Structure and Function Analysis of Cultivated Meconopsis integrifolia Soil Microbial Community Based on High-Throughput Sequencing and Culturability
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. DNA Extraction, PCR, MiSeq Sequencing, and Sequence Data Analysis
2.3. Sequence Processing and Statistical Analysis
2.4. Isolation and Identification of Functional Bacteria
2.4.1. Medium and Reagents
2.4.2. Experimental Methods
Isolation and Identification of Bacteria from Soil Samples
Functional Identification
3. Results
3.1. Soil Microbial Diversity
3.2. Soil Bacterial and Fungal Structure
3.3. Function of Soil Bacterial and Fungal Community
3.3.1. Prediction of Bacterial Community Function
3.3.2. Prediction of Fungal Community Function
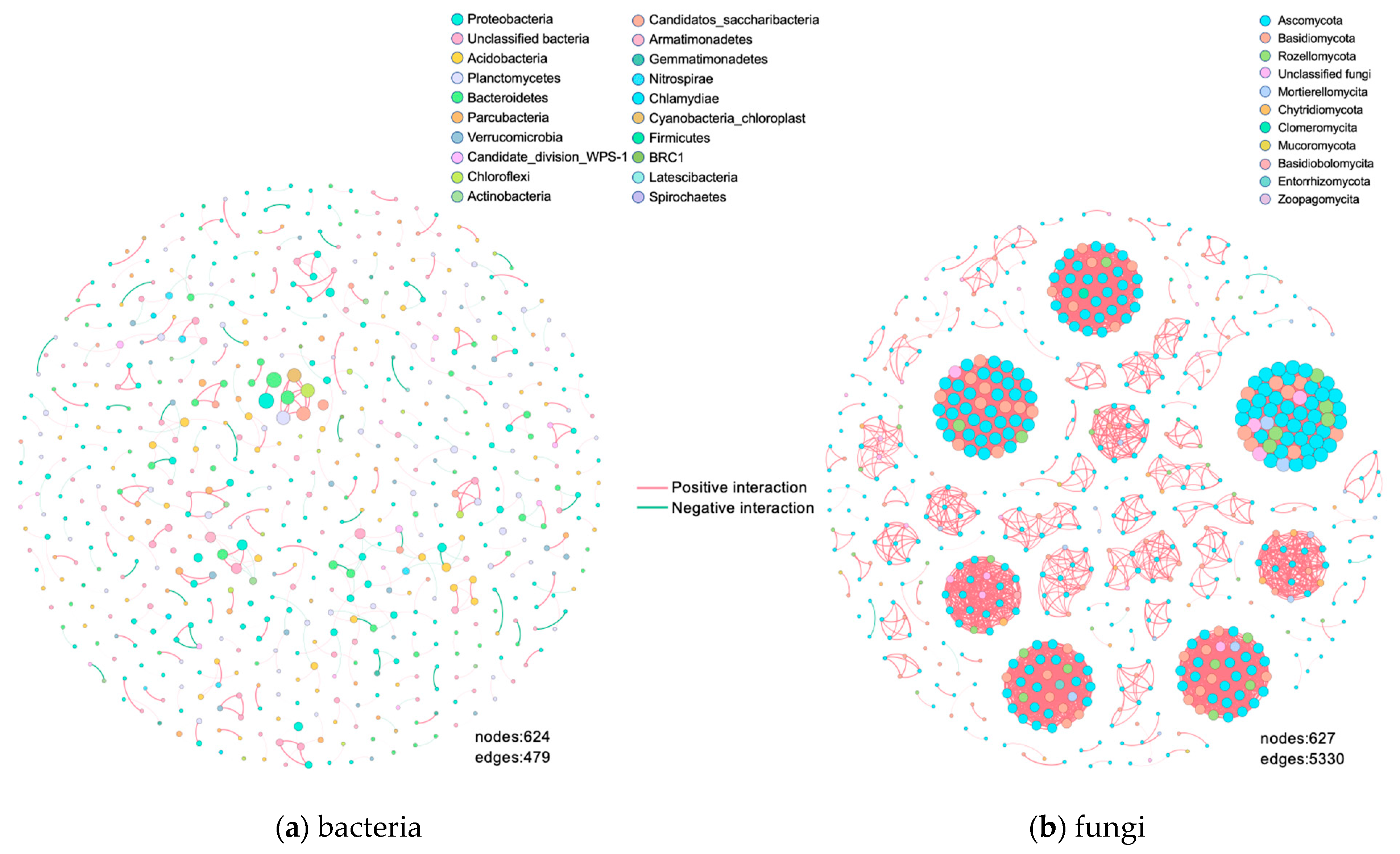
3.4. Analysis of Intra-Domain Co-Occurrence Networks
3.5. Culturable Bacteria
4. Discussion
4.1. Community Structure of Soil Microorganisms
4.1.1. Structural and Functional Prediction of Bacteria
4.1.2. Structural and Functional Prediction of Fungi
4.2. Co-Occurrence Network
4.2.1. Fungal Networks Are More Complex and More Sensitive to External Disturbances Than Bacterial Networks
4.2.2. The Identified Networks Are Mostly Positively Correlated
4.2.3. Importance of Keystone Strains
4.3. Potential Growth-Promoting Bacteria in Culturable Microorganisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Chen, Z.; Sun, X.; Zhong, N.; Liu, Z. Application of Traditional Chinese Medicine and Systems Pharmacology in Drug Prevention and Treatment against COVID-19. Am. J. Chin. Med. 2021, 49, 1045–1061. [Google Scholar] [CrossRef]
- Lin, J.Z. Rare alpine flowers—Meconopsis. Chin. Flower Bonsai 1999, 7, 49. (In Chinese) [Google Scholar]
- Chen, H.G.; Yang, T.; Du, T.; Su, H.F.; Guo, Z.F.; Lang, B.B. Study on seed dormancy and germination characteristics of Meconopsis integrifolia. Chin. Herb. Med. 2014, 37, 1529–1531. (In Chinese) [Google Scholar]
- Wu, Z.Y. Poppies, Citrus family. In Flora of China; Editorial Committee of the Flora of China, Chinese Academy of Sciences: Beijing, China, 1990; Volume 32. (In Chinese) [Google Scholar]
- Wang, M.A.; Chen, Y.Z. Structure of a new alkaloid from Meconopsis quintuplinervia. Regel. Nat. Prod. Res. Dev. 1995, 7, 32–34. (In Chinese) [Google Scholar]
- Chang, Y.; Wang, X.L.; Tang, X.L.; Yuan, L.; Lianhong, C. A new alkaloid from Meconopsis horridula. Nat. Prod. Res. Dev. 2017, 29, 731–734. (In Chinese) [Google Scholar]
- Shang, S.Y.; Li, C.; Zhang, C.Z.; Yang, Y.C.; Shi, J.G. Non-alkaloidal components of the Tibetan medicine Meconopsis quintuplinervia. Regel. Chin. J. Tradit. Chin. Med. 2006, 4, 468–471. (In Chinese) [Google Scholar]
- Shang, X.Y.; Zhang, C.Z.; Li, C.; Yang, Y.C.; Shi, J.G. Isolation and identification of flavonoids in the Tibetan medicine Meconopsis quintuplinervia. Regel. Chin. Herb. Med. 2002, 3, 250–252. (In Chinese) [Google Scholar]
- Guan, Y.L.; Dawa, D.; Kelsang, S.; Bianpa, T.; Bai, B. GC-MS analysis of the essential oil of the flowers of Meconopsis integrifolia in the whole leaf. Chin. J. Pharm. Sci. 2007, 26, 539–540. (In Chinese) [Google Scholar]
- Shang, X.Y.; Li, C.; Zhang, C.Z.; Yang, Y.C.; Shi, J.G. Non-alkaloid constituents from a Tibetan medicine Meconopsis quintuplinervia. China J. Chin. Mater. Med. 2006, 31, 468–471. [Google Scholar]
- Shang, X.Y.; Wang, Y.H.; Li, C.; Zhang, C.Z.; Yang, Y.C.; Shi, J.G. Acetylated flavonol diglucosides from Meconopsis quintuplinervia. Phytochemistry 2006, 67, 511–515. [Google Scholar] [CrossRef]
- Wu, H.F.; Pan, L.; Zou, D.S.; Yang, S.J.; Zhang, X.F. GC-MS analysis of the chemical composition of volatile oils of three species of Meconopsis. Chin. J. Pharm. Sci. 2006, 41, 1298–1300. (In Chinese) [Google Scholar]
- Majid, A.; Ahmad, H.; Saqib, Z. Conservation status assessment of Meconopsis aculeata Royle: A threatened endemic of Pakistan and Kashmir. Pak. J. Bot. 2015, 47, 1–5. [Google Scholar]
- Wang, G.; Baskin, C.C.; Baskin, J.M.; Yang, X.; Liu, G.; Ye, X.; Zhang, X.; Huang, Z. Effects of climate warming and prolonged snow cover on phenology of the early life history stages of four alpine herbs on the southeastern Tibetan Plateau. Am. J. Bot. 2018, 105, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Chen, Y.B. List of National Key Protected Wild Plants (First Batch). Plant Magazine. 1999, 5, 4–11. (In Chinese) [Google Scholar]
- Da, Q.J.; Chen, X.L.; Guan, X.J.; Zhang, Q.W.; Zhang, H.X. Seed dormancy and method of breaking it in Meconopsis Horridula. Bull. Biol. 2018, 53, 6. (In Chinese) [Google Scholar]
- Liao, J.M.; Qin, L.N.; Peng, H.; Wu, Y.; Liu, G.L. Effects of different temperatures on seed germination and antioxidant enzyme activities of Meconopsis integrifolia. Guizhou Agric. Sci. 2016, 44, 4. (In Chinese) [Google Scholar]
- Qu, Y.; Au, Z.; You, S.T.; Wang, G. Effect of different experimental conditions on seed germination characteristics of Meconopsis horridula populations. Seeds 2015, 34, 90–93. (In Chinese) [Google Scholar]
- Zhang, S.B.; Chang, W.; Hu, H. Photosynthetic characteristics of two alpine flowers, Meconopsis integrifolia and Primula sinopurpurea. J. Hortic. Sci. Biotech. 2010, 85, 335–340. [Google Scholar] [CrossRef]
- Zhang, S.B. Temperature Acclimation of Photosynthesis in Meconopsis horridula var. racemosa Prain. Bot. Stud. 2010, 51, 457–464. [Google Scholar]
- Sulaiman, I.M. Ecological studies on five species of endangered Himalayan poppy, Meconopsis (Papaveraceae). Bot. J. Linn. Soc. 1996, 121, 169–176. [Google Scholar]
