Seasonal Patterns of Picocyanobacterial Community Structure in the Kuroshio Current
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Scheme
2.2. Determination of Chl a and Inorganic Nutrient Concentrations
2.3. Determination of Picophytoplankton Abundance
2.4. Picoplanktonic DNA Isolation
2.5. The Diversity of Picoplankton Composition
2.6. Nucleotide Sequence Deposition
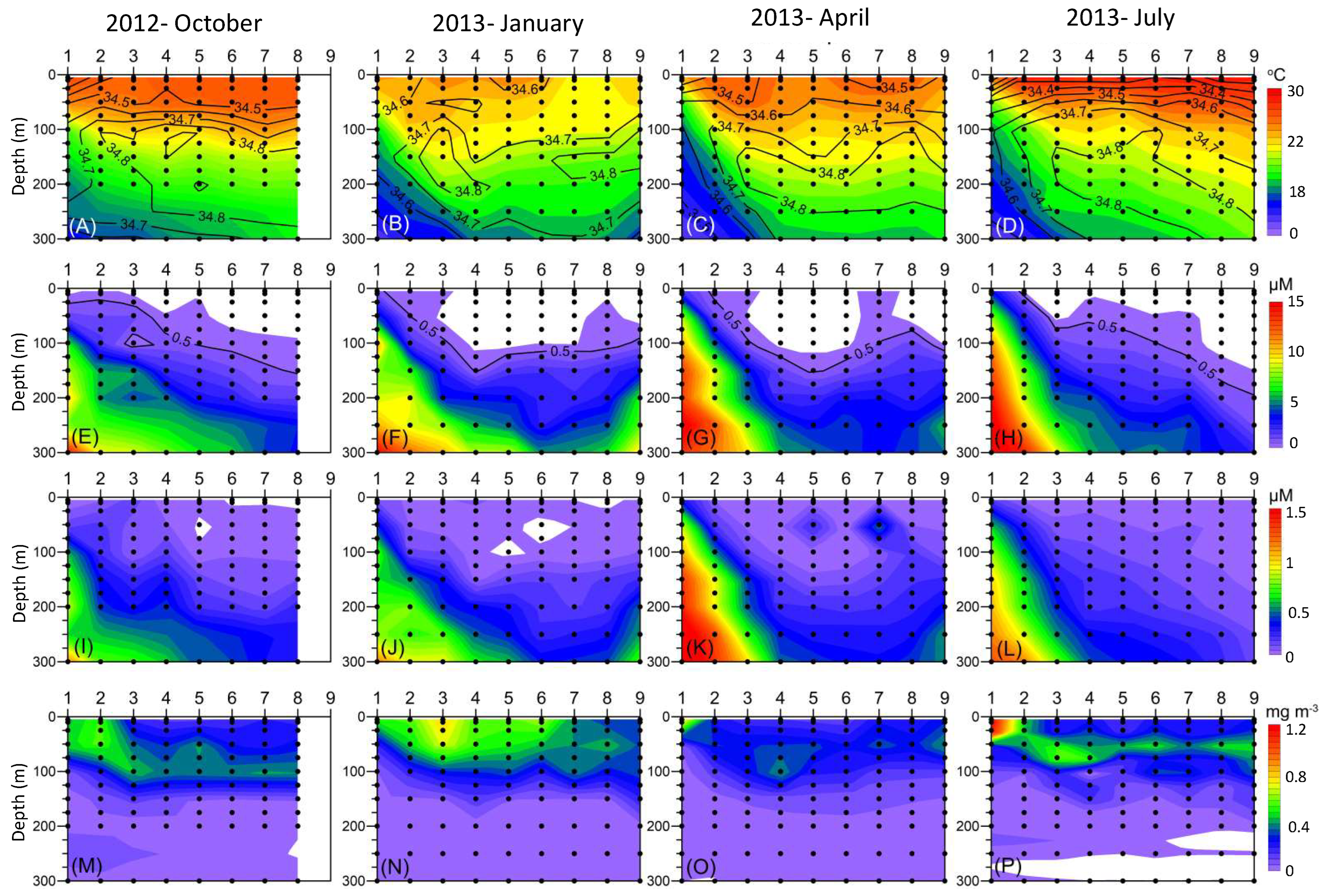
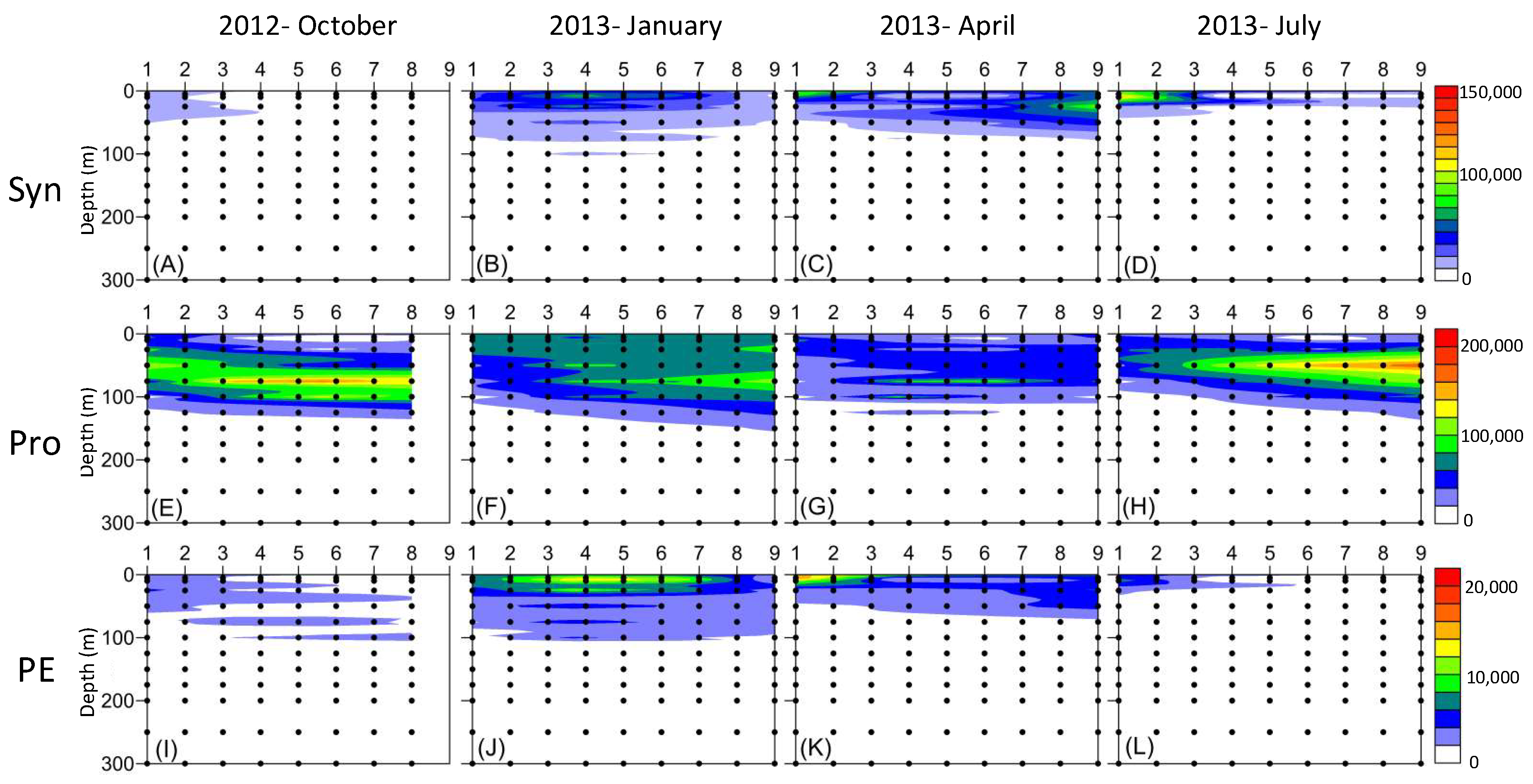
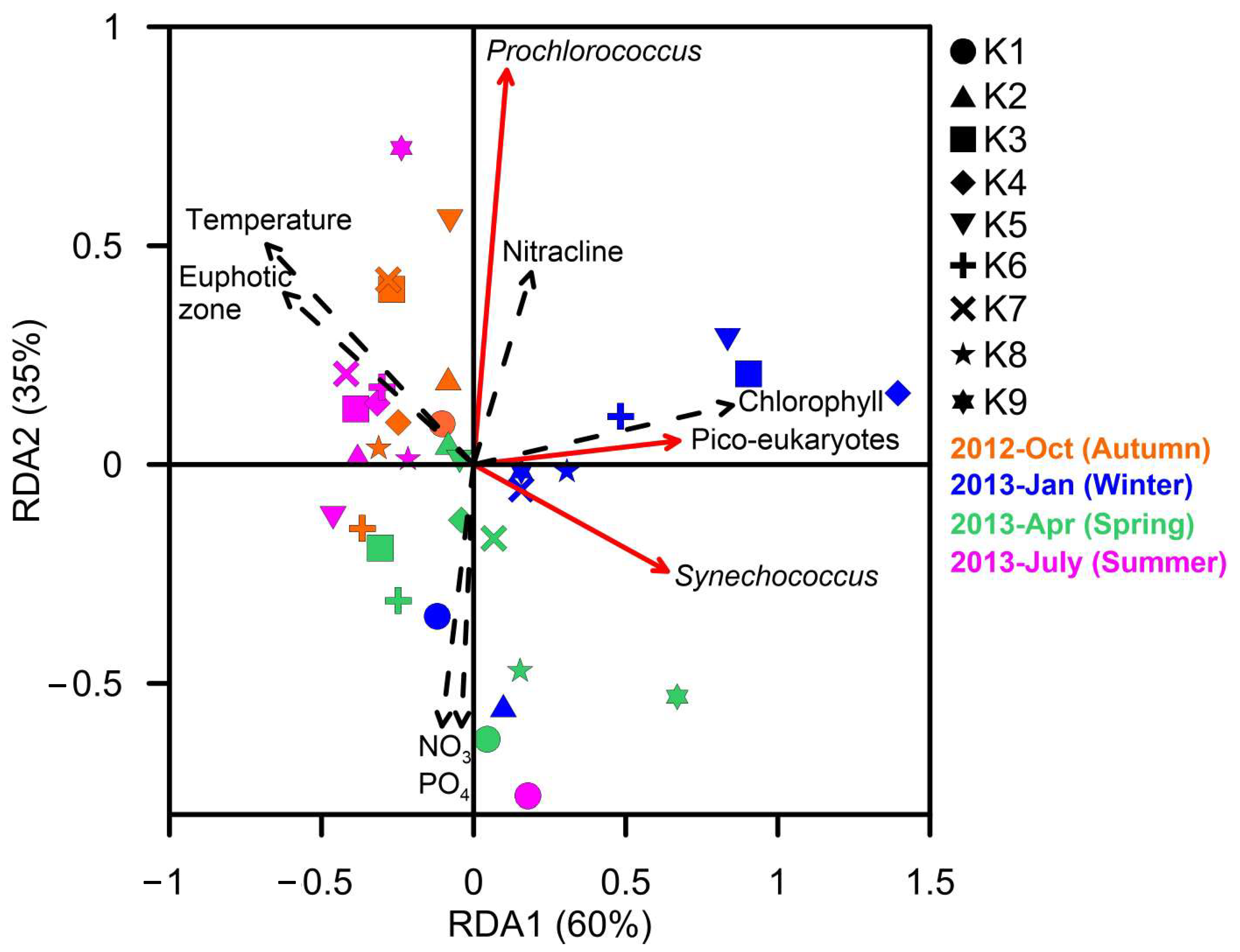
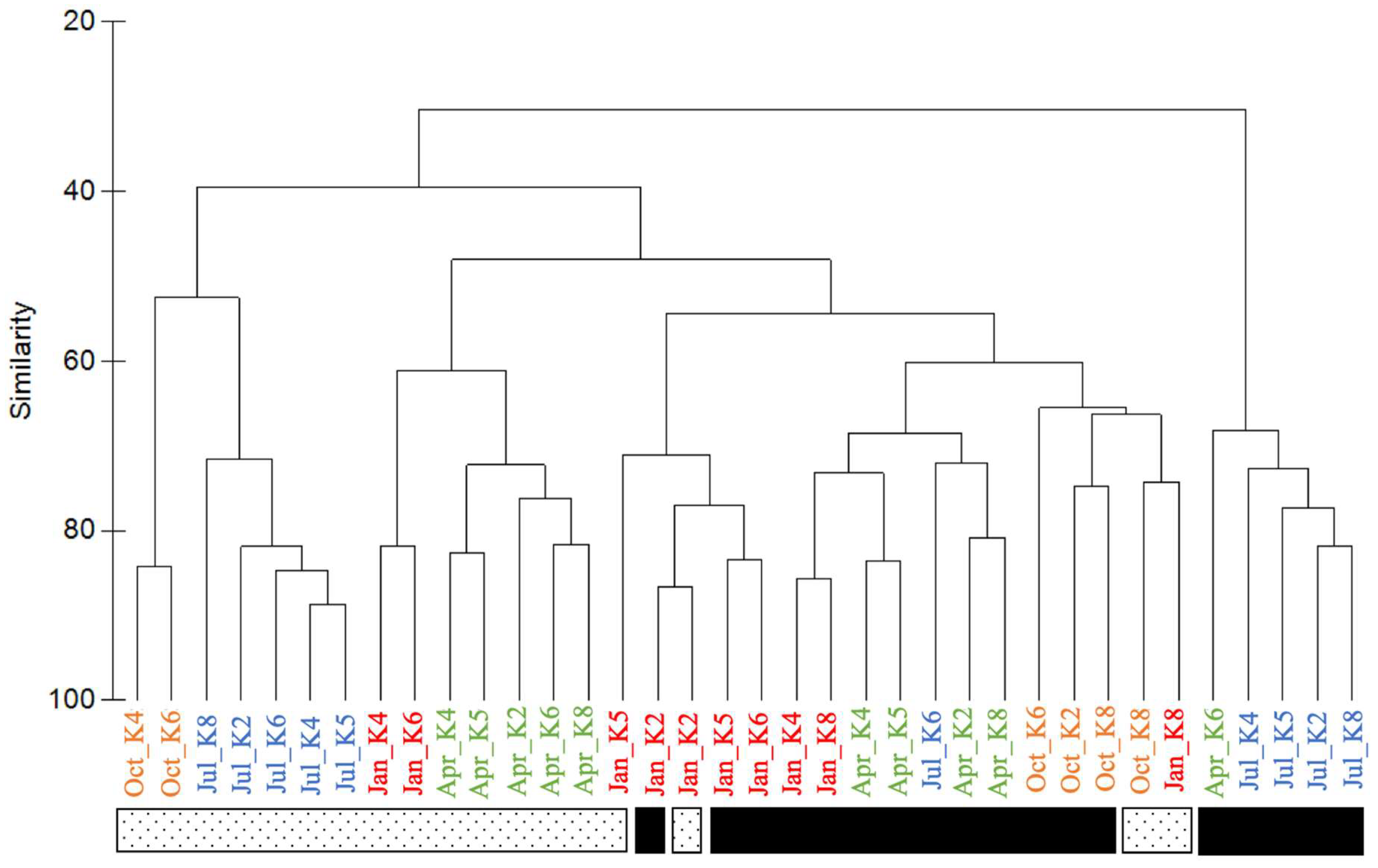
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uitz, J.; Claustre, H.; Gentili, B.; Stramski, D. Phytoplankton class-specific primary production in the world’s oceans: Seasonal and interannual variability from satellite observations. Glob. Biogeochem. Cycles 2010, 24, GB3016. [Google Scholar] [CrossRef]
- Chavez, F.P.; Messié, M.; Pennington, J.T. Marine primary production in relation to climate variability and change. Annu. Rev. Mar. Sci. 2011, 3, 227–260. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, H.; Deng, J.; Zhou, R.; Zeng, Q. Biological interactions with Prochlorococcus: Implications for the marine carbon cycle. Trends Microbiol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.L.; Jackson, G.A.; Ducklow, H.W.; Roman, M.R. Carbon fluxes through food webs of the eastern equatorial Pacific: An inverse approach. Deep Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 1245–1274. [Google Scholar] [CrossRef]
- Magazzu, G.; Decembrini, F. Primary production, biomass and abundance of phototrophic picoplankton in the Mediterranean Sea: A review. Aquat. Microb. Ecol. 1995, 9, 97–104. [Google Scholar] [CrossRef]
- Morán, X.A.G.; López-Urrutia, Á.; Calvo-Díaz, A.; Li, W.K. Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Flombaum, P.; Wang, W.-L.; Primeau, F.W.; Martiny, A.C. Global picophytoplankton niche partitioning predicts overall positive response to ocean warming. Nat. Geosci. 2020, 13, 116–120. [Google Scholar] [CrossRef]
- Zwirglmaier, K.; Jardillier, L.; Ostrowski, M.; Mazard, S.; Garczarek, L.; Vaulot, D.; Not, F.; Massana, R.; Ulloa, O.; Scanlan, D.J. Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages among oceanic biomes. Environ. Microbiol. 2008, 10, 147–161. [Google Scholar] [CrossRef]
- Tai, V.; Palenik, B. Temporal variation of Synechococcus clades at a coastal Pacific Ocean monitoring site. ISME J. 2009, 3, 903–915. [Google Scholar] [CrossRef]
