Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Plan Schedule
2.3. Behavioral Tests and Cognitive Frailty Index
2.4. H. erinaceus (He2) Primordium: Isolation/Cultivation, Extraction Procedures and Analytical Determinations
2.5. Necropsy, Tissue Sampling and Immunohistochemical Analyses
2.5.1. Brain Specimens’ Preparation
2.5.2. Immunohistochemistry and Bright-Field Microscopy
2.5.3. Immunohistochemical Evaluations
2.6. Statistics
3. Results
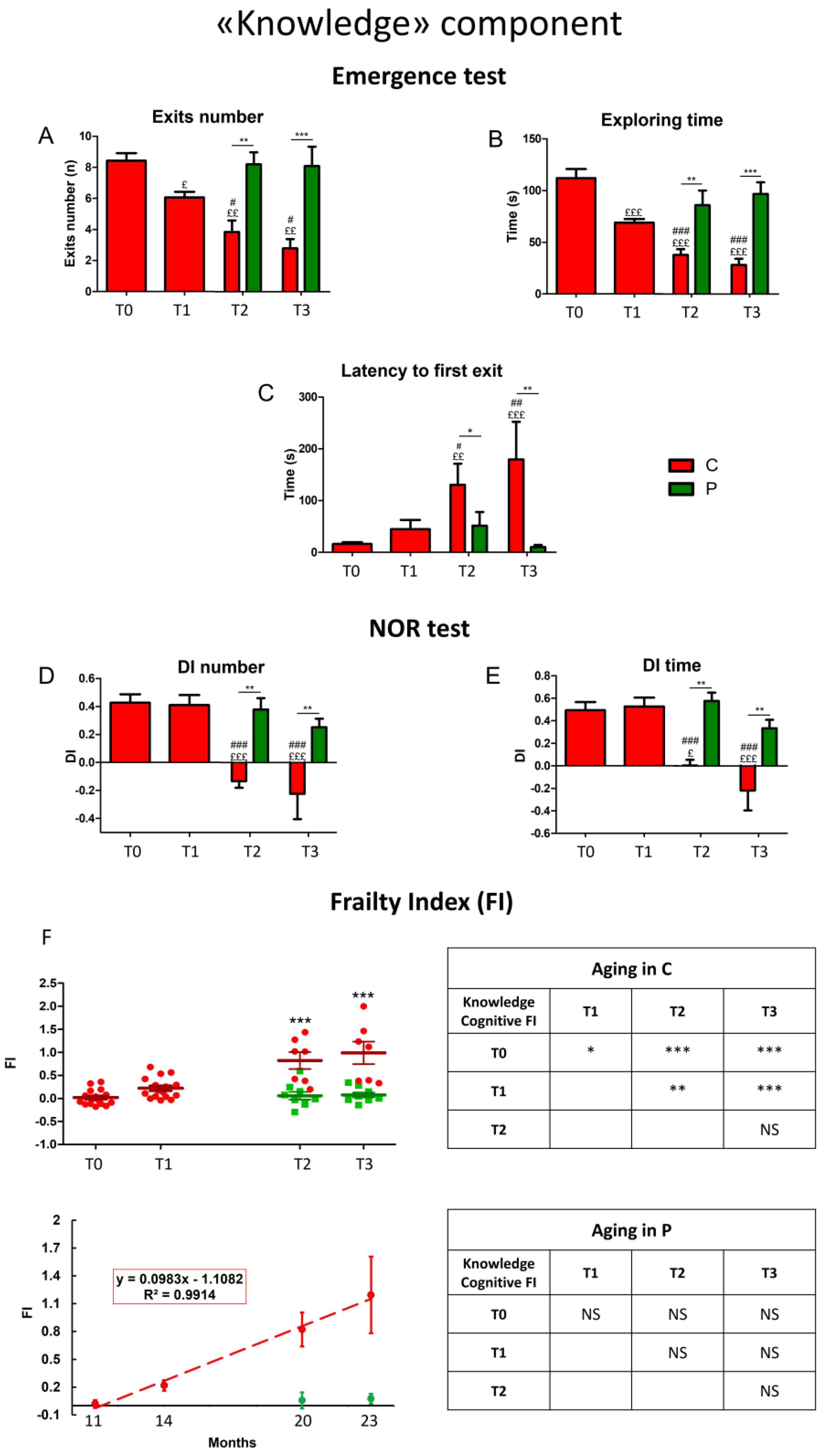
3.1. Cognitive Outcomes
3.1.1. He2 Preventive Effect on Aging Decline of the “Knowledge” Memory Component
3.1.2. He2 Preventive Effect on Aging Decline of the “Remember” Memory Component
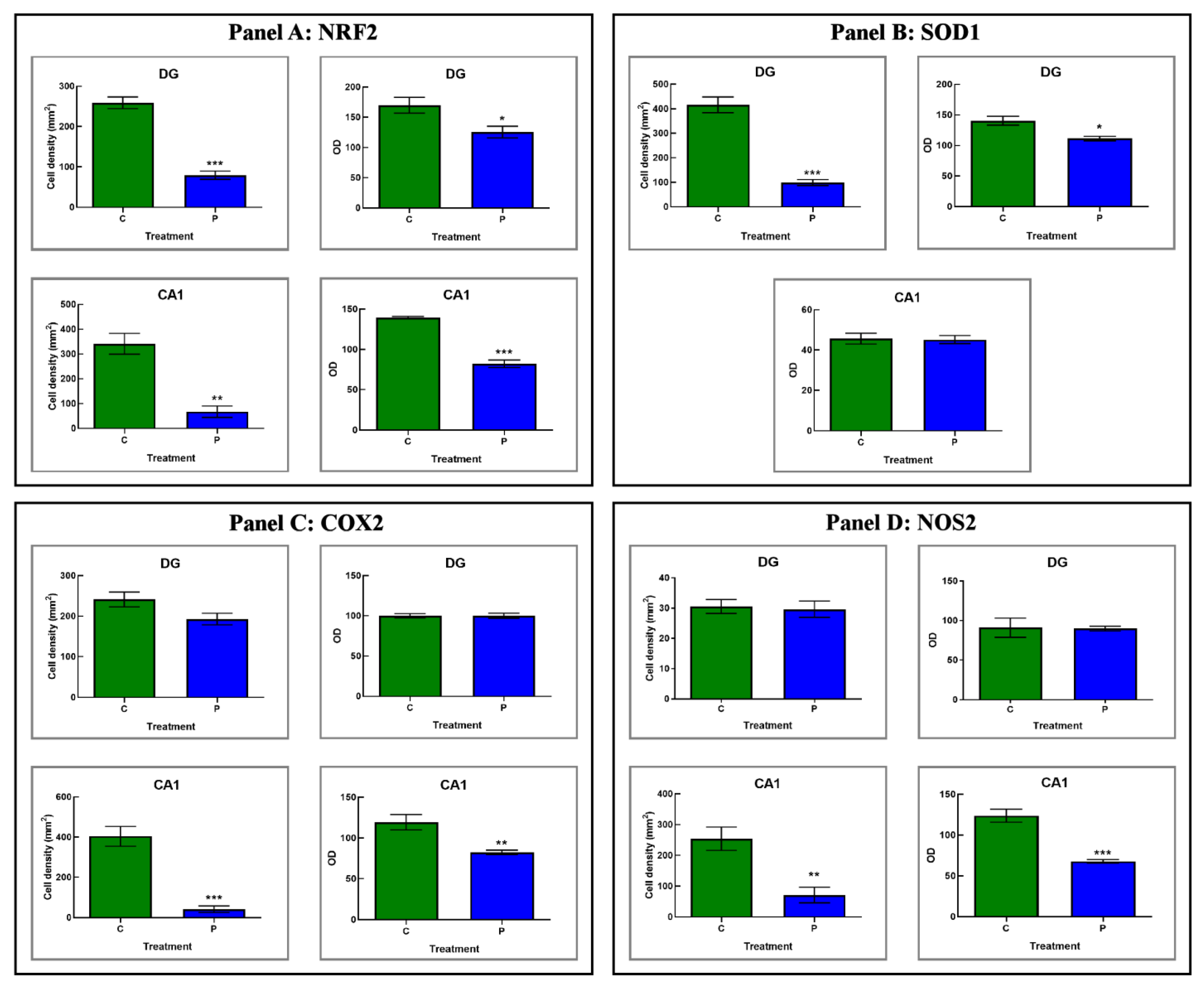
3.2. Histological and Immunohistochemical Data
3.2.1. He2 Supplementation Preserves Hippocampus Healthy Cytoarchitecture
3.2.2. He2 Supplement Decreases Inflammaging
3.2.3. He2 Supplement Diminished Age-Related ROS Levels
3.3. He2 Supplement Improved Glutamate Neurotransmission in Aging
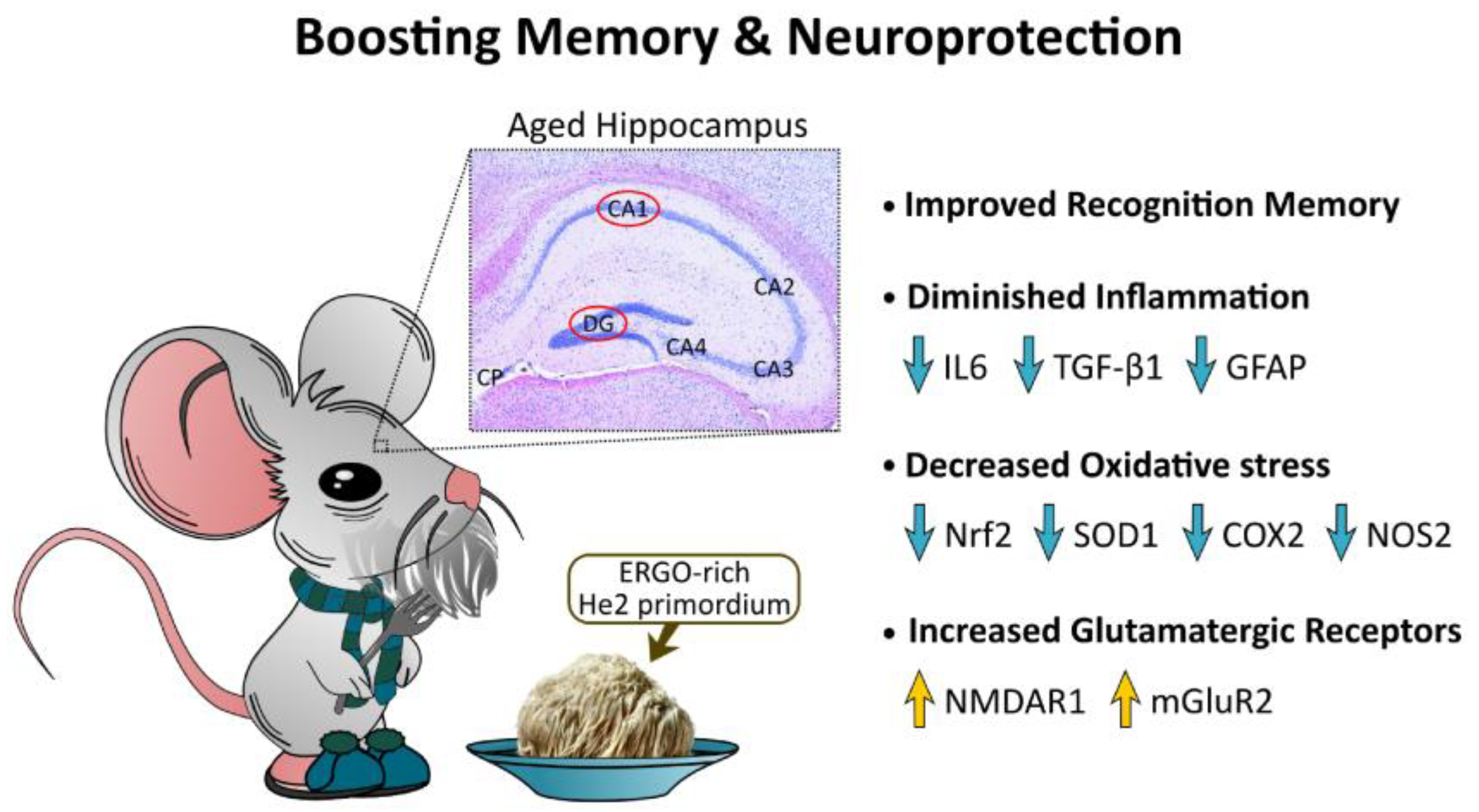
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Rossum, I.A.; Vos, S.; Handels, R.; Visser, P.J. Biomarkers as Predictors for Conversion from Mild Cognitive Impairment to Alzheimer-Type Dementia: Implications for Trial Design. J. Alzheimers Dis. 2010, 20, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.S. Changes in Cognition. Neurobiol. Aging 2011, 32 (Suppl. S1), S58–S63. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, K.; Sehlin, D.; Syvänen, S.; Svedberg, M.M.; Philipson, O.; Söderberg, L.; Tegerstedt, K.; Holmquist, M.; Gellerfors, P.; Tolmachev, V.; et al. Specific Uptake of an Amyloid-β Protofibril-Binding Antibody-Tracer in AβPP Transgenic Mouse Brain. J. Alzheimers Dis. 2013, 37, 29–40. [Google Scholar] [CrossRef]
- Bird, C.M. The Role of the Hippocampus in Recognition Memory. Cortex 2017, 93, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Gasiorowska, A.; Wydrych, M.; Drapich, P.; Zadrozny, M.; Steczkowska, M.; Niewiadomski, W.; Niewiadomska, G. The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front. Aging Neurosci. 2021, 13, 654931. [Google Scholar] [CrossRef]
- Roda, E.; Ratto, D.; De Luca, F.; Desiderio, A.; Ramieri, M.; Goppa, L.; Savino, E.; Bottone, M.G.; Locatelli, C.A.; Rossi, P. Searching for a Longevity Food, We Bump into Hericium Erinaceus Primordium Rich in Ergothioneine: The “Longevity Vitamin” Improves Locomotor Performances during Aging. Nutrients 2022, 14, 1177. [Google Scholar] [CrossRef]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective Metabolites of Hericium Erinaceus Promote Neuro-Healthy Aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef]
