First Trimester Evaluation of Maternal Visceral Fat and Its Relationship with Adverse Pregnancy Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
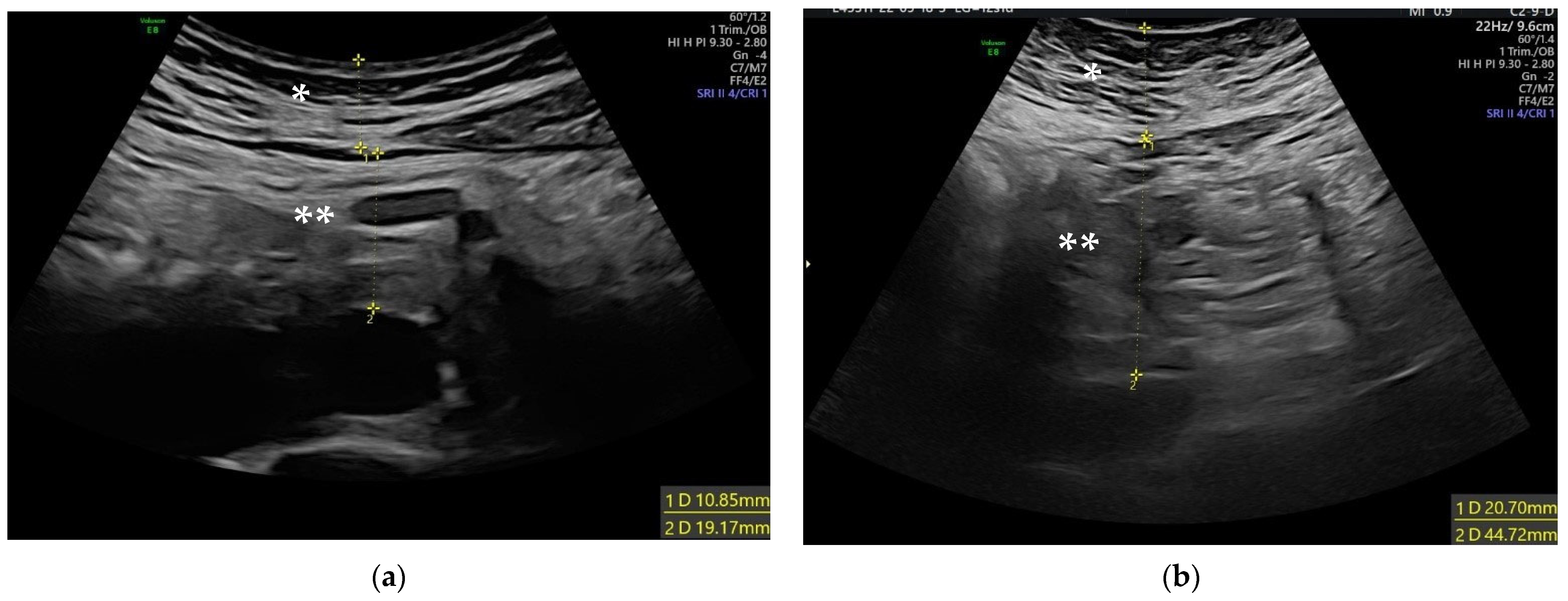
2.2. Ultrasound Measurements
2.3. Biochemical Analysis
2.4. Diagnosis of Adverse Pregnancy Outcomes
2.5. Statistical Analysis
3. Results
3.1. Clinical and Anthropometric Characteristics
3.2. Biochemical Analysis and Clinical Parameters Assessed
3.3. Ultrasound Measurements and Their Relationship with Biochemical and Clinical Parameters
3.4. Logistic Regression Analysis
3.5. Receiver Operating Characteristic (ROC) Curves Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion number 591. Challenges for Overweight and Obese Women. Obstet. Gynecol. 2014, 123, 726–730. [Google Scholar] [CrossRef] [PubMed]
- NMPA Project Team. National Maternity and Perinatal Audit: Clinical Report 2017; RCOG: London, UK, 2017. [Google Scholar]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in Pregnancy: ACOG Practice Bulletin, Number 230. Obstet. Gynecol. 2021, 137, e128–e144. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. Changes in body composition and metabolic disease risk. Eur. J. Clin. Nutr. 2019, 73, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.; Thomas, R.; Fox, B.; Acharya, U.; Wilkin, T. The central issue? Visceral fat mass is a good marker of insulin resistance and metabolic disturbance in women with polycystic ovary syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, B.; Sabir, N.; Kaleli, B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil. Steril. 2003, 79, 1358–1364. [Google Scholar] [CrossRef]
- Giouleka, S.; Tsakiridis, I.; Koutsouki, G.; Kostakis, N.; Mamopoulos, A.; Kalogiannidis, I.; Athanasiadis, A.; Dagklis, T. Obesity in Pregnancy: A Comprehensive Review of Influential Guidelines. Obstet. Gynecol. Surv. 2023, 78, 50–68. [Google Scholar] [CrossRef]
- Aune, D.; Saugstad, O.D.; Henriksen, T.; Tonstad, S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: A systematic review and meta-analysis. JAMA 2014, 311, 1536–1546. [Google Scholar] [CrossRef]
- Vena, F.; D’Ambrosio, V.; Paladini, V.; Saluzzi, E.; Di Mascio, D.; Boccherini, C.; Spiniello, L.; Mondo, A.; Pizzuti, A.; Giancotti, A. Risk of neural tube defects according to maternal body mass index: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 7296–7305. [Google Scholar] [CrossRef]
- Cedergren, M.I. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet. Gynecol. 2004, 103, 219–224. [Google Scholar] [CrossRef]
- Bartha, J.L.; Marín-Segura, P.; González-González, N.L.; Wagner, F.; Aguilar-Diosdado, M.; Hervias-Vivancos, B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity 2007, 15, 2233–2239. [Google Scholar] [CrossRef]
