Crystal Structures Reveal Hidden Domain Mechanics in Protein Kinase A (PKA)
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Set Compilation and Pair-Wise Distance Analysis
2.2. B′-Factor Analysis
3. Results
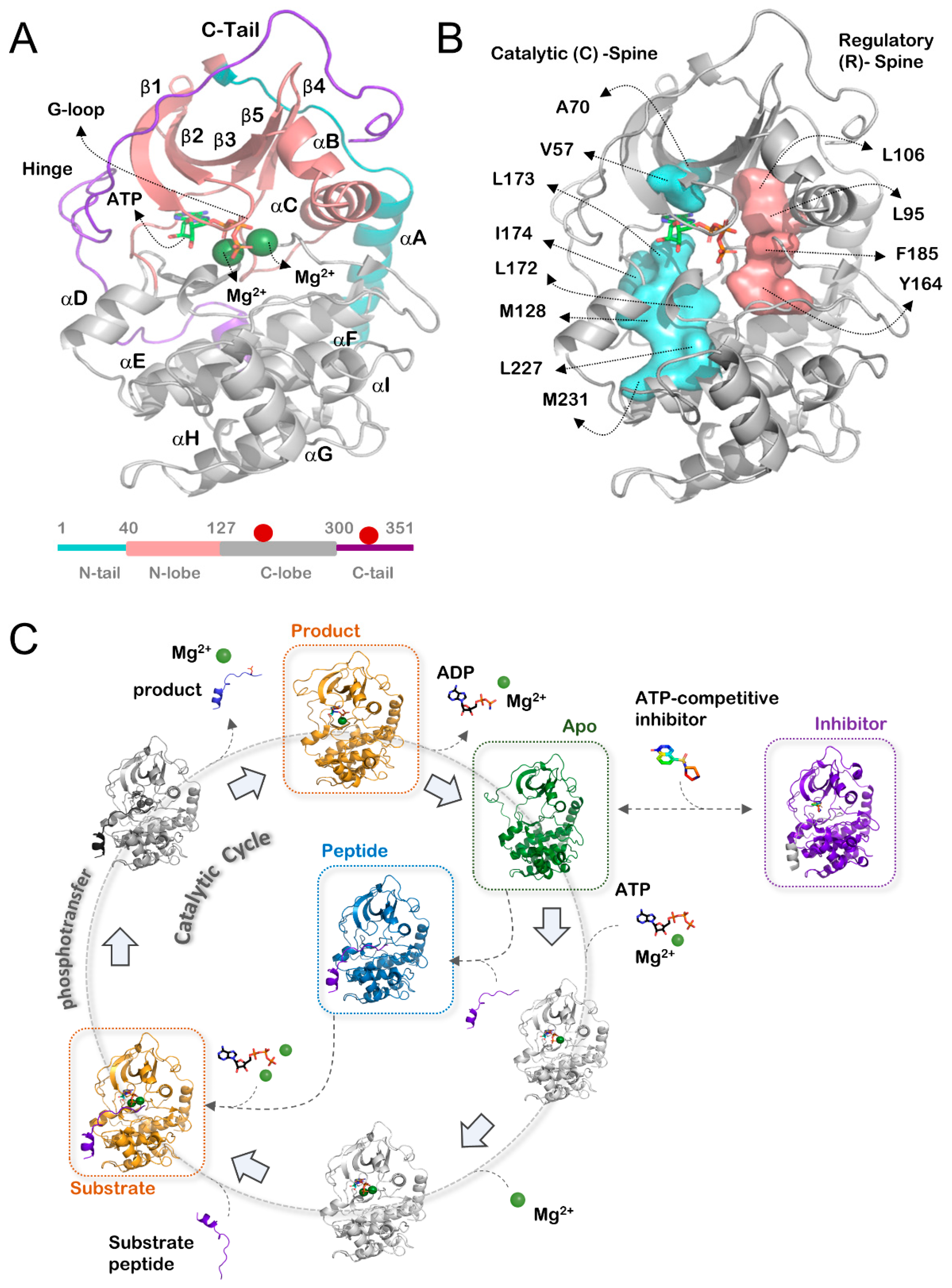
3.1. Catalytic States of PKA
- Substrate/product (n = 37): PKA bound to ATP or ADP, with or without a substrate peptide.
- Inhibitor (n = 60): PKA bound to a small molecule that is unable to be used for phosphotransfer (e.g., AMP-PNP or Type-I inhibitors).
- Apo (n = 15): PKA without a small molecule or peptide in the active site.
- Peptide (n = 3): PKA without a small molecule in the active site and with a peptide bound.
