Low-Dose Ionizing Radiation-Crosslinking Immunoprecipitation (LDIR-CLIP) Identified Irradiation-Sensitive RNAs for RNA-Binding Protein HuR-Mediated Decay
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Cell Culture, Plasmids, Transfection, and Irradiation
2.2. Generation of Human HuR Knockout Cell Lines
2.3. MTS Assays
2.4. Western Blotting
2.5. Phos-Tag SDS-PAGE Electrophoresis
2.6. LDIR-CLIP-Sequencing Analysis
2.7. Ribonucleoprotein Immunoprecipitation
2.8. RNA-Seq
2.9. mRNA Stability Assay
2.10. Quantitative RT-PCR Analysis
2.11. Statistical and Reproducibility Analysis
3. Results
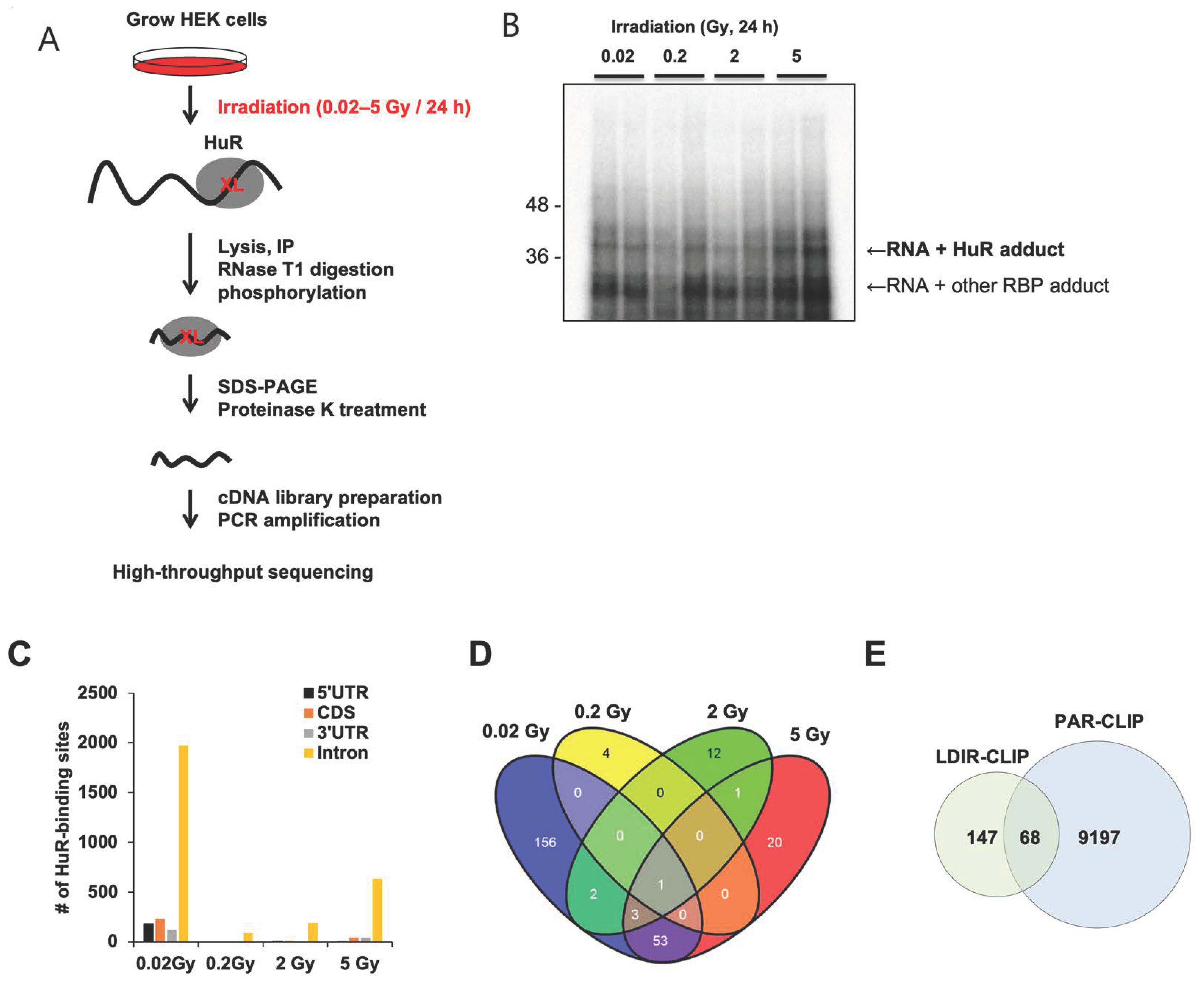
3.1. LDIR Promotes Crosslinking of RNA-Binding Protein HuR with Its Target RNAs
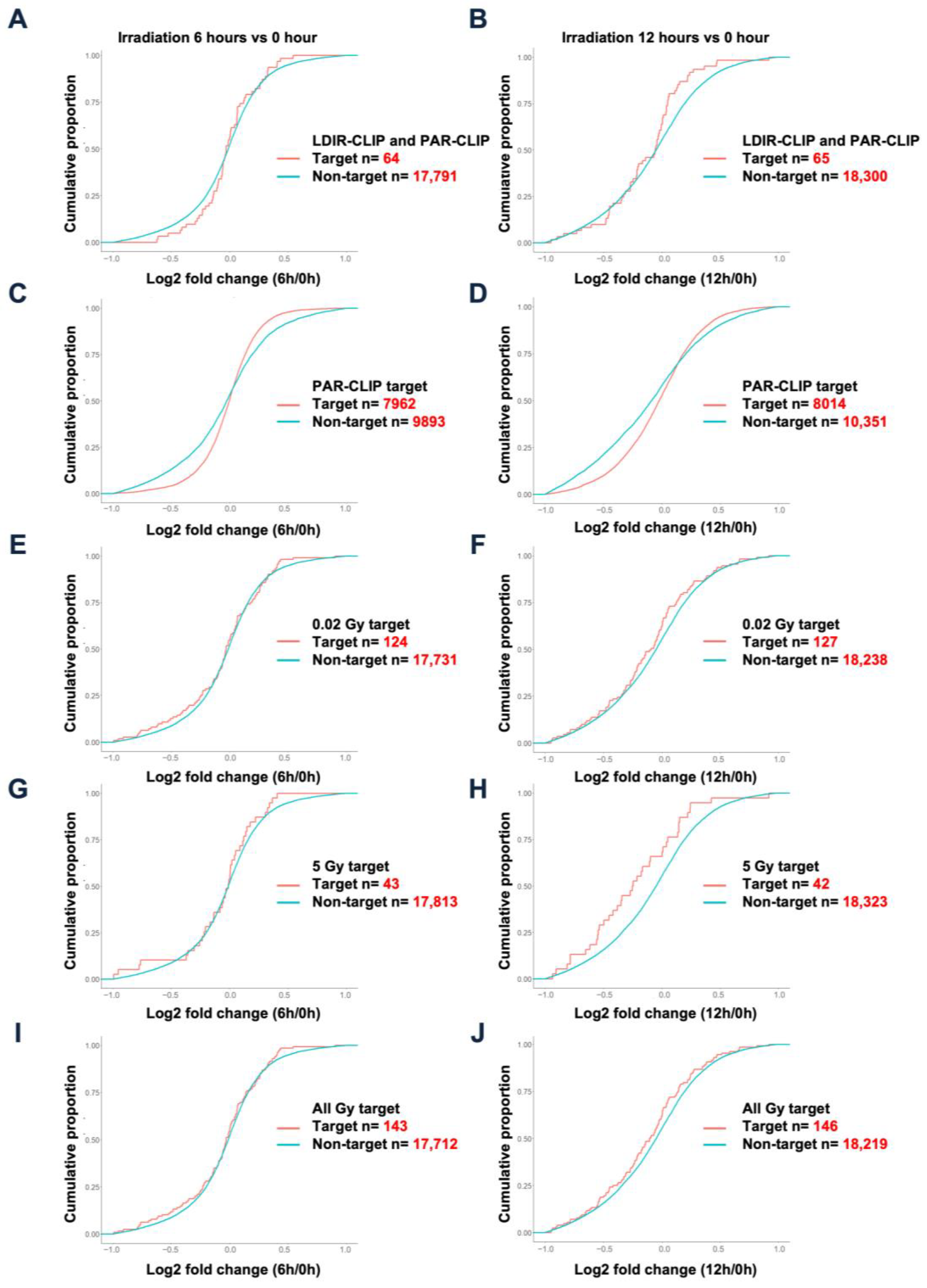
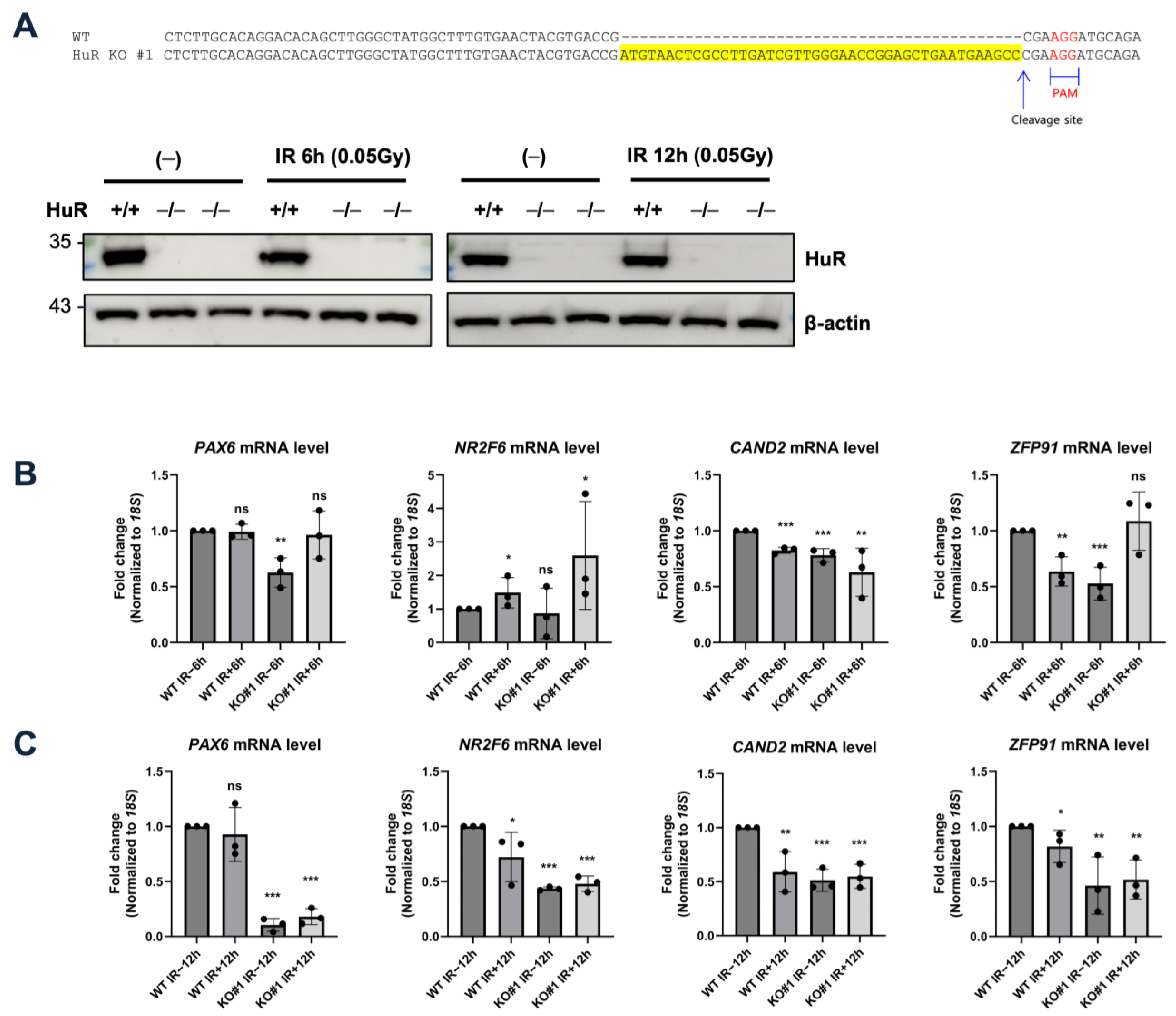
3.2. LDIR Modulates the Stability of HuR-Crosslinked mRNAs
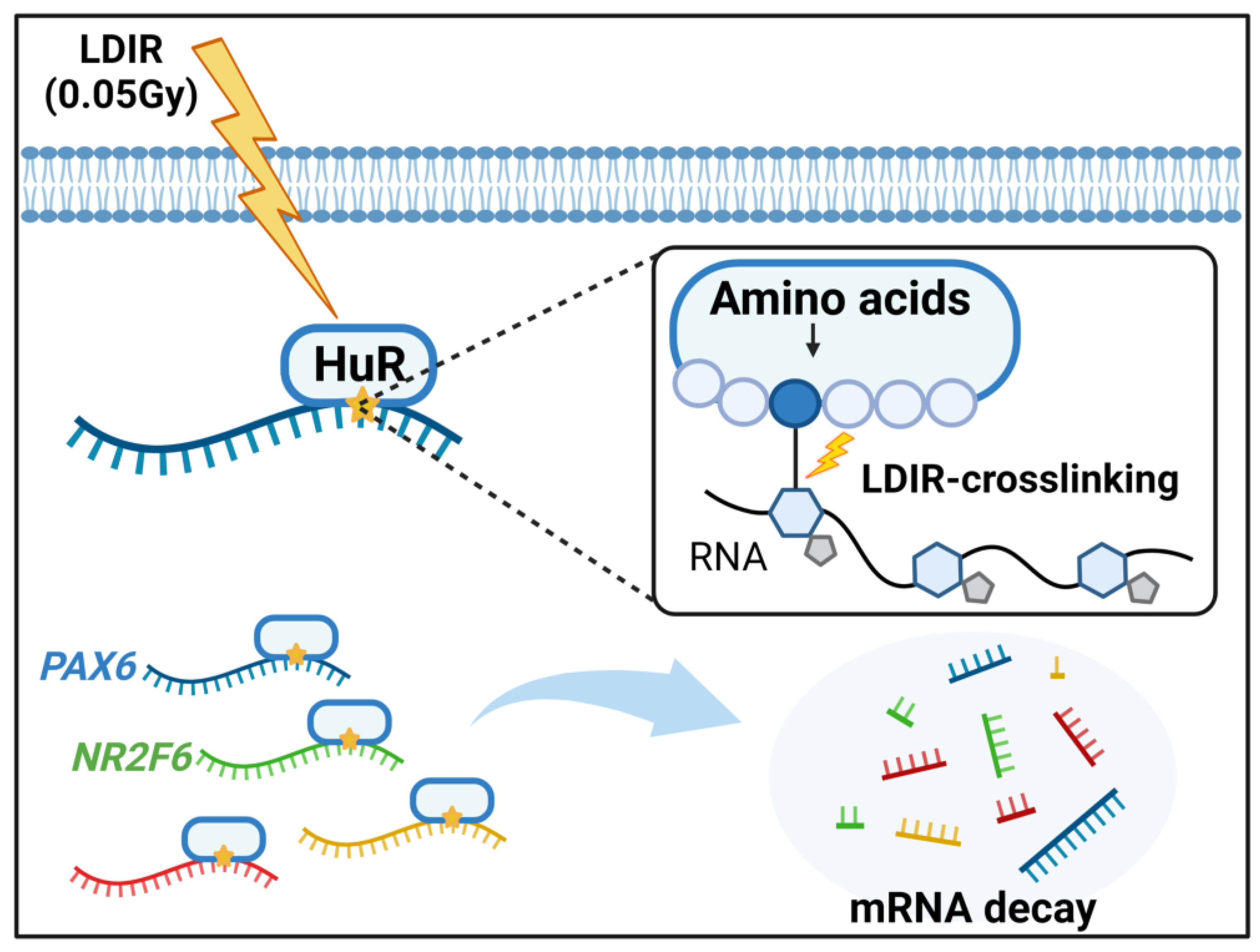
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maqsudur Rashid, A.; Ramalingam, L.; Al-Jawadi, A.; Moustaid-Moussa, N.; Moussa, H. Low dose radiation, inflammation, cancer and chemoprevention. Int. J. Radiat. Biol. 2019, 95, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Mullenders, L.; Atkinson, M.; Paretzke, H.; Sabatier, L.; Bouffler, S. Assessing cancer risks of low-dose radiation. Nat. Rev. Cancer 2009, 9, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Kim, J.A.; Park, S.J.; Kim, J.K.; Heo, K.; Yang, K.M.; Son, T.G. Low-dose radiation activates Nrf1/2 through reactive species and the Ca2+/ERK1/2 signaling pathway in human skin fibroblast cells. BMB Rep. 2013, 46, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Z.; Luo, Q.; Wang, Y.; Li, Q.; Zhou, L.; Zuo, H. Radiotherapy modulates tumor cell fate decisions: A review. Radiat. Oncol. 