- Wang, W.C. Three new species of the genus Meconopsis in the family Poppyidae. Guangxi Plants 2019, 39, 6. (In Chinese) [Google Scholar]
- Wei, Y.; Wang, X.Q. First open field flowering of “Plateau Beauty” Meconopsis on the plain. Green. Life 2020, 7, 33–36. (In Chinese) [Google Scholar]
- Haixia, L. The rare Blooming Flower of Meconopsis. Available online: https://www.tibet3.com/tuku/rdtp/2018-05-17/77399.html (accessed on 17 May 2018).
- Yu, W.Y. Ecological Adaptability and Artificial Cultivation of Two Species of Meconopsis Seedlings in Southeast Tibet. Master’s Thesis, Tibet University, Tibet, China, 2021. (In Chinese). [Google Scholar]
- Chen, X.F.; Liu, J.Y.; Feng, H.Y.; Jiang, Y.M. Tissue culture of Meconopsis horridula. Spec. Econ. Plants Anim. 2018, 21, 8. (In Chinese) [Google Scholar]
- Van Der Heijden, M.G.; Bruin, S.D.; Luckerhoff, L.; Van Logtestijn, R.S.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. Isme J. Emultidisciplinary J. Microb. Ecol. 2016, 10, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockwood, J.L. Soil fungistasis: Mechanisms and relation to biological control of soilborne plant pathogens. Soilborne Crop Dis. Asia. 1984, 26, 159–174. [Google Scholar]
- Carney, K.M.; Hungate, B.A.; Drake, B.G.; Megonigal, J.P. Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc. Natl. Acad. Sci. USA 2007, 104, 4990–4995. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Wan, S.; He, J.; Zhao, X. Foreword to the special issue: Looking into the impacts of global warming from the roof of the world. J. Plant Ecol. 2009, 2, 169–171. [Google Scholar] [CrossRef] [Green Version]
- Freeman, K.R.; Pescador, M.Y.; Reed, S.C.; Costello, E.K.; Schmidt, S.K. Soil CO2 flux and photoautotrophic community composition in high-elevation, ‘barren’ soil. Environ. Microbiol. 2010, 11, 674–686. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Nemergut, D.R.; Miller, A.E.; Freeman, K.R.; King, A.J.; Seimon, A. Microbial activity and diversity during extreme freeze–thaw cycles in periglacial soils, 5400. Extremophiles 2009, 13, 807–816. [Google Scholar] [CrossRef]
- Fan, Z.H.; Zhang, B.; Liao, M.; Li, X.L.; Zhang, X.P.; ChenXiong, C.R. Diversity of endophytic actinomycetes in different habitats of Meconopsis in Aba, Sichuan. Microbiol. Bull. 2018, 45, 81–90. (In Chinese) [Google Scholar]
- Delseny, M.; Han, B.; Hsing, Y.I. High throughput DNA sequencing: The new sequencing revolution. Plant Sci.-Limerick 2010, 179, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Kassian, K.; Tomáš, F.; Alexandros, S. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.; Zaneveld, J.; Caporaso, J.G.; Mcdonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.V.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi:An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the Third International Conference on Weblogs and Social Media, ICWSM 2009, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Xu, X.H.; Zheng, H.Y. Chinese Academy of Sciences, Microbiology Department, Institute of Forestry and Soil Research. In Handbook of Methods for Soil Microbiological Analysis; Science Press: Beijing, China, 1960. [Google Scholar]
- Wu, X.L.; Li, G.B. Microbial Pharmaceutical Technology; China Light Industry Press: Beijing, China, 2015. [Google Scholar]
- Wang, W.X.; Zhou, X.L.; Zhong-Ling, L.I.; Wang, M.P.; Wang, W.W. Detection of siderophore production from hydrogen-oxidizing bacteria with CAS overlay plate method. Microbiol. China 2014, 41, 1692–1697. [Google Scholar]
- Wichner, S.; Libbert, E. Interactions between plantsand epiphyticbacteriaregarding their auxin metabolism II. Physiol. Plant. 1968, 21, 500–509. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Schywn, B.; Nielands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Su, Y.R.; He, X.Y.; Qin, W.C.; Wei, Y.W.; Liang, Y.M.; Wu, J. Effects of different disturbance methods on the dominant taxa of soil bacteria, Aspergillus spp. communities in karst ecosystems. J. Soil Sci. 2012, 49, 10. (In Chinese) [Google Scholar]
- Guan, X.; Wang, J.; Hui, Z.; Wang, J.; Zhao, F. Soil bacterial communities shaped by geochemical factors and land use in a less-explored area, Tibetan Plateau. Bmc Genom. 2013, 14, 820. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Alonso, E.; Pena-Perez, S.; Serrano, S.; Garcia-Lopez, E.; Cid, C. Taxonomic and functional characterization of a microbial community from a volcanic englacial ecosystem in Deception Island, Antarctica. Sci. Rep. 2019, 9, 12158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, S.R.; Männistö, M.K.; Starovoytov, V.; Goodwin, L.; Nolan, M.; Hauser, L.; Land, M.; Davenport, K.W.; Woyke, T.; Häggblom, M.M. Complete genome sequence of Granulicella tundricola type strain MP5ACTX9T, an Acidobacteria from tundra soil. Stand. Genom. Sci. 2014, 9, 449–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.F.; Xiong, J.B.; Hu, C.J.; Shi, Y.; Wang, K.; Zhang, D.M. Temperature sensitivity of soil bacterial community along contrasting warming gradient. Appl. Soil Ecol. 2015, 94, 40–48. [Google Scholar] [CrossRef]
- Pepe-Ranney, C.; Campbell, A.N.; Koechli, C.N.; Berthrong, S.; Buckley, D.H. Unearthing the Ecology of Soil Microorganisms Using a High Resolution DNA-SIP Approach to Explore Cellulose and Xylose Metabolism in Soil. Front. Microbiol. 2016, 7, 703. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.L.; Lu, J.K.; Dou, Y.J.; Zhu, Y.J.; Wang, S.K.; Dun, X.H. Bradyrhizobium Ganzhouense sp.nov. and Application Thereof. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/CN-103981122-A. (accessed on 26 March 2014).
