Using SXRF and LA-ICP-TOFMS to Explore Evidence of Treatment and Physiological Responses to Leprosy in Medieval Denmark
Abstract
Simple Summary
Abstract
1. Introduction
Mineral Imbalances in Leprosy
2. Materials and Methods
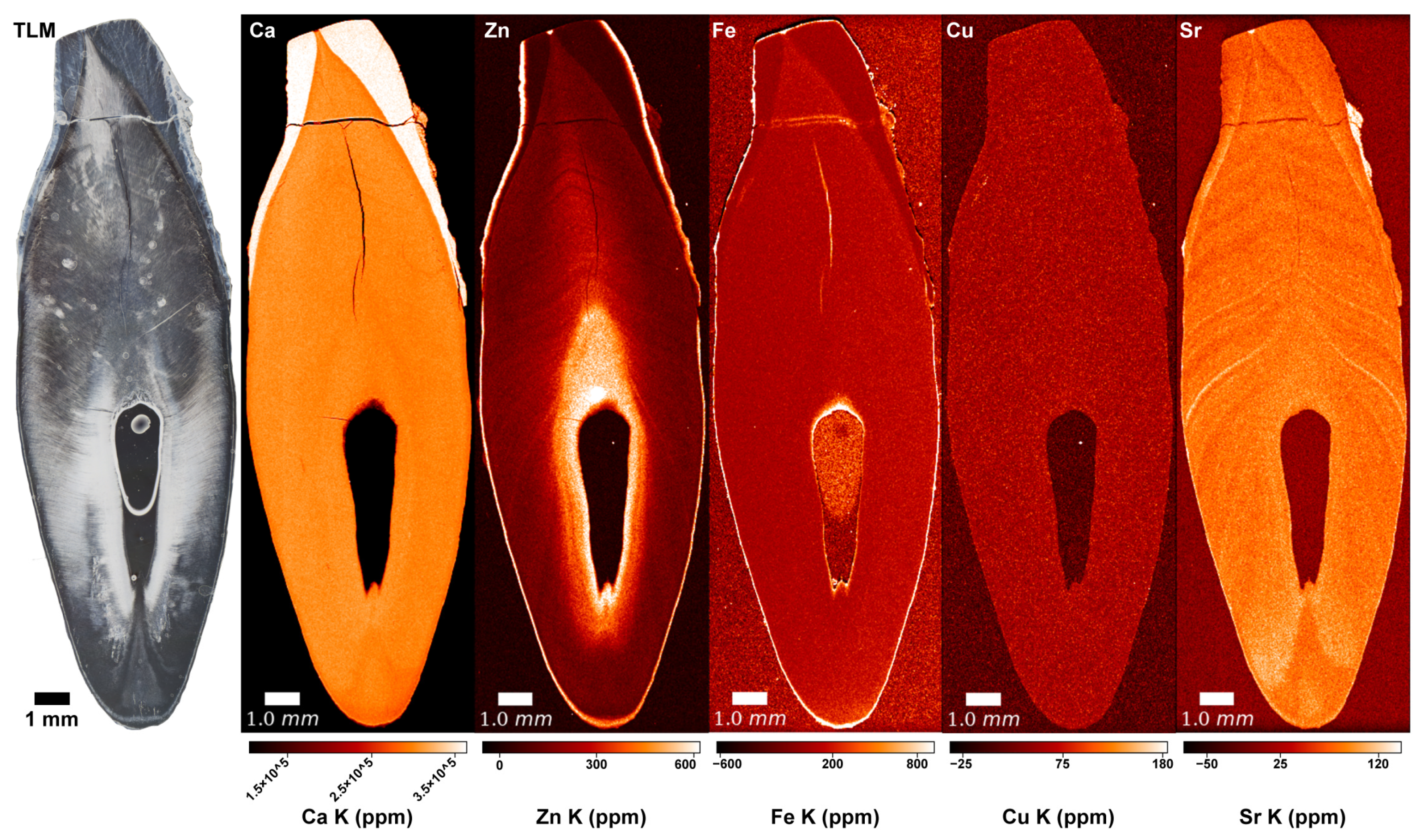
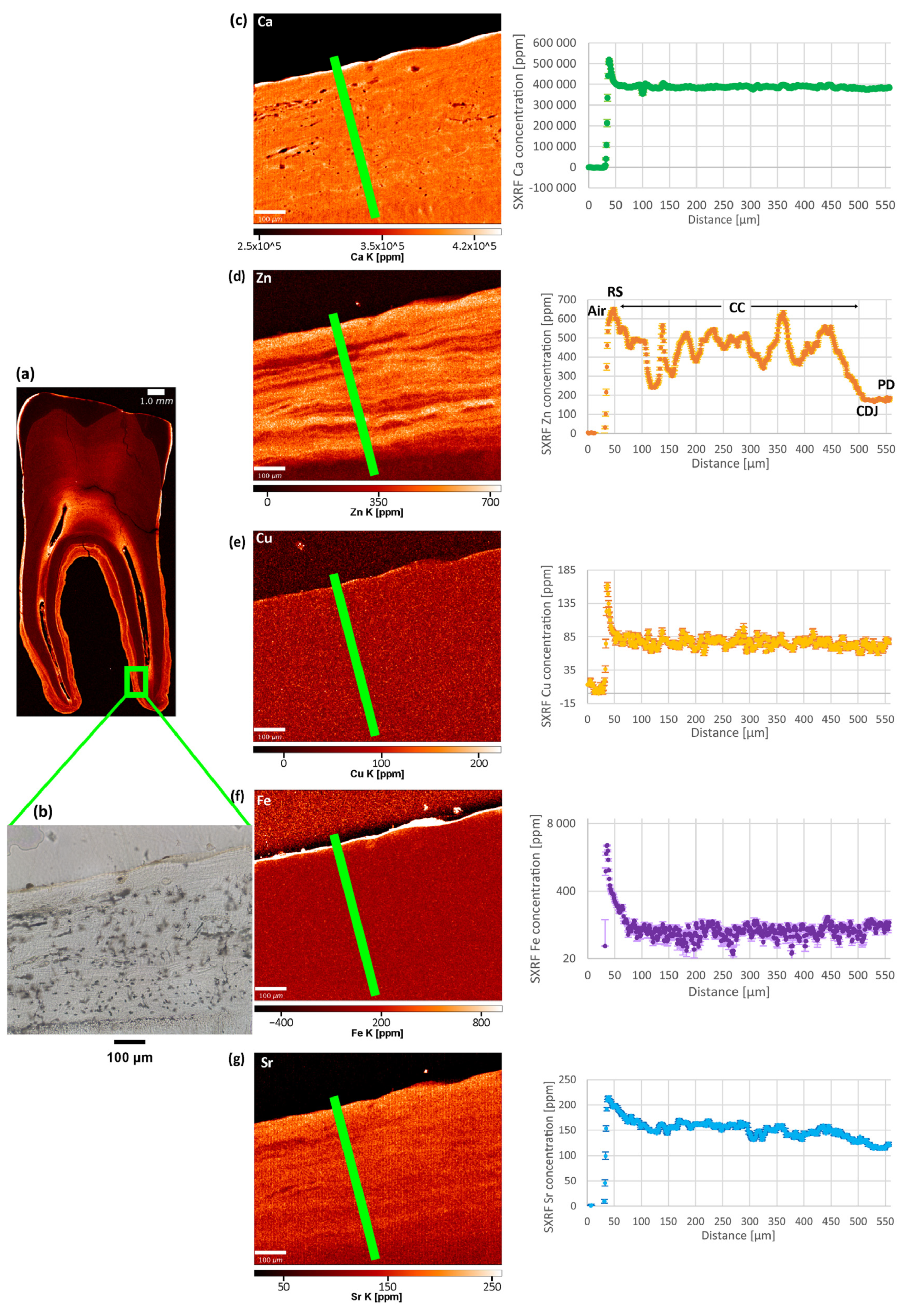
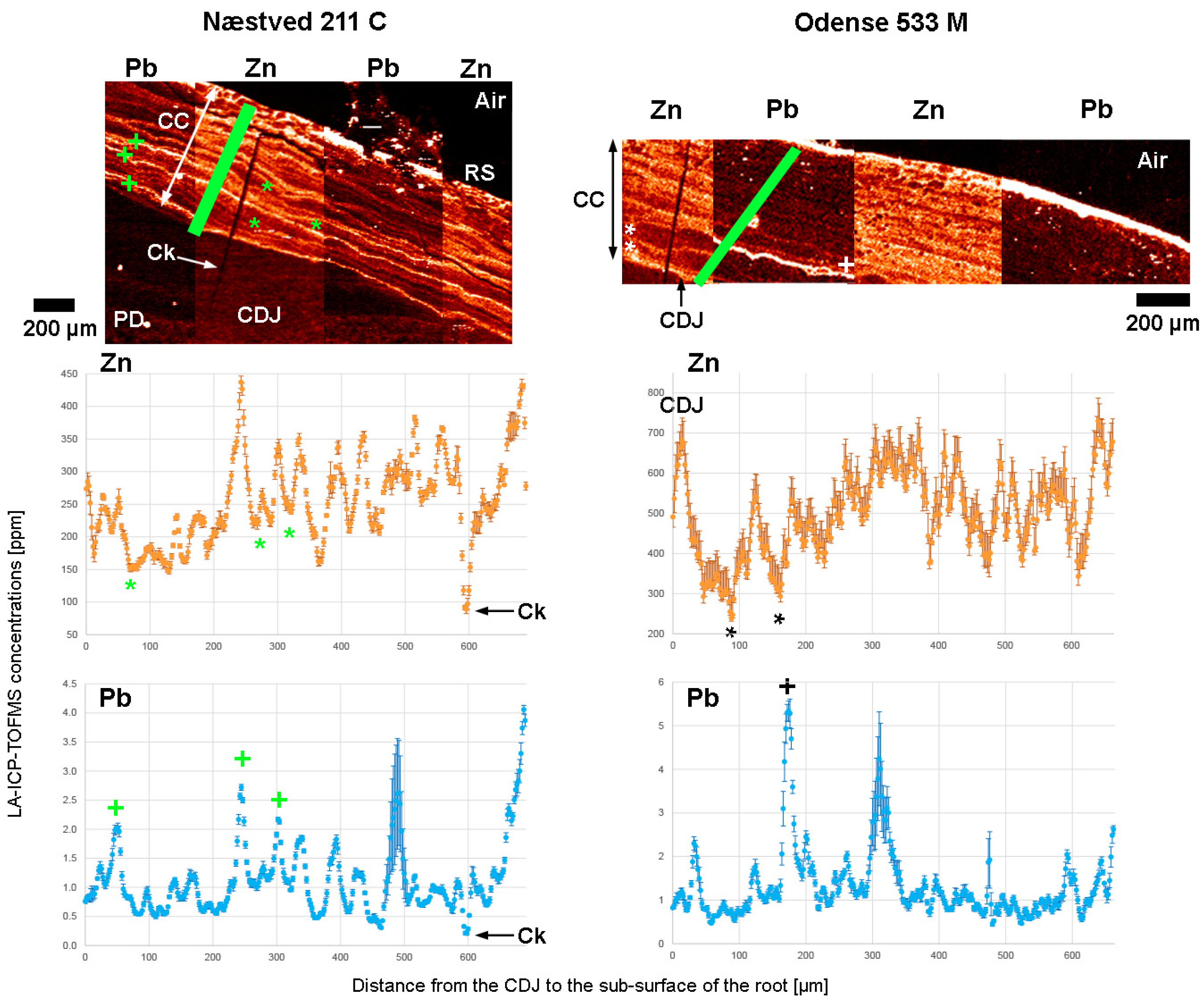
3. Results
3.1. Copper (Cu) and Iron (Fe)
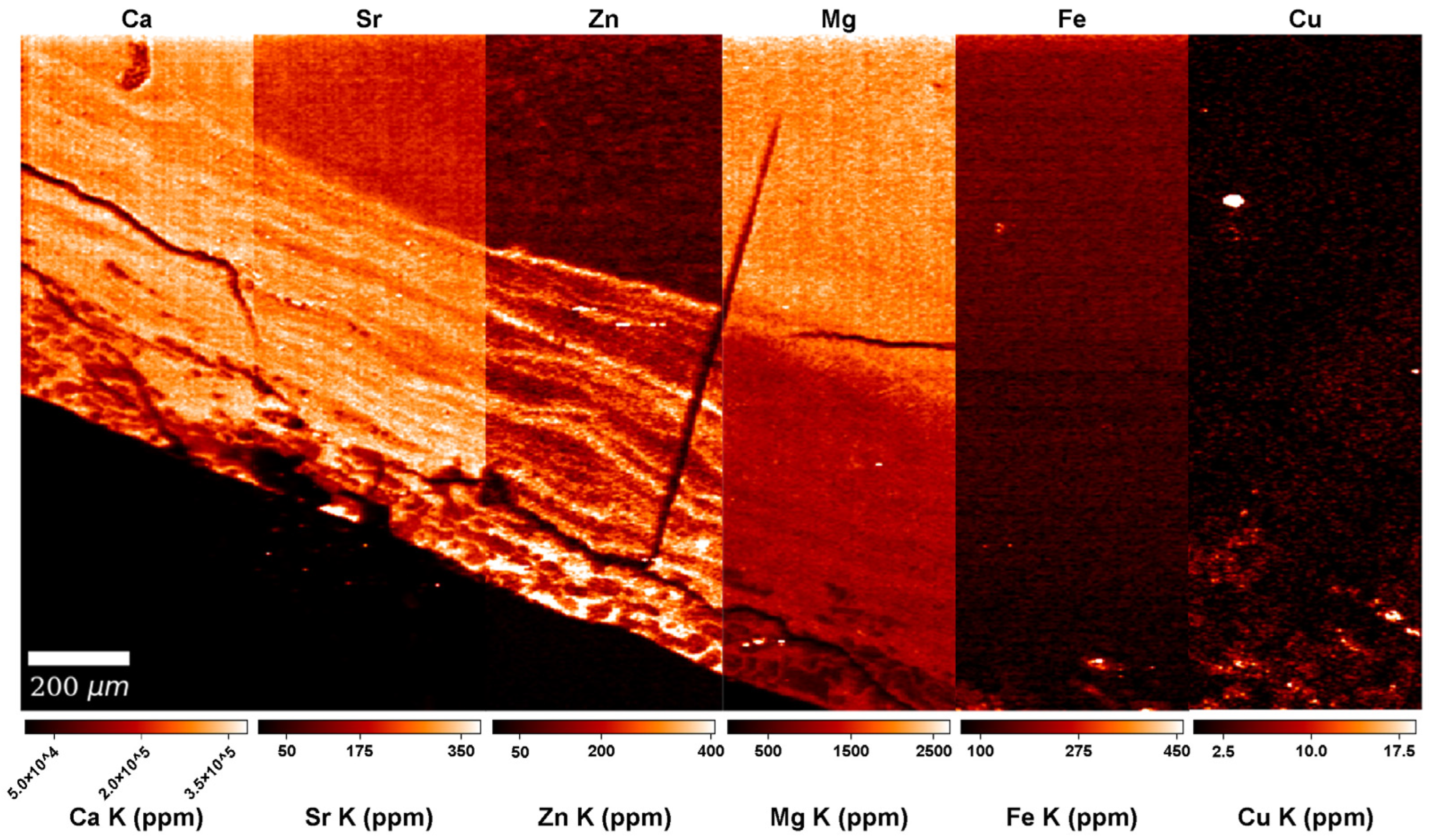
3.2. Calcium (Ca) and Magnesium (Mg)
3.3. Zinc (Zn)
3.4. Strontium (Sr)
3.5. Arsenic (As) and Mercury (Hg)
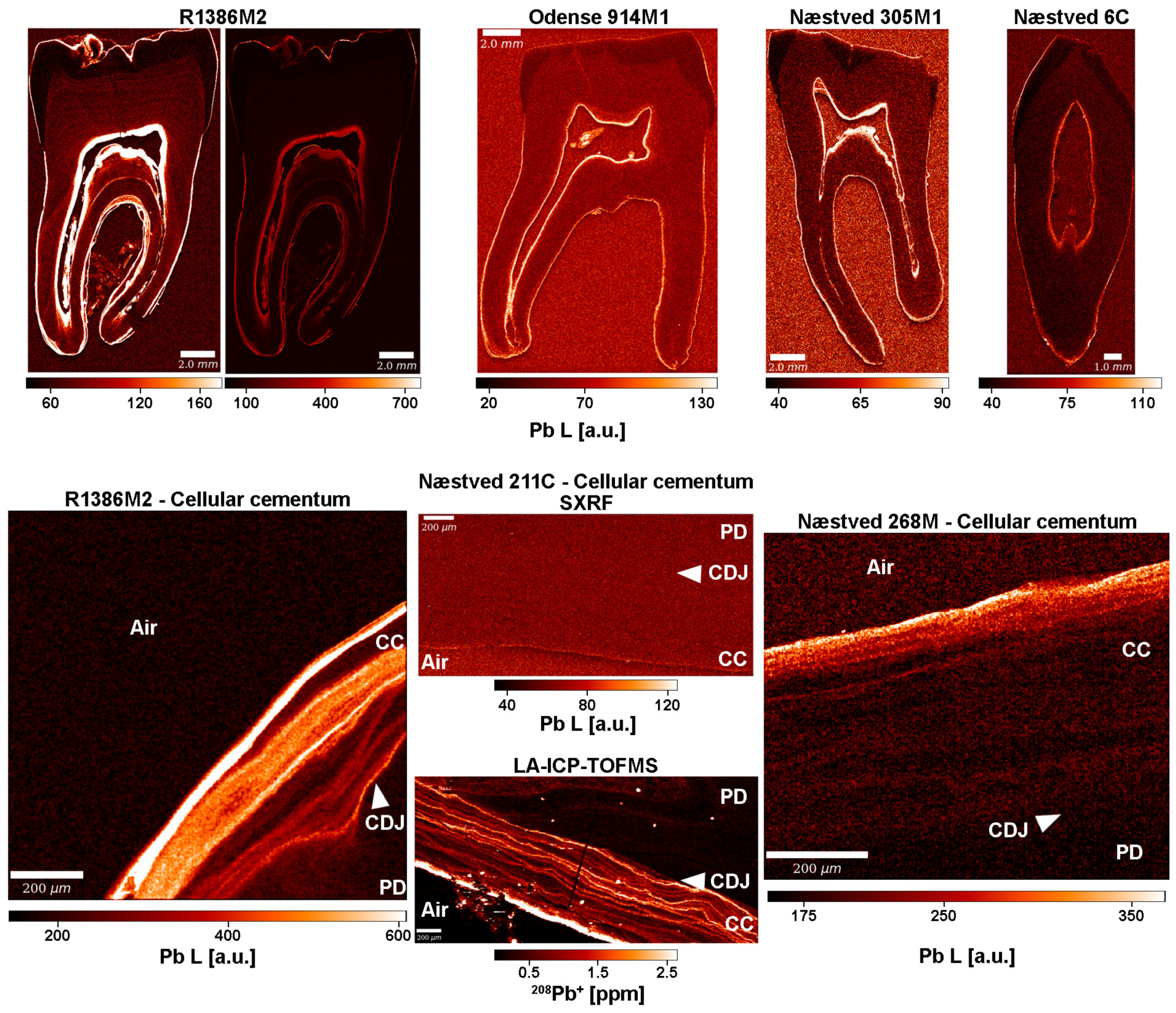
3.6. Lead (Pb)
4. Discussion
4.1. Exploring Leprosy-Induced Mineral Imbalances in Dental Tissues
4.2. Exploring Evidence of Leprosy Treatment by Heavy Metals in Dental Tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global leprosy update, 2018: Moving towards a leprosy-free world–Situation de la lèpre dans le monde, 2018: Parvenir à un monde exempt de lèpre. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2019, 94, 389–412. [Google Scholar]
- Han, X.Y.; Sizer, K.C.; Thompson, E.J.; Kabanja, J.; Li, J.; Hu, P.; Gómez-Valero, L.; Silva, F.J. Comparative Sequence Analysis of Mycobacterium leprae and the New Leprosy-Causing Mycobacterium lepromatosis. J. Bacteriol. 2009, 191, 6067–6074. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Seo, Y.-H.; Sizer, K.C.; Schoberle, T.; May, G.S.; Spencer, J.S.; Li, W.; Nair, R.G. A New Mycobacterium Species Causing Diffuse Lepromatous Leprosy. Am. J. Clin. Pathol. 2008, 130, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Lynnerup, N.; Boldsen, J.L. Leprosy (Hansen’s Disease). In A Companion to Paleopathology; Grauer, A.L., Ed.; Wiley-Blackwell: Chichester, UK, 2012; pp. 458–471. [Google Scholar]
- Ridley, D.; Jopling, W. Classification of Leprosy According to Immunity. A Five-Group System. Int. J. Lepr. Other Mycobact. Dis. 1966, 34, 255–273. [Google Scholar] [PubMed]
- Dwivedi, V.P.; Banerjee, A.; Das, I.; Saha, A.; Dutta, M.; Bhardwaj, B.; Biswas, S.; Chattopadhyay, D. Diet and Nutrition: An Important Risk Factor in Leprosy. Microb. Pathog. 2019, 137, 103714. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, S.G.; Nahar, Q.; Pahan, D.; Oskam, L.; Richardus, J.H. Recent Food Shortage Is Associated with Leprosy Disease in Bangladesh: A Case-Control Study. PLoS Negl. Trop. Dis. 2011, 5, e1029. [Google Scholar] [CrossRef] [PubMed]
- Kerr-Pontes, L.R.; Barreto, M.L.; Evangelista, C.M.; Rodrigues, L.C.; Heukelbach, J.; Feldmeier, H. Socioeconomic, Environmental, and Behavioural Risk Factors for Leprosy in North-East Brazil: Results of a Case–Control Study. Int. J. Epidemiol. 2006, 35, 994–1000. [Google Scholar] [CrossRef]
- Oktaria, S.; Hurif, N.S.; Naim, W.; Thio, H.B.; Nijsten, T.; Richardus, J. Dietary Diversity and Poverty as Risk Factors for Leprosy in Indonesia: A Case-Control Study. PLoS Negl. Trop. Dis. 2018, 12, e0006317. [Google Scholar] [CrossRef]
- Wagenaar, I.; van Muiden, L.; Alam, K.; Bowers, R.; Hossain, M.A.; Kispotta, K.; Richardus, J.H. Diet-Related Risk Factors for Leprosy: A Case-Control Study. PLoS Negl. Trop. Dis. 2015, 9, e0003766. [Google Scholar] [CrossRef]
- Katona, P.; Katona-Apte, J. The Interaction between Nutrition and Infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef]
- Oz, H. Nutrients, Infectious and Inflammatory Diseases. Nutrients 2017, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Sethi, N.; Madadi, A.J.; Bhandari, S. Serum Zinc, Copper, Magnesium, Proteins and Superoxide Dismutase in Leprosy Patients on Multidrug Therapy–a Follow-up Study. Indian J. Lepr. 1996, 68, 325–333. [Google Scholar] [PubMed]
- Fatimah, S.; Rahfiludin, M.Z. The Difference of BMI and Micronutrient Intake Between Multibacillary Leprosy and Non Leprosy (A Study in District Brondong, Lamongan 2013). Adv. Sci. Lett. 2017, 23, 3421–3423. [Google Scholar] [CrossRef]
- Foster, R.; Sanchez, A.; Foulkes, J.; Cameron, L.J. Profile of Blood Elements in Leprosy Patients. Indian J. Lepr. 1991, 63, 12–33. [Google Scholar] [PubMed]
- Khalid, H.N.; Mostafa, M.I.; Attia, N.S.; Bazid, H.A.S.E. Serum Level of Selenium, Zinc, and Vitamin C and Their Relation to the Clinical Spectrum of Leprosy. J. Infect. Dev. Ctries 2022, 16, 491–499. [Google Scholar] [CrossRef]
- Nigam, P.; Dayal, S.; Sriwastava, P.; Joshi, L. Serum Calcium and Magnesium in Leprosy. Asian J. Infect. Dis. 1979, 3, 81–83. [Google Scholar]