- Gur, E.B.; Ince, O.; Turan, G.A.; Karadeniz, M.; Tatar, S.; Celik, E.; Yalcin, M.; Guclu, S. Ultrasonographic visceral fat thickness in the first trimester can predict metabolic syndrome and gestational diabetes mellitus. Endocrine 2014, 47, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Thaware, P.K.; Patterson, C.C.; Young, I.S.; Casey, C.; McCance, D.R. Clinical utility of ultrasonography-measured visceral adipose tissue depth as a tool in early pregnancy screening for gestational diabetes: A proof-of-concept study. Diabet. Med. 2019, 36, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Balani, J.; Hyer, S.; Johnson, A.; Shehata, H. The importance of visceral fat mass in obese pregnant women and relation with pregnancy outcomes. Obstet. Med. 2014, 7, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H. A model for a new pyramid of prenatal care based on the 11 to 13 weeks’ assessment. Prenat. Diagn. 2011, 31, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sonek, J.D.; Kagan, K.O.; Nicolaides, K.H. Inverted Pyramid of Care. Clin. Lab. Med. 2016, 36, 305–317. [Google Scholar] [CrossRef]
- Sattar, N.; Greer, I.A. Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening? BMJ 2002, 325, 157–160. [Google Scholar] [CrossRef]
- Bartha, J.L.; González-Bugatto, F.; Fernández-Macías, R.; González-González, N.L.; Comino-Delgado, R.; Hervías-Vivancos, B. Metabolic syndrome in normal and complicated pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 178–184. [Google Scholar] [CrossRef]
- Horvath, B.; Bodecs, T.; Boncz, I.; Bodis, J. Metabolic syndrome in normal and complicated pregnancies. Metab. Syndr. Relat. Disord. 2013, 11, 185–188. [Google Scholar] [CrossRef]
- International Society of Ultrasound in Obstetrics and Gynecology; Bilardo, C.M.; Chaoui, R.; Hyett, J.A.; Kagan, K.O.; Karim, J.N.; Papageorghiou, A.T.; Poon, L.C.; Salomon, L.J.; Syngelaki, A.; et al. ISUOG Practice Guidelines (updated): Performance of 11–14-week ultrasound scan. Ultrasound Obstet. Gynecol. 2023, 61, 127–143. [Google Scholar] [CrossRef]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Denk, B.; Stepan, H. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 161.e1–161.e11. [Google Scholar] [CrossRef]
- Di Renzo, G.C. The great obstetrical syndromes. J. Matern. Fetal Neonatal Med. 2009, 22, 633–635. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Figueras, F.; Meler, E.; Iraola, A.; Eixarch, E.; Coll, O.; Figueras, J.; Francis, A.; Gratacos, E.; Gardosi, J. Customized birthweight standards for a Spanish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 20–24. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obs. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Heslehurst, N.; Ngongalah, L.; Bigirumurame, T.; Nguyen, G.; Odeniyi, A.; Flynn, A.; Smith, V.; Crowe, L.; Skidmore, B.; Gaudet, L.; et al. Association between maternal adiposity measures and adverse maternal outcomes of pregnancy: Systematic review and meta-analysis. Obes. Rev. 2022, 23, e13449. [Google Scholar] [CrossRef]