- Choi, D.H.; Noh, J.H.; Hahm, M.-S.; Lee, C.M. Picocyanobacterial abundances and diversity in surface water of the northwestern Pacific Ocean. Ocean Sci. J. 2011, 46, 265–271. [Google Scholar] [CrossRef]
- Post, A.F.; Penno, S.; Zandbank, K.; Paytan, A.; Huse, S.M.; Welch, D.M. Long term seasonal dynamics of Synechococcus population structure in the Gulf of Aqaba, Northern Red Sea. Front. Microbiol. 2011, 2, 131. [Google Scholar] [CrossRef] [PubMed]
- Zwirglmaier, K.; Heywood, J.L.; Chamberlain, K.; Woodward, E.M.S.; Zubkov, M.V.; Scanlan, D.J. Basin-scale distribution patterns of picocyanobacterial lineages in the Atlantic Ocean. Environ. Microbiol. 2007, 9, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.G.; Baer, S.E.; Mouginot, C.; Huang, J.S.; Larkin, A.A.; Lomas, M.W.; Martiny, A.C. Parallel phylogeography of Prochlorococcus and Synechococcus. ISME J. 2019, 13, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.J.; Chisholm, S.W.; Zettler, E.R.; Altabet, M.A.; Dusenberry, J.A. Spatial and temporal distributions of prochlorophyte picoplankton in the North Atlantic Ocean. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 1033–1051. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Zinser, E.R.; Coe, A.; McNulty, N.P.; Woodward, E.M.S.; Chisholm, S.W. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 2006, 311, 1737–1740. [Google Scholar] [CrossRef]
- Zinser, E.R.; Coe, A.; Johnson, Z.I.; Martiny, A.C.; Fuller, N.J.; Scanlan, D.J.; Chisholm, S.W. Prochlorococcus ecotype abundances in the North Atlantic Ocean as revealed by an improved quantitative PCR method. Appl. Environ. Microb. 2006, 72, 723–732. [Google Scholar] [CrossRef]
- Zinser, E.R.; Johnson, Z.I.; Coe, A.; Karaca, E.; Veneziano, D.; Chisholm, S.W. Influence of light and temperature on Prochlorococcus ecotype distributions in the Atlantic Ocean. Limnol. Oceanogr. 2007, 52, 2205–2220. [Google Scholar] [CrossRef]
- Malmstrom, R.R.; Coe, A.; Kettler, G.C.; Martiny, A.C.; Frias-Lopez, J.; Zinser, E.R.; Chisholm, S.W. Temporal dynamics of Prochlorococcus ecotypes in the Atlantic and Pacific oceans. ISME J. 2010, 4, 1252–1264. [Google Scholar] [CrossRef]