- Venkatesan, K.; Kannan, K.B.; Bharadwaj, V.P.; Sritharan, V.; Katoch, K.; Usha, R.; Ramu, G. Serum Copper & Zinc in Leprosy & Effect of Oral Zinc Therapy. Indian J. Med. Res. 1983, 78, 37–41. [Google Scholar]
- World Health Organization. Leprosy/Hansen Disease: Management of Reactions and Prevention of Disabilities. In Technical Guidance; World Health Organization, Regional Office for South-East Asia: New Delhi, India, 2020. [Google Scholar]
- MacLeod, J.M.H. Sections of Tropical Diseases and Parasitology, Dermatology, and Therapeutics and Pharmacology. Proc. R. Soc. Med. 1927, 6, 987–1025. [Google Scholar]
- Muir, E. Treatment of Leprosy. A Review. Int. J. Lepr. 1933, 1, 407–458. [Google Scholar]
- Rawcliffe, C. Leprosy in Medieval England; The Boydell Press: Woodbridge, UK, 2006. [Google Scholar]
- Beaumont, J.; Montgomery, J. The Great Irish Famine: Identifying Starvation in the Tissues of Victims Using Stable Isotope Analysis of Bone and Incremental Dentine Collagen. PLoS ONE 2016, 11, e0160065. [Google Scholar] [CrossRef]
- Cerrito, P.; Bailey, S.E.; Hu, B.; Bromage, T.G. Parturitions, Menopause and Other Physiological Stressors Are Recorded in Dental Cementum Microstructure. Sci. Rep. 2020, 10, 5381. [Google Scholar] [CrossRef] [PubMed]
- Colard, T.; Bertrand, B.; Naji, S.; Delannoy, Y.; Bécart, A. Toward the Adoption of Cementochronology in Forensic Context. Int. J. Legal Med. 2018, 132, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.C. Retrieving Chronological Age from Dental Remains of Early Fossil Hominins to Reconstruct Human Growth in the Past. Phil. Trans. R. Soc. B 2010, 365, 3397–3410. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.T.; Reid, D.J.; Dean, M.C.; Zihlman, A.L. A Faithful Record of Stressful Life Events Recorded in the Dental Developmental Record of a Juvenile Gorilla. Int. J. Primatol. 2006, 27, 1201–1219. [Google Scholar] [CrossRef]
- Skinner, M.; Byra, C. Signatures of Stress: Pilot Study of Accentuated Laminations in Porcine Enamel. Am. J. Phys. Anthropol. 2019, 169, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, M.J.; Popowics, T. Dental Embryology, Histology and Anatomy; Elsevier: St. Louis, MI, USA, 2020; ISBN 978-0-323-63990-3. [Google Scholar]
- Nanci, A. Ten Cate’s Oral Histology. Development, Structure and Function; Elsevier: St. Louis, MI, USA, 2018. [Google Scholar]
- Gherase, M.R.; Fleming, D.E.B. Probing Trace Elements in Human Tissues with Synchrotron Radiation. Crystals 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- Prashanth, L.; Kattapagari, K.; Chitturi, R.T.; Baddam, V.R.R.; Prasad, L.K. A Review on Role of Essential Trace Elements in Health and Disease. J. NTR Univ. Health Sci. 2015, 4, 75–85. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.; Hurrell, R.F. Bioavailability of Minerals and Trace Elements: Members of EC Flair Concerted Action No. 10: Measurements of Micronutrient Absorption and Status. Nutr. Res. Rev. 1996, 9, 295–324. [Google Scholar] [CrossRef]
- Soetan, K.; Olaiya, C.O.; Oyewole, O. The Importance of Mineral Elements for Humans, Domestic Animals and Plants: A Review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Wada, O. What Are Trace Elements? Their Deficiency and Excess States. JMAJ 2004, 47, 351–358. [Google Scholar]
- Rao, K.; Gupta, J.D.; Sehgal, V.; Chakrabarti, A.; Saha, K. Trace Elements in the Sera of Leprosy Spectrum. Indian J. Lepr. 1985, 57, 556–561. [Google Scholar] [PubMed]
- Arora, P.; Dhillon, K.; Rajan, S.; Sayal, S.; Das, A. Serum Zinc Levels in Cutaneous Disorders. MJAFI 2002, 58, 304–306. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Bhatia, V.N.; Balakrishnan, S.; Ramu, G. Serum Zinc/Copper Ratio in Subtypes of Leprosy and Effect of Oral Zinc Therapy on Reactional States. Int. J. Lepr. Other Mycobact. Dis. 1991, 59, 20–24. [Google Scholar] [PubMed]
- Mathur, N.K.; Sharma, M.L.; Mangal, H.N.; Rai, S.M. Serum Zinc Levels in Subtypes of Leprosy. Int. J. Lepr. Other Mycobact. Dis. 1984, 52, 327–330. [Google Scholar] [PubMed]
- Pradhan, T.; Kumari, S. Evaluation of Oxidative Status and Zinc Level in Leprosy Patients after Zinc Supplementation. Int. Biol. Med. Res. 2015, 6, 4984–4987. [Google Scholar]
- Saxena, N.; Sharma, R.; Singh, V.S. Study of Serum Zinc Level in Leprosy. Indian J. Lepr. 1988, 60, 556–561. [Google Scholar] [PubMed]
