: https://cran.R-project.Org/package=mumin (accessed on 1 May 2023).
- Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Herman, K.; Jabado, R.W.; Jones, G.C.A.; Liu, K.M.; et al. Carcharodon carcharias (amended version of 2019 assessment). In The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Cambridge, UK, 2022; p. e.T3855A212629880. [Google Scholar]
- Niella, Y.; Peddemors, V.M.; Smoothey, A.F.; Green, M.; Harcourt, R. A “wicked problem” reconciling human-shark conflict, lethal shark control and endangered species. Fron. Cons. Sci. 2021, 2, 720–741. [Google Scholar]
- Barnes, C.; Butcher, P.; Peddemors, V.; Mandleman, J. Movements and mortality of two commercially exploited carcharhinid sharks following longline capture and release off eastern Australia. End Spec. Res. 2016, 30, 193–208. [Google Scholar] [CrossRef]
- Grainger, R.; Raubenheimer, D.; Peddemors, V.M.; Butcher, P.A.; Machovsky-Capuska, G.E. Integrating biologging and behavioural state modelling to identify cryptic behaviours and post-capture recovery processes: New insights from a threatened marine apex predator. Front. Mar. Sci. 2022, 8, 791185. [Google Scholar] [CrossRef]
- Colefax, A.P.; Kelaher, B.P.; Pagendam, D.E.; Butcher, P.A. Assessing white shark (Carcharodon carcharias) behaviour along coastal beaches for conservation-focused shark mitigation. Front. Mar. Sci. 2020, 7, 268. [Google Scholar] [CrossRef]
- Bonfil, R.; Meӱer, M.; Scholl, M.C.; Johnson, R.; O’Brien, S.; Oosthuizen, H.; Swanson, S.; Kotze, D.; Paterson, M. Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science 2005, 310, 100–103. [Google Scholar] [CrossRef]
- Watanabe, Y.Y.; Payne, N.L.; Semmens, J.M.; Fox, A.; Huveneers, C. Swimming strategies and energetics of endothermic white sharks during foraging. J. Exp. Biol. 2019, 222, 185603. [Google Scholar] [CrossRef]
- Semmens, J.M.; Payne, N.L.; Huveneers, C.; Sims, D.W.; Bruce, B.D. Feeding requirements of White sharks may be higher than originally thought. Sci. Rep. 2013, 3, 1471. [Google Scholar] [CrossRef]
- Harasti, D.; Lee, K.; Bruce, B.; Gallen, C.; Bradford, R. Juvenile White sharks Carcharodon carcharias use estuarine environments in south-eastern Australia. Mar. Biol. 2017, 164, 58. [Google Scholar] [CrossRef]
- Lee, K.A.; Roughan, M.; Harcourt, R.G.; Peddemors, V.M. Environmental correlates of relative abundance of potentially dangerous sharks in nearshore areas, southeastern Australia. Mar. Ecol. Prog. Ser. 2018, 599, 157–179. [Google Scholar] [CrossRef]
- Werry, J.M.; Bruce, B.D.; Sumpton, W.; Reid, D.; Mayer, D.G. Beach areas used by juvenile White shark, Carcharodon carcharias, in eastern Australia. In Global Perspectives on the Biology and Life History of the White Shark; Domeier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 225–254. [Google Scholar]
- Domeier, M.L.; Nasby-Lucas, N. Two-year migration of adult female white sharks (Carcharodon carcharias) reveals widely separated nursery areas and conservation concerns. Anim. Biotele. 2013, 1, 2. [Google Scholar] [CrossRef]
- Jorgensen, S.J.; Reeb, C.A.; Chapple, T.K.; Anderson, S.; Perle, C.; Van Sommeran, S.R.; Fritz-Cope, C.; Brown, A.C.; Klimley, A.P.; Block, B.A. Philopatry and migration of Pacific white sharks. Proc. R. Soc. B. 2009, 277, 1155. [Google Scholar] [CrossRef] [PubMed]