- Novikova, A.T.; Knyazeva, V.L.; Kozhemyakov, A.P. Novel Strain of Bradyrhizobium sp. (astragalus). Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:1992389282 (accessed on 3 January 1992).
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Ricke, P.; Liesack, W. NifH and NifD phylogenies: An evolutionary basis for understanding nitrogen fixation capabilities of methanotrophic bacteria. Microbiology 2004, 150, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.M.; Ke, W.H.; Chen, L.L.; Huang, J.W.; Lin, W.X. Diversity of bacterial community in rhizosphere soils under effects of continuously planting burley tobacco. Chin. J. Appl. Ecol. 2010, 21, 1751–1758. [Google Scholar]
- Koblížek, M.; Dachev, M.; Bína, D.; Piwosz, K.; Kaftan, D. Utilization of light energy in phototrophic gemmatimonadetes. J. Photochem. Photobiol. B Biol. 2020, 213, 112085. [Google Scholar] [CrossRef]
- Debruyn, J.M.; Fawaz, M.N.; Peacock, A.D.; Dunlap, J.R.; Nixon, L.T.; Cooper, K.E.; Radosevich, M. Gemmatirosa kalamazoonesis gen. nov., sp. nov., a member of the rarely-cultivated bacterial phylum Gemmatimonadetes. J. Gen. Appl. Microbiol. 2013, 59, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onyango, M.; Wang, Y.; Nickel, O.; Zhao, C.; Zhang, X.; Hartke, A.; Hemberger, J.; Cemic, F. First Genome Sequence of Potential Mycotoxin-Degrading Bacterium Devosia nanyangense DDB001. Genome Announc. 2014, 2, e00922-14. [Google Scholar] [CrossRef] [PubMed]
- Rivett, D.W.; Bell, T. Abundance determines the functional role of bacterial phylotypes in complex communities. Nat. Microbiol. 2018, 3, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhang, T.; Su, J.; Zhao, L.L.; Yu, L.Y. Structural variation of the bacterial community associated with airborne particulate matter in Beijing during haze and non-haze days. Appl. Environ. Microbiol. 2018, 84, e00004-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Yu, Y.; Ye, Z.; Li, G.; An, T. Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens from typical area, Pearl River Estuary and their exposure risk to human. Environ. Int. 2020, 143, 105934. [Google Scholar] [CrossRef]
- Hu, J.W.; Zhu, R.G.; Wang, H.B.; Deng, Z.Y.; Zhang, L. Free Amino Acids in Pine Needles Response to Environment Nitrogen. Biol. Chem. Eng. 2017, 3, 5–8. (In Chinese) [Google Scholar]
- Gao, M.L. Study on the Effect of Phosphorus on Yield and Quality of Tomato. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2003. (In Chinese). [Google Scholar]
- Ying, Z.; Zhang, C.; Wang, Z.; Chen, Y.; Gang, C.; Ru, A.; Li, J. Vegetation dynamics and its driving forces from climate change and human activities in the Three-River Source Region, China from 1982 to 2012. Sci. Total Environ. 2016, 563–564, 210–220. [Google Scholar]
- Freeman, K.R.; Martin, A.P.; Karki, D.; Lynch, R.C.; Mitter, M.S.; Meyer, A.F.; Longcore, J.E.; Simmons, D.R.; Schmidt, S.K. Evidence that chytrids dominate fungal communities in high-elevation soils. Proc. Natl. Acad. Sci. USA 2009, 106, 18315–18320. [Google Scholar] [CrossRef] [Green Version]
- Letcher, P.M.; Powell, M.J.; Barr, D.J.S.; Churchill, P.F.; Picard, K.T. Rhizophlyctidales-a new order in Chytridiomycota. Mycol. Res. 2008, 112, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Letcher, P.M.; Longcore, J.E.; Mozley-Standridge, S.E.; Porter, D.; Powell, M.J.; Griffith, G.W.; Vilgalys, R. A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 2006, 98, 860. [Google Scholar] [CrossRef]
- Gleason, F.H.; Letcher, P.M.; Mcgee, P.A. Some Chytridiomycota in soil recover from drying and high temperatures. Mycol. Res. 2004, 108, 583–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Mehrotra, B.S.; Baijal, U. Species of Mortierella from india-III. Mycopathol. Mycol. Appl. 1963, 20, 49. [Google Scholar] [CrossRef]
- Mitchel, D. Survey of the Grassland Fungi of County Clare; Heritage Council: Dublin, Ireland, 2006. [Google Scholar]
- Vidovic, S.; Zekovic, Z.; Jokic, S. Clavaria Mushrooms and Extracts: Investigation on Valuable Components and Antioxidant Properties. Int. J. Food Prop. 2014, 17, 2072–2081. [Google Scholar] [CrossRef]
- Dong, J.Y.; Li, J.; Chen, Y.H.; Feng, X.F. Progress in the study of chemical components and their biological activities in secondary metabolites of fungi of the genus Dictyostelium. Chin. Herb. Med. 2021, 52, 12. (In Chinese) [Google Scholar]
- Keswani, C.; Singh, S.P.; García-Estrada, C.; Borriss, R.; Rajput, V.D.; Minkina, T.M.; Ortiz, A.; Sansinenea, E.; Mezaache-Aichour, S.; Glare, T.R. Biosynthesis and beneficial effects of microbial gibberellins on crops for sustainable agriculture. J. Appl. Microbiol. 2022, 132, 1597–1615. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Li, S.; Tan, G. New Humicola A695 Used for Preparing Anti-Bacterial Drug against Staphylococcus Aureus or Bacillus Subtilis, is Preserved in Guangdong Microbial Culture Collection Center. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:2016788942 (accessed on 8 January 2016).