- Saxena, N.; Sharma, R.; Singh, V.S. Serum Iron and Total Iron Binding Capacity in Leprosy Patients. Indian J. Lepr. 1990, 62, 219–222. [Google Scholar] [PubMed]
- Bhattacharya, R.N.; Goswami, K.; Bandyopadhyay, A. Copper and Ascorbic Acid Status in Children Suffering from Leprosy. EJBPS 2020, 7, 421–423. [Google Scholar]
- Jain, P.; Khare, V.; Koshti, A.; Malik, R.; Bhimte, B. Serum Zinc Level Estimation- Comparison between Normal Control and in Leprosy Patients. Int. J. Biol. Med. Res. 2014, 5, 3847–3849. [Google Scholar]
- Oon, B.B.; Khong, K.Y.; Greaves, M.W.; Plummer, V.M. Trophic Skin Ulceration of Leprosy: Skin and Serum Zinc Concentrations. BMJ 1974, 2, 531–533. [Google Scholar] [CrossRef]
- Mennen, U.; Howells, C.; Wiese, A. Serum Zinc, Sodium, Calcium, Magnesium and Potassium Levels and Standard Diet in Leprosy Patients. Indian J. Lepr. 1993, 65, 415–421. [Google Scholar] [PubMed]
- Rao, K.N.; Saha, K. Undernutrition in Lepromatous Leprosy, Part II. Altered Levels of Serum Elements. Their Association with the Disease and Not with Food Deprivation. Lepr. Rev. 1986, 57, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Sher, R.; Shulman, G.; Baily, P.; Politzer, W.M. Serum Trace Elements and Vitamin A in Leprosy Subtypes. Am. J. Clin. Nutr. 1981, 34, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Wannemacher, R.W.; Pekarek, R.S.; Klainer, A.S.; Bartelloni, P.J.; Dupont, H.L.; Hornick, R.B.; Beisel, W.R. Detection of a Leukocytic Endogenous Mediator-like Mediator of Serum Amino Acid and Zinc Depression during Various Infectious Illnesses. Infect. Immun. 1975, 11, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Malhotra, V.; Singh, R.; Vij, J.; Anand, B. Structure and Function of the Small Bowel in Lepromatous Leprosy. Int. J. Lepr. Other Mycobact. Dis. 1982, 50, 148–151. [Google Scholar] [PubMed]
- Bhattacharya, R.N.; Goswami, K.; Bandyopadhyay, A. Plasma Copper and Ascorbic Acid Status in Leprosy. EJBPS 2020, 7, 248–252. [Google Scholar]
- Bharadwaj, V.P.; Venkatesan, K.; Ramu, G.; Desikan, K.V. Serum Iron and Total Iron Binding Capacity in Leprosy Patients. Leprosy in India 1978, 50, 11–17. [Google Scholar] [PubMed]
- Shwe, T.; Than-Toe; Tu, A.T.B. Serum Iron and Total Iron Binding Capacity in Burmese Patients with Leprosy. Lepr. Rev. 1976, 47, 190–287. [Google Scholar] [CrossRef]
- Tamara, R.; Muchtar, S.V.; Amin, S.; Seweng, A.; Sjahril, R.; Adam, A.M. Serum Iron, Total Iron Binding Capacity and Transferrin Saturation Levels in Leprosy Patients before Multi Drug Therapy - World Health Organization (MDT-WHO) Compared with Healthy Control Group. Int. J. Med. Rev. Case Rep. 2018, 2, 105–108. [Google Scholar]
- Jain, A.; Mukherjee, A.; Chattopadhya, D.; Saha, K. Biometals in Skin and Sera of Leprosy Patients and Their Correlation to Trace Element Contents of M. Leprae and Histological Types of the Disease, a Comparative Study with Cutaneous Tuberculosis. Int. J. Lepr. Other Mycobact. Dis. 1995, 63, 249. [Google Scholar]
- Swathi, M.; Tagore, R. Study of Oxidative Stress in Different Forms of Leprosy. Indian J. Dermatol. 2015, 60, 321. [Google Scholar] [CrossRef] [PubMed]
- Skaar, E.P.; Raffatellu, M. Metals in Infectious Diseases and Nutritional Immunity. Metallomics 2015, 7, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Nutritional Immunity: Host’s Attempt to Withhold Iron from Microbial Invaders. JAMA 1975, 231, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Botella, H.; Stadthagen, G.; Lugo-Villarino, G.; de Chastellier, C.; Neyrolles, O. Metallobiology of Host–Pathogen Interactions: An Intoxicating New Insight. Trends in Microbiology 2012, 20, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.I.; Skaar, E.P. Nutritional Immunity: Transition Metals at the Pathogen–Host Interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Neyrolles, O.; Mintz, E.; Catty, P. Zinc and Copper Toxicity in Host Defense against Pathogens: Mycobacterium Tuberculosis as a Model Example of an Emerging Paradigm. Front. Cell. Infect. Microbiol. 2013, 3, 89. [Google Scholar] [CrossRef] [PubMed]
- Møller-Christensen, V. Hvad de Døde Fortalte. Historisk Samfund for Præstø Amt 1954, 4, 86–101. [Google Scholar]
- Madsen, K. Spedalskhed Og Sct. Jørgensgård; Næstved Museum: Næstved, Denmark, 1990. [Google Scholar]
- Arentoft, E. De spedalskes hospital: Udgravning af Sankt Jørgensgården i Odense; Odense Bys Museer: Odense, Denmark, 1999; ISBN 87-7838-500-8. [Google Scholar]