- Rowaiye, A.; Wilfred, O.I.; Onuh, O.A.; Bur, D.; Oni, S.; Nwonu, E.J.; Ibeanu, G.; Oli, A.N.; Wood, T.T. Modulatory Effects of Mushrooms on the Inflammatory Signaling Pathways and Pro-Inflammatory Mediators. Clin. Complement. Med. Pharmacol. 2022, 2, 100037. [Google Scholar] [CrossRef]
- Su, X.; Liu, K.; Xie, Y.; Zhang, M.; Wu, X.; Zhang, Y.; Wang, J. Mushroom Inonotus Sanghuang Alleviates Experimental Pulmonary Fibrosis: Implications for Therapy of Pulmonary Fibrosis. Biomed. Pharmacother. 2021, 133, 110919. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A. Regulation of GFAP Expression. ASN Neuro 2021, 13, 1759091420981206. [Google Scholar] [CrossRef]
- Messing, A.; Brenner, M. GFAP at 50. ASN Neuro 2020, 12, 1759091420949680. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Walker, R.; McGeer, E.G. Patterns of Gliosis in Alzheimer’s Disease and Aging Cerebrum. Glia 1989, 2, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.W. Glial Reaction in Aging and Alzheimer’s Disease. Microsc. Res. Tech. 1998, 43, 24–28. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Y. P53, Oxidative Stress, and Aging. Antioxid. Redox Signal 2011, 15, 1669–1678. [Google Scholar] [CrossRef]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; d’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef] [Green Version]
- Pandya, C.D.; Lee, B.; Toque, H.A.; Mendhe, B.; Bragg, R.T.; Pandya, B.; Atawia, R.T.; Isales, C.; Hamrick, M.; Caldwell, R.W.; et al. Age-Dependent Oxidative Stress Elevates Arginase 1 and Uncoupled Nitric Oxide Synthesis in Skeletal Muscle of Aged Mice. Oxid. Med. Cell. Longev. 2019, 2019, 1704650. [Google Scholar] [CrossRef] [Green Version]
- Teniou, S.; Bensegueni, A.; Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M.; Chovelon, B.; Bensouici, C.; Boumendjel, A.; Hininger-Favier, I. Biodriven Investigation of the Wild Edible Mushroom Pleurotus Eryngii Revealing Unique Properties as Functional Food. J. Funct. Foods 2022, 89, 104965. [Google Scholar] [CrossRef]
- Drozdz-Afelt, J.M.; Koim-Puchowska, B.B.; Kaminski, P. Analysis of Oxidative Stress Indicators in Polish Patients with Prostate Cancer. Environ. Sci. Pollut. Res. Int. 2022, 29, 4632–4640. [Google Scholar] [CrossRef]
- Gonçalves, S.; Yin, K.; Ito, Y.; Chan, A.; Olan, I.; Gough, S.; Cassidy, L.; Serrao, E.; Smith, S.; Young, A.; et al. COX2 Regulates Senescence Secretome Composition and Senescence Surveillance through PGE2. Cell Rep. 2021, 34, 108860. [Google Scholar] [CrossRef]
- Strauss, K.I. Antiinflammatory and Neuroprotective Actions of COX2 Inhibitors in the Injured Brain. Brain Behav. Immun. 2008, 22, 285–298. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arami, K.M.; Jameie, B.; AkbarMoosavi, S.; Arami, K.M.; Jameie, B.; AkbarMoosavi, S. Neuronal Nitric Oxide Synthase; IntechOpen, 2017; ISBN 978-953-51-3164-9. [Google Scholar]