- Lang, J.; Hu, J.; Ran, W.; Xu, Y.; Shen, Q. Control of cotton Verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biol. Fertil. Soils 2012, 48, 191–203. [Google Scholar] [CrossRef]
- Ko, W.H.; Yang, C.H.; Lin, M.J.; Chen, C.Y.; Tsou, Y.J. Humicola phialophoroides sp. nov. from soil with potential for biological control of plant diseases. Bot. Stud. 2011, 52, 197–202. [Google Scholar]
- Faust, K.; Raes, J.; Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat Rev Microbiol 10: 538–550. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Heijden, M. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Publ. Group 2019, 10, 4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vries; Franciska, T.; Wallenstein; Matthew, D. Below-ground connections underlying above-ground food production: A framework for optimising ecological connections in the rhizosphere. J. Ecol. 2017, 105, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, K.S.; Geisen, S.; Morrin, E.; Snoek, B.L.; Putten, W. Network Analyses Can Advance Above-Belowground Ecology. Trends Plant Sci. 2018, 23, 759–768. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Erratum: Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2014, 6, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.H.; Wang, J.Y.; Meng, Z.X.; He, J.; Dong, Z.H.; Liu, C.; Chen, W.Q. Soil microbial diversity characterizes alpine meadow ecosystem multifunctionality through co-occurrence network complexity. J. Ecol. 2022, 42, 17. (In Chinese) [Google Scholar]
- Chen, W.; Wang, J.; Meng, Z.; Xu, R.; Chen, J.; Zhang, Y.; Hu, T. Fertility-related interplay between fungal guilds underlies plant richness–productivity relationships in natural grasslands. New Phytol. 2020, 226, 1129–1143. [Google Scholar] [CrossRef]
- Yu, G.; Lu, C.; Xie, H. Seasonal dynamics of ecosystem service functions in grasslands of Qinghai-Tibet Plateau. J. Appl. Ecol. 2007, 18, 5. (In Chinese) [Google Scholar]
- Morri, N.E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; De Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephen, C.; Kenneth, A.; Scott, B.; Reinette, B.; William, B.; Anne-Sophie, C.; Gustav, E.M.; Carl, F.; Terry, H.; Nils, K. General Resilience to Cope with Extreme Events. Sustainability 2012, 4, 3248–3259. [Google Scholar]
- Ye, D.; Ping, Z.; Qin, Y.; Tu, Q.; Yang, Y.; He, Z.; Schadt, C.W.; Zhou, J. Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ. Microbiol. 2016, 18, 205–218. [Google Scholar]
- Ghoul, M.; Mitri, S. The Ecology and Evolution of Microbial Competition. Trends Microbiol 2016, 24, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G. A new vision of immunity: Homeostasis of the superorganism. Mucosal Immunol. 2010, 3, 450–460. [Google Scholar] [CrossRef]
- Verstraete, P.; Wiele, V.D.; Abbeele, V.D. The host selects mucosal and luminal associations of coevolved gut microorganisms: A novel concept. FEMS Microbiol. Rev. 2011, 35, 681–704. [Google Scholar]
- Oliveira, N.M.; Niehus, R.; Foster, K.R. Evolutionary limits to cooperation in microbial communities. Proc. Natl. Acad. Sci. USA 2014, 111, 17941. [Google Scholar] [CrossRef] [Green Version]
- Fiegna, F.; Velicer, G.J. Exploitative and Hierarchical Antagonism in a Cooperative Bacterium. PLoS Biol. 2005, 3, e370. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.; Sonnenburg, J.; Peterson, D.; Gordon, J. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Cagno, R.D.; Angelis, M.D.; Pasquale, I.D.; Ndagijimana, M.; Francavilla, R. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef] [Green Version]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling Interactions in the Microbiome: A Network Perspective. Trends Microbiol. 2016, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; Van, D. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Song, Y.S.; Seo, D.J. Pedobacter Species PR-M6 Strain Used Environment-Friendly Microbial Agent, for Producing Chitinolytic Enzyme and Controlling Plant Diseases, is Preserved in Korean Agricultural Culture Collection and Exhibits Activity at Low Temperature. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:201849564K (accessed on 26 July 2018).
- Dahal, R.H.; Chaudhary, D.K.; Kim, D.U.; Kim, J. Nine novel psychrotolerant species of the genus Pedobacter isolated from Arctic soil with potential antioxidant activities. Int. J. Syst. Evol. Microbiol. 2020, 70, 2537–2553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, Y.; Kot, W.; Zhang, F.; Zheng, S.; Wang, G.; Hansen, L.H.; Rensing, C. Genome Sequence of Selenium-Solubilizing Bacterium Caulobacter vibrioides T5M6. Genome Announc. 2016, 4, e01715–e01721. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Si, M.; Zhu, L.; Li, C.; Wei, Y.; Shen, X. Draft Genome Sequence of Nafulsella turpanensis ZLM-10T, a Novel Member of the Family Flammeovirgaceae. Genome Announc. 2013, 2, e00263-14. [Google Scholar]
- Fuerst, J.A.; Sagulenko, E. Beyond the bacterium: Planctomycetes challenge our concepts of microbial structure and function. Nat. Rev. Microbiol. 2011, 9, 403–413. [Google Scholar] [CrossRef]
- Guo, M.; Zhou, Q.; Zhou, Y.; Yang, L.; Liu, T.; Yang, J.; Chen, Y.; Su, L.; Xu, J.; Chen, J. Genomic Evolution of 11 Type Strains within Family Planctomycetaceae. PLoS ONE 2014, 9, e86752. [Google Scholar] [CrossRef]
- Makhalanyane, T.; Valverde, A.; Velázquez, D.; Gunnigle, E.; Van Goethem, M.W.; Quesada, A.; Cowan, D. Ecology and biogeochemistry of cyanobacteria in soils, permafrost, aquatic and cryptic polar habitats. Biodivers. Conserv. 2015, 24, 819–840. [Google Scholar] [CrossRef]
- Yung, C.C.M.; Chan, Y.; Lacap, D.C.; Pérez-Ortega, S.; Rios-Murillo, A.; Lee, C.K.; Cary, S.C.; Pointing, S.B. Characterization of Chasmoendolithic Community in Miers Valley, McMurdo Dry Valleys, Antarctica. Microb. Ecol. 2014, 68, 351–359. [Google Scholar] [CrossRef]
- Stomeo, F.; Makhalanyane, T.P.; Valverde, A.; Pointing, S.B.; Stevens, M.I.; Cary, C.S.; Tuffin, I.M.; Cowan, D.A. Adelaide Research and Scholarship: Abiotic factors influence microbial diversity in permanently cold soil horizons of a maritime-associated Antarctic Dry Valley. FEMS Microbiol. Ecol. 2012, 82, 326–340. [Google Scholar] [CrossRef]
- Rios, A.D.L.; Cary, C.; Cowan, D. The spatial structures of hypolithic communities in the Dry Valleys of East Antarctica. Polar Biol. 2014, 37, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.; Lacap, D.C.; Lau, M.C.; Ha, K.Y.; Warren-Rhodes, K.A.; Cockell, C.S.; Cowan, D.A.; McKay, C.P.; Pointing, S.B. Hypolithic microbial communities: Between a rock and a hard place. Env. Microbiol 2012, 14, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O.; Kirby, B.M.; Roes-Hill, M.L.; Cook, A.E.; Cary, S.C.; Burton, S.G.; Cowan, D.A. Phylogenetic analysis of actinobacterial populations associated with Antarctic Dry Valley mineral soils. Environ. Microbiol. 2010, 11, 566–576. [Google Scholar] [CrossRef]
- Aislabie, J.M.; Chhour, K.-L.; Saul, D.J.; Miyauchi, S.; Ayton, J.; Paetzold, R.F.; Balks, M.R. Dominant bacteria in soils of Marble Point and Wright Valley, Victoria Land, Antarctica. Soil Biol. Biochem. 2006, 38, 3041–3056. [Google Scholar] [CrossRef]
- Carrión, V.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; Raaijmakers, J.M. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, J.; Ling, J.; Long, L.; Pan, H.; Zhang, S.; Zhang, Y. New Xylariaceae Species DPZ-SY43 Used for Generating Cellulase, and for Producing Bacterial Fertilizer Capable of Degrading Biological Organic Substance. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:2012D63754 (accessed on 14 March 2012).