- Nielsen, E. Beretning for Udgravningen 1980-81 Af Sct. Jørgensgården, Odense, 1981.
- Michelsen, F. St. Jørgensgården i Aaderup Ved Næstved. Årbog Hist. Samf. Præstø 1954, 4, 72–85. [Google Scholar]
- Ehlers, E. Danske St. Jørgensgaarde i Middelalderen.; FR. Bagges Bogtrykkeri: Copenhagen, Denmark, 1898. [Google Scholar]
- Erslev, K. Testamenter fra Danmarks Middelalder indtil 1450; Den Gyldendalske Boghandel: Copenhagen, Denmark, 1901. [Google Scholar]
- Møller-Christensen, V. Location and Excavation of the First Danish Leper Graveyard from the Middle Ages – St. Jørgen’s Farm, Næstved. Bull. Hist. Med. 1953, 27, 112–123. [Google Scholar] [PubMed]
- Nielsen, E. Sct. Jørgensgården i Odense. Fynske Minder 1982 1983, 61–74. [Google Scholar]
- Richards, P. The Medieval Leper and His Northern Heirs; Butler & Tanner Ltd.: Cambridge, UK, 1977. [Google Scholar]
- Boldsen, J.L.; Mollerup, L. Outside St. Jørgen: Leprosy in the Medieval Danish City of Odense. Am. J. Phys. Anthropol. 2006, 130, 344–351. [Google Scholar] [CrossRef]
- Møller-Christensen, V. Bone Changes in Leprosy; Munksgaard: Copenhagen, Denmark, 1961. [Google Scholar]
- Møller-Christensen, V. Ten Lepers from Næstved in Denmark. A Study of Skeletons from a Medieval Danish Leper Hospital; Danish Science Press: Copenhagen, Denmark, 1953. [Google Scholar]
- Schuurs, A. Pathology of the Hard Dental Tissues; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Boesenberg, U.; Ryan, C.G.; Kirkham, R.; Siddons, D.P.; Alfeld, M.; Garrevoet, J.; Núñez, T.; Claussen, T.; Kracht, T.; Falkenberg, G. Fast X-Ray Microfluorescence Imaging with Submicrometer-Resolution Integrating a Maia Detector at Beamline P06 at PETRA III. J. Synchrotron Rad. 2016, 23, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Schroer, C.; Boye, P.; Feldkamp, J.; Patommel, J.; Samberg, D.; Schropp, A.; Schwab, A.; Stephan, S.; Falkenberg, G.; Wellenreuther, G.; et al. Hard X-Ray Nanoprobe at Beamline P06 at PETRA III. Nucl. Instrum. Meth. A 2010, 616, 93–97. [Google Scholar] [CrossRef]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief Communication: The London Atlas of Human Tooth Development and Eruption. Am. J. Phys. Anthropol. 2010, 142, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Gundlach-Graham, A.; Allner, S.; Schwarz, G.; Wang, H.A.O.; Gyr, L.; Burgener, S.; Hattendorf, B.; Grolimund, D.; Günther, D. High-Speed, High-Resolution, Multielemental LA-ICP-TOFMS Imaging: Part II. Critical Evaluation of Quantitative Three-Dimensional Imaging of Major, Minor, and Trace Elements in Geological Samples. Anal. Chem. 2015, 87, 8259–8267. [Google Scholar] [CrossRef] [PubMed]
- Gundlach-Graham, A.; Günther, D. Toward Faster and Higher Resolution LA–ICPMS Imaging: On the Co-Evolution of LA Cell Design and ICPMS Instrumentation. Anal. Bioanal. Chem. 2016, 408, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Dean, M.C.; Spiers, K.M.; Garrevoet, J.; Le Cabec, A. Synchrotron X-Ray Fluorescence Mapping of Ca, Sr and Zn at the Neonatal Line in Human Deciduous Teeth Reflects Changing Perinatal Physiology. Arch. Oral Biol. 2019, 104, 90–102. [Google Scholar] [CrossRef]
- Humphrey, L.T.; Jeffries, T.E.; Dean, M.C. Micro Spatial Distributions of Lead and Zinc in Human Deciduous Tooth Enamel. In Technique and application in Dental Anthropology; Irish, J.D., Nelson, G.C., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 87–110. [Google Scholar]
- Müller, W.; Nava, A.; Evans, D.; Rossi, P.F.; Alt, K.W.; Bondioli, L. Enamel Mineralization and Compositional Time-Resolution in Human Teeth Evaluated via Histologically-Defined LA-ICPMS Profiles. Geochim. Cosmochim. Acta 2019, 255, 105–126. [Google Scholar] [CrossRef]
- Barbosa, F.; Tanus-Santos, J.E.; Gerlach, R.F.; Parsons, P.J. A Critical Review of Biomarkers Used for Monitoring Human Exposure to Lead: Advantages, Limitations, and Future Needs. Environ. Health Perspect. 2005, 113, 1669–1674. [Google Scholar] [CrossRef]
- Bercovitz, K.; Laufer, D. Age and Gender Influence on Lead Accumulation in Root Dentine of Human Permanent Teeth. Arch. Oral Biol. 1991, 36, 671–673. [Google Scholar] [CrossRef]
- Mahajan, P.; Jadhav, V.; Patki, A.H.; Jogaikar, D.G.; Mehta, J. Oral Zinc Therapy in Recurrent Erythema Nodosum Leprosum: A Clinical Study. Indian J. Lepr. 1994, 66, 51–57. [Google Scholar] [PubMed]