3.2. Ligand-Induced Closing of the N- and C-Lobes
3.3. PKA Preferred a DFGin Conformation
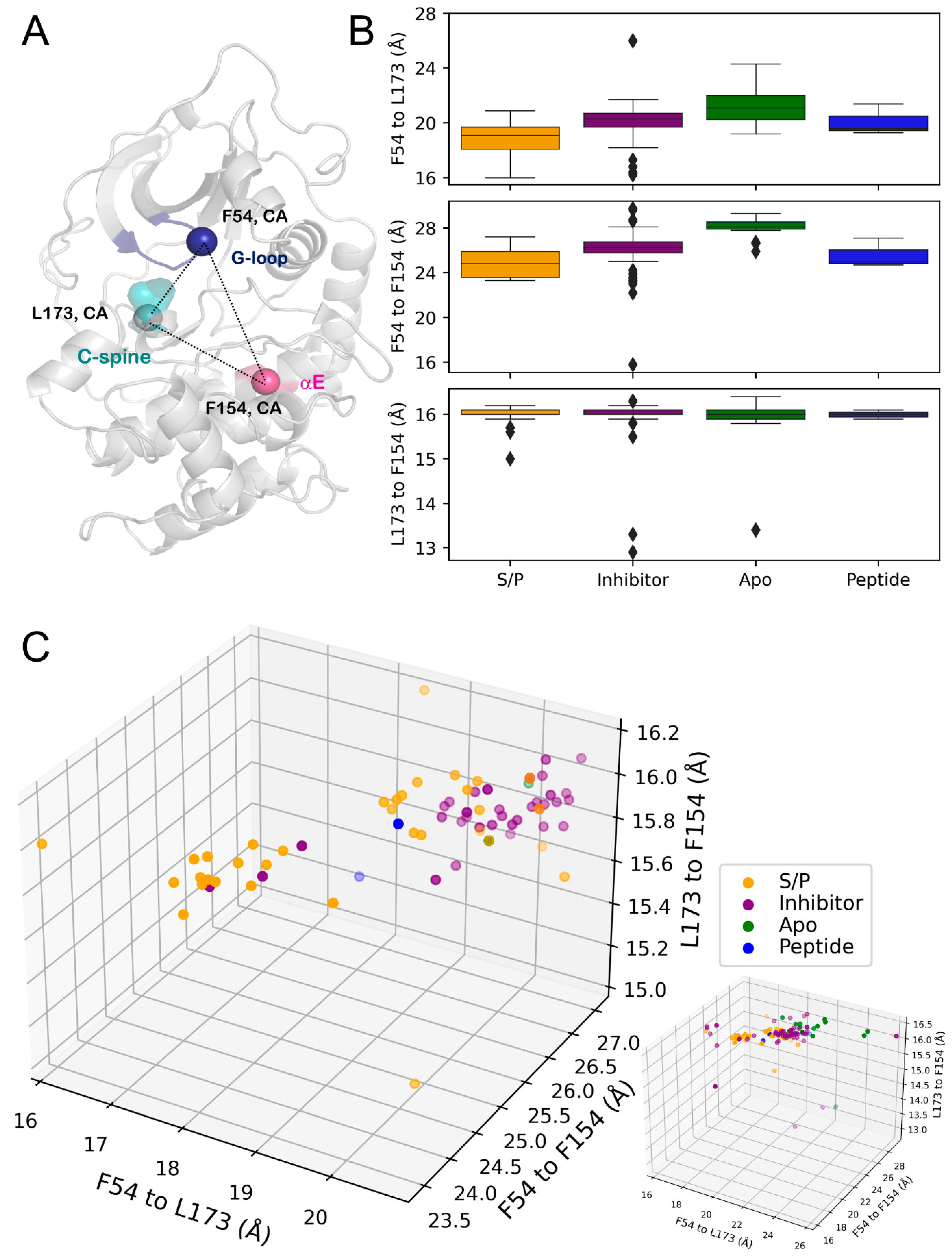
3.4. G-Loop Conformation Was Sensitive to Ligand Type
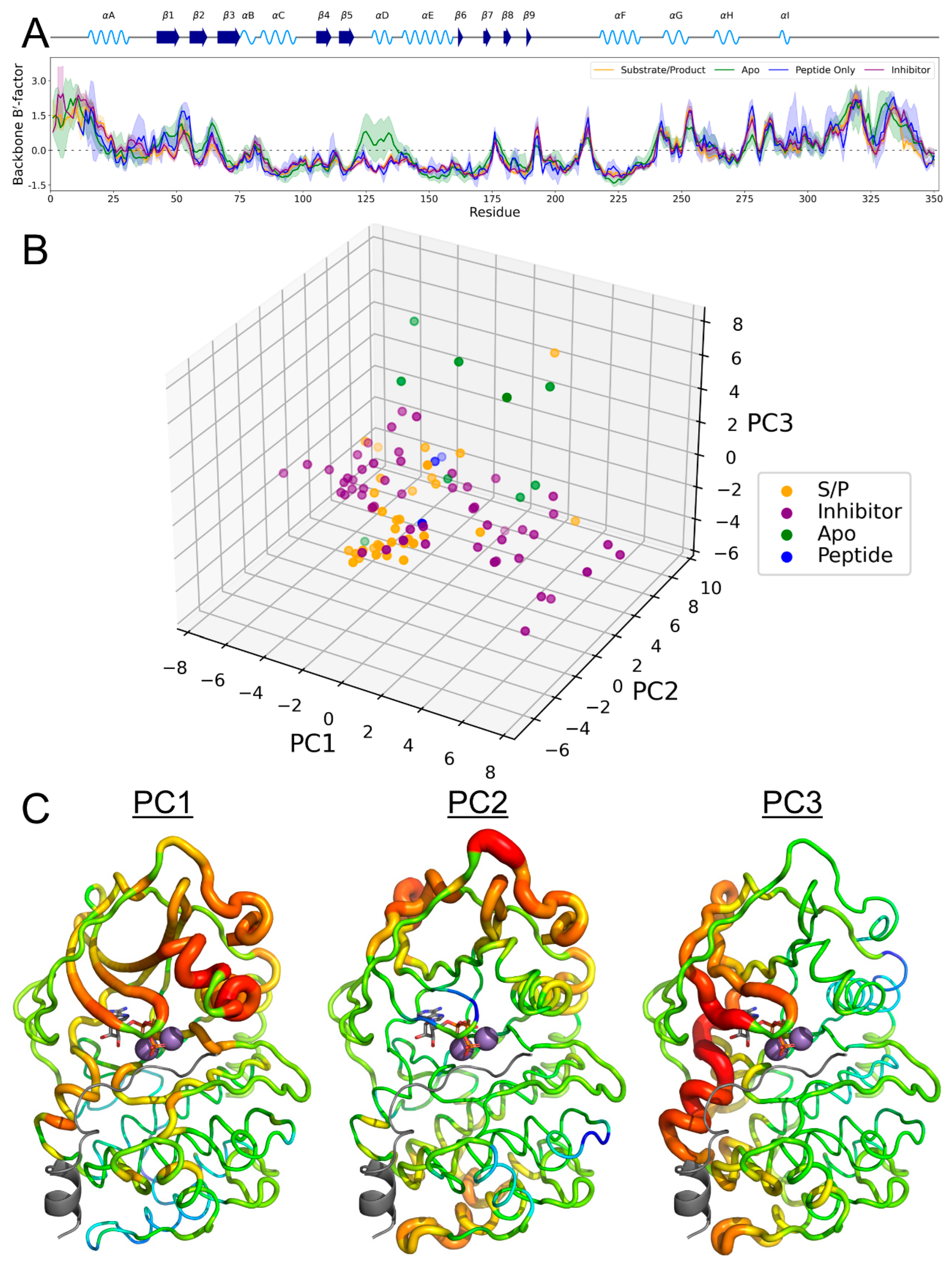
3.5. B-Factor Variations Based on State
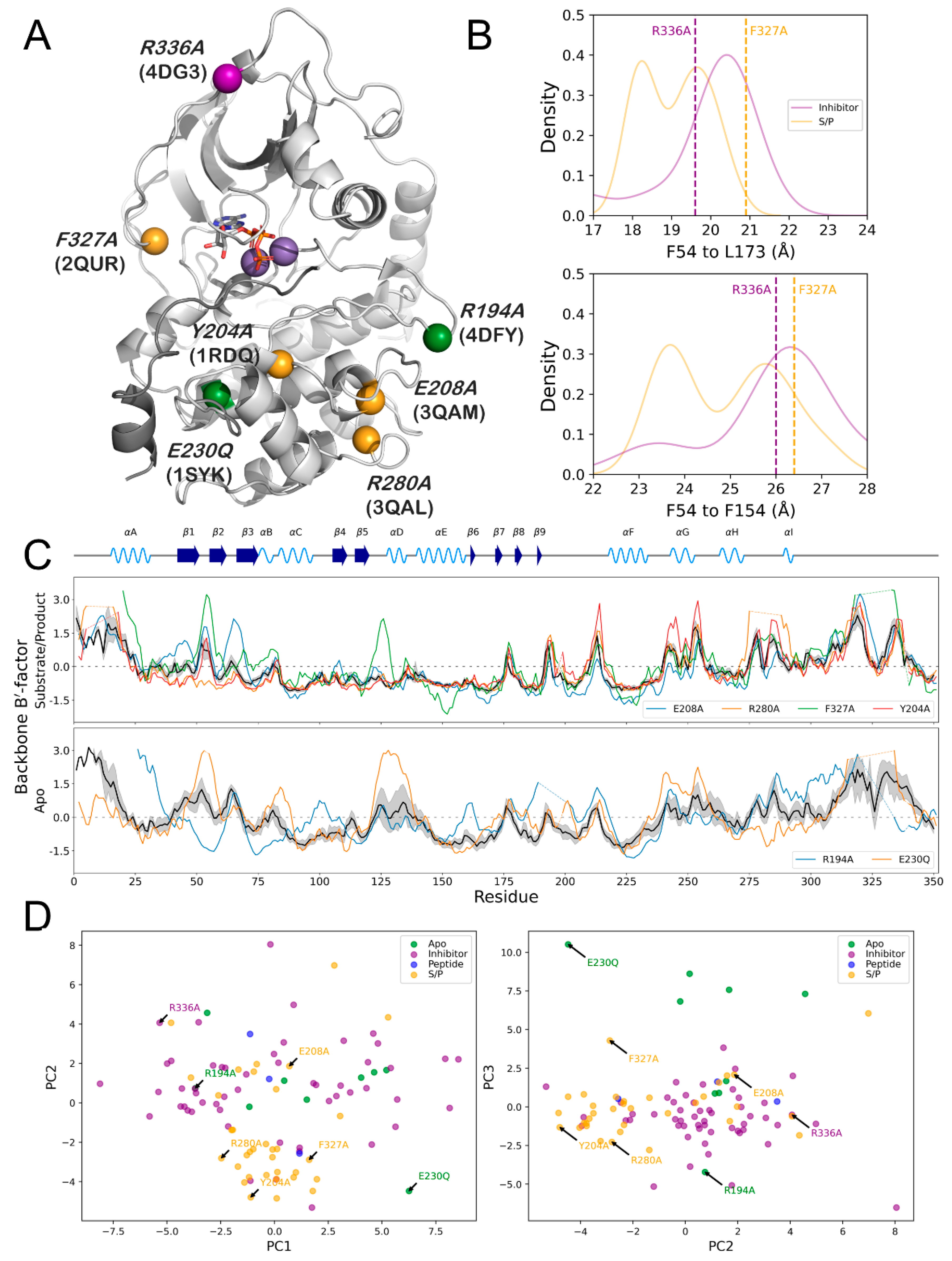
3.6. PKA Mutants Changed Conformational Preference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Zalyte, E.; Bairoch, A.; Gaudet, P. Kinases and Cancer. Cancers 2018, 10, 63. [Google Scholar] [CrossRef]
- Ahuja, L.G. Protein Tyrosine Phosphatases; De Gruyter: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2023 update. Pharmacol. Res. 2023, 187, 106552. [Google Scholar] [CrossRef]
- Taylor, S.S.; Kornev, A.P. Protein kinases: Evolution of dynamic regulatory proteins. Trends Biochem. Sci. 2011, 36, 65–77. [Google Scholar] [CrossRef]
- Taylor, S.S.; Wu, J.; Bruystens, J.G.H.; Del Rio, J.C.; Lu, T.-W.; Kornev, A.P.; Ten Eyck, L.F. From structure to the dynamic regulation of a molecular switch: A journey over 3 decades. J. Biol. Chem. 2021, 296, 100746. [Google Scholar] [CrossRef]
- Taylor, S.S.; Ilouz, R.; Zhang, P.; Kornev, A.P. Assembly of allosteric macromolecular switches: Lessons from PKA. Nat. Rev. Mol. Cell Biol. 2012, 13, 646–658. [Google Scholar] [CrossRef]
- Lu, T.-W.; Aoto, P.C.; Weng, J.-H.; Nielsen, C.; Cash, J.N.; Hall, J.; Zhang, P.; Simon, S.M.; Cianfrocco, M.A.; Taylor, S.S. Structural analyses of the PKA RIIβ holoenzyme containing the oncogenic DnaJB1-PKAc fusion protein reveal protomer asymmetry and fusion-induced allosteric perturbations in fibrolamellar hepatocellular carcinoma. PLoS Biol. 2020, 18, e3001018. [Google Scholar] [CrossRef]
- Zhang, P.; Knape, M.J.; Ahuja, L.G.; Keshwani, M.M.; King, C.C.; Sastri, M.; Herberg, F.W.; Taylor, S.S. Single Turnover Autophosphorylation Cycle of the PKA RIIβ Holoenzyme. PLoS Biol. 2015, 13, e1002192. [Google Scholar] [CrossRef]
- Wong, W.; Scott, J.D. AKAP signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004, 5, 959–970. [Google Scholar]
- Amer, Y.O.; Hebert-Chatelain, E. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim. Biophys. Acta BBA—Bioenerg. 2018, 1859, 868–877. [Google Scholar]
- Zheng, L.; Yu, L.; Tu, Q.; Zhang, M.; He, H.; Chen, W.; Gao, J.; Yu, J.; Wu, Q.; Zhao, S. Cloning and mapping of human PKIB and PKIG, and comparison of tissue expression patterns of three members of the protein kinase inhibitor family, including PKIA. Biochem. J. 2000, 349, 403–407. [Google Scholar] [PubMed]
- Grisan, F.; Iannucci, L.F.; Surdo, N.C.; Gerbino, A.; Zanin, S.; Di Benedetto, G.; Pozzan, T.; Lefkimmiatis, K. PKA compartmentalization links cAMP signaling and autophagy. Cell Death Differ. 2021, 28, 2436–2449. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-W.; Wu, J.; Aoto, P.C.; Weng, J.-H.; Ahuja, L.G.; Sun, N.; Cheng, C.Y.; Zhang, P.; Taylor, S.S. Two PKA RIα holoenzyme states define ATP as an isoform-specific orthosteric inhibitor that competes with the allosteric activator, cAMP. Proc. Natl. Acad. Sci. USA 2019, 116, 16347–16356. [Google Scholar] [CrossRef]