- Mayer, M.L.; Westbrook, G.L. The Physiology of Excitatory Amino Acids in the Vertebrate Central Nervous System. Prog Neurobiol. 1987, 28, 197–276. [Google Scholar] [CrossRef] [PubMed]
- Segovia, G.; Porras, A.; Del Arco, A.; Mora, F. Glutamatergic Neurotransmission in Aging: A Critical Perspective. Mech. Ageing Dev. 2001, 122, 1–29. [Google Scholar] [CrossRef]
- Michaelis, E.K. Molecular Biology of Glutamate Receptors in the Central Nervous System and Their Role in Excitotoxicity, Oxidative Stress and Aging. Prog. Neurobiol. 1998, 54, 369–415. [Google Scholar] [CrossRef]
- Ménard, C.; Quirion, R. Group 1 Metabotropic Glutamate Receptor Function and Its Regulation of Learning and Memory in the Aging Brain. Front. Pharmacol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, T.C.; Kyritsopoulos, C.; Kumar, A. Central Role for NMDA Receptors in Redox Mediated Impairment of Synaptic Function during Aging and Alzheimer’s Disease. Behav. Brain Res. 2017, 322, 223–232. [Google Scholar] [CrossRef]
- Gramuntell, Y.; Klimczak, P.; Coviello, S.; Perez-Rando, M.; Nacher, J. Effects of Aging on the Structure and Expression of NMDA Receptors of Somatostatin Expressing Neurons in the Mouse Hippocampus. Front. Aging Neurosci. 2021, 13, 782737. [Google Scholar] [CrossRef]
- Lee, H.-K.; Min, S.S.; Gallagher, M.; Kirkwood, A. NMDA Receptor-Independent Long-Term Depression Correlates with Successful Aging in Rats. Nat. Neurosci. 2005, 8, 1657–1659. [Google Scholar] [CrossRef]
- Mecca, A.P.; Rogers, K.; Jacobs, Z.; McDonald, J.W.; Michalak, H.R.; DellaGioia, N.; Zhao, W.; Hillmer, A.T.; Nabulsi, N.; Lim, K.; et al. Effect of Age on Brain Metabotropic Glutamate Receptor Subtype 5 Measured with [18F]FPEB PET. Neuroimage 2021, 238, 118217. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal Mushrooms in Human Clinical Studies. Part I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-F.; Chen, J.-H.; Teng, C.-C.; Shen, C.-H.; Hsieh, M.-C.; Lu, C.-C.; Lee, K.-C.; Lee, L.-Y.; Chen, W.-P.; Chen, C.-C.; et al. Protective Effects of Hericium Erinaceus Mycelium and Its Isolated Erinacine A against Ischemia-Injury-Induced Neuronal Cell Death via the Inhibition of INOS/P38 MAPK and Nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Fabrizi, C.; Fornai, F. Potential Antidepressant Effects of Scutellaria Baicalensis, Hericium Erinaceus and Rhodiola Rosea. Antioxidants 2020, 9, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Cuzzocrea, S.; Di Paola, R.; Fusco, R.; et al. Key Mechanisms and Potential Implications of Hericium Erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants 2021, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Beelman, R.B.; Kalaras, M.D.; Phillips, A.T.; Richie, J.P. Is Ergothioneine a “longevity Vitamin” Limited in the American Diet? J. Nutr. Sci. 2020, 9, e52. [Google Scholar] [CrossRef] [PubMed]