- Naafs, B.; Noto, S. Reactions in Leprosy. In Leprosy. A Practical Guide.; Nunzi, E., Massone, C., Eds.; Springer-Verlag: Milan, Italy, 2012; pp. 219–240. [Google Scholar]
- Cool, S.M.; Forwood, M.R.; Campbell, P.; Bennett, M.B. Comparisons between Bone and Cementum Compositions and the Possible Basis for Their Layered Appearances. Bone 2002, 30, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.R.; Naftel, S.J.; Nelson, A.J.; Feilen, A.B.; Narvaez, A. Metal Distributions in the Cementum Rings of Human Teeth: Possible Depositional Chronologies and Diagenesis. J. Archaeol. Sci. 2007, 34, 936–945. [Google Scholar] [CrossRef]
- Martin, R.R.; Naftel, S.J.; Nelson, A.J.; Feilen, A.B.; Narvaez, A. Synchrotron X-Ray Fluorescence and Trace Metals in the Cementum Rings of Human Teeth. J. Environ. Monit. 2004, 6, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.; Le Cabec, A.; Spiers, K.; Zhang, Y.; Garrevoet, J. Incremental Distribution of Strontium and Zinc in Great Ape and Fossil Hominin Cementum Using Synchrotron X-Ray Fluorescence Mapping. J. R. Soc. Interface. 2018, 15, 20170626. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.R.; Finney, L.A.; Telser, A.; Maxey, E.; Vogt, S.; Okasinski, J.S. Cementum Structure in Beluga Whale Teeth. Acta Biomater. 2017, 48, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.R.; Deymier-Black, A.C.; Veis, A.; Telser, A.; Lux, E.; Cai, Z. Bovine and Equine Peritubular and Intertubular Dentin. Acta Biomater. 2014, 10, 3969–3977. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.D.; Clayton, E.; Ainsworth, T. Preliminary Investigations of Trace Element Concentrations in Human Teeth. Nucl. Instrum. Methods Phys. Res., B 1981, 188, 203–209. [Google Scholar] [CrossRef]
- Dean, C.; Le Cabec, A.; Van Malderen, S.J.M.; Garrevoet, J. Synchrotron X-Ray Fluorescence Imaging of Strontium Incorporated into the Enamel and Dentine of Wild-Shot Orangutan Canine Teeth. Arch. Oral Biol. 2020, 104879. [Google Scholar] [CrossRef]
- Rautray, T.R.; Das, S.; Rautray, A.C. In Situ Analysis of Human Teeth by External PIXE. Nucl. Instrum. Methods Phys. Res., B 2010, 268, 2371–2374. [Google Scholar] [CrossRef]
- Carvalho, M.L.; Casaca, C.; Marques, J.P.; Pinheiro, T.; Cunha, A.S. Human Teeth Elemental Profiles Measured by Synchrotron X-Ray Fluorescence: Dietary Habits and Environmental Influence. X-Ray Spectrom. 2001, 30, 190–193. [Google Scholar] [CrossRef]
- Bouillon, R.; Suda, T. Vitamin D: Calcium and Bone Homeostasis during Evolution. BoneKEy Reports 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc Homeostasis in Humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, T.; von Blanckenburg, F. Natural Iron Isotope Variations in Human Blood. Science 2002, 295, 2065–2066. [Google Scholar] [CrossRef] [PubMed]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, Calcium Homeostasis and Aging. Bone Research 2016, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Calcium and Bone Metabolism during Pregnancy and Lactation. J. Mammary Gland Biol. Neoplasia 2015, 10. [Google Scholar] [CrossRef]
- Rawcliffe, C. Medicine and Society in Later Medieval England, 2nd ed.; Sandpiper Books Ltd.: London, UK, 1999. [Google Scholar]
- Demaitre, L. Leprosy in Premodern Medicine. A Malady of the Whole Body; The Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Brenner, E. Leprosy and Charity in Medieval Rouen; The Boydell Press: Woodbridge, VA, USA, 2015. [Google Scholar]
- Jáuregui, C. Inside the Leprosarium: Illness in the Daily Life of 14th Century Rouen. In New Approaches to Disease, Disability, and Medicine in Medieval Europe; Connelly, E., Künzel, S., Eds.; The Holywell Press: Oxford, UK, 2018. [Google Scholar]
- Pedersen, C. En Nøttelig Legebog; Pedersen: Malmø, Sweden, 1533. [Google Scholar]
- Smit, H. En Skøn Nyttelig Lægebog Ind Hollendis Atskillige Mange Skøne Forfarne Lægedommer Huilcke Som Tiæne Bartskerrerne, Oc Dem Som Ville Læge Ferske Oc Gamle Saar. Desligiste Oc Om Bad Aare Ladelse Oc Koppe Settelse, Och Om de Lagedomme Som Findis i Apotecken Fale.; Vingaard, Hans: Malmø, Sweden, 1557. [Google Scholar]
- Norse Medical and Herbal Healing. A Medical Book from Medieval Iceland; Troth Publications: New Haven, CT, USA, 2011. [Google Scholar]
- Arrizabalaga, J.; Henderson, J.; French, R. The Great Pox. The French Disease in Renaissance Europe; Yale University Press: New Haven, CT, USA, 1997. [Google Scholar]