- Kim, C.; Cheng, C.Y.; Saldanha, S.A.; Taylor, S.S. PKA-I Holoenzyme Structure Reveals a Mechanism for cAMP-Dependent Activation. Cell 2007, 130, 1032–1043. [Google Scholar] [CrossRef]
- Boettcher, A.J.; Wu, J.; Kim, C.; Yang, J.; Bruystens, J.; Cheung, N.; Pennypacker, J.K.; Blumenthal, D.A.; Kornev, A.P.; Taylor, S.S. Realizing the Allosteric Potential of the Tetrameric Protein Kinase A RIα Holoenzyme. Structure 2011, 19, 265–276. [Google Scholar] [CrossRef]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP–PKA–CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Yang, J.; Ten Eyck, L.F.; Xuong, N.-H.; Taylor, S.S. Crystal Structure of a cAMP-dependent Protein Kinase Mutant at 1.26 Å: New Insights into the Catalytic Mechanism. J. Mol. Biol. 2004, 336, 473–487. [Google Scholar] [CrossRef]
- Akamine, P.; Madhusudan; Wu, J.; Xuong, N.-H.; Eyck, L.F.; Taylor, S.S. Dynamic Features of cAMP-dependent Protein Kinase Revealed by Apoenzyme Crystal Structure. J. Mol. Biol. 2003, 327, 159–171. [Google Scholar] [CrossRef]
- Taylor, S.S.; Søberg, K.; Kobori, E.; Wu, J.; Pautz, S.; Herberg, F.W.; Skålhegg, B.S. The Tails of Protein Kinase A. Mol. Pharmacol. 2022, 101, 219–225. [Google Scholar] [CrossRef]
- Meharena, H.S.; Fan, X.; Ahuja, L.G.; Keshwani, M.M.; McClendon, C.L.; Chen, A.M.; Adams, J.A.; Taylor, S.S. Decoding the Interactions Regulating the Active State Mechanics of Eukaryotic Protein Kinases. PLoS Biol. 2016, 14, e2000127. [Google Scholar] [CrossRef]
- Carrera, A.C.; Alexandrov, K.; Roberts, T.M. The conserved lysine of the catalytic domain of protein kinases is actively involved in the phosphotransfer reaction and not required for anchoring ATP. Proc. Natl. Acad. Sci. USA 1993, 90, 442–446. [Google Scholar] [CrossRef]
- Masterson, L.R.; Cembran, A.; Shi, L.; Veglia, G. Allostery and Binding Cooperativity of the Catalytic Subunit of Protein Kinase A by NMR Spectroscopy and Molecular Dynamics Simulations. Adv. Protein Chem. Struct. Biol. 2012, 87, 363–389. [Google Scholar] [CrossRef]
- Zheng, J.; Knighton, D.R.; Xuong, N.H.; Taylor, S.S.; Sowadski, J.M.; Ten Eyck, L.F. Crystal structures of the myristylated catalytic subunit of cAMP-dependent protein kinase reveal open and closed conformations. Protein Sci. 1993, 2, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Lauber, B.S.; Hardegger, L.A.; Alam, K.A.; Lund, B.A.; Dumele, O.; Harder, M.; Kuhn, B.; Engh, R.A.; Diederich, F. Addressing the Glycine-Rich Loop of Protein Kinases by a Multi-Facetted Interaction Network: Inhibition of PKA and a PKB Mimic. Chem.—Eur. J. 2016, 22, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.F. Post-translational modifications at the ATP-positioning G-loop that regulate protein kinase activity. Pharmacol. Res. 2018, 135, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Aimes, R.T.; Hemmer, W.; Taylor, S.S. Serine-53 at the Tip of the Glycine-Rich Loop of cAMP-Dependent Protein Kinase: Role in Catalysis, P-Site Specificity, and Interaction with Inhibitors. Biochemistry 2000, 39, 8325–8332. [Google Scholar] [CrossRef]
