Investigating the Link between Eating Attitudes, Taste and Odour Preferences and the Chemical Senses
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Pathological Eating and Olfactory Dysfunction
1.2. Pathological Eating and Gustatory Dysfunction
1.3. Pathological Eating and Odour/Taste Preferences
1.4. Limitations of Previous Research
1.5. The Present Study Aims and Hypotheses
2. Method
2.1. Participants
2.2. Measures
2.2.1. Hunger
2.2.2. Health Questionnaire
2.2.3. Eating Attitudes Test (EAT-26)
2.2.4. Olfactory Function (Threshold and Discrimination)
2.2.5. Food Odour Rating Task
2.2.6. Liquid Tastant Rating Task
2.3. Procedure
2.4. Data Analyses
3. Results
3.1. Assumption Testing
3.2. Descriptive Statistics and Correlations
3.2.1. Hypothesis 1: Were Higher EAT-26 Scores Associated with Lower Olfactory Ability?
3.2.2. Hypothesis 2: Were Higher EAT-26 Scores Associated with Higher Perceived Pleasantness of High Sugar, Healthy Food Smells?
3.2.3. Hypothesis 3: Were Higher EAT-26 Scores Associated with Lower Psychophysical Ratings of Taste Stimuli?
3.2.4. Hypothesis 4: Were Higher EAT-26 Scores Associated with Higher Perceived Pleasantness of Sweet Tastes?
3.3. Exploratory Analyses: Did the Higher EAT-26 Group Differ from the Lower EAT-26 Group on Any Measures Employed?
3.3.1. Did Lower and Higher EAT Groups Differ in Terms of BMI or Olfactory Ability?
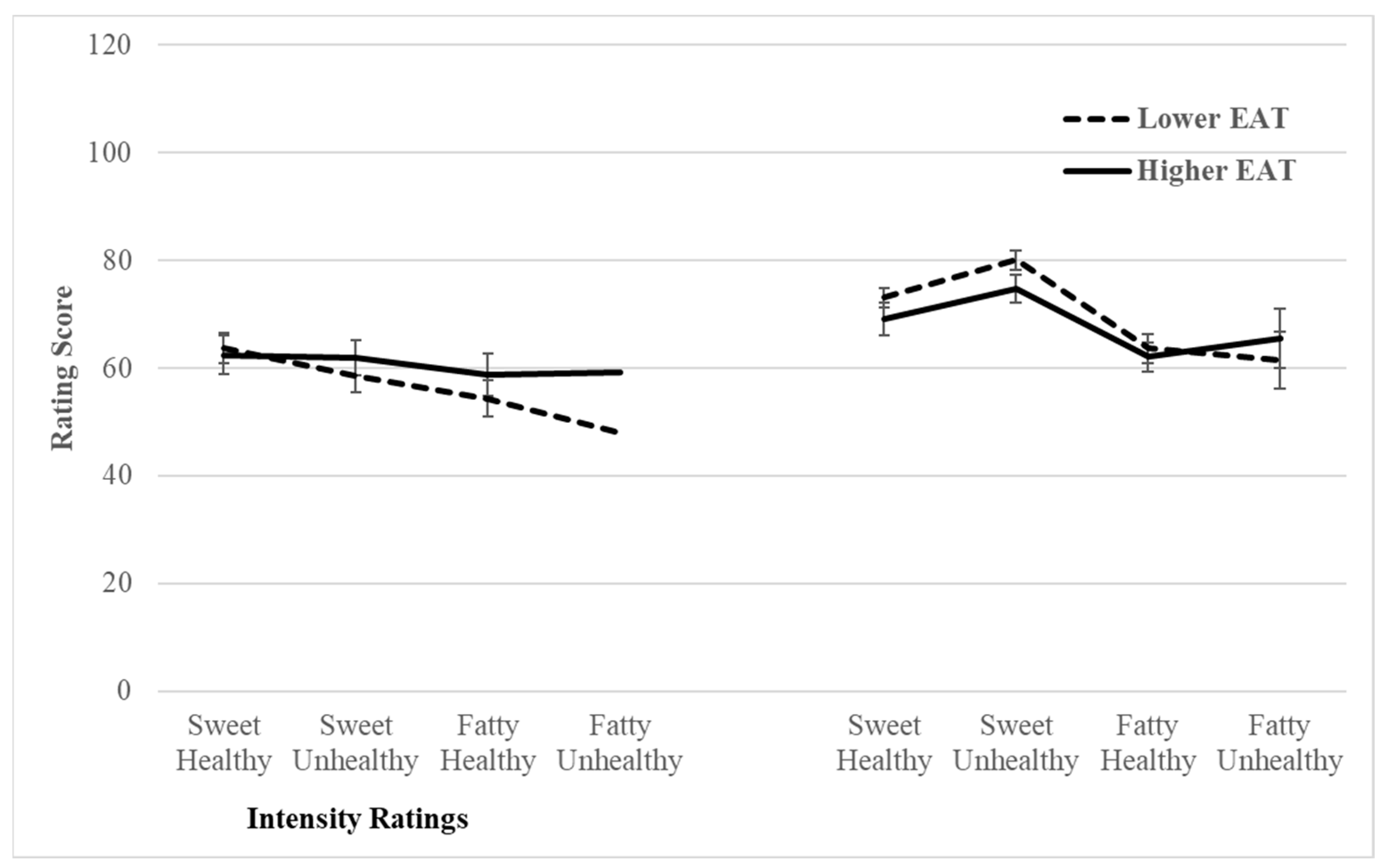
3.3.2. Did Lower and Higher EAT Median Groups Rate the Food Odours Differently Depending on the Food Type?
3.3.3. Did Lower and Higher EAT Median Groups Rate the Liquid Tastant Differently Depending on the Tastant Type?
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Eeden, A.E.; van Hoeken, D.; Hoek, H.W. Incidence, prevalence and mortality of anorexia and bulimia nervosa. Curr. Opin. Psychiatry 2021, 34, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F. Mortality in anorexia nervosa. Am. J. Psychiatry 1995, 152, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, B.; Lederhendler, I. Research on eating disorders: Current status and future prospects. Biol. Psychiatry 2000, 47, 777–786. [Google Scholar] [CrossRef]
- Aschenbrenner, K.; Scholze, N.; Joraschky, P.; Hummel, T. Gustatory and olfactory sensitivity in patients with anorexia and bulimia in the course of treatment. J. Psychiatr. Res. 2008, 43, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.D.; Tucker, M.; Gerstner, N. A bitter sweet asynchrony. The relation between eating attitudes, dietary restraint on smell and taste function. Appetite 2013, 70, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Birmingham, C. Zinc supplementation in the treatment of anorexia nervosa. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2002, 7, 20–22. [Google Scholar] [CrossRef]
- Roessner, V.; Bleich, S.; Banaschewski, T.; Rothenberger, A. Olfactory deficits in anorexia nervosa. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 255, 6–9. [Google Scholar] [CrossRef]