- Apparoo, Y.; Phan, C.W.; Kuppusamy, U.R.; Sabaratnam, V. Ergothioneine and Its Prospects as an Anti-Ageing Compound. Exp. Gerontol. 2022, 170, 111982. [Google Scholar] [CrossRef]
- Kondoh, H.; Teruya, T.; Kameda, M.; Yanagida, M. Decline of Ergothioneine in Frailty and Cognition Impairment. FEBS Lett. 2022, 596, 1270–1278. [Google Scholar] [CrossRef]
- Nakamichi, N.; Tsuzuku, S.; Shibagaki, F. Ergothioneine and Central Nervous System Diseases. Neurochem. Res. 2022, 47, 2513–2521. [Google Scholar] [CrossRef]
- Corana, F.; Cesaroni, V.; Mannucci, B.; Baiguera, R.M.; Picco, A.M.; Savino, E.; Ratto, D.; Perini, C.; Kawagishi, H.; Girometta, C.E.; et al. Array of Metabolites in Italian Hericium Erinaceus Mycelium, Primordium, and Sporophore. Molecules 2019, 24, 3511. [Google Scholar] [CrossRef] [Green Version]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium Erinaceus Improves Recognition Memory and Induces Hippocampal and Cerebellar Neurogenesis in Frail Mice during Aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef]
- Brandalise, F.; Cesaroni, V.; Gregori, A.; Repetti, M.; Romano, C.; Orrù, G.; Botta, L.; Girometta, C.; Guglielminetti, M.L.; Savino, E.; et al. Dietary Supplementation of Hericium Erinaceus Increases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice. Evid. Based Complement Alternat. Med. 2017, 2017, 3864340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, P.; Cesaroni, V.; Brandalise, F.; Occhinegro, A.; Ratto, D.; Perrucci, F.; Lanaia, V.; Girometta, C.; Orrù, G.; Savino, E. Dietary Supplementation of Lion’s Mane Medicinal Mushroom, Hericium Erinaceus (Agaricomycetes), and Spatial Memory in Wild-Type Mice. Int. J. Med. Mushrooms 2018, 20, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Cesaroni, V.; Brusoni, M.; Cusaro, C.M.; Girometta, C.; Perini, C.; Picco, A.M.; Rossi, P.; Salerni, E.; Savino, E. Phylogenetic Comparison between Italian and Worldwide Hericium Species (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 943–954. [Google Scholar] [CrossRef]
- Gerbec, B.; Tavčar, E.; Gregori, A.; Kreft, S.; Berovic, M. Solid State Cultivation of Hericium Erinaceus Biomass and Erinacine: A Production. J. Bioprocess. Biotech. 2015, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Roda, E.; Bottone, M.G.; Biggiogera, M.; Milanesi, G.; Coccini, T. Pulmonary and Hepatic Effects after Low Dose Exposure to Nanosilver: Early and Long-Lasting Histological and Ultrastructural Alterations in Rat. Toxicol. Rep. 2019, 6, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Kasapoglu, M.; Ozben, T. Alterations of Antioxidant Enzymes and Oxidative Stress Markers in Aging. Exp. Gerontol. 2001, 36, 209–220. [Google Scholar] [CrossRef]