2022, 17, 196. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Valerie, K.; Yacoub, A.; Hagan, M.P.; Curiel, D.T.; Fisher, P.B.; Grant, S.; Dent, P. Radiation-induced cell signaling: Inside-out and outside-in. Mol. Cancer Ther. 2007, 6, 789–801. [Google Scholar] [CrossRef]
- Iliakis, G.; Wang, Y.; Guan, J.; Wang, H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 2003, 22, 5834–5847. [Google Scholar] [CrossRef]
- Santivasi, W.L.; Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid. Redox Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef]
- Sage, E.; Shikazono, N. Radiation-induced clustered DNA lesions: Repair and mutagenesis. Free Radic. Biol. Med. 2017, 107, 125–135. [Google Scholar] [CrossRef]
- Dutertre, M.; Lambert, S.; Carreira, A.; Amor-Guéret, M.; Vagner, S. DNA damage: RNA-binding proteins protect from near and far. Trends Biochem. Sci. 2014, 39, 141–149. [Google Scholar] [CrossRef]
- Chen, J.-K.; Lin, W.-L.; Chen, Z.; Liu, H.-W. PARP-1–dependent recruitment of cold-inducible RNA-binding protein promotes double-strand break repair and genome stability. Proc. Natl. Acad. Sci. USA 2018, 115, E1759–E1768. [Google Scholar] [CrossRef]
- Klaric, J.A.; Wüst, S.; Panier, S. New Faces of Old Friends: Emerging New Roles of RNA-Binding Proteins in the DNA Double-Strand Break Response. Front. Mol. Biosci. 2021, 8, 668821. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Abdelmohsen, K.; Kim, M.M.; Srikantan, S.; Lee, E.K.; Tominaga, K.; Selimyan, R.; Martindale, J.L.; Yang, X.; Lehrmann, E.; et al. Global dissociation of HuR-mRNA complexes promotes cell survival after ionizing radiation. EMBO J. 2011, 30, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.; Jaskiewicz, L.; Burger, L.; Hausser, J.; Khorshid, M.; Zavolan, M. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat. Methods 2011, 8, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Hafner, M.; Landthaler, M.; Burger, L.; Khorshid, M.; Hausser, J.; Berninger, P.; Rothballer, A.; Ascano, M., Jr.; Jungkamp, A.C.; Munschauer, M.; et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 2010, 141, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, E.L.; Pratt, G.A.; Shishkin, A.A.; Gelboin-Burkhart, C.; Fang, M.Y.; Sundararaman, B.; Blue, S.M.; Nguyen, T.B.; Surka, C.; Elkins, K.; et al. Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nat. Methods 2016, 13, 508–514. [Google Scholar] [CrossRef]
- Anastasakis, D.G.; Jacob, A.; Konstantinidou, P.; Meguro, K.; Claypool, D.; Cekan, P.; Haase, A.D.; Hafner, M. A non-radioactive, improved PAR-CLIP and small RNA cDNA library preparation protocol. Nucleic Acids Res. 2021, 49, e45. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.F.; Miao, W.; Yang, X.; Goda, G.A.; Ji, A.L.; Donohue, L.K.H.; Aleman, M.M.; Dominguez, D.; Khavari, P.A. easyCLIP analysis of RNA-protein interactions incorporating absolute quantification. Nat. Commun. 2021, 12, 1569. [Google Scholar] [CrossRef] [PubMed]