- Clark, J.E. Taste and flavour: Their importance in food choice and acceptance. Proc. Nutr. Soc. 1998, 57, 639–643. [Google Scholar] [CrossRef]
- Hurwitz, T.; Kopala, L.; Clark, C.; Jones, B. Olfactory deficits in schizophrenia. Biol. Psychiatry 1988, 23, 123–128. [Google Scholar] [CrossRef]
- Kopala, L.; Clark, C.; A Hurwitz, T. Sex differences in olfactory function in schizophrenia. Am. J. Psychiatry 1989, 146, 1320–1322. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Settle, R.; Doty, R.L.; Abelman, E.; Winokur, A. Taste and smell perception in depression. Biol. Psychiatry 1987, 22, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Kopala, L.C.; Good, K.; Goldner, E.M.; Birmingham, C.L. Olfactory identification ability in anorexia nervosa. J. Psychiatry Neurosci. 1995, 20, 283. [Google Scholar] [PubMed]
- Doty, R.L.; Shaman, P.; Dann, M. Development of the university of pennsylvania smell identification test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 1984, 32, 489–502. [Google Scholar] [CrossRef]
- Fedoroff, I.C.; Stoner, S.A.; Andersen, A.E.; Doty, R.L.; Rolls, B.J. Olfactory Dysfunction in anorexia and bulimia nervosa. Int. J. Eat. Disord. 1995, 18, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Sekinger, B.; Wolf, S.; Pauli, E.; Kobal, G. ‘Sniffin’ Sticks’: Olfactory performance assessed by the combined testing of odour identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Rapps, N.; Giel, K.E.; Söhngen, E.; Salini, A.; Enck, P.; Bischoff, S.C.; Zipfel, S. Olfactory deficits in patients with anorexia nervosa. Eur. Eat. Disord. Rev. 2010, 18, 385–389. [Google Scholar] [CrossRef]
- Bentz, M.; Guldberg, J.; Vangkilde, S.; Pedersen, T.; Plessen, K.J.; Jepsen, J.R.M. Heightened olfactory sensitivity in young females with recent-onset anorexia nervosa and recovered individuals. PLoS ONE 2017, 12, e0169183. [Google Scholar] [CrossRef]
- Churnin, I.; Qazi, J.; Fermin, C.R.; Wilson, J.H.; Payne, S.C.; Mattos, J.L. Association Between Olfactory and Gustatory Dysfunction and Cognition in Older Adults. Am. J. Rhinol. Allergy 2019, 33, 170–177. [Google Scholar] [CrossRef]
- Casper, R.C.; Kirschner, B.; Sandstead, H.H.; A Jacob, R.; Davis, J.M. An evaluation of trace metals, vitamins, and taste function in anorexia nervosa. Am. J. Clin. Nutr. 1980, 33, 1801–1808. [Google Scholar] [CrossRef]
- Nakai, Y.; Kinoshita, F.; Koh, T.; Tsujii, S.; Tsukada, T. Taste function in patients with anorexia nervosa and bulimia nervosa. Int. J. Eat. Disord. 1987, 6, 257–265. [Google Scholar] [CrossRef]
