Prediction of Potential Suitable Distribution Areas of Quasipaa spinosa in China Based on MaxEnt Optimization Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
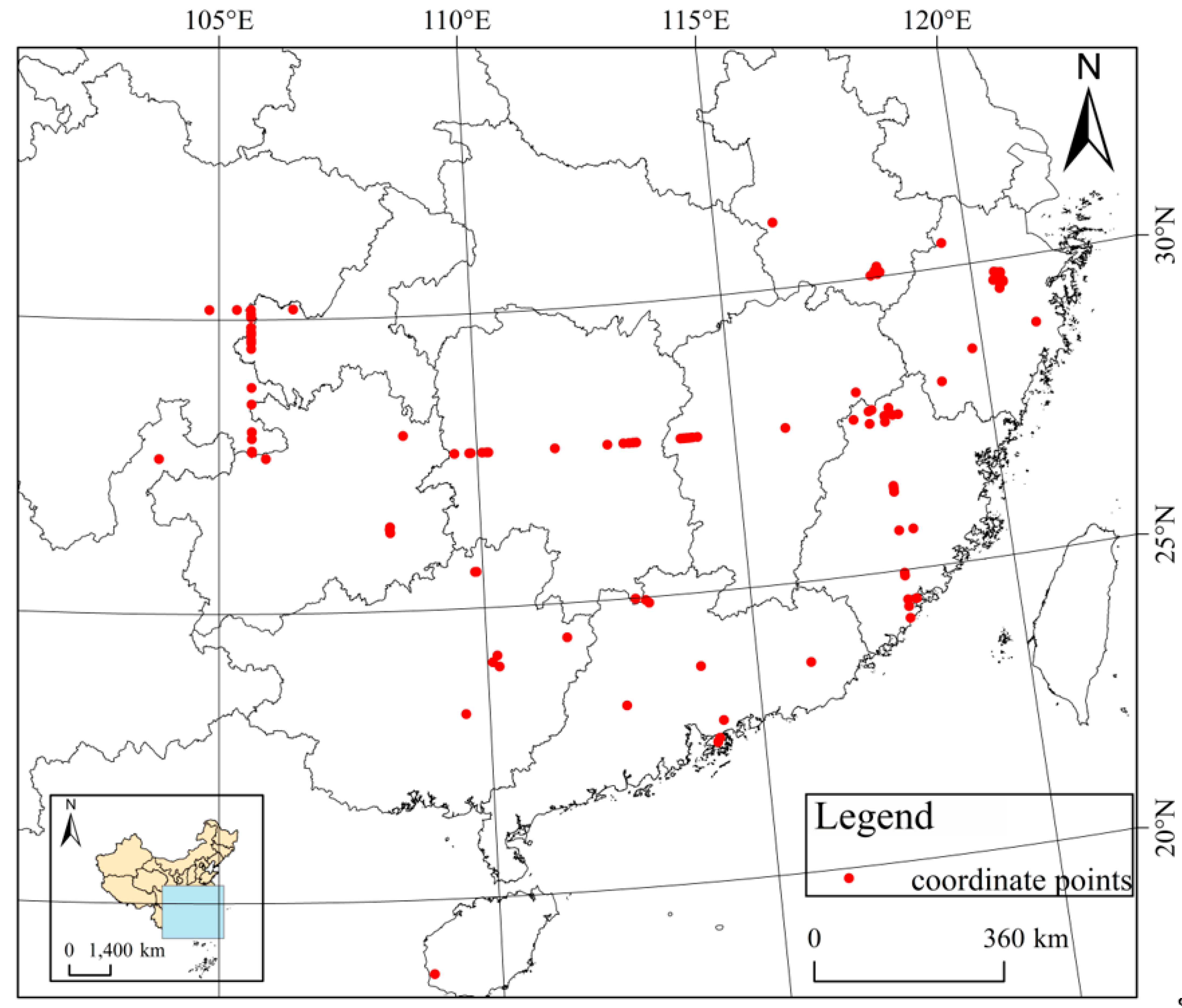
2.1. Data Collection and Processing
2.2. Species Distribution Modeling, Optimization, and Evaluation
2.3. Classification of Suitable Living Grade of Q. spinosa
3. Results
3.1. Model Optimization and Accuracy Evaluation
3.2. The Importance of Environmental Variables
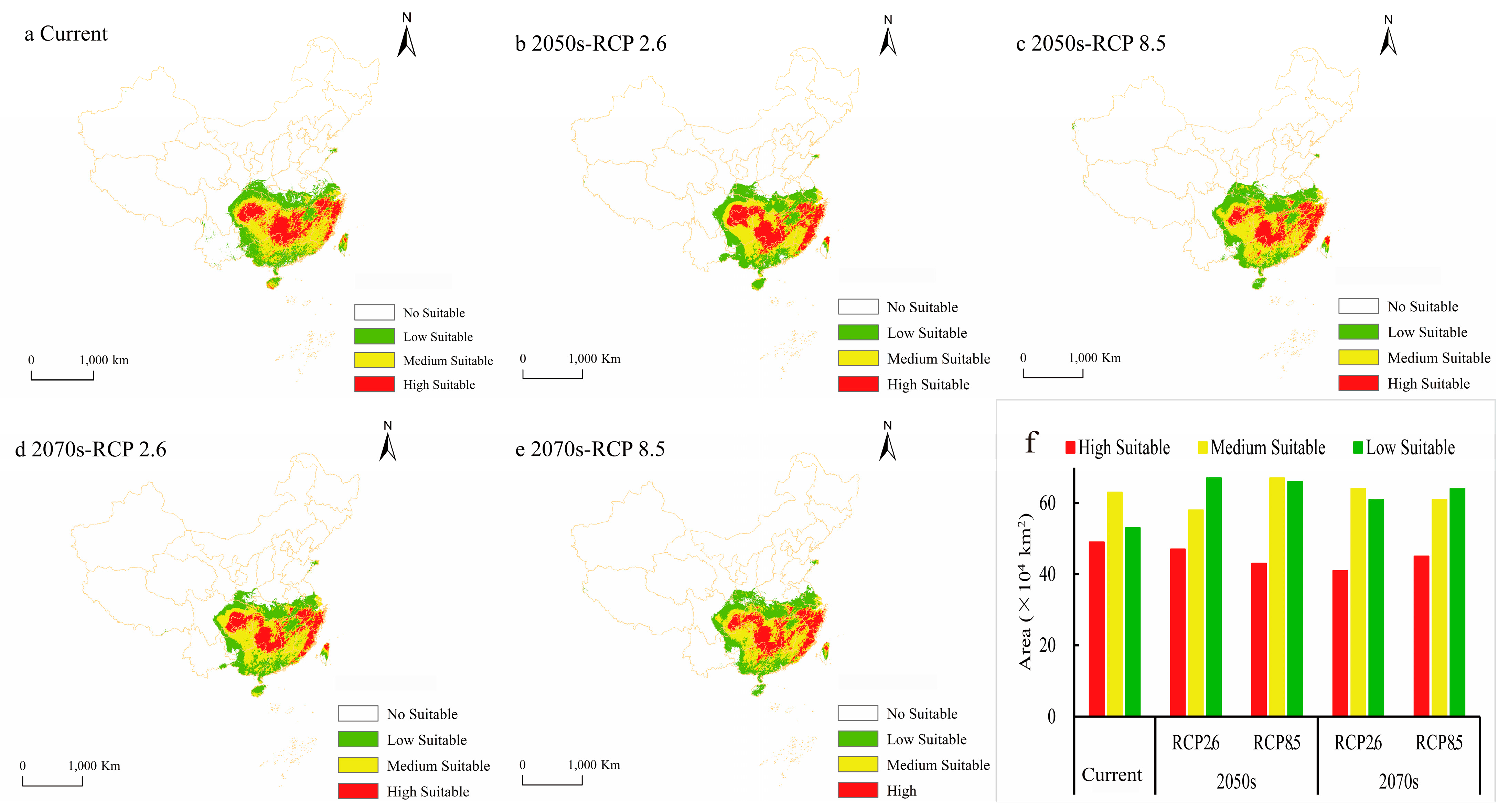
3.3. Current and Future Potential Suitable Areas and Their Spatiotemporal Changes
4. Discussion
4.1. Rationality of Model
4.2. Main Environmental Factors Affecting the Distribution of Q. spinosa
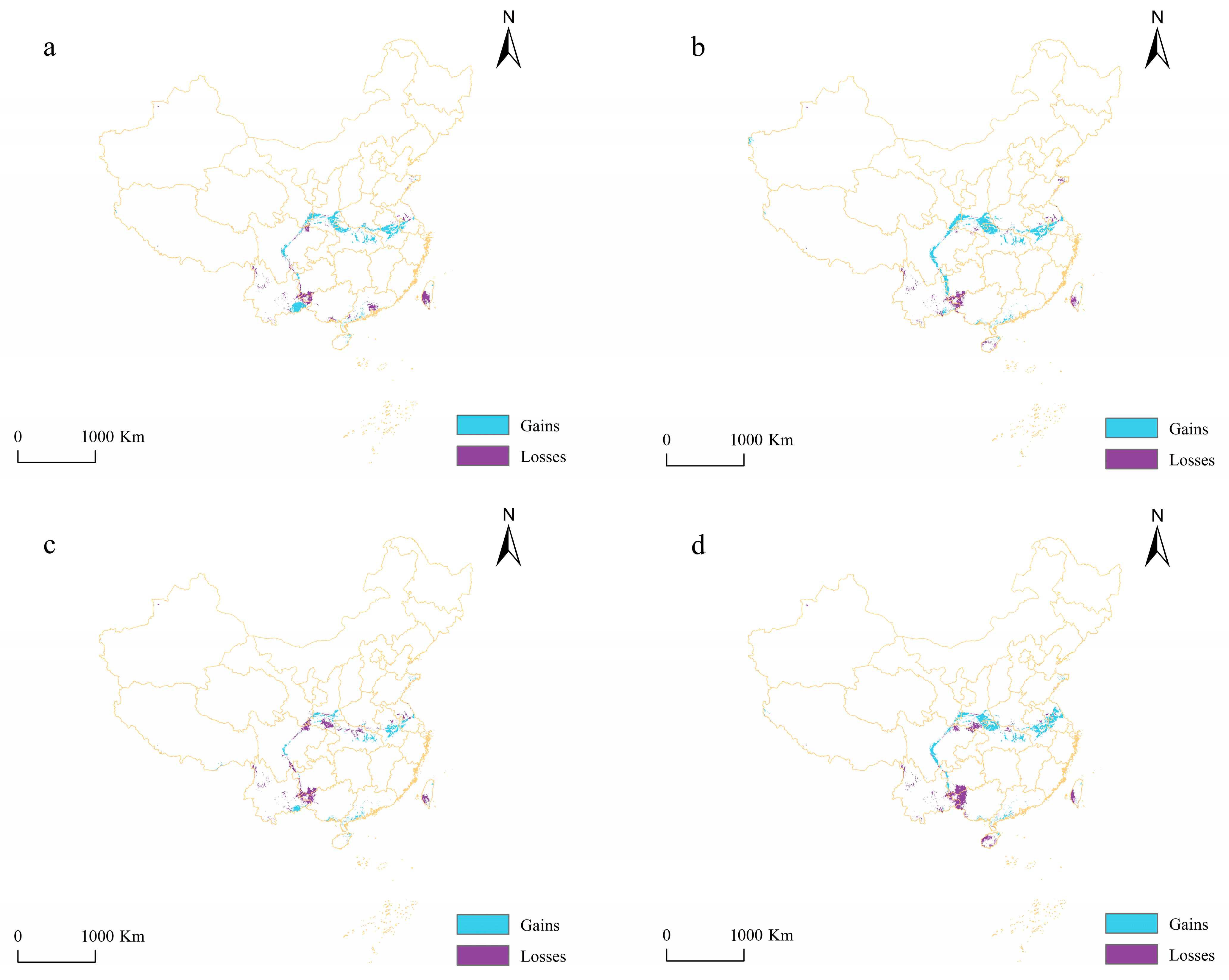
4.3. Changes in Potential Suitable Areas
4.4. Resource Conservation of Q. spinosa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soberon, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, J.; Wang, F.; Lin, Y.; Liu, L.; Mi, H.; Fangxu, M.; Fang, J.; Jialing, L. Predicting the potential geographical distribution of Hainan odorous frog (Odorrana hainanensis) in Hainan province. Chin. J. Zool. 2017, 1, 30–41. [Google Scholar] [CrossRef]
- Ward, D.F. Modelling the potential geographic distribution of invasive ant species in New Zealand. Biol. Invasions 2007, 9, 723–735. [Google Scholar] [CrossRef]