- Abramson, S.B. Nitric Oxide in Inflammation and Pain Associated with Osteoarthritis. Arthritis Res. Ther. 2008, 10 (Suppl. S2), S2. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, S.; Nakajima, Y.; Masu, M.; Ueda, Y.; Nakahara, K.; Watanabe, D.; Yamaguchi, S.; Kawabata, S.; Okada, M. Glutamate Receptors: Brain Function and Signal Transduction. Brain Res. Brain Res. Rev. 1998, 26, 230–235. [Google Scholar] [CrossRef]
- Magnusson, K.R. Aging of the NMDA Receptor: From a Mouse’s Point of View. Future Neurol. 2012, 7, 627–637. [Google Scholar] [CrossRef]
- Pang, S.; Lu, Z.; Jiang, J.; Zhao, L.; Lin, L.; Li, X.; Lian, T.; Huang, M.; Yang, W.; Feng, Q. Hippocampus Segmentation Based on Iterative Local Linear Mapping With Representative and Local Structure-Preserved Feature Embedding. IEEE Trans. Med. Imaging 2019, 38, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Chao, O.Y.; de Souza Silva, M.A.; Yang, Y.-M.; Huston, J.P. The Medial Prefrontal Cortex—Hippocampus Circuit That Integrates Information of Object, Place and Time to Construct Episodic Memory in Rodents: Behavioral, Anatomical and Neurochemical Properties. Neurosci. Biobehav. Rev. 2020, 113, 373–407. [Google Scholar] [CrossRef] [PubMed]
- Lana, L.G.; de Araújo, L.M.; Silva, T.F.; Modolo, L.V. Interplay between Gasotransmitters and Potassium Is a K+ey Factor during Plant Response to Abiotic Stress. Plant Physiol. Biochem. 2021, 169, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, J.; Mamelak, A.N.; Birch, K.; Mosher, C.P.; Tagliati, M.; Rutishauser, U. Novelty-Sensitive Dopaminergic Neurons in the Human Substantia Nigra Predict Success of Declarative Memory Formation. Curr. Biol. 2018, 28, 1333–1343.e4. [Google Scholar] [CrossRef] [Green Version]
- Gardner, R.S.; Newman, L.A.; Mohler, E.G.; Tunur, T.; Gold, P.E.; Korol, D.L. Aging Is Not Equal across Memory Systems. Neurobiol. Learn Mem. 2020, 172, 107232. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal Aging Induces A1-like Astrocyte Reactivity. Proc Natl Acad Sci U S A 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative Stress Response and Nrf2 Signaling in Aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [Green Version]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Helgueta Romero, S.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Shafiq, K.; Sanghai, N.; Guo, Y.; Kong, J. Implication of Post-Translationally Modified SOD1 in Pathological Aging. Geroscience 2021, 43, 507–515. [Google Scholar] [CrossRef]