- Klein, D.A.; Ba, G.S.B.; Devlin, M.J.; Walsh, B.T. Artificial sweetener use among individuals with eating disorders. Int. J. Eat. Disord. 2006, 39, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.F.; Ahene, P.; Lacey, J.H. Salinophagia in anorexia nervosa: Case resports. Int. J. Eat. Disord. 2010, 43, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, S.-I.; Masuda, A.; Naruo, T.; Soejima, Y.; Nagai, N.; Tanaka, H. Changes in taste responsiveness in patients with anorexia nervosa during behavior therapy. Physiol. Behav. 1996, 59, 549–553. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Shott, M.E.; Keffler, C.; Cornier, M.-A. Extremes of eating are associated with reduced neural taste discrimination. Int. J. Eat. Disord. 2016, 49, 603–612. [Google Scholar] [CrossRef]
- Brand-Gothelf, A.; Parush, S.; Eitan, Y.; Admoni, S.; Gur, E.; Stein, D. Sensory modulation disorder symptoms in anorexia nervosa and bulimia nervosa: A pilot study. Int. J. Eat. Disord. 2016, 49, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; A Halmi, K.; Pierce, B.; Gibbs, J.; Smith, G.P. Taste and eating disorders. Am. J. Clin. Nutr. 1987, 46, 442–450. [Google Scholar] [CrossRef]
- Hartman-Petrycka, M.; Klimacka-Nawrot, E.; Ziora, K.; Suchecka, W.; Gorczyca, P.; Rojewska, K.; Błońska-Fajfrowska, B. Sweet, Salty, and umami taste sensitivity and the hedonic perception of taste sensations in adolescent females with anorexia nervosa. Nutrients 2022, 14, 1042. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Cowart, B.J. Development of sweet taste. In Sweetness: ILSI Human Nutrition Reviews; Dobbing, J., Ed.; Springer: London, UK, 1987; pp. 127–140. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Bitter taste receptors and human bitter taste perception. Cell. Mol. Life Sci. 2006, 63, 1501–1509. [Google Scholar] [CrossRef]
- Sunday, S.R.; A Einhorn, A.; A Halmi, K. Relationship of perceived macronutrient and caloric content to affective cognitions about food in eating-disordered, restrained, and unrestrained subjects. Am. J. Clin. Nutr. 1992, 55, 362–371. [Google Scholar] [CrossRef]
- Drewnowski, A. Taste responsiveness in eating disorders. Ann. N. Y. Acad. Sci. 1989, 595, 399–409. [Google Scholar] [CrossRef]
- Koritar, P.; Philippi, S.T.; Alvarenga, M.d.S. Attitudes toward health and taste of food among women with bulimia nervosa and women of a non-clinical sample. Appetite 2017, 113, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W. Neurobiology of anorexia and bulimia nervosa. Physiol. Behav. 2008, 94, 121–135. [Google Scholar] [CrossRef]
- Simon, Y.; Bellisle, F.; Monneuse, M.-O.; Samuel-Lajeunesse, B.; Drewnowski, A. Taste Responsiveness in Anorexia Nervosa. Br. J. Psychiatry 1993, 162, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Keating, C.; Tilbrook, A.J.; Rossell, S.L.; Enticott, P.G.; Fitzgerald, P.B. Reward processing in anorexia nervosa. Neuropsychologia 2012, 50, 567–575. [Google Scholar] [CrossRef]