- Cui, Y.; Sun, G. Structural versatility that serves the function of the HRD motif in the catalytic loop of protein tyrosine kinase, Src. Protein Sci. 2019, 28, 533–542. [Google Scholar] [CrossRef]
- La Sala, G.; Riccardi, L.; Gaspari, R.; Cavalli, A.; Hantschel, O.; De Vivo, M. HRD Motif as the Central Hub of the Signaling Network for Activation Loop Autophosphorylation in Abl Kinase. J. Chem. Theory Comput. 2016, 12, 5563–5574. [Google Scholar] [CrossRef]
- Steichen, J.M.; Kuchinskas, M.; Keshwani, M.M.; Yang, J.; Adams, J.A.; Taylor, S.S. Structural Basis for the Regulation of Protein Kinase A by Activation Loop Phosphorylation. J. Biol. Chem. 2012, 287, 14672–14680. [Google Scholar] [CrossRef] [PubMed]
- Keshwani, M.M.; Klammt, C.; von Daake, S.; Ma, Y.; Kornev, A.P.; Choe, S.; Insel, P.A.; Taylor, S.S. Cotranslational cis-phosphorylation of the COOH-terminal tail is a key priming step in the maturation of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 2012, 109, E1221–E1229. [Google Scholar] [CrossRef]
- Cox, S.; Taylor, S.S. Kinetic Analysis of cAMP-Dependent Protein Kinase: Mutations at Histidine 87 Affect Peptide Binding and pH Dependence. Biochemistry 1995, 34, 16203–16209. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.M.; Deal, M.S.; Taylor, S.S. Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J. Biol. Chem. 2005, 280, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Kornev, A.P.; Haste, N.M.; Taylor, S.S.; Ten Eyck, L.F. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc. Natl. Acad. Sci. USA 2006, 103, 17783–17788. [Google Scholar] [CrossRef]
- Hu, J.; Ahuja, L.G.; Meharena, H.S.; Kannan, N.; Kornev, A.P.; Taylor, S.S.; Shaw, A.S. Kinase Regulation by Hydrophobic Spine Assembly in Cancer. Mol. Cell. Biol. 2015, 35, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, L.G.; Kornev, A.P.; McClendon, C.L.; Veglia, G.; Taylor, S.S. Mutation of a kinase allosteric node uncouples dynamics linked to phosphotransfer. Proc. Natl. Acad. Sci. USA 2017, 114, E931–E940. [Google Scholar] [CrossRef]
- Ahuja, L.G.; Aoto, P.C.; Kornev, A.P.; Veglia, G.; Taylor, S.S. Dynamic allostery-based molecular workings of kinase:peptide complexes. Proc. Natl. Acad. Sci. USA 2019, 116, 15052–15061. [Google Scholar] [CrossRef]
- Wang, Y.; Manu, V.S.; Kim, J.; Li, G.; Ahuja, L.G.; Aoto, P.; Taylor, S.S.; Veglia, G. Globally correlated conformational entropy underlies positive and negative cooperativity in a kinase’s enzymatic cycle. Nat. Commun. 2019, 10, 799. [Google Scholar] [CrossRef]
- Khavrutskii, I.V.; Grant, B.; Taylor, S.S.; McCammon, J.A. A Transition Path Ensemble Study Reveals a Linchpin Role for Mg2+ during Rate-Limiting ADP Release from Protein Kinase A. Biochemistry 2009, 48, 11532–11545. [Google Scholar] [CrossRef][Green Version]