- Zhu, G.; Bu, W.; Gao, Y.; Liu, G. Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLoS ONE 2012, 7, e31246. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A.J.J.o.b. ORIGINAL ARTICLE: Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, N.; Liu, G.; Li, J. Prediction of the potential geographical distribution of five specices of Scutiger in the south of hrngduan Mountains Biodiversity Conservation Priority Zone. Acta Ecol. Sin. 2022, 42, 2636–2647. [Google Scholar]
- Zhang, K.; Yang, K.; Wo, Y.; Tong, H.; Jin, Y.; Jin, Y. Suitability evaluation of potential geographic distribution for Rana zhenhaiensis based on MaxEnt. Chin. J. Ecol. 2018, 37, 164–170. [Google Scholar] [CrossRef]
- Mu, S.; Qi, X.; Xie, L.; Qian, T.; Zhou, Z.; Lu, Y.; Mo, Y.; Li, P. MaxEnt-based prediction on the geographical distribution of Hainan stream treefrog (Buergeria oxycephala) in Hainan island. Chin. J. Wildl. 2021, 42, 809–816. [Google Scholar] [CrossRef]
- Zhou, W.W.; Zhang, B.L.; Chen, H.M.; Jin, J.Q.; Yang, J.X.; Wang, Y.Y.; Jiang, K.; Murphy, R.W.; Zhang, Y.P.; Che, J. DNA barcodes and species distribution models evaluate threats of global climate changes to genetic diversity: A case study from Nanorana parkeri (Anura: Dicroglossidae). PLoS ONE 2014, 9, e103899. [Google Scholar] [CrossRef]