- Kock, A.C.; Photopoulou, T.; Durbach, I.; Mauff, K.; Meӱer, M.; Kotze, D.; Griffiths, C.L.; O’Riain, M.J. Summer at the beach: Spatio-temporal patterns of white shark occurrence along the inshore areas of False Bay, South Africa. Mov. Ecol. 2018, 6, 7. [Google Scholar] [CrossRef]
- Skomal, G.B.; Braun, C.D.; Chisholm, J.H.; Thorrold, S.R. Movements of the white shark Carcharodon carcharias in the North Atlantic Ocean. Mar. Ecol. Prog. Ser. 2017, 580, 1–6. [Google Scholar] [CrossRef]




| Shark ID | Date | Location Released | Sex | Total Length (cm) | Time from Hooking to Release | Time from Secured at the Boat to Release | Days to First Detection (All Classes) | Days to First Detection in First 10 Days (Class 1–3) | Distance (km) from Release Location to First SLRT Detection (Class 1–3) | Distance (km) from Coast to First SLRT Detection (Class 1–3) | Days to Last SLRT Detection | Days to First Acoustic Detection on VR4G | Distance (km) from Release Location to First Acoustic Detection on VR4G | Days to Last Acoustic Detection Anywhere |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 31 May 2016 | Main Beach, Evans Head | F | 235 | 44 | 29 | 0.19 | - | - | - | 273 | 12 | 34 | 477 |
| 16 | 31 May 2016 | Main Beach, Evans Head | M | 265 | 59 | 30 | 1.19 | - | - | - | 480 | 17 | 34 | 573 |
| 17 | 31 May 2016 | Main Beach, Evans Head | M | 245 | 34 | 20 | 0.66 | - | - | - | 4 | 60 | 200 | 404 |
| 18 | 2 June 2016 | Main Beach, Evans Head | F | 280 | 58 | 30 | 1.19 | 2.18 | 89.0 | 12.2 | 18 | 26 | 34 | 74 |
| 25 | 4 July 2016 | South Ballina Beach, Ballina | M | 298 | 49 | 20 | 2.44 | 7.51 | 29.1 | 22.4 | 791 | 22 | 30 | 1073 |
| 26 | 4 July 2016 | South Ballina Beach, Ballina | F | 268 | 37 | 19 | 0.62 | - | - | - | 109 | 24 | 65 | 45 |
| 27 | 5 July 2016 | South Ballina Beach, Ballina | F | 360 | 63 | 23 | 1.13 | 1.13 | 40.8 | 36.2 | 508 | - | - | - |
| 28 | 5 July 2016 | South Ballina Beach, Ballina | M | 306 | 47 | 20 | 0.17 | 0.40 | 15.2 | 14.7 | 920 | 350 | 9 | 568 |
| 30 | 21 July 2016 | Tuncurry Beach, Tuncurry | F | 220 | 46 | 25 | 0.16 | 0.24 | 7.8 | 7.6 | 292 | 7 | 86 | 967 |
| 31 | 21 July 2016 | Tuncurry Beach, Tuncurry | M | 267 | 43 | 32 | 0.10 | 0.21 | 8.6 | 7.8 | 85 | 112 | 75 | 1011 |
| 33 | 22 July 2016 | Tuncurry Beach, Tuncurry | F | 290 | 56 | 26 | 0.80 | 0.80 | 40.0 | 15.9 | 165 | 17 | 413 | 108 |
| 34 | 27 July 2016 | Crowdy Beach, Crowdy Head | F | 228 | 52 | 30 | 32.32 | - | - | - | 46 | 4 | 45 | 4 |
| 35 | 1 August 2016 | Boambee Beach, Coffs Harbour | M | 293 | 64 | 39 | 1.62 | 1.62 | 24.1 | 6.1 | 425 | 428 | 130 | 804 |
| 36 | 1 August 2016 | Boambee Beach, Coffs Harbour | F | 214 | 48 | 29 | 0.51 | 0.51 | 9.8 | 9.0 | 546 | 280 | 140 | 967 |
| 37 | 2 August 2016 | Boambee Beach, Coffs Harbour | M | 264 | 67 | 30 | 4.09 | - | - | - | 193 | 44 | 140 | 114 |
| 38 | 9 August 2016 | Sharpes Beach, Ballina | F | 215 | 57 | 29 | 3.41 | 9.24 | 78.5 | 8.5 | 310 | 28 | 0 | 773 |
| 39 | 9 August 2016 | South Ballina Beach, Ballina | F | 305 | 63 | 42 | 4.65 | 4.65 | 21.4 | 6.0 | 4 | 45 | 30 | 171 |
| 40 | 9 August 2016 | Sharpes Beach, Ballina | F | 259 | 54 | 29 | 0.83 | 0.83 | 18.8 | 15.5 | 871 | 28 | 0 | 860 |
| 41 | 10 August 2016 | South Ballina Beach, Ballina | F | 350 | 50 | 25 | 9.28 | - | - | - | 1061 | 31 | 5 | 860 |