- Liu, G.; Shang, H.; Wang, Q.; Gu, H.; Bai, J.; Wu, L. New Acremonium Nepalense Strain LZ1 with Preservation Number CGMCC No. 7703 or Helotiales (BH5) with Preservation Number CGMCC No. 7704 for Promoting Plant Growth and/or Drought Stress Resistance. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:2015169015 (accessed on 2 January 2015).
- Oberhofer, M.; Malfent, F.; Zehl, M.; Urban, E.; Wackerlig, J.; Reznicek, G.; Vignolle, G.; Rückert, C.; Busche, T.; Wibberg, D.; et al. Biosynthetic Potential of the Endophytic Fungus Helotiales sp. BL73 Revealed via Compound Identification and Genome Mining. Appl. Environ. Microbiol. 2022, 88, aem0251021. [Google Scholar] [CrossRef] [PubMed]
- Du, T.F.; Jiang, S.P.; Wang, M.R.; Dai, Y.J.; Li, J. Classification and identification of fusidic acid producing strain SIIA06-05-201 and spectral analysis of its secondary metabolite FA-3. Chin. J. Antibiot. 2008, 33, 145. [Google Scholar]
- Khan, A.L.; Asaf, S.; Khan, A.R.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. First draft genome sequencing of indole acetic acid producing and plant growth promoting fungus Preussia sp BSL10. J. Biotechnol. 2016, 225, 44–45. [Google Scholar] [CrossRef]
- Elsebai, M.F. Secondary metabolites from the marine-derived fungus Phaeosphaeria spartinae. Nat. Prod. Res. 2021, 35, 1504–1509. [Google Scholar] [CrossRef]
- Park, Y.I.; Kim, W.J.; Park, J.W. New Coniochaeta Velutina Strain Capable of Degrading Galacto-Fucoidan to Galacto Fuco-oligosaccharide Used for Promoting Blood Clotting, Treating Stress and Cancer, and Lowering Blood Cholesterol. KR1448802-B1. 2014. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:2014T31534 (accessed on 3 January 2014).
- Kim, C.J.; Lee, H.B. Novel Myrothecium Roridum Tode ex Fries F000252(KCTC 0980BP) is Useful as a Biocontrol Agent. KR2002086812-A.; KR427410-B1. 2003. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:2003741800 (accessed on 3 January 2003).
- Fang, F.; Gao, X.; Li, J.; Li, M. New Myrothecium Roridum is Used as Biological Herbicide for Barnyard Bristlegrass, Crabgrass or Amaranthus. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:201623038R (accessed on 8 January 2016).
- K.K.KOJIN. Antibiotic from Cultivation of Myrothecium Verucaria A-H-14. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:197014479R (accessed on 4 September 2022).
- Margesin, R.; Neuner, G.; Storey, K.B. Cold-loving microbes, plants, and animals—Fundamental and applied aspects. Die Nat. 2007, 94, 77–99. [Google Scholar] [CrossRef]
- Teplyakova, T.V.; Repin, V.E.; Ryabchikova, E.I.; Sviridenko, A.N. Fungi Strain Duddingtonia Flagrans with Activity against Plant Gall Nematode and Animal Parasitic Nematode, Also Stimulates Plant Growth and Development. Available online: https://www.webofscience.com/wos/alldb/full-record/DIIDW:2005454771 (accessed on 7 January 2005).
- Le, D.Q.; Shin, T.S.; Park, M.S.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Kim, I.S.; Kim, J.C. Antimicrobial activities of novel mannosyl lipids isolated from the biocontrol fungus Simplicillium lamellicola BCP against phytopathogenic bacteria. J. Agric. Food Chem. 2014, 62, 3363–3370. [Google Scholar]