- Zhang, Y.; Ikeno, Y.; Bokov, A.; Gelfond, J.; Jaramillo, C.; Zhang, H.-M.; Liu, Y.; Qi, W.; Hubbard, G.; Richardson, A.; et al. Dietary Restriction Attenuates the Accelerated Aging Phenotype of Sod1(-/-) Mice. Free Radic. Biol. Med. 2013, 60, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Zhao, Z.; Liu, T.; Zhang, Z.; Wang, P.; Xu, S.; Lei, X.G.; Shan, A. Oxidative Stress Induced by Se-Deficient High-Energy Diet Implicates Neutrophil Dysfunction via Nrf2 Pathway Suppression in Swine. Oncotarget 2017, 8, 13428–13439. [Google Scholar] [CrossRef] [Green Version]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2021, 19, 114–126. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, H.S.; Lee, J.; Chung, J.; Lee, J.S.; Choi, J.S.; Yoon, T.R.; Kim, H.K.; Chung, H.Y. Down-Regulation of Oxidative Stress and COX-2 and INOS Expressions by Dimethyl Lithospermate in Aged Rat Kidney. Arch. Pharm. Res. 2014, 37, 1032–1038. [Google Scholar] [CrossRef]
- Munhoz, C.D.; García-Bueno, B.; Madrigal, J.L.M.; Lepsch, L.B.; Scavone, C.; Leza, J.C. Stress-Induced Neuroinflammation: Mechanisms and New Pharmacological Targets. Braz. J. Med. Biol. Res. 2008, 41, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Heumann, D.; Leuba, G. Neuronal Death in the Development and Aging of the Cerebral Cortex of the Mouse. Neuropathol. Appl. Neurobiol. 1983, 9, 297–311. [Google Scholar] [CrossRef]
- Shi, L.; Adams, M.; Brunso-Bechtold, J.K. Subtle Alterations in Glutamatergic Synapses Underlie the Aging-Related Decline in Hippocampal Function. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-3818-2. [Google Scholar]
- Bukke, V.N.; Archana, M.; Villani, R.; Romano, A.D.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Beggiato, S.; Serviddio, G.; Cassano, T. The Dual Role of Glutamatergic Neurotransmission in Alzheimer’s Disease: From Pathophysiology to Pharmacotherapy. Int. J. Mol. Sci. 2020, 21, 7452. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-Induced Excitotoxicity in Parkinson’s Disease: The Role of Glial Cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Cox, M.F.; Hascup, E.R.; Bartke, A.; Hascup, K.N. Friend or Foe? Defining the Role of Glutamate in Aging and Alzheimer’s Disease. Front. Aging 2022, 3, 929474. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Mai, D.; Qu, S. Molecular Mechanisms of Glutamate Toxicity in Parkinson’s Disease. Front. Neurosci. 2020, 14, 585584. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Peineau, S.; Howland, J.G.; Wang, Y.T. Long-Term Depression in the CNS. Nat. Rev. Neurosci. 2010, 11, 459–473. [Google Scholar] [CrossRef]
- Cotman, C.W.; Lynch, G.S. The Neurobiology of Learning and Memory. Cognition 1989, 33, 201–241. [Google Scholar] [CrossRef]
- Magnusson, K.R.; Brim, B.L.; Das, S.R. Selective Vulnerabilities of N-Methyl-D-Aspartate (NMDA) Receptors During Brain Aging. Front. Aging Neurosci. 2010, 2, 11. [Google Scholar] [CrossRef]
- Mechanisms of Memory—2nd Edition. Available online: https://www.elsevier.com/books/mechanisms-of-memory/sweatt/978-0-12-374951-2 (accessed on 19 December 2022).