- Garfinkel, P.E.; Moldofsky, H.; Garner, D.M.; Stancer, H.C.; Coscina, D.V. Body awareness in anorexia nervosa: Disturbances in “body image” and “satiety”. Psychosom. Med. 1978, 40, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Sunday, S.R.; Halmi, K.A. Taste perceptions and hedonics in eating disorders. Physiol. Behav. 1990, 58, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Wichstrøm, L. Social, psychological and physical correlates of eating problems. A study of the general population in Norway. Psychol. Med. 1995, 25, 567–579. [Google Scholar] [CrossRef]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The eating attitudes test: Psychometric features and clinical correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef]
- Banasiak, S.J.; Wertheim, E.H.; Koerner, J.; Voudouris, N.J. Test-retest reliability and internal consistency of a variety of measures of dietary restraint and body concerns in a sample of adolescent girls. Int. J. Eat. Disord. 2001, 29, 85–89. [Google Scholar] [CrossRef]
- Eiber, R.; Berlin, I.; de Brettes, B.; Foulon, C.; Guelfi, J.D. Hedonic response to sucrose solutions and the fear of weight gain in patients with eating disorders. Psychiatry Res. 2002, 113, 173–180. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.-W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Mahmut, M.K.; Banzer, B. Exploring the relationship between psychopathy and taste perception. Chemosens. Percept. 2021, 14, 47–56. [Google Scholar] [CrossRef]
- Prescott, J.; Laing, D.; Bell, G.; Yoshida, M.; Gillmore, R.; Allen, S.; Yamazaki, K.; Ishii, R. Hedonic responses to taste solutions: A cross-cultural study of Japanese and Australians. Chem. Senses 1992, 17, 801–809. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Version 28.0; IBM Corp.: Armonk, NY, USA, 2021. [Google Scholar]
- Kumar, S.; Singh, A.K.; Kaur, M.; Sharma, A. The prevalence of eating disorder among young girls and boys by using eating attitude Test. Int. J. Phys. Educ. Sports Health 2016, 3, 378–381. [Google Scholar]
- Sanlier, N.; Varli, S.N.; Macit, M.S.; Mortas, H.; Tatar, T. Evaluation of disordered eating tendencies in young adults. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2017, 22, 623–631. [Google Scholar] [CrossRef]
- Goldzak-Kunik, G.; Friedman, R.; Spitz, M.; Sandler, L.; Leshem, M. Intact sensory function in anorexia nervosa. Am. J. Clin. Nutr. 2012, 95, 272–282. [Google Scholar] [CrossRef]
- Machado, P.P.; Machado, B.C.; Gonçalves, S.; Hoek, H.W. The prevalence of eating disorders not otherwise specified. Int. J. Eat. Disord. 2007, 40, 212–217. [Google Scholar] [CrossRef]
- Peebles, R.; Hardy, K.K.; Wilson, J.L.; Lock, J.D. Are diagnostic criteria for eating disorders markers of medical severity? Pediatrics 2010, 125, e1193–e1201. [Google Scholar] [CrossRef]
- Luce, K.H.; Crowther, J.H. The reliability of the eating disorder examination: Self-report questionnaire version. Int. J. Eat. Disord. 1999, 25, 349–351. [Google Scholar] [CrossRef]
- Deloitte Access Economics. Investing in Need: Cost-Effective Interventions for Eating Disorders. The Butterfly Foundation. 2015. Available online: https://www.deloitteaccesseconomics.com.au/uploads/File/Butterfly_Report_Paying%20the%20Price_online.pdf (accessed on 20 January 2023).