- Nottingham, S.; Pelletier, T.A. The impact of climate change on western Plethodon salamanders’ distribution. Ecol. Evol. 2021, 11, 9370–9384. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, N.; Shen, M.; Li, J. Comparison between optimized MaxEnt and random forest modeling in predicting potential distribution: A case study with Quasipaa boulengeri in China. Sci. Total Env. 2022, 842, 156867. [Google Scholar] [CrossRef]
- Long, J.; Hou, J.; Zhou, W.; Xiang, J.; Pan, W. Analysis of karyotype in Quasipaa spinosa. J. Anhui Agric. Sci. 2021, 49, 95–97+103. [Google Scholar]
- Long, J.; Xiang, J.; He, T.; Zhang, N.; Pan, W. Gut microbiota differences during metamorphosis in sick and healthy giant spiny frogs (Paa spinosa) tadpoles. Lett. Appl. Microbiol. 2020, 70, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.G.; He, T.Y.; Wang, P.P.; Xie, M.; Xiang, J.; Ni, J.J. Opportunistic pathogens are abundant in the gut of cultured giant spiny frog (Paa spinosa). Aquac. Res. 2018, 49, 2033–2041. [Google Scholar] [CrossRef]
- Ye, C. Rare and Economical Amphibians of China; Sichuan Science and Technology Press: Sichuan, Chian, 1993. [Google Scholar]
- Zhou, W.; Li, J.; Hou, J.; Xiang, J.; Pan, W. Culture technology of Quasipaa spinosa (first)—Site selection and design. Curr. Fish. 2021, 46, 80–81. [Google Scholar]