- Blue, S.M.; Yee, B.A.; Pratt, G.A.; Mueller, J.R.; Park, S.S.; Shishkin, A.A.; Starner, A.C.; Van Nostrand, E.L.; Yeo, G.W. Transcriptome-wide identification of RNA-binding protein binding sites using seCLIP-seq. Nat. Protoc. 2022, 17, 1223–1265. [Google Scholar] [CrossRef]
- Hafner, M.; Katsantoni, M.; Köster, T.; Marks, J.; Mukherjee, J.; Staiger, D.; Ule, J.; Zavolan, M. CLIP and complementary methods. Nat. Rev. Methods Prim. 2021, 1, 20. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, A.; Zhang, A.; Zhou, C. HuR promotes breast cancer cell proliferation and survival via binding to CDK3 mRNA. Biomed. Pharmacother. 2017, 91, 788–795. [Google Scholar] [CrossRef]
- Kim, H.H.; Abdelmohsen, K.; Lal, A.; Pullmann, R., Jr.; Yang, X.; Galban, S.; Srikantan, S.; Martindale, J.L.; Blethrow, J.; Shokat, K.M.; et al. Nuclear HuR accumulation through phosphorylation by Cdk1. Genes Dev. 2008, 22, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Hinman, M.N.; Lou, H. Diverse molecular functions of Hu proteins. Cell. Mol. Life Sci. 2008, 65, 3168–3181. [Google Scholar] [CrossRef]
- Rajasingh, J. The many facets of RNA-binding protein HuR. Trends Cardiovasc. Med. 2015, 25, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Tang, P.W.; Welles, J.E.; Pan, W.; Javed, Z.; Elhaw, A.T.; Mythreye, K.; Kimball, S.R.; Hempel, N. HuR-dependent SOD2 protein synthesis is an early adaptation to anchorage-independence. Redox Biol. 2022, 53, 102329. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Song, J.; Gao, Y.; Huang, S.; Dou, R.; Zhong, P.; Huang, G.; Han, L.; Zheng, J.; Zhang, X.; et al. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022, 52, 102312. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Pullmann, R., Jr.; Lal, A.; Kim, H.H.; Galban, S.; Yang, X.; Blethrow, J.D.; Walker, M.; Shubert, J.; Gillespie, D.A.; et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol. Cell 2007, 25, 543–557. [Google Scholar] [CrossRef]