- Dong, Q.L.; Dong, R.Z.; Xing, X.Y.; Miao, J.X.; Yu-Kuan, L.I. Isolation, purification and preliminary characterization of antibiotic from Simplicillium lanosoniveum. Sci. Technol. Food Ind. 2017, 38, 119–129. [Google Scholar]
- Xie, L.; Yin, W.; Li, W.; Gao, W. Advance on natural products and their pharmacological activities and biosynthesis from Beauveria species. Nat. Prod. Res. Dev. 2021, 33, 150–164. [Google Scholar]
- Hallasgo, A.M.; Spangl, B.; Steinkellner, S.; Hage-Ahmed, K. The Fungal Endophyte Serendipita williamsii Does Not Affect Phosphorus Status but Carbon and Nitrogen Dynamics in Arbuscular Mycorrhizal Tomato Plants. J. Fungi—Open Access Mycol. J. 2020, 6, 233. [Google Scholar] [CrossRef]
- Walker, J.F.; Miller, O.K.; Horton, J.L. Hyperdiversity of ectomycorrhizal fungus assemblages on oak seedlings in mixed forests in the southern Appalachian Mountains. Mol. Ecol. 2005, 14, 829–838. [Google Scholar] [CrossRef]
- Gong, S.; Feng, B.; Jian, S.P.; Wang, G.S.; Yang, Z.L. Elevation Matters More than Season in Shaping the Heterogeneity of Soil and Root Associated Ectomycorrhizal Fungal Community. Microbiol. Spectr. 2022, 10, e01950-21. [Google Scholar] [CrossRef] [PubMed]
- Yumiko, M.; Yoshie, T.; Kazuhide, N. Temperature niche position and breadth of ectomycorrhizal fungi: Reduced diversity under warming predicted by a nested community structure. Glob. Change Biol. 2018, 24, 5724–5737. [Google Scholar]
- Morgado, L.N.; Semenova, T.A.; Welker, J.M.; Walker, M.; Smets, E.; Geml, J. Summer temperature increase has distinct effects on the ectomycorrhizal fungal communities of moist tussock and dry tundra in Arctic Alaska. Glob. Change Biol. 2015, 21, 959–972. [Google Scholar] [CrossRef] [Green Version]
- Lilleskov, E.A.; Hobbie, E.A.; Horton, T.R. Conservation of ectomycorrhizal fungi: Exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol. 2011, 4, 174–183. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Fahey, T.J.; Horton, T.R.; Lovett, G.M. Esa Online Journals-Belowground Ectomycorrhizal Fungal Community Change Over a Nitrogen Deposition Gradient in Alaska. Ecol. Soc. Am. 2002, 83, 104–105. [Google Scholar]
- Shao, M.W.; Kong, L.C.; Jiang, D.H.; Zhang, Y.L. Phytotoxic and Antimicrobial Metabolites from Paraphaeosphaeria sp. QTYC11 Isolated from the Gut of Pantala flavescens Larvae. Rec. Nat. Prod. 2016, 10, 326–331. [Google Scholar]
- Zhang, K.; Bonito, G.; Hsu, C.M.; Hameed, K.; Liao, H.L. Mortierella elongata Increases Plant Biomass among Non-Leguminous Crop Species. Agronomy 2020, 10, 754. [Google Scholar] [CrossRef]
- Ozimek, E.; Jaroszuk-Ściseł, J.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Tyśkiewicz, R.; Słomka, A.; Nowak, A.; Hanaka, A. Synthesis of Indoleacetic Acid, Gibberellic Acid and ACC-Deaminase by Mortierella Strains Promote Winter Wheat Seedlings Growth under Different Conditions. Int. J. Mol. Sci. 2018, 19, 3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Zhang, Y.; Mi, F.; Tang, X.; Xu, J. Recent advances in population genetics of ectomycorrhizal mushrooms Russula spp. Mycology 2015, 6, 110–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutzendubel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. journal of experimental botany 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant–microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [Green Version]