- Whitmore, C.A.; Haynes, J.R.; Behof, W.J.; Rosenberg, A.J.; Tantawy, M.N.; Hachey, B.C.; Wadzinski, B.E.; Spiller, B.W.; Peterson, T.E.; Paffenroth, K.C.; et al. Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2022, 15, 742. [Google Scholar] [CrossRef]
- Nakamichi, N.; Nakao, S.; Nishiyama, M.; Takeda, Y.; Ishimoto, T.; Masuo, Y.; Matsumoto, S.; Suzuki, M.; Kato, Y. Oral Administration of the Food-Derived Hydrophilic Antioxidant Ergothioneine Enhances Object Recognition Memory in Mice. Curr. Mol. Pharmacol. 2021, 14, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-Y.; Lin, H.-C.; Chen, C.-L.; Wu, J.-H.; Liao, J.-W.; Hu, M.-L. Ergothioneine and Melatonin Attenuate Oxidative Stress and Protect against Learning and Memory Deficits in C57BL/6J Mice Treated with D-Galactose. Free Radic. Res. 2014, 48, 1049–1060. [Google Scholar] [CrossRef]
- Song, T.-Y.; Chen, C.-L.; Liao, J.-W.; Ou, H.-C.; Tsai, M.-S. Ergothioneine Protects against Neuronal Injury Induced by Cisplatin Both in Vitro and in Vivo. Food Chem. Toxicol. 2010, 48, 3492–3499. [Google Scholar] [CrossRef]
- Yang, N.-C.; Lin, H.-C.; Wu, J.-H.; Ou, H.-C.; Chai, Y.-C.; Tseng, C.-Y.; Liao, J.-W.; Song, T.-Y. Ergothioneine Protects against Neuronal Injury Induced by β-Amyloid in Mice. Food Chem. Toxicol. 2012, 50, 3902–3911. [Google Scholar] [CrossRef]







| Spontaneous Behavioral Test | Selected Cognitive Parameters |
|---|---|
| Emergence | Exit Number (n) |
| Latency of First Exit (s) | |
| Time of Exploration (s) | |
| NOR | Number of Approaches: DI |
| Time of Approaches: DI | |
| OL | Number of Approaches: DI |
| Time of Approaches: DI | |
| Y maze | Alternation % |
| Antigen | Immunogen | Manufacturer, Species, Mono-Polyclonal, Cat./Lot. No., RRID | Dilution | |
|---|---|---|---|---|
| Primary antibodies | Anti-Interleukin-6 (M-19) | Purified antibody raised against a peptide mapping at the C-terminus of murine IL6 | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Goat polyclonal IgG, Cat# sc-1265, RRID: AB_2127470 | 1:100 |
| Anti-Transforming Growth Factor β1 (V) | Purified antibody raised against a peptide mapping at the C-terminus of TGF-β1 of human origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Rabbit polyclonal IgG, Cat# sc-146, RRID: AB_632486 | 1:100 | |
| Anti-Glial fibrillary acidic protein (C-19) | Purified antibody raised against a peptide mapping at the C-terminus of GFAP of human origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Goat polyclonal IgG, Cat# sc-6170, RRID: AB_641021 | 1:100 | |
| Anti-Nuclear factor erythroid 2–related factor 2 | Purified antibody raised against a peptide within Human Nrf2 aa 550 to the C-terminus | Abcam (Cambridge, UK), Rabbit polyclonal IgG, Cat# ab31163, RRID: AB_881705 | 1:100 | |
| Anti-Superoxide Dismutase-1 (FL-154) | Purified antibody raised against amino acids 1–154 representing full-length human SOD-1 | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Rabbit polyclonal IgG, Cat# sc-11407, RRID: AB_2193779 | 1:100 | |
| Anti- Cyclooxygenase-2 (M-19) | Purified antibody raised against a peptide mapping at the C-terminus of COX2 of mouse origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Goat polyclonal IgG, Cat# sc-1747, RRID: AB_2084976 | 1:100 | |
| Anti-Nitric Oxide Synthases-2 (M19) | Purified antibody raised against a peptide mapping at the C-terminus of NOS2 of mouse origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Rabbit polyclonal IgG, Cat# sc-650, RRID: AB_631831 | 1:100 | |