- Armstrong, R.N.; Kondo, H.; Granot, J.; Kaiser, E.T.; Mildvan, A.S. Magnetic resonance and kinetic studies of the manganese(II) ion and substrate complexes of the catalytic subunit of adenosine 3’,5’-monophosphate dependent protein kinase from bovine heart. Biochemistry 1979, 18, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, Y.; McCammon, J.A. How Does the cAMP-Dependent Protein Kinase Catalyze the Phosphorylation Reaction: An ab Initio QM/MM Study. J. Am. Chem. Soc. 2005, 127, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.C.; Sheppard, D.W.; Hillier, I.H.; Burton, N.A. What is the mechanism of phosphoryl transfer in protein kinases? A hybrid quantum mechanical/molecular mechanical study. Chem. Commun. 1999, 79–80. [Google Scholar] [CrossRef]
- Sheppard, D.; Burton, N.; Hillier, I. Ab initio hybrid quantum mechanical/molecular mechanical studies of the mechanisms of the enzymes protein kinase and thymidine phosphorylase. J. Mol. Struct. Theochem. 2000, 506, 35–44. [Google Scholar] [CrossRef]
- Valiev, M.; Kawai, R.; Adams, J.A.; Weare, J.H. The Role of the Putative Catalytic Base in the Phosphoryl Transfer Reaction in a Protein Kinase: First-Principles Calculations. J. Am. Chem. Soc. 2003, 125, 9926–9927. [Google Scholar] [CrossRef][Green Version]
- Díaz, N.; Field, M.J. Insights into the Phosphoryl-Transfer Mechanism of cAMP-Dependent Protein Kinase from Quantum Chemical Calculations and Molecular Dynamics Simulations. J. Am. Chem. Soc. 2004, 126, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; LaBute, M.X.; Tung, C.-S.; Fenimore, P.W.; McMahon, B.H. Conformational dependence of a protein kinase phosphate transfer reaction. Proc. Natl. Acad. Sci. USA 2005, 102, 15347–15351. [Google Scholar] [CrossRef]
- Valiev, M.; Yang, J.; Adams, J.A.; Taylor, S.S.; Weare, J.H. Phosphorylation Reaction in cAPK Protein Kinase-Free Energy Quantum Mechanical/Molecular Mechanics Simulations. J. Phys. Chem. B 2007, 111, 13455–13464. [Google Scholar] [CrossRef]
- Montenegro, M.; Garcia-Viloca, M.; Lluch, J.M.; González-Lafont, A. A QM/MM study of the phosphoryl transfer to the Kemptide substrate catalyzed by protein kinase A. The effect of the phosphorylation state of the protein on the mechanism. Phys. Chem. Chem. Phys. 2011, 13, 530–539. [Google Scholar] [CrossRef]
- Adams, J.A. Kinetic and Catalytic Mechanisms of Protein Kinases. Chem. Rev. 2001, 101, 2271–2290. [Google Scholar] [CrossRef]
- Arter, C.; Trask, L.; Ward, S.; Yeoh, S.; Bayliss, R. Structural features of the protein kinase domain and targeted binding by small-molecule inhibitors. J. Biol. Chem. 2022, 298, 102247. [Google Scholar] [CrossRef]
- Vijayan, R.S.K.; He, P.; Modi, V.; Duong-Ly, K.C.; Ma, H.; Peterson, J.R.; Dunbrack, R.L., Jr.; Levy, R.M. Conformational Analysis of the DFG-Out Kinase Motif and Biochemical Profiling of Structurally Validated Type II Inhibitors. J. Med. Chem. 2015, 58, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Modi, V.; Dunbrack, R.L. Defining a new nomenclature for the structures of active and inactive kinases. Proc. Natl. Acad. Sci. USA 2019, 116, 6818–6827. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, Version 1.8; Schrödinger, Inc.: Cambridge, MA, USA, 2015.