- MacGregor-Chatwin, C.; Jackson, P.; Sener, M.; Chidgey, J.; Hitchcock, A.; Qian, P.; Mayneord, G.; Johnson, M.; Luthey-Schulten, Z.; Dickman, M. Membrane organization of photosystem I complexes in the most abundant phototroph on Earth. Nat. Plants 2019, 5, 879–889. [Google Scholar] [CrossRef]
- Penno, S.; Lindell, D.; Post, A.F. Diversity of Synechococcus and Prochlorococcus populations determined from DNA sequences of the N-regulatory gene ntcA. Environ. Microbiol. 2006, 8, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.J.; Berube, P.M.; Berta-Thompson, J.W.; Kelly, L.; Roggensack, S.E.; Awad, L.; Roache-Johnson, K.H.; Ding, H.; Giovannoni, S.J.; Rocap, G. Genomes of diverse isolates of the marine cyanobacterium Prochlorococcus. Sci. Data 2014, 1, 140034. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-C.; Chang, J.; Gong, G.-C.; Hsu, S.-C.; Chiang, K.-P.; Liao, C.-W. Effects of Asian dust storms on Synechococcus populations in the subtropical Kuroshio Current. Mar. Biotechnol. 2011, 13, 751–763. [Google Scholar] [CrossRef]
- Chung, C.-C.; Gong, G.-C.; Huang, C.-Y.; Lin, J.-Y.; Lin, Y.-C. Changes in the Synechococcus assemblage composition at the surface of the east China Sea due to flooding of the Changjiang river. Microb. Ecol. 2015, 70, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Lukas, R. Seasonal and interannual variability of the North Equatorial Current, the Mindanao Current, and the Kuroshio along the Pacific western boundary. J. Geophys. Res. Ocean. 1996, 101, 12315–12330. [Google Scholar] [CrossRef]
- Qiu, B. Kuroshio and Oyashio currents. In Encyclopedia of Ocean Sciences; Elsevier: Amsterdam, The Netherlands, 2001; pp. 61–72. [Google Scholar]
- Rudnick, D.L.; Jan, S.; Centurioni, L.; Lee, C.M.; Lien, R.-C.; Wang, J.; Lee, D.-K.; Tseng, R.-S.; Kim, Y.Y.; Chern, C.-S. Seasonal and mesoscale variability of the Kuroshio near its origin. Oceanography 2011, 24, 52–63. [Google Scholar] [CrossRef]
- Guo, Y. The Kuroshio. Part II. Primary productivity and phytoplankton. Oceanogr. Mar. Biol. Annu. Rev. 1991, 29, 155–189. [Google Scholar]
- Chen, C.-C.; Meng, P.-J.; Hsieh, C.-H.; Jan, S. Plankton Community Respiration and Particulate Organic Carbon in the Kuroshio East of Taiwan. Plants 2022, 11, 2909. [Google Scholar] [CrossRef]
- Shen, M.-L.; Tseng, Y.-H.; Jan, S. The formation and dynamics of the cold-dome off northeastern Taiwan. J. Mar. Syst. 2011, 86, 10–27. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsu, S.-C.; Jan, S.; Gong, G.-C. Episodic events imposed on the seasonal nutrient dynamics of an upwelling system off northeastern Taiwan. J. Mar. Syst. 2015, 141, 128–135. [Google Scholar] [CrossRef]