- Goldwater, L.J. Mercury. A History of Quicksilver; York Press: Baltimore, MD, USA, 1972. [Google Scholar]
- Lev, E. Drugs Held and Sold by Pharmacists of the Jewish Community of Medieval (11–14th Centuries) Cairo According to Lists of Materia Medica Found at the Taylor–Schechter Genizah Collection, Cambridge. J. Ethnopharmacol. 2007, 110, 275–293. [Google Scholar] [CrossRef]
- Nriagu, J.O. Saturnine Drugs and Medicinal Exposure to Lead: An Historical Outline. In Human Lead Exposure; Needleman, H.L., Ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 3–22. [Google Scholar]
- Dawson, W.R. A Leechbook or Collection of Medical Recipes of the Fifteenth Century; MacMillan and Co., Limited: London, UK, 1934. [Google Scholar]
- Ogden, M.S. The Liber de Diversis Medicinis; Oxford University Press: London, UK, 1969. [Google Scholar]
- Harward, C.; Holder, N.; Phillpotts, C.; Thomas, C. The Medieval Priory and Hospital of St Mary Spital and the Bishopsgate Suburb. Excavations at Spitalfields Market, London E1, 1991–2007; MOLA Monograph 59; MOLA: London, UK, 2019. [Google Scholar]
- Buckingham, J. Leprosy in Colonial South India: Medicine and Confinement.; Palgrave Macmillan: New York, NY, USA, 2002; ISBN 1-349-42530-3. [Google Scholar]
- Rasmussen, K.L.; Boldsen, J.L.; Kristensen, H.K.; Skytte, L.; Hansen, K.L.; Mølholm, L.; Grootes, P.M.; Nadeau, M.-J.; Eriksen, K.M.F. Mercury Levels in Danish Medieval Human Bones. J. Archaeol. Sci. 2008, 35, 2295–2306. [Google Scholar] [CrossRef]
- Rasmussen, K.L.; Skytte, L.; Jensen, A.J.; Boldsen, J.L. Comparison of Mercury and Lead Levels in the Bones of Rural and Urban Populations in Southern Denmark and Northern Germany during the Middle Ages. J. Archaeol. Sci. Rep. 2015, 3, 358–370. [Google Scholar] [CrossRef]
- Rasmussen, E.G. Antimony, Arsenic, Bromine and Mercury in Enamel from Human Teeth. Eur. J. Oral Sci. 1974, 82, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.K.; Skytte, L.; D’imporzano, P.; Thomsen, O.P.; Søvsø, M.; Lier Boldsen, J. On the Distribution of Trace Element Concentrations in Multiple Bone Elements in 10 Danish Medieval and Post-Medieval Individuals. Am. J. Phys. Anthropol. 2016, 162, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.; Millard, A.; Chenery, C.; Lucy, S.; Roberts, C. Investigating Population Movement by Stable Isotope Analysis: A Report from Britain. Antiquity 2004, 78, 127–141. [Google Scholar] [CrossRef]
- Le Roux, G.; Weiss, D.; Grattan, J.; Givelet, N.; Krachler, M.; Cheburkin, A.; Rausch, N.; Kober, B.; Shotyk, W. Identifying the Sources and Timing of Ancient and Medieval Atmospheric Lead Pollution in England Using a Peat Profile from Lindow Bog, Manchester. J. Environ. Monit. 2004, 6, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Lessler, M.A. Lead and Lead Poisoning from Antiquity to Modern Times. Ohio J. Sci. 1988, 88, 78–84. [Google Scholar]
- Rasmussen, K.L.; Delbey, T.; d’Imporzano, P.; Skytte, L.; Schiavone, S.; Torino, M.; Tarp, P.; Thomsen, P.O. Comparison of Trace Element Chemistry in Human Bones Interred in Two Private Chapels Attached to Franciscan Friaries in Italy and Denmark: An Investigation of Social Stratification in Two Medieval and Post-Medieval Societies. Herit. Sci. 2020, 8, 65. [Google Scholar] [CrossRef]
- Alexandrovskaya, E.I.; Panova, T. History of the Soil, Cultural Layer, and People in Medieval Moscow. Rev. Mex. Cienc. 2003, 20, 289–294. [Google Scholar] [CrossRef]
- Critical Approaches to the History of Western Herbal Medicine: From Classical Antiquity to the Early Modern Period; Francia, S., Stobart, A., Eds.; Bloomsbury: London, UK, 2014; ISBN 978-1-4411-8418-4. [Google Scholar]
- Nielsen, O.V.; Grandjean, P.; Bennike, P. Chemical Analyses of Archaeological Bone-Samples: Evidence for High Lead Exposure on the Faroe Islands. J. Dan. Archaeol. 1982, 1, 145–148. [Google Scholar] [CrossRef]
- Bigi, A.; Gandolfi, M.; Gazzano, M.; Ripamonti, A.; Roveri, N.; Thomas, S.A. Structural Modifications of Hydroxyapatite Induced by Lead Substitution for Calcium. J. Chem. Soc. Dalton Trans. 1991, 2883–2886. [Google Scholar] [CrossRef]
- Carvalho, M.L.; Casaca, C.; Pinheiro, T.; Marques, J.P.; Chevallier, P.; Cunha, A.S. Analysis of Human Teeth and Bones from the Chalcolithic Period by X-Ray Spectrometry. Nucl. Instrum. Methods Phys. Res. B 2000, 168, 559–565. [Google Scholar] [CrossRef]