- Rivas, T.; Bersabé, R.; Jiménez, M.; Berrocal, C. The eating attitudes test: Reliability and validity in Spanish female samples. Span. J. Psychol. 2010, 13, 1044–1056. [Google Scholar] [CrossRef]
| Concentration Level | ||||
|---|---|---|---|---|
| Tastant | Very low mM (g/L) | Low mM (g/L) | Medium mM (g/L) | High mM (g/L) |
| Sucrose (sweet) | 20 (6.85) | 40 (13.7) | 145 (49.6) | 420 (143.8) |
| Caffeine (bitter) | 0.7 (0.136) | 1.9 (0.369) | 6.5 (1.26) | 39 (7.5) |
| Variable | Descriptives | Correlations | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Min-Max | BMI | Hunger Mean | Odour Threshold | Odour Discrim. | |
| EAT-26 score | 8.1 (8.9) | 0.0–42.0 | −0.05 | −0.04 | 0.07 | −0.18 |
| Body Mass Index | 22.5 (4.3) | 14.5–35.1 | −0.11 | 0.12 | 0.24 * | |
| Hunger Mean | 5.0 (1.9) | 1.3–9.0 | 0.08 | −0.11 | ||
| Odour Threshold | 6.9 (2.9) | 1.0–13.0 | ||||
| Odour Discrimination | 9.2 (2.1) | 3.0–14.0 | ||||
| Food Odour Ratings | Descriptives | Correlations | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Min-Max | EAT-26 | BMI | Hunger Mean | Odour Threshold | Odour Discrim. | |
| High Sugar, Healthy | |||||||
| Odour Intensity | 63.1 (21.56) | 25–113 | −0.11 | 0.06 | 0.04 | −0.03 | −0.01 |
| Odour Pleasantness | 71.3 (16.29) | 29–114 | −0.10 | 0.20 | −0.01 | 0.13 | 0.12 |
| High Sugar, Unhealthy | |||||||
| Odour Intensity | 60.15 (20.01) | 19.5–112.5 | 0.02 | 0.02 | 0.03 | −0.11 | −0.05 |
| Odour Pleasantness | 77.65 (15.165) | 31–107 | −0.06 | 0.02 | 0.18 | 0.17 | 0.12 |
| High Fat, Healthy | |||||||
| Odour Intensity | 56.35 (19.98) | 11–107 | 0.10 | 0.07 | 0.12 | −0.08 | −0.06 |
| Odour Pleasantness | 62.9 (13.63) | 32.5–95.5 | −0.15 | −0.10 | 0.08 | 0.16 | 0.11 |
| High Fat, Unhealthy | |||||||
| Odour Intensity | 53.2 (23.595) | 8–116.5 | 0.18 | 0.19 | 0.01 | 0.14 | 0.13 |
| Odour Pleasantness | 63.35 (16.985) | 14–95.5 | 0.08 | 0.05 | 0.15 | 0.07 | 0.04 |
| Liquid Tastant Ratings | Descriptives | Correlations | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Min-Max | EAT-26 | BMI | Hunger Mean | Odour Threshold | Odour Discrim. | |
| Sweet tastant | |||||||
| Sweetness | 44.9 (17.91) | 1.75–97 | 0.00 | 0.04 | 0.05 | 0.21 | 0.13 |
| Intensity | 40.4 (15.6) | 6.75–94.5 | 0.10 | −0.02 | −0.03 | 0.10 | 0.06 |
| Pleasantness | 62 (17.31) | 3–116.25 | −0.10 | −0.08 | 0.09 | −0.07 | 0.09 |
| Bitter tastant | |||||||