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Smith, D.K.; Radivojac, P.; Obradovic, Z.; Dunker, A.K.; Zhu, G. Improved amino acid flexibility parameters. Protein Sci. 2003, 12, 1060–1072. [Google Scholar] [CrossRef]
- Barthels, F.; Schirmeister, T.; Kersten, C. BANΔIT: B’-Factor Analysis for Drug Design and Structural Biology. Mol. Inform. 2021, 40, 2000144. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Antao, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Yang, J.; Wu, J.; Steichen, J.M.; Kornev, A.P.; Deal, M.S.; Li, S.; Sankaran, B.; Woods, V.L.; Taylor, S.S. A Conserved Glu–Arg Salt Bridge Connects Coevolved Motifs That Define the Eukaryotic Protein Kinase Fold. J. Mol. Biol. 2012, 415, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, J.; Kannan, N.; Madhusudan; Xuong, N.-H.; Ten Eyck, L.F.; Taylor, S.S. Crystal structure of the E230Q mutant of cAMP-dependent protein kinase reveals an unexpected apoenzyme conformation and an extended N-terminal A helix. Protein Sci. 2005, 14, 2871–2879. [Google Scholar] [CrossRef]
- Yang, J.; Kennedy, E.J.; Wu, J.; Deal, M.S.; Pennypacker, J.; Ghosh, G.; Taylor, S.S. Contribution of Non-catalytic Core Residues to Activity and Regulation in Protein Kinase A. J. Biol. Chem. 2009, 284, 6241–6248. [Google Scholar] [CrossRef] [PubMed]
- Möbitz, H. The ABC of protein kinase conformations. Biochim. Biophys. Acta BBA—Proteins Proteom. 2015, 1854, 1555–1566. [Google Scholar] [CrossRef]
- Ung, P.M.-U.; Rahman, R.; Schlessinger, A. Redefining the Protein Kinase Conformational Space with Machine Learning. Cell Chem. Biol. 2018, 25, 916–924.e2. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.D.; Caron, P.R.; Hare, B.J. Classifying protein kinase structures guides use of ligand-selectivity profiles to predict inactive conformations: Structure of lck/imatinib complex. Proteins Struct. Funct. Bioinform. 2007, 70, 1451–1460. [Google Scholar] [CrossRef]
- Brooijmans, N.; Chang, Y.-W.; Mobilio, D.; Denny, R.A.; Humblet, C. An enriched structural kinase database to enable kinome-wide structure-based analyses and drug discovery. Protein Sci. 2010, 19, 763–774. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Q.; Qu, G.; Feng, Y.; Reetz, M.T. Utility of B-Factors in Protein Science: Interpreting Rigidity, Flexibility, and Internal Motion and Engineering Thermostability. Chem. Rev. 2019, 119, 1626–1665. [Google Scholar] [CrossRef]



| Distance (Å) | T51 (Ca) to A223 (Ca) | T51 (Ca) to H87 (Ca) | H87 (Ca) to A223 (Ca) |
|---|---|---|---|
| Substrate/Product | |||
| Average | 19.72 | 14.62 | 19.48 |
| Median | 19.00 | 14.60 | 19.40 |
| Std Dev | 1.48 | 0.34 | 0.34 |
| N | 37.00 | 37.00 | 37.00 |
| Min Value | 18.30 | 14.10 | 18.60 |
| 1st Quartile | 18.80 | 14.40 | 19.40 |
| 2nd Quartile | 19.00 | 14.60 | 19.40 |
| 3rd Quartile | 20.90 | 14.80 | 19.60 |
| Max Value | 23.80 | 15.90 | 20.90 |
| Inhibitor | |||
| Average | 21.24 | 14.54 | 19.76 |
| Median | 20.85 | 14.40 | 19.50 |
| Std Dev | 1.52 | 0.69 | 0.76 |
| N | 60.00 | 60.00 | 60.00 |
| Min Value | 17.30 | 13.10 | 18.80 |
| 1st Quartile | 20.50 | 14.10 | 19.40 |
| 2nd Quartile | 20.85 | 14.40 | 19.50 |
| 3rd Quartile | 22.03 | 14.80 | 19.73 |
| Max Value | 25.00 | 16.40 | 22.80 |
| Apo | |||
| Average | 25.15 | 15.59 | 21.31 |
| Median | 25.30 | 15.40 | 21.00 |
| Std Dev | 2.69 | 1.32 | 1.83 |
| N | 15.00 | 15.00 | 15.00 |
| Min Value | 20.70 | 14.30 | 19.50 |
| 1st Quartile | 24.20 | 14.55 | 20.15 |
| 2nd Quartile | 25.30 | 15.40 | 21.00 |
| 3rd Quartile | 26.75 | 16.45 | 21.60 |
| Max Value | 29.20 | 17.90 | 25.40 |
| Peptide | |||
| Average | 20.23 | 14.40 | 19.53 |