- Filippova, N.; Yang, X.; King, P.; Nabors, L.B. Phosphoregulation of the RNA-binding protein Hu antigen R (HuR) by Cdk5 affects centrosome function. J. Biol. Chem. 2012, 287, 32277–32287. [Google Scholar] [CrossRef]
- Yoon, J.H.; Abdelmohsen, K.; Srikantan, S.; Guo, R.; Yang, X.; Martindale, J.L.; Gorospe, M. Tyrosine phosphorylation of HuR by JAK3 triggers dissociation and degradation of HuR target mRNAs. Nucleic Acids Res. 2014, 42, 1196–1208. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Srikantan, S.; Yang, X.; Lal, A.; Kim, H.H.; Kuwano, Y.; Galban, S.; Becker, K.G.; Kamara, D.; de Cabo, R.; et al. Ubiquitin-mediated proteolysis of HuR by heat shock. EMBO J. 2009, 28, 1271–1282. [Google Scholar] [CrossRef]
- Mukherjee, N.; Corcoran, D.L.; Nusbaum, J.D.; Reid, D.W.; Georgiev, S.; Hafner, M.; Ascano, M.; Tuschl, T.; Ohler, U.; Keene, J.D. Integrative Regulatory Mapping Indicates that the RNA-Binding Protein HuR Couples Pre-mRNA Processing and mRNA Stability. Mol. Cell 2011, 43, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wei, J.; Cui, X.; Yu, C.; Ni, W.; Bungert, J.; Wu, L.; He, C.; Qian, Z. Post-translational modification of RNA m6A demethylase ALKBH5 regulates ROS-induced DNA damage response. Nucleic Acids Res. 2021, 49, 5779–5797. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.J.T.; Pan, T.; Kalsotra, A. RNA modifications and structures cooperate to guide RNA–protein interactions. Nat. Rev. Mol. Cell Biol. 2017, 18, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Mendel, M.; Delaney, K.; Pandey, R.R.; Chen, K.-M.; Wenda, J.M.; Vågbø, C.B.; Steiner, F.A.; Homolka, D.; Pillai, R.S. Splice site m6A methylation prevents binding of U2AF35 to inhibit RNA splicing. Cell 2021, 184, 3125–3142.e3125. [Google Scholar] [CrossRef] [PubMed]
- Mazan-Mamczarz, K.; Galbán, S.; de Silanes, I.L.; Martindale, J.L.; Atasoy, U.; Keene, J.D.; Gorospe, M. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc. Natl. Acad. Sci. USA 2003, 100, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Bergmeier, A.P.; Liao, Y.; Wu, S.; Tong, L. CIRP Sensitizes Cancer Cell Responses to Ionizing Radiation. Radiat. Res. 2021, 195, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Wang, Y.; Li, B. Suppression of PAX6 promotes cell proliferation and inhibits apoptosis in human retinoblastoma cells. Int. J. Mol. Med. 2014, 34, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Shen, Y.C.; Yeh, L.K.; Li, W.; Coyle, B.M.; Liu, C.Y.; Fini, M.E. Pax6 overexpression suppresses cell proliferation and retards the cell cycle in corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2397–2407. [Google Scholar] [CrossRef]
- Olson, W.J.; Jakic, B.; Labi, V.; Woelk, J.; Derudder, E.; Baier, G.; Hermann-Kleiter, N. A role for the nuclear receptor NR2F6 in peritoneal B cell homeostasis. Front. Immunol. 2022, 13, 845235. [Google Scholar] [CrossRef]