- Wong, G.T.; Chao, S.-Y.; Li, Y.-H.; Shiah, F.-K. The Kuroshio edge exchange processes (KEEP) study—An introduction to hypotheses and highlights. Cont. Shelf Res. 2000, 20, 335–347. [Google Scholar] [CrossRef]
- Hung, C.-C.; Gong, G.-C. Biogeochemical responses in the southern East China Sea after typhoons. Oceanography 2011, 24, 42–51. [Google Scholar] [CrossRef]
- Gong, G.-C.; Shiah, F.-K.; Liu, K.-K.; Chuang, W.-S.; Chang, J. Effect of the Kuroshio intrusion on the chlorophyll distribution in the southern East China Sea during spring 1993. Cont. Shelf Res. 1997, 17, 79–94. [Google Scholar] [CrossRef]
- Gong, G.-C.; Chang, J.; Wen, Y.-H. Estimation of annual primary production in the Kuroshio waters northeast of Taiwan using a photosynthesis-irradiance model. Deep Sea Res. Part I Oceanogr. Res. Pap. 1999, 46, 93–108. [Google Scholar] [CrossRef]
- Lai, C.; Wu, C.; Chuang, C.; Tai, J.; Lee, K.; Kuo, H.; Shiah, F. Phytoplankton and Bacterial Responses to Monsoon-Driven Water Masses Mixing in the Kuroshio Off the East Coast of Taiwan. Front. Mar. Sci. 2021, 8, 707807. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lu, C.-Y.; Jan, S.; Hsieh, C.-h.; Chung, C.-C. Effects of the coastal uplift on the Kuroshio ecosystem, Eastern Taiwan, the western boundary current of the North Pacific Ocean. Front. Mar. Sci. 2022, 9, 796187. [Google Scholar] [CrossRef]
- Chen, Y.-L.L. Comparisons of primary productivity and phytoplankton size structure in the marginal regions of southern East China Sea. Cont. Shelf Res. 2000, 20, 437–458. [Google Scholar] [CrossRef]
- Visintini, N.; Martiny, A.C.; Flombaum, P. Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton abundances in the global ocean. Limnol. Oceanogr. Lett. 2021, 6, 207–215. [Google Scholar] [CrossRef]
- Mioduchowska, M.; Pawłowska, J.; Mazanowski, K.; Weydmann-Zwolicka, A. Contrasting Marine Microbial Communities of the Fram Strait with the First Confirmed Record of Cyanobacteria Prochlorococcus marinus in the Arctic Region. Biology 2023, 12, 1246. [Google Scholar] [CrossRef]
- Fan, L.-F.; Chow, C.H.; Gong, G.-C.; Chou, W.-C. Surface Seawater p CO2 Variation after a Typhoon Passage in the Kuroshio off Eastern Taiwan. Water 2022, 14, 1326. [Google Scholar] [CrossRef]