- Bugatto, F.; Quintero-Prado, R.; Visiedo, F.M.; Vilar-Sánchez, J.M.; Figueroa-Quiñones, A.; López-Tinoco, C.; Torrejón, R.; Bartha, J.L. The Influence of Lipid and Proinflammatory Status on Maternal Uterine Blood Flow in Women With Late Onset Gestational Diabetes. Reprod. Sci. 2018, 25, 837–843. [Google Scholar] [CrossRef]
- Bugatto, F.; Fernández-Deudero, Á.; Bailén, Á.; Fernández-Macías, R.; Hervías-Vivancos, B.; Bartha, J.L. Second-trimester amniotic fluid proinflammatory cytokine levels in normal and overweight women. Obstet. Gynecol. 2010, 115, 127–133. [Google Scholar] [CrossRef]
- López-Tinoco, C.; Roca, M.; Fernández-Deudero, A.; García-Valero, A.; Bugatto, F.; Aguilar-Diosdado, M.; Bartha, J.L. Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine 2012, 58, 14–19. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Oton-Gonzalez, L.; Selvatici, R.; Rizzo, P.; Pavasini, R.; Campo, G.C.; Lanzillotti, C.; Mazziotta, C.; De Mattei, M.; Tognon, M.; et al. SERPINA1 Gene Promoter Is Differentially Methylated in Peripheral Blood Mononuclear Cells of Pregnant Women. Front. Cell Dev. Biol. 2020, 8, 550543. [Google Scholar] [CrossRef]
- Jacobs, M.; Nassar, N.; Roberts, C.L.; Hadfield, R.; Morris, J.M.; Ashton, A.W. Levels of soluble fms-like tyrosine kinase one in first trimester and outcomes of pregnancy: A systematic review. Reprod. Biol. Endocrinol. 2011, 9, 77. [Google Scholar] [CrossRef]
- Benevides, F.T.; Araujo Júnior, E.; Maia, C.S.C.; Montenegro Junior, R.M.; Carvalho, F.H.C. Ultrasound evaluation of subcutaneous and visceral abdominal fat as a predictor of gestational diabetes mellitus: A systematic review. J. Matern. Fetal Neonatal Med. 2022, 35, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.R.; Berger, H.; Retnakaran, R.; Maguire, J.L.; Nathens, A.B.; Connelly, P.W.; Ray, J.G. First-Trimester Maternal Abdominal Adiposity Predicts Dysglycemia and Gestational Diabetes Mellitus in Midpregnancy. Diabetes Care 2016, 39, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Berger, H.; Nisenbaum, R.; Lausman, A.Y.; MacGarvie, S.; Crerar, C.; Ray, J.G. Abdominal visceral adiposity in the first trimester predicts glucose intolerance in later pregnancy. Diabetes Care 2009, 32, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Silva Rocha, A.D.; Rombaldi Bernardi, J.; Matos, S.; Cortés Kretzer, D.; Carvalhal Schöffel, A.; Zubaran Goldani, M.; Azevedo Magalhães, J.A. Maternal adipose tissue to early preeclampsia risk detection: Is the time to maternal ultrasound beyond fetal evaluation? J. Obstet. Gynaecol. Res. 2021, 47, 2021–2030. [Google Scholar] [CrossRef]
- Alser, M.; Elrayess, M.A. From an Apple to a Pear: Moving Fat around for Reversing Insulin Resistance. Int. J. Environ. Res. Public Health 2022, 19, 14251. [Google Scholar] [CrossRef]