- Benito, B.; González-Guerrero, M. Unravelling potassium nutrition inectomycorrhizal associations. New Phytol. 2014, 201, 707–709. [Google Scholar] [CrossRef]
- Hobbie, E. Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecology 2006, 87, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, F.D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Mazzaferro, L.; Piuel, L.; Minig, M.; Breccia, J.D. Extracellular monoenzyme deglycosylation system of 7-O-linked flavonoid beta-rutinosides and its disaccharide transglycosylation activity from Stilbella fimetaria. Arch. Microbiol. 2011, 193, 461. [Google Scholar] [CrossRef] [Green Version]
- Puder, C.; Wagner, K.; Vettermann, R.; Hauptmann, R.; Potterat, O. Terphenylquinone inhibitors of the src protein tyrosine kinase from Stilbella sp. J. Nat. Prod. 2005, 323, 326. [Google Scholar]
- Lakshmanan, V.; Kitto, S.L.; Caplan, J.L.; Hsueh, Y.H.; Kearns, D.B.; Bais, W. Microbe-Associated Molecular Patterns-Triggered Root Responses Mediate Beneficial Rhizobacterial Recruitment in Arabidopsis. Plant Physiol. 2012, 160, 1642–1661. [Google Scholar] [CrossRef] [Green Version]
- Shetty, S.A.; Hugenholtz, F.; Lahti, L.; Smidt, H.; Vos, W. Intestinal microbiome landscaping: Insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol. Rev. 2017, 41, 182–199. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- Herren, C.M.; Mcmahon, K.D. Keystone taxa predict compositional change in microbial communities. Environ. Microbiol. 2018, 20, 2207–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.H. Endophytic and Inter-Rhizospheric Actinomycete Diversity of Artemisia Annua in Aba. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2016. (In Chinese). [Google Scholar]
- Shan-Shan, Q.I.; Zhou, L.H.; Jiu-Ping, H.U.; Liu, M.; Zhao, H.; Xiong, Y. Isolation, Identification and Diversity of Soil Bacteria in Multiple Regions from Tibetan Plateau. Southwest China J. Agric. Sci. 2017, 30, 1629–1635. [Google Scholar]
- Teale, W.; Paponov, I.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Sun, H.; Wang, C.; Dongxue, L.U.; Liu, J.; Yue, S.; Yang, M. Research Progress of Phosphate Dissolving Microorganisms and Their Promotion Effect on Plant Growth. J. Henan Agric. Sci. 2016, 1, 3–7. [Google Scholar]
- Wu, X.; Rensing, C.; Han, D.; Xiao, K.Q.; Dai, Y.; Tang, Z.; Liesack, W.; Peng, J.; Cui, Z.; Zhang, F. Genome-Resolved Metagenomics Reveals Distinct Phosphorus Acquisition Strategies between Soil Microbiomes. mSystems. 2022, 7, e0110721. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
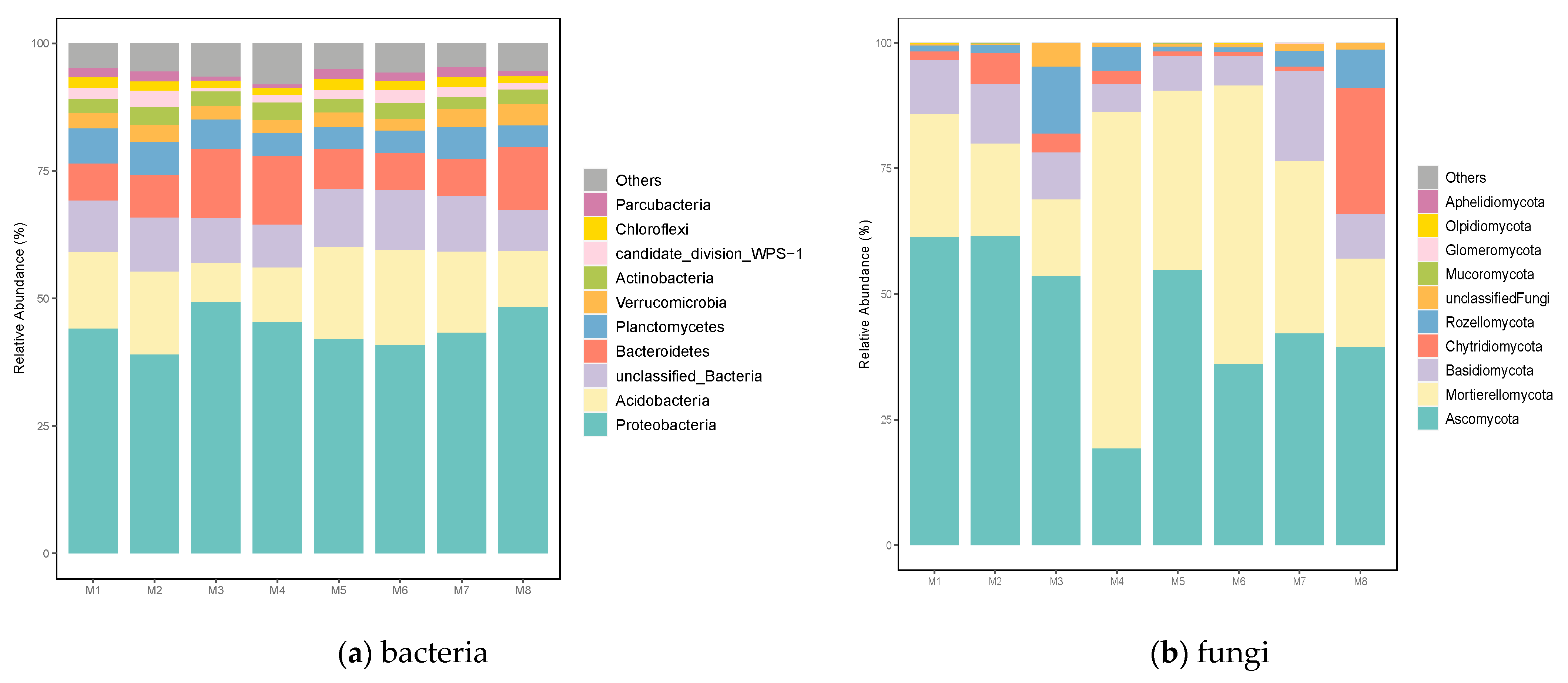
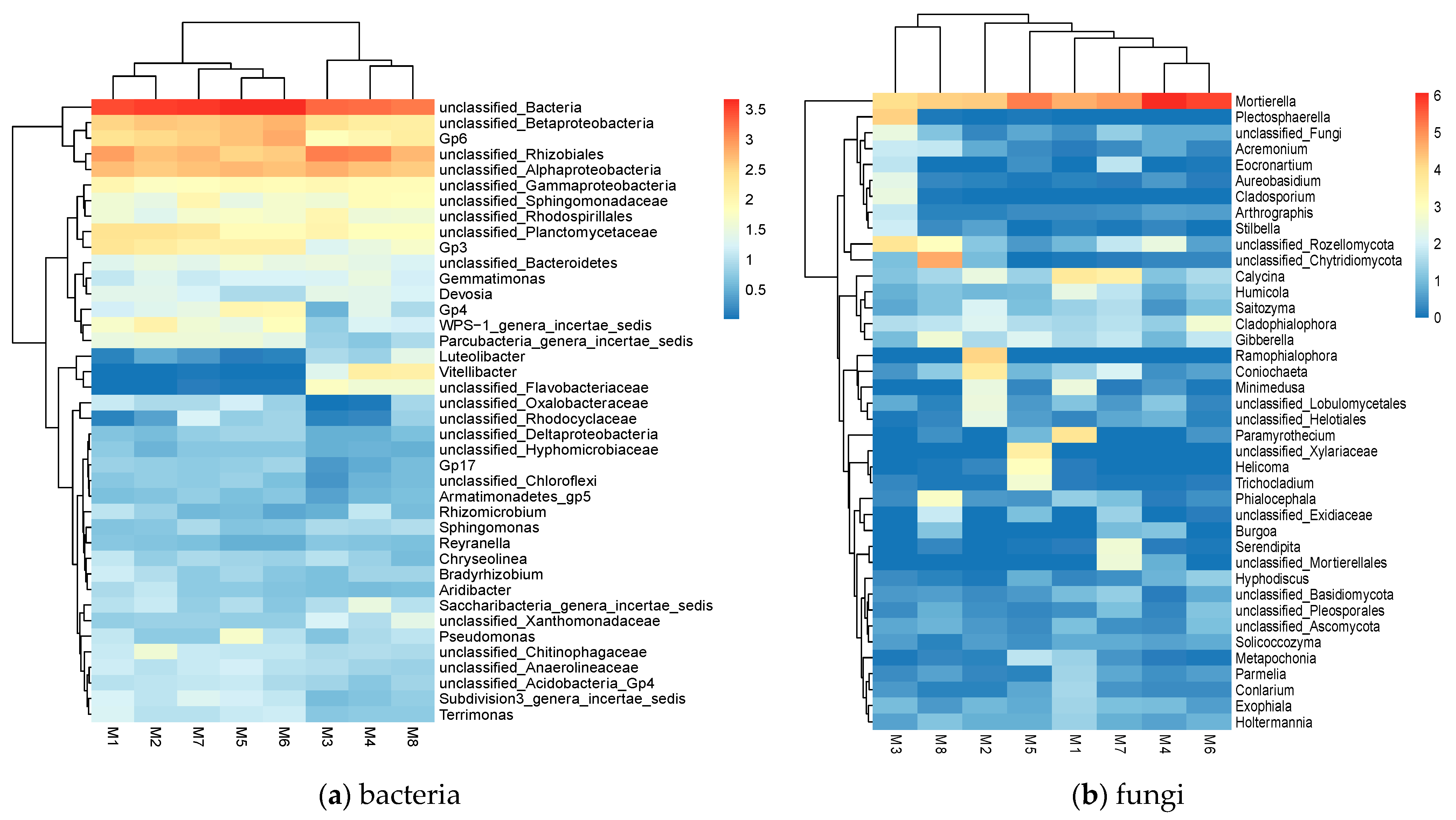
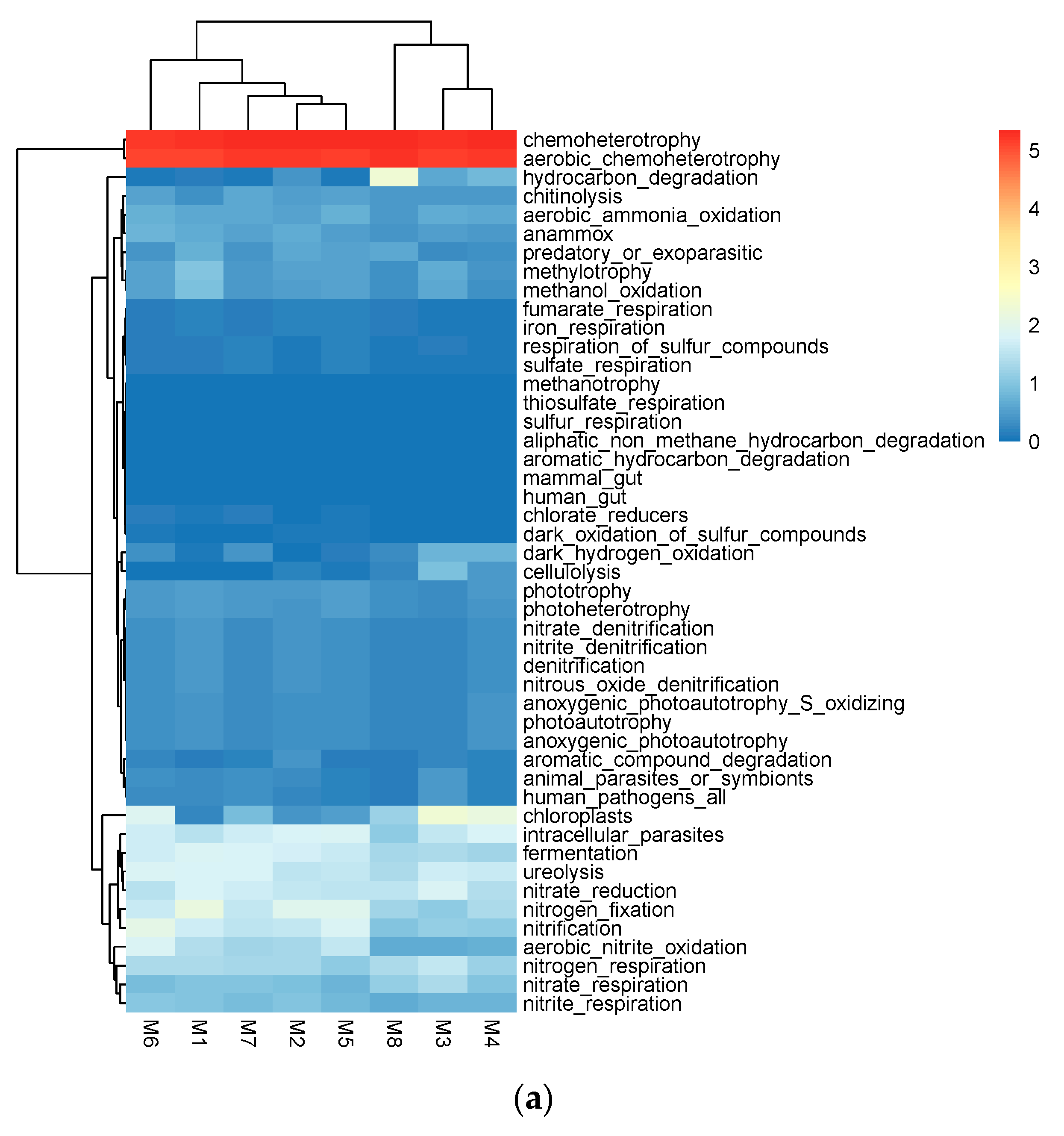
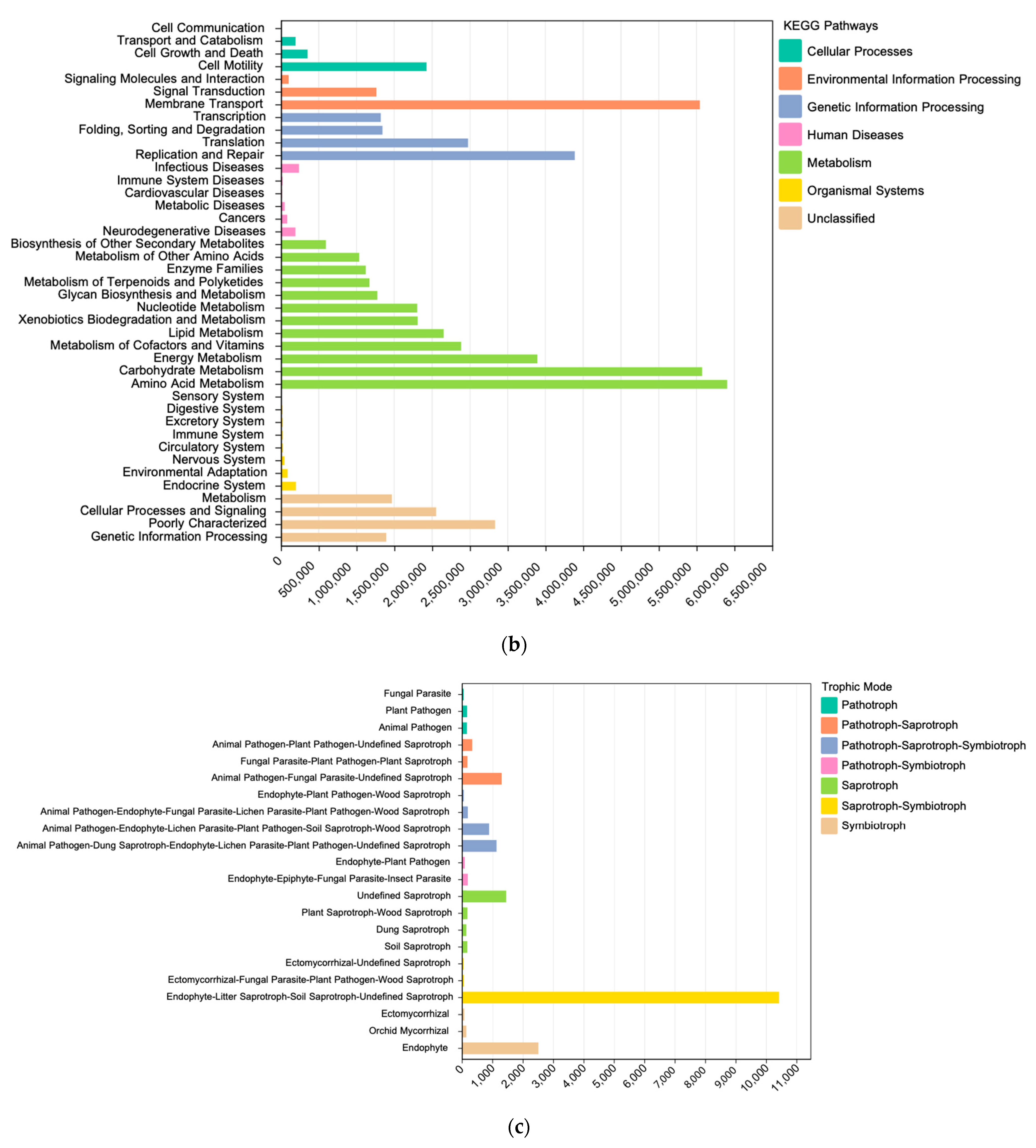
| Classified | Sample | Number of Sequences | Number of OTUs | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|---|---|
| Bacteria | M1 | 105,298 | 2759 | 3049.37 | 3020.17 | 6.80 | 0.002747 |
| M2 | 87,264 | 2756 | 3059.27 | 3065.06 | 6.93 | 0.002269 | |