| Median | 19.80 | 14.40 | 19.50 |
| Std Dev | 1.21 | 0.10 | 0.06 |
| N | 3.00 | 3.00 | 3.00 |
| Min Value | 19.30 | 14.30 | 19.50 |
| 1st Quartile | 19.55 | 14.35 | 19.50 |
| 2nd Quartile | 19.80 | 14.40 | 19.50 |
| 3rd Quartile | 20.70 | 14.45 | 19.55 |
| Max Value | 21.60 | 14.50 | 19.60 |
| Distance (Å) | K72 (Ca) to F185 (Cz) | F185 (Cz) to L95 (Ca) | K72 (Ca) to L95 (Ca) |
|---|---|---|---|
| Substrate/Product | |||
| Average | 14.45 | 5.88 | 12.51 |
| Median | 14.40 | 5.80 | 12.50 |
| Std Dev | 0.27 | 0.29 | 0.19 |
| N | 37.00 | 37.00 | 37.00 |
| Min Value | 14.00 | 5.40 | 11.80 |
| 1st Quartile | 14.30 | 5.70 | 12.40 |
| 2nd Quartile | 14.40 | 5.80 | 12.50 |
| 3rd Quartile | 14.60 | 6.10 | 12.60 |
| Max Value | 15.30 | 6.60 | 13.00 |
| Inhibitor | |||
| Average | 14.12 | 6.16 | 12.62 |
| Median | 14.30 | 6.10 | 12.55 |
| Std Dev | 0.82 | 0.63 | 0.32 |
| N | 60.00 | 60.00 | 60.00 |
| Min Value | 11.10 | 4.60 | 12.10 |
| 1st Quartile | 14.00 | 5.70 | 12.40 |
| 2nd Quartile | 14.30 | 6.10 | 12.55 |
| 3rd Quartile | 14.60 | 6.40 | 12.70 |
| Max Value | 15.00 | 7.80 | 13.90 |
| Apo | |||
| Average | 14.91 | 6.81 | 13.14 |
| Median | 14.80 | 6.70 | 13.30 |
| Std Dev | 0.83 | 0.53 | 0.46 |
| N | 13.00 | 13.00 | 15.00 |
| Min Value | 13.40 | 6.00 | 12.40 |
| 1st Quartile | 14.40 | 6.40 | 12.85 |
| 2nd Quartile | 14.80 | 6.70 | 13.30 |
| 3rd Quartile | 15.30 | 7.30 | 13.50 |
| Max Value | 16.20 | 7.60 | 13.60 |
| Peptide | |||
| Average | 14.57 | 6.57 | 12.47 |
| Median | 14.50 | 6.40 | 12.40 |
| Std Dev | 0.21 | 0.38 | 0.12 |
| N | 3.00 | 3.00 | 3.00 |
| Min Value | 14.40 | 6.30 | 12.40 |
| 1st Quartile | 14.45 | 6.35 | 12.40 |
| 2nd Quartile | 14.50 | 6.40 | 12.40 |
| 3rd Quartile | 14.65 | 6.70 | 12.50 |
| Max Value | 14.80 | 7.00 | 12.60 |
| Distance (Å) | D328 (Ca) to E127 (Ca) | D328 (Ca) to G52 (Ca) | G52 (Ca) to E127 (Ca) |
|---|---|---|---|
| Substrate/Product | |||
| Average | 9.95 | 13.44 | 13.12 |
| Median | 9.60 | 13.40 | 12.90 |
| Std Dev | 1.52 | 0.61 | 0.91 |
| N | 37.00 | 37.00 | 37.00 |
| Min Value | 9.30 | 12.50 | 11.70 |
| 1st Quartile | 9.50 | 13.10 | 12.60 |
| 2nd Quartile | 9.60 | 13.40 | 12.90 |
| 3rd Quartile | 9.60 | 13.60 | 13.20 |
| Max Value | 17.40 | 16.40 | 16.10 |
| Inhibitor | |||
| Average | 9.87 | 13.80 | 14.55 |
| Median | 9.70 | 13.80 | 14.55 |
| Std Dev | 0.81 | 0.50 | 0.80 |
| N | 58.00 | 58.00 | 58.00 |
| Min Value | 7.50 | 12.90 | 12.00 |
| 1st Quartile | 9.50 | 13.50 | 14.20 |
| 2nd Quartile | 9.70 | 13.80 | 14.55 |
| 3rd Quartile | 9.90 | 14.00 | 15.10 |
| Max Value | 13.30 | 15.00 | 16.50 |
| Apo | |||
| Average | 15.00 | 15.33 | 16.70 |
| Median | 13.85 | 14.50 | 16.10 |
| Std Dev | 6.08 | 2.00 | 2.13 |
| N | 6.00 | 6.00 | 9.00 |
| Min Value | 9.70 | 13.70 | 14.20 |
| 1st Quartile | 9.73 | 14.13 | 15.20 |
| 2nd Quartile | 13.85 | 14.50 | 16.10 |
| 3rd Quartile | 18.88 | 15.85 | 16.80 |
| Max Value | 23.70 | 19.00 | 20.20 |
| Peptide | |||
| Average | 9.67 | 13.70 | 14.17 |
| Median | 9.70 | 13.50 | 13.70 |
| Std Dev | 0.25 | 0.53 | 1.36 |
| N | 3.00 | 3.00 | 3.00 |
| Min Value | 9.40 | 13.30 | 13.10 |
| 1st Quartile | 9.55 | 13.40 | 13.40 |
| 2nd Quartile | 9.70 | 13.50 | 13.70 |
| 3rd Quartile | 9.80 | 13.90 | 14.70 |
| Max Value | 9.90 | 14.30 | 15.70 |
| Distance (Å) | F54 (Ca) to L173 (Ca) | F54 (Ca) to F154 (Ca) | L173 (Ca) to F154 (Ca) |
|---|---|---|---|
| Substrate/Product | |||
| Average | 18.99 | 24.92 | 15.98 |
| Median | 19.20 | 25.30 | 16.00 |
| Std Dev | 0.98 | 1.23 | 0.21 |
| N | 33.00 | 33.00 | 33.00 |
| Min Value | 16.00 | 23.30 | 15.00 |
| 1st Quartile | 18.20 | 23.60 | 16.00 |
| 2nd Quartile | 19.20 | 25.30 | 16.00 |