- Chan, Y.F.; Chung, C.C.; Gong, G.C.; Hsu, C.W. Spatial variation of abundant picoeukaryotes in the subtropical Kuroshio Current in winter. Mar. Ecol. 2020, 41, e12579. [Google Scholar] [CrossRef]
- Morel, A.; Ahn, Y.-H.; Partensky, F.; Vaulot, D.; Claustre, H. Prochlorococcus and Synechococcus: A comparative study of their optical properties in relation to their size and pigmentation. J. Mar. Res. 1993, 51, 617–649. [Google Scholar] [CrossRef]
- Pai, S.-C.; Yang, C.-C.; Riley, J.P. Formation kinetics of the pink azo dye in the determination of nitrite in natural waters. Anal. Chim. Acta 1990, 232, 345–349. [Google Scholar] [CrossRef]
- Gong, G.-C.; Liu, K.-K.; Pai, S.-C. Prediction of nitrate concentration from two end member mixing in the southern East China Sea. Cont. Shelf Res. 1995, 15, 827–842. [Google Scholar] [CrossRef]
- Gong, G.-C.; Chen, Y.-L.L.; Liu, K.-K. Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: Implications in nutrient dynamics. Cont. Shelf Res. 1996, 16, 1561–1590. [Google Scholar] [CrossRef]
- Marañón, E.; Van Wambeke, F.; Uitz, J.; Boss, E.S.; Dimier, C.; Dinasquet, J.; Engel, A.; Haëntjens, N.; Pérez-Lorenzo, M.; Taillandier, V. Deep maxima of phytoplankton biomass, primary production and bacterial production in the Mediterranean Sea. Biogeosciences 2021, 18, 1749–1767. [Google Scholar] [CrossRef]
- Crombet, Y.; Leblanc, K.; Queguiner, B.; Moutin, T.; Rimmelin, P.; Ras, J.; Claustre, H.; Leblond, N.; Oriol, L.; Pujo-Pay, M. Deep silicon maxima in the stratified oligotrophic Mediterranean Sea. Biogeosciences 2011, 8, 459–475. [Google Scholar] [CrossRef]
- Marañón, E.; Behrenfeld, M.J.; González, N.; Mouriño, B.; Zubkov, M.V. High variability of primary production in oligotrophic waters of the Atlantic Ocean: Uncoupling from phytoplankton biomass and size structure. Mar. Ecol. Prog. Ser. 2003, 257, 1–11. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Martins, I.; Bettencourt, R.; Colaco, A.; Sarradin, P.M.; Santos, R.S.; Cosson, R. The influence of nutritional conditions on metal uptake by the mixotrophic dual symbiosis harboring vent mussel Bathymodiolus azoricus. Comp. Biochem. Phys. C 2011, 153, 40–52. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Fuller, N.J.; Marie, D.; Partensky, F.; Vaulot, D.; Post, A.F.; Scanlan, D.J. Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl. Environ. Microb. 2003, 69, 2430–2443. [Google Scholar] [CrossRef] [PubMed]