| 42 | 6 September 2016 | Tuncurry Beach, Tuncurry | F | 220 | 59 | 38 | 0.85 | 0.92 | 17.0 | 3.5 | 344 | 51 | 75 | 1075 |
| 44 | 7 September 2016 | Tuncurry Beach, Tuncurry | M | 214 | 41 | 24 | 4.20 | 4.20 | 66.4 | 2.6 | 168 | 270 | 220 | 774 |
| 45 | 7 September 2016 | Tuncurry Beach, Tuncurry | M | 262 | 54 | 29 | 0.15 | 0.15 | 6.8 | 4.3 | 388 | 56 | 0 | 399 |
| 47 | 8 September 2016 | Tuncurry Beach, Tuncurry | M | 197 | 56 | 30 | 16.36 | - | - | - | 223 | 23 | 390 | 53 |
| 48 | 27 September 2016 | Angels Beach, Ballina | M | 291 | 71 | 49 | 5.28 | 5.29 | 27.6 | 3.5 | 758 | 5 | 25 | 786 |
| 49 | 28 September 2016 | Lighthouse Beach, Ballina | M | 172 | 59 | 30 | 5.45 | 5.47 | 36.9 | 16.9 | 262 | 606 | 296 | 1001 |
| 50 | 1 October 2016 | Sharpes Beach, Ballina | M | 213 | 63 | 44 | 0.30 | 0.36 | 14.3 | 13.8 | 232 | 6 | 0 | 311 |
| 51 | 2 October 2016 | Lighthouse Beach, Ballina | M | 256 | 51 | 35 | 0.79 | - | - | - | 362 | 235 | 265 | 676 |
| 52 | 2 October 2016 | Sharpes Beach, Ballina | M | 300 | 121 | 48 | 14.14 | - | - | - | 670 | - | - | 86 |
| 53 | 2 October 2016 | Sharpes Beach, Ballina | M | 232 | 71 | 44 | 1.23 | 1.23 | 53.5 | 18.0 | 328 | 113 | 301 | 411 |
| 54 | 4 October 2016 | Trestles Headland, Ballina | F | 267 | 78 | 42 | 0.81 | 3.20 | 107.9 | 10.9 | 639 | - | - | - |
| 55 | 4 October 2016 | Lighthouse Beach, Ballina | F | 223 | 37 | 28 | 0.23 | 0.30 | 15.3 | 14.1 | 150 | - | - | - |
| 56 | 6 October 2016 | Lighthouse Beach, Ballina | M | 281 | 60 | 36 | 0.62 | 5.35 | 25.5 | 24.2 | 180 | 43 | 230 | 398 |
| 57 | 8 October 2016 | South Ballina Beach, Ballina | F | 250 | 63 | 38 | 0.27 | - | - | - | 182 | 572 | 296 | 1056 |
| 58 | 8 October 2016 | South Ballina Beach, Ballina | F | 222 | 54 | 28 | 10.23 | - | - | - | 147 | 28 | 65 | 486 |
| 59 | 8 October 2016 | Lighthouse Beach, Ballina | F | 213 | 48 | 28 | 1.46 | - | - | - | 43 | - | - | - |
| 60 | 15 October 2016 | Lighthouse Beach, Ballina | F | 313 | 65 | 45 | - | - | - | - | - | 48 | 230 | 960 |
| Model | df | ΔAICc | AICc Weight |
|---|---|---|---|
| Null | 3 | 0.00 | 0.35 |
| Sex | 4 | 1.64 | 0.15 |
| TL | 4 | 1.77 | 0.14 |
| Time since tagged | 4 | 1.80 | 0.14 |
| Sex + TL | 5 | 3.13 | 0.07 |
| Sex + Time since tagged | 5 | 3.57 | 0.06 |
| Time since tagged + TL | 5 | 3.61 | 0.06 |
| Time since tagged + TL + sex | 6 | 5.15 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butcher, P.A.; Lee, K.A.; Brand, C.P.; Gallen, C.R.; Green, M.; Smoothey, A.F.; Peddemors, V.M. Capture Response and Long-Term Fate of White Sharks (Carcharodon carcharias) after Release from SMART Drumlines. Biology 2023, 12, 1329. https://doi.org/10.3390/biology12101329
Butcher PA, Lee KA, Brand CP, Gallen CR, Green M, Smoothey AF, Peddemors VM. Capture Response and Long-Term Fate of White Sharks (Carcharodon carcharias) after Release from SMART Drumlines. Biology. 2023; 12(10):1329. https://doi.org/10.3390/biology12101329
Chicago/Turabian StyleButcher, Paul A., Kate A. Lee, Craig P. Brand, Christopher R. Gallen, Marcel Green, Amy F. Smoothey, and Victor M. Peddemors. 2023. "Capture Response and Long-Term Fate of White Sharks (Carcharodon carcharias) after Release from SMART Drumlines" Biology 12, no. 10: 1329. https://doi.org/10.3390/biology12101329