| 3rd Quartile | 19.70 | 25.90 | 16.10 |
| Max Value | 20.60 | 27.20 | 16.20 |
| Inhibitor | |||
| Average | 20.08 | 25.94 | 15.94 |
| Median | 20.30 | 26.25 | 16.00 |
| Std Dev | 1.70 | 2.07 | 0.55 |
| N | 60.00 | 60.00 | 60.00 |
| Min Value | 16.20 | 15.80 | 12.90 |
| 1st Quartile | 19.70 | 25.78 | 16.00 |
| 2nd Quartile | 20.30 | 26.25 | 16.00 |
| 3rd Quartile | 20.70 | 26.75 | 16.10 |
| Max Value | 26.00 | 29.70 | 16.30 |
| Apo | |||
| Average | 20.12 | 24.48 | 15.14 |
| Median | 20.90 | 28.00 | 15.90 |
| Std Dev | 2.33 | 7.99 | 1.35 |
| N | 13.00 | 13.00 | 15.00 |
| Min Value | 15.30 | 6.60 | 13.00 |
| 1st Quartile | 20.10 | 26.60 | 13.60 |
| 2nd Quartile | 20.90 | 28.00 | 15.90 |
| 3rd Quartile | 21.50 | 28.10 | 16.10 |
| Max Value | 22.50 | 29.00 | 16.40 |
| Peptide | |||
| Average | 20.10 | 25.60 | 20.10 |
| Median | 19.60 | 25.00 | 19.60 |
| Std Dev | 1.14 | 1.31 | 1.14 |
| N | 3.00 | 3.00 | 3.00 |
| Min Value | 19.30 | 24.70 | 19.30 |
| 1st Quartile | 19.45 | 24.85 | 19.45 |
| 2nd Quartile | 19.60 | 25.00 | 19.60 |
| 3rd Quartile | 20.50 | 26.05 | 20.50 |
| Max Value | 21.40 | 27.10 | 21.40 |
| PDB | 4DG3 | 3QAM | 3QAL | 2QUR | 1RDQ | 4DFY | 4DFY | 1SYK | 1SYK |
|---|---|---|---|---|---|---|---|---|---|
| Chain ID | A | E | E | A | E | A | B | A | B |
| Mutants | R336A | E208A | R280A | F327A | Y204A | R194A | R194A | E230A | E230A |
| Distance (Å) | INHIBITOR | SUBSTRATE/PRODUCT | APO | ||||||
| T51 to A223 | 18.9 | 19 | 18.8 | 22.6 | 18.5 | 27.4 | 27.4 | 29.2 | 29.2 |
| T51 to H87 | 14.6 | 14.6 | 14.7 | 15.9 | 14.8 | 17.9 | 17.9 | 17.2 | 17.2 |
| H87 to A223 | 19.5 | 19.3 | 19.4 | 19.7 | 19.3 | 25.4 | 25.4 | 20.7 | 20.7 |
| D328 to E127 | 9.4 | 9.4 | 9.6 | 12.2 | 9.4 | - | - | 17.9 | - |
| D328 to G52 | 13.3 | 12.9 | 13.1 | 14 | 12.9 | - | - | 16.2 | - |
| G52 to E127 | 12.7 | 13.1 | 12.7 | 16.1 | 12.1 | 20.2 | 20.2 | 16 | 16.1 |
| K72 to F185 | 14.4 | 14.2 | 14.4 | 14.6 | 14.5 | - | - | 15.3 | 15.3 |
| F185 to L95 | 6.0 | 5.6 | 5.6 | 6.6 | 5.7 | - | - | 6.6 | 6.6 |
| K72 to L95 | 12.6 | 12.4 | 12.6 | 12.7 | 12.5 | 13 | 13 | 13.6 | 13.6 |
| F54 to L173 | 18.5 | 18.2 | 18.1 | 20.9 | 17.8 | 24.2 | 24.3 | 20.3 | 20.3 |
| F54 to F154 | 27.1 | 23.6 | 23.6 | 26.4 | 23.4 | 29 | 29.3 | 28.2 | 28.2 |
| L173 to F154 | 16.2 | 16 | 16 | 15.7 | 16 | 16 | 16.1 | 15.9 | 16 |
| S53 to A223 | 19.5 | 19.5 | 19.2 | 23.5 | 18.7 | 28.1 | 27.9 | 28 | 28 |
| S53 to H87 | 13.1 | 13.5 | 13.1 | 14.9 | 13.1 | 13.4 | 13.4 | 14.5 | 14.5 |
| H87 to A223 | 19.5 | 19.3 | 19.4 | 19.7 | 19.3 | 25.4 | 25.4 | 20.7 | 20.7 |
| K81 to V104 | 27.5 | 27.2 | 27 | 27.4 | 26.9 | 28.8 | 28.8 | 26.5 | 26.5 |
| K81 to N113 | 16.6 | 16.4 | 16.5 | 16.4 | 16.4 | 16.4 | 16.5 | 16.3 | 16.3 |
| N113 to V104 | 26.5 | 26.4 | 26.4 | 26.5 | 21 | 27.2 | 27.2 | 26.3 | 26.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welsh, C.L.; Conklin, A.E.; Madan, L.K. Crystal Structures Reveal Hidden Domain Mechanics in Protein Kinase A (PKA). Biology 2023, 12, 1370. https://doi.org/10.3390/biology12111370
Welsh CL, Conklin AE, Madan LK. Crystal Structures Reveal Hidden Domain Mechanics in Protein Kinase A (PKA). Biology. 2023; 12(11):1370. https://doi.org/10.3390/biology12111370
Chicago/Turabian StyleWelsh, Colin L., Abigail E. Conklin, and Lalima K. Madan. 2023. "Crystal Structures Reveal Hidden Domain Mechanics in Protein Kinase A (PKA)" Biology 12, no. 11: 1370. https://doi.org/10.3390/biology12111370
APA StyleWelsh, C. L., Conklin, A. E., & Madan, L. K. (2023). Crystal Structures Reveal Hidden Domain Mechanics in Protein Kinase A (PKA). Biology, 12(11), 1370. https://doi.org/10.3390/biology12111370