| M3 | 89,772 | 2469 | 2826.53 | 2819.84 | 6.57 | 0.003527 | |
| M4 | 93,596 | 2612 | 2939.35 | 2923.72 | 6.65 | 0.004035 | |
| M5 | 76,373 | 2784 | 3076.36 | 3056.26 | 6.88 | 0.002544 | |
| M6 | 107,230 | 2840 | 3098.08 | 3114.25 | 6.89 | 0.002408 | |
| M7 | 94,746 | 2877 | 3147.46 | 3132.49 | 6.89 | 0.002575 | |
| M8 | 127,350 | 2873 | 3190.22 | 3156.52 | 6.70 | 0.003976 | |
| Fungi | M1 | 39,471 | 319 | 433.62 | 435.68 | 3.81 | 0.060223 |
| M2 | 82,544 | 590 | 633.25 | 644.05 | 3.72 | 0.065641 | |
| M3 | 34,032 | 478 | 530.93 | 518.58 | 3.98 | 0.05309 | |
| M4 | 40,498 | 492 | 574.20 | 571.94 | 2.83 | 0.227684 | |
| M5 | 46,018 | 509 | 546.35 | 557.46 | 3.83 | 0.064865 | |
| M6 | 45,850 | 483 | 517.35 | 522.06 | 3.53 | 0.143607 | |
| M7 | 36,850 | 474 | 521.56 | 513.72 | 4.11 | 0.046816 | |
| M8 | 84,407 | 552 | 588.28 | 588.98 | 3.78 | 0.065266 |
| Network Indexes | Bacteria | Fungi |
|---|---|---|
| Total nodes | 624 | 627 |
| Total links | 479 | 5330 |
| Average degree | 1.535 | 17.002 |
| Diameter | 9 | 2 |
| Density | 0.002 | 0.027 |
| Average clustering coefficient | 0.622 | 0.967 |
| Average path distance | 1.973 | 1.033 |
| modularity | 0.986 | 0.84 |
| Measuring Item | CK (Sterile Water) | CK (IAA) | N1-12 | N1-21 | N1-73 |
|---|---|---|---|---|---|
| Qualitative color |  | 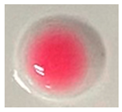 | 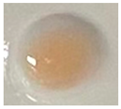 |  | 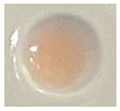 |
| Quantitative determination (IAA μg/mL) | 0.00 | 20 | 1.57 | 2.82 | 3.84 |
| Genus | Strain Name | Average Dissolved Organic Phosphorus Rate (D/d) | Average Iron Production Carrier Rate (D/d) | Average Dissolved Inorganic Phosphorus Rate (D/d) |
|---|---|---|---|---|
| Streptomyces | N1-26 | 1.64 | 1.64 | - |
| R2-10 | 2.06 | 1.28 | - | |
| R2-13 | 1.72 | - | - | |
| R2-18 | 1.69 | 1.63 | - | |
| Peribacillus | N1-13 | 1.27 | - | - |
| N1-73 | 1.95 | 1.82 | - | |
| Ensifer | N1-6 | 1.71 | 1.74 | - |
| Bacillus | N1-18 | 1.24 | - | - |
| Pedobacter | N1-17 | 1.61 | - | - |
| Flavobacterium | N1-4 | 1.49 | - | - |
| Pseudomonas | N1-12 | 1.36 | 1.36 | 1.37 |
| Microbacterium | N1-72 | 1.75 | 2.14 | - |
| N1-21 | - | 1.24 | - | |
| N1-15 | - | 1.24 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, Q.; Wang, L.; Hu, J.; Xue, H.; Han, D.; Xing, Z.; Ruan, Z. Structure and Function Analysis of Cultivated Meconopsis integrifolia Soil Microbial Community Based on High-Throughput Sequencing and Culturability. Biology 2023, 12, 160. https://doi.org/10.3390/biology12020160
Wang Y, Ma Q, Wang L, Hu J, Xue H, Han D, Xing Z, Ruan Z. Structure and Function Analysis of Cultivated Meconopsis integrifolia Soil Microbial Community Based on High-Throughput Sequencing and Culturability. Biology. 2023; 12(2):160. https://doi.org/10.3390/biology12020160
Chicago/Turabian StyleWang, Yan, Qingyun Ma, Lingling Wang, Jingkuo Hu, Huiying Xue, Dongfei Han, Zhen Xing, and Zhiyong Ruan. 2023. "Structure and Function Analysis of Cultivated Meconopsis integrifolia Soil Microbial Community Based on High-Throughput Sequencing and Culturability" Biology 12, no. 2: 160. https://doi.org/10.3390/biology12020160