| Variable | Uncomplicated Group (n = 260) | Adverse Pregnancy Outcomes Group (n = 123) | p Value |
|---|---|---|---|
| Maternal age (years) | 33 (29–36) | 34 (30–37) | p = 0.05 * |
| Height (m) | 1.63 ± 0.06 | 1.63 ± 0.06 | p = 0.93 |
| Pregestational weight (Kg) | 62 (57–70.7) | 66.3 (58–76) | p = 0.005 * |
| Pregestational BMI (Kg/m2) | 23.3 (21.1–26.5) | 24.9 (21.9–28.5) | p = 0.003 * |
| Nulliparous (n, %) | 145 (56%) | 67 (54.5%) | p = 0.34 |
| Weight gain (kg) | 11 (8.5–15) | 11.5 (7.5–14) | p = 0.56 |
| Gestational age at birth (weeks) | 40 (39–40) | 39 (37–40) | p = 0.001 * |
| Newborn male sex (n, %) | 129 (50.4%) | 58 (49.6%) | p = 0.88 |
| Newborn weight (Kg) | 3363 ± 365 | 2957 ± 668 | p = 0.001 * |
| Newborn centile | 47 (30–65.7) | 23 (6–67) | p = 0.001 * |
| SBP (mm Hg) | 113 (105–122) | 120 (110–128) | p = 0.001 * |
| DBP (mmHg) | 70 (60–75) | 72 (65.5–80) | p = 0.001 * |
| MAP (mmHg) | 83.5 ± 9.3 | 88.1 ± 10 | p = 0.001 * |
| Smoking (N, %) | 22 (8.7%) | 20 (16.3%) | p = 0.02 * |
| Variable | Uncomplicated Group (n = 260) | Adverse Pregnancy Outcomes Group (n = 123) | p Value |
|---|---|---|---|
| Biochemical Parameters | |||
| Glucose (mg/dL) | 77 (72–80) | 78.5 (74–84) | p = 0.11 |
| Insulin (µUI/mL) | 5.8 (4.3–8.1) | 6.3 (4.6–9.1) | p = 0.44 |
| HOMA-IR index | 1 (0.7–1.3) | 1 (0.71–1.60) | p = 0.69 |
| Triglycerides (mg/dL) | 77 (67–95) | 95 (74–123) | p = 0.001 * |
| Total Cholesterol (mg/dL) | 171.8 ± 21.9 | 178.8 ± 31.4 | p = 0.16 |
| HDL Cholesterol (mg/dL) | 65.1 ± 10.8 | 65.1 ± 10.9 | p = 0.99 |
| LDL Cholesterol (mg/dL) | 56.1 ± 19 | 63.9 ± 20 | p = 0.02 * |
| CRP (mg/L) | 2.6 (1.5–4.7) | 3.45 (2–8) | p = 0.04 * |
| Leukocyte count (×103/μL) | 8.29 ± 1.86 | 8.66 ± 1.97 | p = 0.08 |
| PlGF (pg/mL) | 31 (25.5–42.7) | 30.7 (23.1–44.3) | p = 0.95 |
| sFlt-1 (pg/mL) | 1579 (1257–2036) | 1379 (1032–1666) | p = 0.005 * |
| sFlt1/PlGF ratio | 48.4 (36.2–62.1) | 43.2 (31.3–54.7) | p = 0.03 * |
| Ultrasound Parameters | |||
| SFT (mm) | 17.1 (13.1–21.4) | 18.9 (14.3–24.2) | p = 0.03 * |
| VFT (mm) | 26.5 (19.5–35.9) | 30 (23.4–45) | p = 0.001 * |
| Ratio of VFT-to-SFT | 1.5 (1.1–2) | 1.6 (1.2–2.1) | p = 0.24 |
| Mean Uterine Arteries PI | 1.58 (1.33–2.02) | 1.58 (1.23–2) | p = 0.47 |
| Variable | Visceral Fat Thickness | Subcutaneous Fat Thickness |
|---|---|---|
| Glucose | r = 0.11; p = 0.16 | r = 0.08; p = 0.28 |
| Insulin | r = 0.37; p < 0.001 * | r = 0.30; p < 0.001 * |
| HOMA-IR index | r = 0.33; p < 0.001 * | r = 0.34; p < 0.001 * |
| Triglycerides | r = 0.24; p = 0.02 * | r = 0.12; p = 0.11 |
| Total Cholesterol | r = 0.08; p = 0.29 | r = 0.10; p = 0.18 |
| HDL Cholesterol | r = −0.09; p = 0.22 | r = −0.03; p = 0.64 |
| LDL Cholesterol | r = 0.11; p = 0.17 | r = 0.12; p = 0.14 |
| CRP | r = 0.47; p < 0.001 * | r = 0.28; p = 0.001 * |
| Leukocyte count | r = 0.09; p = 0.06 | r = 0.07; p = 0.14 |
| PlGF | r = −0.007; p = 0.92 | r = -0.06; p = 0.45 |