| Anti- N-methyl-D-aspartate Receptors 1 | Purified antibody raised against a peptide corresponding to the C-terminus of rat NMDA receptor subunit | Millipore—Merck KGaA (Darmstadt, Germany), Rabbit monoclonal IgG, Cat# AB9864, RRID: AB_2112158 | 1:500 | |
| Anti-Glutamate Receptor 2 and 3 | Purified antibody raised against a peptide mapping at the C-terminus of rat GluR2 | Millipore—Merck KGaA (Darmstadt, Germany), Rabbit polyclonal IgG, Cat# AB1506, RRID: AB_ 90710 | 1:100 | |
| Secondary Antibodies | Biotinylated goat anti-rabbit IgG | Gamma immunoglobulin | Vector Laboratories (Burlingame, CA, USA), Goat, lot# PK-6101, RRID: AB_2336820 | 1:200 |
| Biotinylated rabbit anti-goat IgG | Gamma immunoglobulin | Vector Laboratories (Burlingame, CA, USA), Rabbit, Cat# PK-6105, RRID: AB_2336824 | 1:200 |
| IL6 | TGFbeta1 | GFAP | |||||
|---|---|---|---|---|---|---|---|
| Cell Density | OD | Cell Density | OD | Cell Density | OD | ||
| DG | C | 310.79 ± 17.86 | 178.50 ± 9.40 | 728.24 ± 33.81 | 142.20 ± 2.96 | 298.24 ± 19.37 | 137.69 ± 17.17 |
| P | 42.26 ± 6.27 | 120.00 ± 4.21 | 119.01 ± 35.41 | 121.06 ± 4.62 | 165.69 ± 12.69 | 86.78 ± 4.49 | |
| CA1 | C | 289.13 ± 19.61 | 127.80 ± 6.63 | n.c. | 30.49 ± 1.44 | 109.87 ± 13.47 | 163.84 ± 3.68 |
| P | 44.48 ± 21.82 | 96.68 ± 3.47 | n.c. | 30.97 ± 0.56 | 89.71 ± 7.53 | 138.49 ± 1.55 | |
| Nrf2 | SOD1 | ||||
|---|---|---|---|---|---|
| Cell Density | OD | Cell Density | OD | ||
| DG | C | 258.84 ± 14.41 | 169.78 ± 13.00 | 415.97 ± 32.21 | 140.49 ± 7.22 |
| P | 79.24 ± 10.42 | 125.38 ± 9.66 | 98.74 ± −12.40 | 111.20 ± 3.67 | |
| CA1 | C | 341.02 ± 41.94 | 139.38 ± 1.37 | n.c. | 45.68 ± 2.70 |
| P | 66.72 ± 23.05 | 82.01 ± 4.72 | n.c. | 45.19 ± 2.03 | |
| COX2 | NOS2 | ||||
| Cell Density | OD | Cell Density | OD | ||
| DG | C | 241.23 ± 18.33 | 100.20 ± 2.58 | 30.52 ± 2.32 | 90.91 ± 12.17 |
| P | 192.81 ± 14.11 | 100.01 ± 3.23 | 29.63 ± 2.69 | 89.84 ± 2.97 | |
| CA1 | C | 404.04 ± 49.32 | 119.39 ± 9.47 | 253.78 ± 38.06 | 123.79 ± 7.98 |
| P | 40.77 ± 16.44 | 82.39 ± 2.65 | 70.78 ± 25.40 | 67.95 ± 2.18 | |
| NMDAR1 | mGluR2 | ||||
|---|---|---|---|---|---|
| Cell Density | OD | Cell Density | OD | ||
| DG | C | 127.78 ± 25.13 | 160.44 ± 3.59 | 113.08 ± 41.06 | 193.14 ± 12.01 |
| P | 397.74 ± 32.78 | 178.13 ± 5.42 | 753.86 ± 102.81 | 201.83 ± 4.28 | |
| CA1 | C | 64.26 ± 23.40 | 69.65 ± 5.44 | 30.15 ± 16.29 | 142.90 ± 8.70 |
| P | 287.26 ± 16.33 | 177.71 ± 7.80 | 275.16 ± 19.83 | 153.10 ± 4.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda, E.; De Luca, F.; Ratto, D.; Priori, E.C.; Savino, E.; Bottone, M.G.; Rossi, P. Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract. Biology 2023, 12, 196. https://doi.org/10.3390/biology12020196
Roda E, De Luca F, Ratto D, Priori EC, Savino E, Bottone MG, Rossi P. Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract. Biology. 2023; 12(2):196. https://doi.org/10.3390/biology12020196
Chicago/Turabian StyleRoda, Elisa, Fabrizio De Luca, Daniela Ratto, Erica Cecilia Priori, Elena Savino, Maria Grazia Bottone, and Paola Rossi. 2023. "Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract" Biology 12, no. 2: 196. https://doi.org/10.3390/biology12020196
APA StyleRoda, E., De Luca, F., Ratto, D., Priori, E. C., Savino, E., Bottone, M. G., & Rossi, P. (2023). Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract. Biology, 12(2), 196. https://doi.org/10.3390/biology12020196