- Linacre, L.; Lara-Lara, R.; Camacho-Ibar, V.; Herguera, J.C.; Bazán-Guzmán, C.; Ferreira-Bartrina, V. Distribution pattern of picoplankton carbon biomass linked to mesoscale dynamics in the southern Gulf of Mexico during winter conditions. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 106, 55–67. [Google Scholar] [CrossRef]
- Linacre, L.; Durazo, R.; Camacho-Ibar, V.; Selph, K.; Lara-Lara, J.; Mirabal-Gómez, U.; Bazán-Guzmán, C.; Lago-Lestón, A.; Fernández-Martín, E.; Sidón-Ceseña, K. Picoplankton carbon biomass assessments and distribution of Prochlorococcus ecotypes linked to Loop Current Eddies during summer in the southern Gulf of Mexico. J. Geophys. Res. Ocean. 2019, 124, 8342–8359. [Google Scholar] [CrossRef]
- Selph, K.E.; Swalethorp, R.; Stukel, M.R.; Kelly, T.B.; Knapp, A.N.; Fleming, K.; Hernandez, T.; Landry, M.R. Phytoplankton community composition and biomass in the oligotrophic Gulf of Mexico. J. Plankton Res. 2022, 44, 618–637. [Google Scholar] [CrossRef]
- Liu, K.; Suzuki, K.; Chen, B.; Liu, H. Are temperature sensitivities of Prochlorococcus and Synechococcus impacted by nutrient availability in the subtropical northwest Pacific? Limnol. Oceanogr. 2021, 66, 639–651. [Google Scholar] [CrossRef]
- Chung, C.-C.; Gong, G.-C. Attribution of the growth of a distinct population of Synechococcus to the coverage of lateral water on an upwelling. Terr. Atmos. Ocean. Sci. 2019, 30, 575–587. [Google Scholar] [CrossRef]
- Paerl, R.W.; Johnson, K.S.; Welsh, R.M.; Worden, A.Z.; Chavez, F.P.; Zehr, J.P. Differential distributions of Synechococcus subgroups across the California current system. Front. Microbiol. 2011, 2, 59. [Google Scholar] [CrossRef]
- Choi, D.H.; Noh, J.H.; Shim, J. Seasonal changes in picocyanobacterial diversity as revealed by pyrosequencing in temperate waters of the East China Sea and the East Sea. Aquat. Microb. Ecol. 2013, 71, 75–90. [Google Scholar] [CrossRef]
- Choi, D.H.; Selph, K.E.; Noh, J.H. Niche partitioning of picocyanobacterial lineages in the oligotrophic northwestern Pacific Ocean. Algae 2015, 30, 223–232. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, R.C.; Kong, F.Z.; Wei, C.J.; Liu, Z.; Geng, H.X.; Dai, L.; Zhou, Z.X.; Zhang, Q.C.; Zhou, M.J. Distribution patterns of picosized and nanosized phytoplankton assemblages in the East China Sea and the Yellow Sea: Implications on the impacts of Kuroshio intrusion. J. Geophys. Res. Ocean. 2019, 124, 1262–1276. [Google Scholar] [CrossRef]