- Yang, S.L.; Guan, H.Q.; Yang, H.B.; Chen, Y.; Huang, X.Y.; Chen, L.; Shen, Z.F.; Wang, L.X. The expression and biological effect of NR2F6 in non-small cell lung cancer. Front. Oncol. 2022, 12, 940234. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Mun, H.; Kim, J.-H.; Ko, S.; Kim, Y.-K.; Shim, M.J.; Kim, K.; Ho, C.W.; Park, H.B.; Kim, M.; et al. Low-Dose Ionizing Radiation-Crosslinking Immunoprecipitation (LDIR-CLIP) Identified Irradiation-Sensitive RNAs for RNA-Binding Protein HuR-Mediated Decay. Biology 2023, 12, 1533. https://doi.org/10.3390/biology12121533
Lee JW, Mun H, Kim J-H, Ko S, Kim Y-K, Shim MJ, Kim K, Ho CW, Park HB, Kim M, et al. Low-Dose Ionizing Radiation-Crosslinking Immunoprecipitation (LDIR-CLIP) Identified Irradiation-Sensitive RNAs for RNA-Binding Protein HuR-Mediated Decay. Biology. 2023; 12(12):1533. https://doi.org/10.3390/biology12121533
Chicago/Turabian StyleLee, Ji Won, Hyejin Mun, Jeong-Hyun Kim, Seungbeom Ko, Young-Kook Kim, Min Ji Shim, Kyungmin Kim, Chul Woong Ho, Hyun Bong Park, Meesun Kim, and et al. 2023. "Low-Dose Ionizing Radiation-Crosslinking Immunoprecipitation (LDIR-CLIP) Identified Irradiation-Sensitive RNAs for RNA-Binding Protein HuR-Mediated Decay" Biology 12, no. 12: 1533. https://doi.org/10.3390/biology12121533
APA StyleLee, J. W., Mun, H., Kim, J.-H., Ko, S., Kim, Y.-K., Shim, M. J., Kim, K., Ho, C. W., Park, H. B., Kim, M., Lee, C., Choi, S. H., Kim, J.-W., Jeong, J.-H., Yoon, J.-H., Min, K.-W., & Son, T. G. (2023). Low-Dose Ionizing Radiation-Crosslinking Immunoprecipitation (LDIR-CLIP) Identified Irradiation-Sensitive RNAs for RNA-Binding Protein HuR-Mediated Decay. Biology, 12(12), 1533. https://doi.org/10.3390/biology12121533