| Bitterness | 47 (21.48) | 1.75–98 | 0.13 | −0.05 | 0.02 | 0.11 | 0.00 |
| Intensity | 45.5 (18.44) | 2.25–91.75 | 0.17 | 0.07 | −0.17 | 0.22 # | 0.01 |
| Pleasantness | 39.2 (13.37) | 5–75.25 | −0.18 | −0.05 | 0.22 * | 0.04 | 0.23 * |
| Fatty tastant | |||||||
| Fattiness | 56.8 (22.44) | 5.25–111.75 | 0.04 | 0.21 | −0.01 | 0.06 | −0.06 |
| Intensity | 45.1 (19.78) | 4.5–92.75 | 0.13 | 0.03 | −0.02 | 0.01 | −0.21 |
| Pleasantness | 51 (18.02) | 5.75–96.25 | −0.15 | −0.18 | 0.22 * | −0.16 | −0.07 |
| Variable | Lower EAT (n = 43) | Higher EAT (n = 37) | ||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| BMI | 22.3 (3.66) | 22.7 (4.98) | ||
| Odour Threshold | 6.8 (2.76) | 7.0 (2.89) | ||
| Odour Discrimination | 9.4 (2.12) | 9.0 (2.18) | ||
| Food Category | Intensity | Pleasantness | Intensity | Pleasantness |
| High sugar-healthy | 63.65 (20.405) | 73.1 (16.83) | 62.45 (23.095) | 69.2 (15.6) |
| High sugar-unhealthy | 58.65 (18.64) | 80.1 (11.505) | 61.85 (21.63) | 74.75 (18.285) |
| High fat, healthy | 54.35 (20.33) | 63.65 (11.91) | 58.7 (19.58) | 62.05 (15.52) |
| High fat, unhealthy | 48 (22.165) | 61.45 (17.32) | 59.2 (24.075) | 65.55 (16.555) |
| Liquid Tastant type | Intensity | Pleasantness | Intensity | Pleasantness |
| Sweet mean | 38.4 (13.98) | 63.5 (12.1) | 42.7 (17.21) | 60.2 (21.93) |
| Bitter mean | 45.0 (19.11) | 40.4 (11.01) | 46.2 (17.87) | 37.8 (15.72) |
| Fatty mean | 43.5 (19.37) | 52.3 (14.19) | 47 (20.34) | 49.7 (21.78) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
St Clair, L.; Grady, A.; Mahmut, M.K. Investigating the Link between Eating Attitudes, Taste and Odour Preferences and the Chemical Senses. Biology 2023, 12, 1415. https://doi.org/10.3390/biology12111415
St Clair L, Grady A, Mahmut MK. Investigating the Link between Eating Attitudes, Taste and Odour Preferences and the Chemical Senses. Biology. 2023; 12(11):1415. https://doi.org/10.3390/biology12111415
Chicago/Turabian StyleSt Clair, Layla, Alyssa Grady, and Mehmet K. Mahmut. 2023. "Investigating the Link between Eating Attitudes, Taste and Odour Preferences and the Chemical Senses" Biology 12, no. 11: 1415. https://doi.org/10.3390/biology12111415
APA StyleSt Clair, L., Grady, A., & Mahmut, M. K. (2023). Investigating the Link between Eating Attitudes, Taste and Odour Preferences and the Chemical Senses. Biology, 12(11), 1415. https://doi.org/10.3390/biology12111415