- Chan, Y.-F.; Chiang, P.-W.; Tandon, K.; Rogozin, D.; Degermendzhi, A.; Zykov, V.; Tang, S.-L. Spatiotemporal Changes in the Bacterial Community of the Meromictic Lake Uchum, Siberia. Microb. Ecol. 2020, 81, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Partensky, F.; Garczarek, L. Prochlorococcus: Advantages and limits of minimalism. Annu. Rev. Mar. Sci. 2010, 2, 305–331. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Composition of ultraphytoplankton in the central North Atlantic. Mar. Ecol. Prog. Ser. 1995, 122, 1–8. [Google Scholar] [CrossRef]
- Landry, M.R.; Selph, K.E.; Stukel, M.R.; Swalethorp, R.; Kelly, T.B.; Beatty, J.L.; Quackenbush, C.R. Microbial food web dynamics in the oceanic Gulf of Mexico. J. Plankton Res. 2022, 44, 638–655. [Google Scholar] [CrossRef]
- Otero-Ferrer, J.L.; Cermeño, P.; Bode, A.; Fernández-Castro, B.; Gasol, J.M.; Morán, X.A.G.; Marañon, E.; Moreira-Coello, V.; Varela, M.M.; Villamaña, M. Factors controlling the community structure of picoplankton in contrasting marine environments. Biogeosciences 2018, 15, 6199–6220. [Google Scholar] [CrossRef]
- Larkin, A.A.; Blinebry, S.K.; Howes, C.; Lin, Y.; Loftus, S.E.; Schmaus, C.A.; Zinser, E.R.; Johnson, Z.I. Niche partitioning and biogeography of high light adapted Prochlorococcus across taxonomic ranks in the North Pacific. ISME J. 2016, 10, 1555–1567. [Google Scholar] [CrossRef]
- Berube, P.M.; Coe, A.; Roggensack, S.E.; Chisholm, S.W. Temporal dynamics of P rochlorococcus cells with the potential for nitrate assimilation in the subtropical A tlantic and P acific oceans. Limnol. Oceanogr. 2016, 61, 482–495. [Google Scholar] [CrossRef]
- Berube, P.M.; Rasmussen, A.; Braakman, R.; Stepanauskas, R.; Chisholm, S.W. Emergence of trait variability through the lens of nitrogen assimilation in Prochlorococcus. eLife 2019, 8, e41043. [Google Scholar] [CrossRef]
- Martiny, A.C.; Tai, A.P.; Veneziano, D.; Primeau, F.; Chisholm, S.W. Taxonomic resolution, ecotypes and the biogeography of Prochlorococcus. Environ. Microbiol. 2009, 11, 823–832. [Google Scholar] [CrossRef]