- Liang, Z.; Xu, Q.; Jiang, Y.; Qin, J.; Deng, W. Breeding situation and development strategy of Rana spinosa in Yongfu County. Guangxi J. Anim. Husb. Vet. Med. 2013, 29, 244–246. [Google Scholar]
- Chan, H.K.; Shoemaker, K.T.; Karraker, N.E. Demography of Quasipaa frogs in China reveals high vulnerability to widespread harvest pressure. Biol. Conserv. 2014, 170, 3–9. [Google Scholar] [CrossRef]
- Gao, S. Annals of Animal Medicine in China; Jilin Science and Technology Publishing House: Jilin, China, 1996. [Google Scholar]
- Mei, Y.; Zheng, R.; Zheng, S.; Yan, H.; Liu, Z.; Hong, Y. Gonad differentiation and the effects of temperature on sex determination in Quasipaa spinosa. Acta Ecol. Sin. 2018, 38, 4809–4816. [Google Scholar]
- Yu, Z.; Ma, B. Status of the Quasipaa spinosa industry in Jiangxi. Jiangxi Fish. Sci. Technol. 2012, 130, 4–8. [Google Scholar] [CrossRef]
- Hou, J.; Long, J.; Xiang, J.; Pan, W.; Li, D.; Liu, X. Ontogenetic characteristics of the intestinal microbiota of Quasipaa spinosa revealed by 16S rRNA gene sequencing. Lett. Appl. Microbiol. 2022, 75, 1182–1192. [Google Scholar] [CrossRef]
- Yu, S.S.; Zhao, Z.H.; Gong, X.F.; Fan, X.L.; Lin, Z.H.; Chen, J. Antimicrobial and immunomodulatory activity of beta-defensin from the Chinese spiny frog (Quasipaa spinosa). Dev. Comp. Immunol. 2022, 126, 104264. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.P.; Yi, G.; Wang, K.Y.; OuYang, P.; Chen, D.F.; Huang, X.L.; Huang, C.; Lai, W.M.; Zhong, Z.J.; Huo, C.L.; et al. Elizabethkingia miricola infection in Chinese spiny frog (Quasipaa spinosa). Transbound Emerg. Dis. 2019, 66, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.P.; Gu, W.B.; Wang, S.Y.; Wang, L.Z.; Zhou, Y.L.; Dong, W.R.; Shu, M.A. Functional differences of three CXCL10 homologues in the giant spiny frog Quasipaa spinosa. Dev. Comp. Immunol. 2020, 109, 103719. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Xie, Y.G.; Hu, W.; Wei, Z.Y.; Wei, X.Y.; Yuan, H.; Yao, H.Y.; Dunxue, C. Transcriptome characterization and SSR discovery in the giant spiny frog Quasipaa spinosa. Gene 2022, 842, 146793. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, W.; Li, J.; Xiang, J.; Pan, W. Culture technology of Quasipaa spinosa (V)—Commercial frog farming. Curr. Fish. 2021, 6, 68. [Google Scholar]
- Zhou, W.; Li, J.; Hou, J.; Xiang, J.; Pan, W. Breeding technology of Quasipaa spinosa (III)—Tadpole breeding. Curr. Fish. 2021, 46, 80+82. [Google Scholar]
- Li, J.; Zhou, W.; Hou, J.; Xiang, J.; Pan, W. Breeding technology of Quasipaa spinosa (IV)—Feeding and management during metamorphosis. Curr. Fish. 2021, 46, 75–79+81. [Google Scholar]
- Global Biodiversity Information Facility (GBIF) Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0173717-210914110416597 (accessed on 6 March 2022).