- Pounds, G.J.; Long, G.J.; Rosen, J.F. Cellular and Molecular Toxicity of Lead in Bone. Environ. Health Perspect. 1991, 91, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, M.B.; Leviton, A.; Bellinger, D. Relationships between Serial Blood Lead Levels and Exfoliated Tooth Dentin Lead Levels: Models of Tooth Lead Kinetics. Calcif. Tissue Int. 1993, 53, 338–341. [Google Scholar] [CrossRef]
- Steenhout, A. Kinetics of Lead Storage in Teeth and Bones: An Epidemiologic Approach. Arch. Environ. Health 1982, 37, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.L.; Gillings, B.R. Lead Exchange in Teeth and Bone – a Pilot Study Using Stable Lead Isotopes. Environ. Health Perspect. 1997, 105, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.Y.; Chan, S.W.; Kennedy, B.J.; Sharma, A.; Crisante, D.; Murray Walker, D. Spatial Distribution of Lead in the Roots of Human Primary Teeth. J. Trace Elem. Med. Biol. 2004, 18, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Keenan, F.; Cooke, M.; Appleton, J. Trace Element Profiling of Dental Tissues Using Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Fresen. J. Anal. Chem. 1996, 354, 254–258. [Google Scholar] [CrossRef]
- Gulson, B.L. Tooth Analyses of Sources and Intensity of Lead Exposure in Children. Environ. Health Perspect. 1996, 104, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Steenhout, A.; Pourtois, M. Lead Accumulation in Teeth as a Function of Age with Different Exposures. Br. J. Ind. Med. 1981, 38, 297. [Google Scholar] [CrossRef]
- Shapiro, I.M.; Mitchell, G.; Davidson, I.; Katz, S.H. The Lead Content of Teeth. Arch. Environ. Health 1975, 30, 483–486. [Google Scholar] [CrossRef]
- Shepherd, T.J.; Dirks, W.; Manmee, C.; Hodgson, S.; Banks, D.A.; Averley, P.; Pless-Mulloli, T. Reconstructing the Life-Time Lead Exposure in Children Using Dentine in Deciduous Teeth. Sci. Total Environ. 2012, 425, 214–222. [Google Scholar] [CrossRef]
- Pascu, G. Le Patrimoine Industriel-Minier Facteur de Développement Territorial: Complexité et Enjeux en Roumanie, en Comparaison Avec la France et la Grande-Bretagne. Master’s Thesis, Université Jean Monnet (Saint-Etienne, France) and Universitatea Politehnica (Timisoara, Romania), Timisoara, Romania, 2015. [Google Scholar]
- Ide-Ektessabi, A.; Shirasawa, K.; Koizumi, A.; Azechi, M. Application of Synchrotron Radiation Microbeams to Environmental Monitoring. Nucl. Instrum. Methods Phys. Res. B 2004, 213, 761–765. [Google Scholar] [CrossRef]
- Wang, Y.; Specht, A.; Liu, Y.; Finney, L.; Maxey, E.; Vogt, S.; Zheng, W.; Weisskopf, M.; Nie, L.H. Microdistribution of Lead in Human Teeth Using Microbeam Synchrotron Radiation X-Ray Fluorescence (μ-SRXRF): Microdistribution of Lead in Human Teeth Using μ-SRXRF. X-Ray Spectrom. 2016, 46, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Talpur, S.; Afridi, H.I.; Kazi, T.G.; Talpur, F.N. Interaction of Lead with Calcium, Iron, and Zinc in the Biological Samples of Malnourished Children. Biol. Trace Elem. Res. 2018, 183, 209–217. [Google Scholar] [CrossRef]
- Miller, G.D.; Massaro, T.; Massaro, E.J. Interactions between Lead and Essential Elements: A Review. Neurotoxicology 1990, 11, 99–119. [Google Scholar]
- Bartoń, H. Advantages of the Use of Deciduous Teeth, Hair, and Blood Analysis for Lead and Cadmium Bio-Monitoring in Children. A Study of 6-Year-Old Children from Krakow (Poland). Biol. Trace Elem. Res. 2011, 143, 637–658. [Google Scholar] [CrossRef]
- Brudevold, F.; Steadman, L.T.; Smith, F.A. Inorganic and Organic Components of Tooth Structure. Ann. N. Y. Acad. Sci. 1960, 85, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Okada, M. Hard Tissues of Animal Body: Highly Interesting Details of Nippon Studies in Periodic Patterns of Hard Tissues Are Described. Shanghai Evening Post 1943, recreation and medical progress., 15–31. [Google Scholar]
- Papakyrikos, A.M.; Arora, M.; Austin, C.; Boughner, J.C.; Capellini, T.D.; Dingwall, H.L.; Greba, Q.; Howland, J.G.; Kato, A.; Wang, X.-P.; et al. Biological Clocks and Incremental Growth Line Formation in Dentine. J. Anat. 2020, 237, 367–378. [Google Scholar] [CrossRef]
- Macchiarelli, R.; Bondioli, L.; Debénath, A.; Mazurier, A.; Tournepiche, J.-F.; Birch, W.; Dean, M.C. How Neanderthal Molar Teeth Grew. Nature 2006, 444, 748–751. [Google Scholar] [CrossRef]