- Day, R.H. The quantitative distribution and characteristics of neuston plastic in the North Pacific Ocean, 1985–1988. In Proceedings of the Second International Conference on Marine Debris, Honolulu, HI, USA, 2–7 April 1989; pp. 247–266. [Google Scholar]
- Nagai, T.; Durán, G.S.; Otero, D.A.; Mori, Y.; Yoshie, N.; Ohgi, K.; Hasegawa, D.; Nishina, A.; Kobari, T. How the Kuroshio Current delivers nutrients to sunlit layers on the continental shelves with aid of near-inertial waves and turbulence. Geophys. Res. Lett. 2019, 46, 6726–6735. [Google Scholar] [CrossRef]
- Sen Gupta, A.; Stellema, A.; Pontes, G.M.; Taschetto, A.S.; Vergés, A.; Rossi, V. Future changes to the upper ocean Western Boundary Currents across two generations of climate models. Sci. Rep. 2021, 11, 9538. [Google Scholar] [CrossRef] [PubMed]


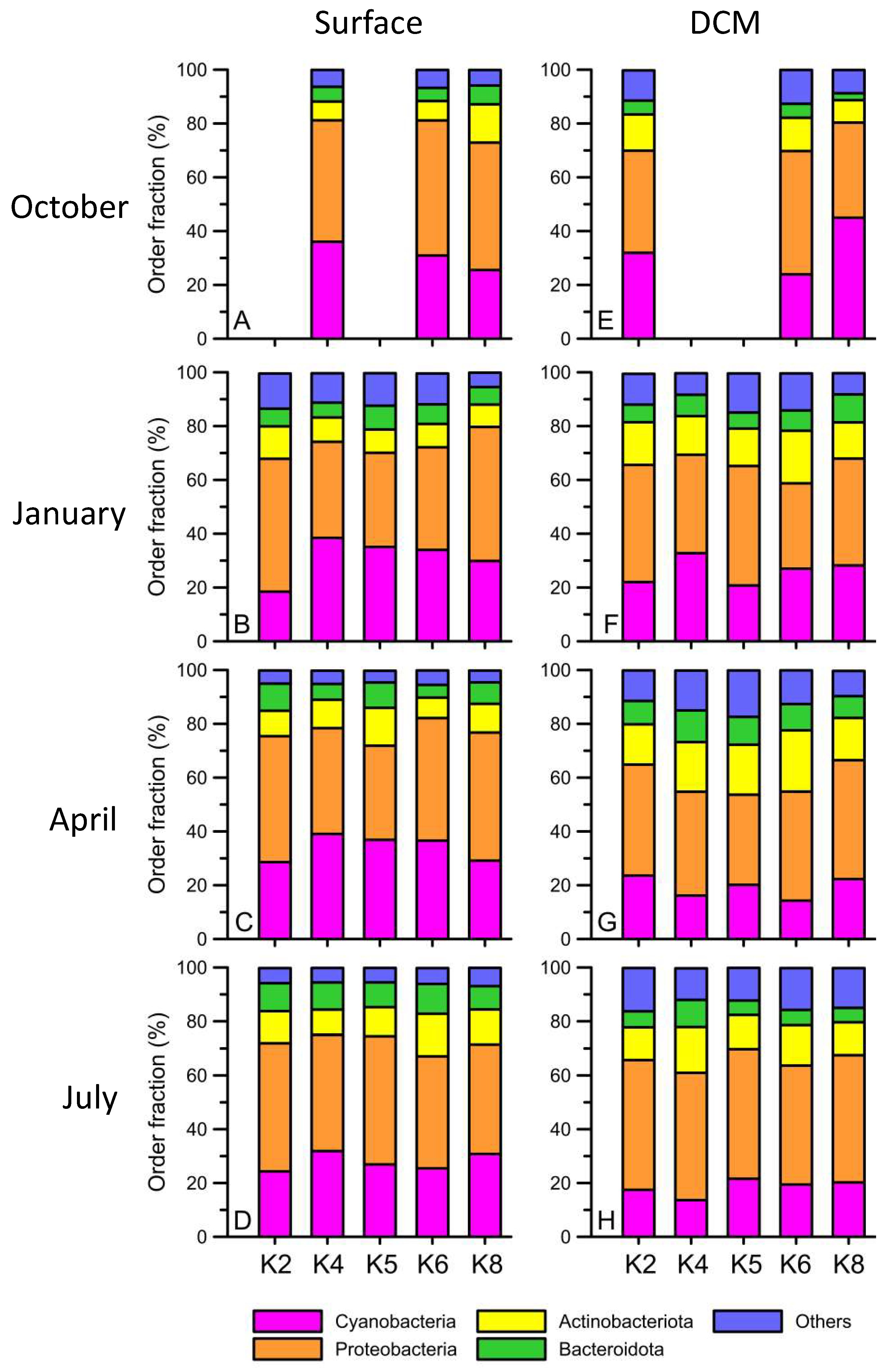
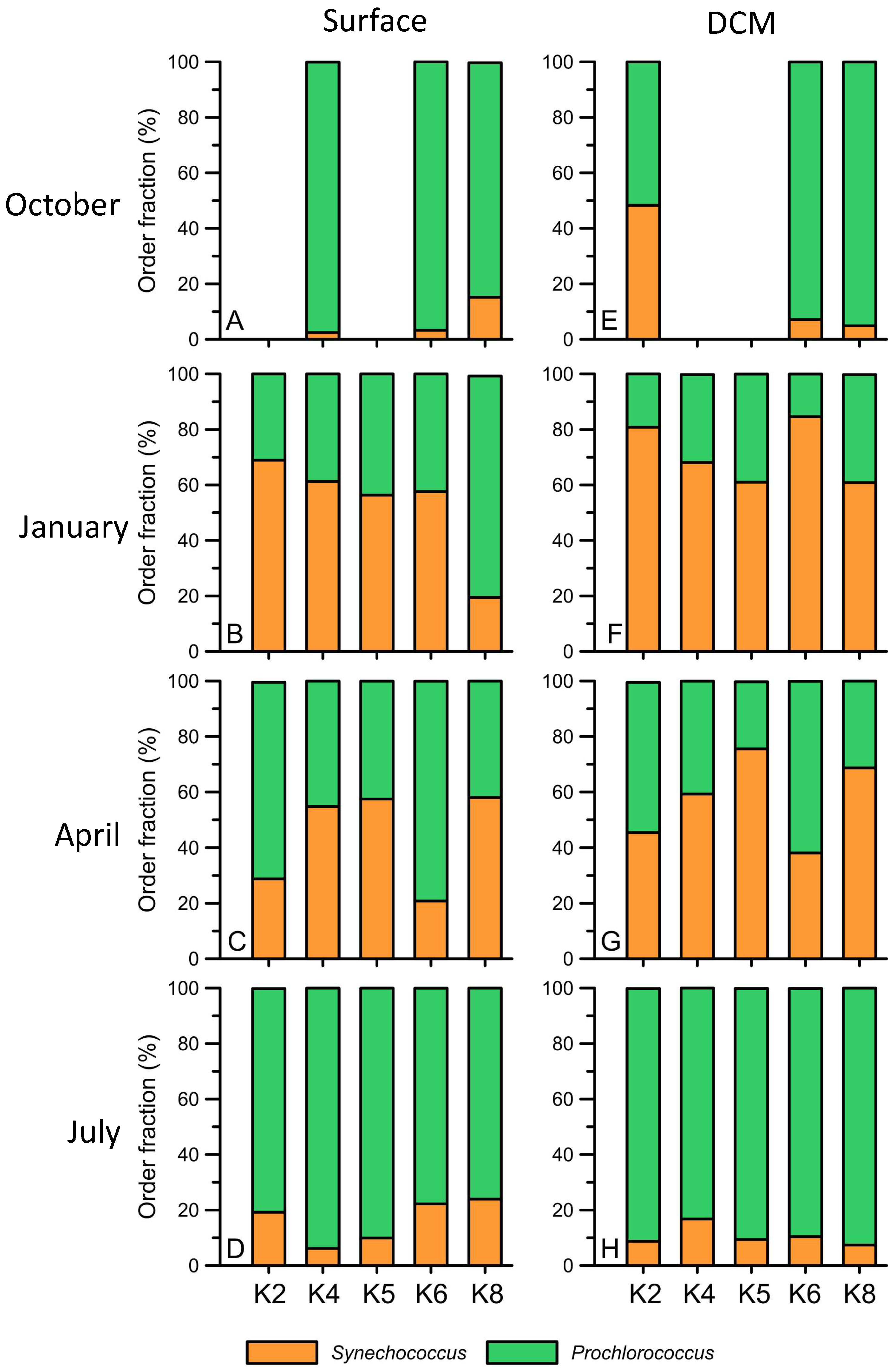

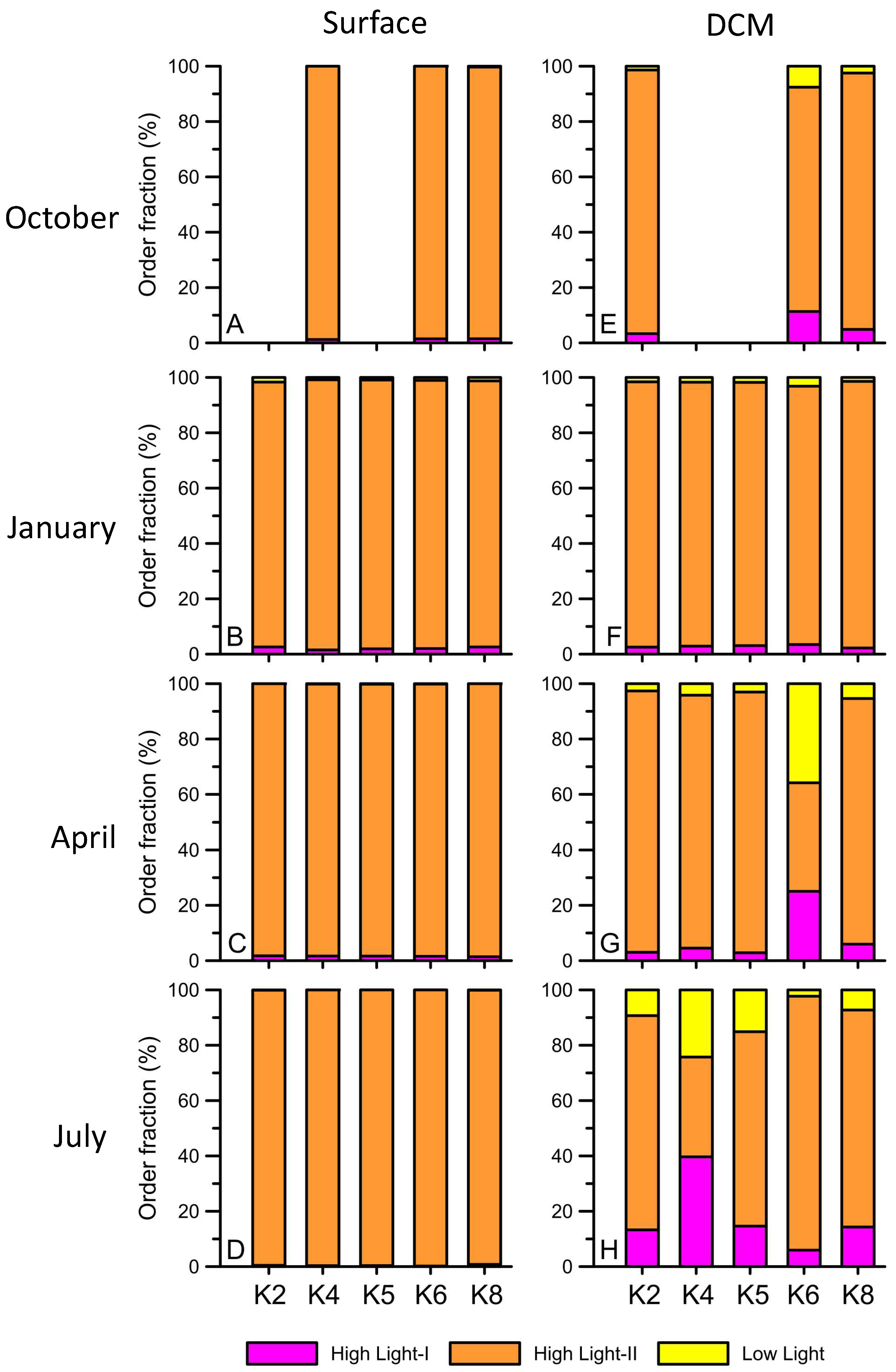
| Time | Station | Reads | ASV | ACE | Shannon |
|---|---|---|---|---|---|
| October 2021 | K4 | 161,662 | 996 | 997 | 4.74 |
| K6 | 199,278 | 1162 | 1162 | 4.95 | |
| K8 | 319,142 | 1551 | 1552 | 5.11 | |
| January 2013 | K2 | 427,192 | 2082 | 2083 | 5.71 |
| K4 | 407,216 | 1807 | 1808 | 5.01 | |
| K5 | 976,914 | 3012 | 3014 | 5.38 | |
| K6 | 272,391 | 1590 | 1590 | 5.22 | |
| K8 | 367,891 | 1764 | 1765 | 5.36 | |
| April 2013 | K2 | 291,525 | 1335 | 1335 | 5.07 |
| K4 | 206,446 | 1233 | 1233 | 5.16 | |
| K5 | 312,912 | 1409 | 1409 | 4.73 | |
| K6 | 294,990 | 1235 | 1235 | 4.65 | |
| K8 | 222,253 | 1201 | 1201 | 4.88 | |
| July 2013 | K2 | 251,552 | 1235 | 1236 | 4.97 |
| K4 | 283,202 | 1139 | 1139 | 4.75 | |
| K5 | 289,476 | 1134 | 1134 | 1.88 | |
| K6 | 278,805 | 1161 | 1162 | 4.85 | |
| K8 | 626,538 | 1898 | 1899 | 5.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, Y.-F.; Chung, C.-C.; Gong, G.-C.; Lin, I.-J.; Hsu, C.-W. Seasonal Patterns of Picocyanobacterial Community Structure in the Kuroshio Current. Biology 2023, 12, 1424. https://doi.org/10.3390/biology12111424
Chan Y-F, Chung C-C, Gong G-C, Lin I-J, Hsu C-W. Seasonal Patterns of Picocyanobacterial Community Structure in the Kuroshio Current. Biology. 2023; 12(11):1424. https://doi.org/10.3390/biology12111424
Chicago/Turabian StyleChan, Ya-Fan, Chih-Ching Chung, Gwo-Ching Gong, I-Jung Lin, and Ching-Wei Hsu. 2023. "Seasonal Patterns of Picocyanobacterial Community Structure in the Kuroshio Current" Biology 12, no. 11: 1424. https://doi.org/10.3390/biology12111424