| sFlt-1 | r = −0.06; p = 0.43 | r = 0.04; p = 0.58 |
| sFlt1/PlGF ratio | r = −0.05; p = 0.49 | r = 0.09; p = 0.26 |
| SAP | r = 0.16; p = 0.003 * | r = 0.32; p < 0.001 * |
| DAP | r = 0.20; p < 0.001 * | r = 0.30; p < 0.001 * |
| MAP | r = 0.20; p < 0.001 * | r = 0.27; p < 0.001 * |
| BMI | r = 0.57; p < 0.001 * | r = 0.60 p < 0.001 * |
| Mean Uterine Arteries PI | r = −0.03; p = 0.61 | r = −0.16; p = 0.01 * |
| Variable | aOR | 95% Confidence Interval | p Value |
|---|---|---|---|
| Composite of Adverse Pregnancy Outcomes | |||
| Subcutaneous fat thickness (mm) | 0.99 | 0.96–1.03 | p = 0.91 |
| Visceral fat thickness (mm) | 1.03 | 1.01–1.04 | p < 0.001 * |
| Ratio of VFT-to-SFT | 1.37 | 1.01–1.85 | p = 0.04 * |
| BMI (Kg/m2) | 1.03 | 0.98–1.08 | p = 0.2 |
| Metabolic Complications | |||
| Subcutaneous fat thickness (mm) | 1.04 | 1.004–1.08 | p = 0.03 * |
| Visceral fat thickness (mm) | 1.07 | 1.04–1.09 | p < 0.001 * |
| Ratio of VFT-to-SFT | 2.09 | 1.35–3.25 | p = 0.001 * |
| BMI (Kg/m2) | 1.11 | 1.03–1.19 | p = 0.03 * |
| Placental Vascular Dysfunction Complications | |||
| Subcutaneous fat thickness (mm) | 0.95 | 0.91–1.004 | p = 0.07 |
| Visceral fat thickness (mm) | 0.98 | 0.96–1.005 | p = 0.13 |
| Ratio of VFT-to-SFT | 1.11 | 0.76–1.62 | p = 0.56 |
| BMI (Kg/m2) | 0.97 | 0.91–1.03 | p = 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenes-Martín, F.; Melero-Jiménez, V.; López-Guerrero, M.Á.; Calero-Ruiz, M.M.; Vázquez-Fonseca, L.; Ábalos-Martínez, J.; Quintero-Prado, R.; Torrejón, R.; Visiedo, F.; Bugatto, F. First Trimester Evaluation of Maternal Visceral Fat and Its Relationship with Adverse Pregnancy Outcomes. Biology 2023, 12, 144. https://doi.org/10.3390/biology12020144
Brenes-Martín F, Melero-Jiménez V, López-Guerrero MÁ, Calero-Ruiz MM, Vázquez-Fonseca L, Ábalos-Martínez J, Quintero-Prado R, Torrejón R, Visiedo F, Bugatto F. First Trimester Evaluation of Maternal Visceral Fat and Its Relationship with Adverse Pregnancy Outcomes. Biology. 2023; 12(2):144. https://doi.org/10.3390/biology12020144
Chicago/Turabian StyleBrenes-Martín, Francisco, Victoria Melero-Jiménez, Miguel Ángel López-Guerrero, María Mercedes Calero-Ruiz, Luis Vázquez-Fonseca, Jessica Ábalos-Martínez, Rocío Quintero-Prado, Rafael Torrejón, Francisco Visiedo, and Fernando Bugatto. 2023. "First Trimester Evaluation of Maternal Visceral Fat and Its Relationship with Adverse Pregnancy Outcomes" Biology 12, no. 2: 144. https://doi.org/10.3390/biology12020144
APA StyleBrenes-Martín, F., Melero-Jiménez, V., López-Guerrero, M. Á., Calero-Ruiz, M. M., Vázquez-Fonseca, L., Ábalos-Martínez, J., Quintero-Prado, R., Torrejón, R., Visiedo, F., & Bugatto, F. (2023). First Trimester Evaluation of Maternal Visceral Fat and Its Relationship with Adverse Pregnancy Outcomes. Biology, 12(2), 144. https://doi.org/10.3390/biology12020144