- Grandjean, P. Lead in Danes. In Lead; Griffin, T.B., Knelson, J.H., Eds.; Georg Thieme Publishers: Stuttgart, Germany, 1975; pp. 6–75. [Google Scholar]
- Franklin, C.A.; Inskip, M.J.; Baccanale, C.L.; Edwards, C.M.; Manton, W.I.; Edwards, E.; O’Flaherty, E.J. Use of Sequentially Administered Stable Lead Isotopes to Investigate Changes in Blood Lead during Pregnancy in a Nonhuman Primate (Macaca Fascicularis). Fundam. Appl. Toxicol. 1997, 39, 109–119. [Google Scholar] [CrossRef]
- Gulson, B.; Mizon, K.; Korsch, M.; Taylor, A. Revisiting Mobilisation of Skeletal Lead during Pregnancy Based on Monthly Sampling and Cord/Maternal Blood Lead Relationships Confirm Placental Transfer of Lead. Arch. Toxicol. 2016, 90, 805–816. [Google Scholar] [CrossRef]
- Austin, C.; Smith, T.M.; Farahani, R.M.Z.; Hinde, K.; Carter, E.A.; Lee, J.; Lay, P.A.; Kennedy, B.J.; Sarrafpour, B.; Wright, R.J.; et al. Uncovering System-Specific Stress Signatures in Primate Teeth with Multimodal Imaging. Sci. Rep. 2016, 6, 18802. [Google Scholar] [CrossRef]
- Cleymaet, R.; Collys, K.; Retief, D.H.; Michotte, Y.; Slop, D.; Taghon, E.; Maex, W.; Coomans, D. Relation between Lead in Surface Tooth Enamel, Blood, and Saliva from Children Residing in the Vicinity of a Non-Ferrous Metal Plant in Belgium. Br. J. Ind. Med. 1991, 48, 702. [Google Scholar] [CrossRef]
- Habercam, J.W.; Keil, J.E.; Routt Reigart, J.; Croft, H.W. Lead Content of Human Blood, Hair, and Deciduous Teeth: Correlation with Environmental Factors and Growth. J. Dent. Res. 1974, 53, 1160–1163. [Google Scholar] [CrossRef]
- Fewtrell, L.; Kaufmann, R.; Prüss-Üstün, A. Lead: Assessing the Environmental Burden of Disease at National and Local Levels; WHO Environmental Burden of Disease Series, No. 2; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- World Health Organization Childhood Lead Poisoning; WHO Document Production Services: Geneva, Switzerland, 2010.

| Site | Gr. Nr. | Teeth | SXRF Overview Scan | SXRF High-Resolution Scan | LA-ICP-TOFMS | |||
|---|---|---|---|---|---|---|---|---|
| Spot Size (μm) | Exposure Time (ms) | Tissue | Spot Size (μm) | Exposure Time (ms) | ||||
| Odense | 533 | LLM1 | 10 | 3 | AC | − | − | 2 µm |
| CC | 1 | 1 | 1 µm | |||||
| SD | 1 | 3 | 2 µm | |||||
| Odense | 896 | LLC | 10 | 3 | − | − | − | − |
| LLM1 | 10 | 2.5 | TC | 1.5 | 3 | − | ||
| Odense | 914 | ULM1 | 10 | 3 | CC | 1 | 3 | − |
| Odense | 1149 | URC | 10 | 4 | AC | 1 | 10 | − |
| ULM1 | 10 | 4 | CMSC? | 1 | 3 | − | ||
| Næstved | 6 | ULC | 10 | 4 | AC | 1 | 10 | − |
| Næstved | 211 | URC | 10 | 3 | CC | 1 | − | 20 μm overview + 2 µm |
| LRM1 | 10 | 2.5 | AC | 1.5 | 3 | − | ||
| Næstved | 268 | LLM1 | 10 | 4 | CC | 1.5 | 3 | − |
| Næstved | 305 | LLC | 10 | 3 | − | − | − | − |
| LRM1 | 10 | 2.5 | CC | 1.5 | 3 | − | ||
| Romania | A1651 | URC | 10 | 3 | CC | 1 | 3 | − |
| LRM1 | 10 | 2.5 | TC | 1.5 | 3 | − | ||
| Romania | R1386 | LRM2 | 10 | 2.5 | CC | 1.5 | 3 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brozou, A.; Mannino, M.A.; Van Malderen, S.J.M.; Garrevoet, J.; Pubert, E.; Fuller, B.T.; Dean, M.C.; Colard, T.; Santos, F.; Lynnerup, N.; et al. Using SXRF and LA-ICP-TOFMS to Explore Evidence of Treatment and Physiological Responses to Leprosy in Medieval Denmark. Biology 2023, 12, 184. https://doi.org/10.3390/biology12020184
Brozou A, Mannino MA, Van Malderen SJM, Garrevoet J, Pubert E, Fuller BT, Dean MC, Colard T, Santos F, Lynnerup N, et al. Using SXRF and LA-ICP-TOFMS to Explore Evidence of Treatment and Physiological Responses to Leprosy in Medieval Denmark. Biology. 2023; 12(2):184. https://doi.org/10.3390/biology12020184
Chicago/Turabian StyleBrozou, Anastasia, Marcello A. Mannino, Stijn J. M. Van Malderen, Jan Garrevoet, Eric Pubert, Benjamin T. Fuller, M. Christopher Dean, Thomas Colard, Frédéric Santos, Niels Lynnerup, and et al. 2023. "Using SXRF and LA-ICP-TOFMS to Explore Evidence of Treatment and Physiological Responses to Leprosy in Medieval Denmark" Biology 12, no. 2: 184. https://doi.org/10.3390/biology12020184
APA StyleBrozou, A., Mannino, M. A., Van Malderen, S. J. M., Garrevoet, J., Pubert, E., Fuller, B. T., Dean, M. C., Colard, T., Santos, F., Lynnerup, N., Boldsen, J. L., Jørkov, M. L., Soficaru, A. D., Vincze, L., & Le Cabec, A. (2023). Using SXRF and LA-ICP-TOFMS to Explore Evidence of Treatment and Physiological Responses to Leprosy in Medieval Denmark. Biology, 12(2), 184. https://doi.org/10.3390/biology12020184