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Last of the Wild Project, Version 3 (LWP-3): 2009 Human Footprint, 2018 Relisades; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2018. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, E.; Wang, Y.; Ma, F.; He, Q.; Yi, C. Prediction of potentially suitable distribution area of Propomacrus davidi Deyrolle in China based on MaxEnt model. Chin. J. Ecol. 2021, 40, 2836–2944. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Carlson, C.J.; Burgio, K.R.; Dougherty, E.R.; Phillips, A.J.; Bueno, V.M.; Clements, C.F.; Castaldo, G.; Dallas, T.A.; Cizauskas, C.A.; Cumming, G.S.; et al. Parasite biodiversity faces extinction and redistribution in a changing climate. Sci. Adv. 2017, 3, e1602422. [Google Scholar] [CrossRef]
- Chen, Q.H.; Yin, Y.J.; Zhao, R.; Yang, Y.; da Silva, J.A.T.; Yu, X.N. Incorporating local adaptation into species distribution modeling of paeonia mairei, an endemic plant to China. Front. Plant Sci. 2020, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, T.; Polsky, A.; Golan, D.; Danilevsky, A.; Shapira, G.; Raff, C.; Basel-Salmon, L.; Matar, R.T.; Shomron, N. Bayesian-based noninvasive prenatal diagnosis of single-gene disorders. Genome Res. 2019, 29, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.D.; Tang, X.G.; Liu, M.Y.; Liu, X.F.; Tao, J. Species distribution models of the Spartina alterniflora loisel in its origin and invasive country reveal an ecological niche shift. Front. Plant Sci. 2021, 12, 738–769. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Ruan, T.; Wei, W. Maxent model-based evaluation og habitat suitability of Chinese red panda in Qionglai mountains. J. Guizhou Norm. Univ. Nat. Sci. 2022, 40, 34–39. [Google Scholar] [CrossRef]
- Tin, A.; Marten, J.; Kuhns, V.L.H.; Li, Y.; Wuttke, M.; Kirsten, H.; Sieber, K.B.; Qiu, C.X.; Gorski, M.; Yu, Z.; et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat. Genet. 2019, 51, 1459–1474. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.J.; Zhang, Y.L.; Xu, X.Q. Current Situation, Global Potential Distribution and Evolution of Six Almond Species in China. Front. Plant Sci. 2021, 12, 619883. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.J.E. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Bull, C.M.; Burzacott, D. Changes in climate and in the timing of pairing of the Australian lizard, Tiliqua rugosa: A 15-year study. J. Zool. 2002, 256, 383–387. [Google Scholar] [CrossRef]
- Lin, W. Studies on the Physiology and Biochemistry of Juvenile Quasipaa Spinosa and the Growth and Breeding Technology of Year-Round Phenology; Fuzhou University: Fuzhou, China, 2017. [Google Scholar]
- Soares, C.; Brito, J.C. Environmental correlates for species richness among amphibians and reptiles in a climate transition area. Biodivers. Conserv. 2007, 16, 1087–1102. [Google Scholar] [CrossRef]
- Kong, W.; Li, X.; Zou, H. Optimizing MaxEnt model in the prediction of species distribution. Chin. J. Appl. Ecol. 2019, 6, 2116–2128. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Lai, W.; Yang, M.; Fan, H.; Zhang, G.; Chen, S.; Liu, B. Prediction of potential suitable distribution of Phoebe bournei based on MaxEnt optimization model. Acta Ecol. Sin. 2021, 41, 8135–8144. [Google Scholar]
- Chen, Q. Physiological and Ecological Studies and the Annual Growth and Culture Techniques of Rana spinosa Tadpoles; Fuzhou University: Fuzhou, China, 2017. [Google Scholar]
- Kong, S. Interspecific Hybridization of Quasipaa spinosa and Hybrid Tadpole Fitness Research; Zhejiang Normal University: Jinhua, China, 2017. [Google Scholar]
- Xie, Y.; Wei, Z.; Wei, X.; Luo, H.; Chen, D. Acute Toxicity of four conventional disinfectants in aquaculture on tadpoles of Quasipaa spinosa. Fish. Sci. 2021, 1–10. [Google Scholar] [CrossRef]
- Zhang, D. Molecular Basis of Local Adaptation and Speciation of Quasipaa spinosa; Zhejiang Normal University: Jinhua, China, 2020. [Google Scholar] [CrossRef]
- Liu, C.; Hu, S. The Tailless Amphibians of China; Science Press: Beijing, China, 1961. [Google Scholar]
- Tao, Z.; Ma, B.; Yu, Z.; Huang, J.; Zhang, H.; Zhang, A.; Li, Z.; Zhu, G. Effects of environmental factors on the growth of Rana spinosa tadpole. Hunan Agric. Sci. 2015, 2, 55–56+59. [Google Scholar]
- Xia, X.; Li, Y.; Yang, D.; Pi, Y. Potential geographical distribution of Rana hanluica in China under climate change. Chin. J. Appl. Ecol. 2021, 32, 4307–4314. [Google Scholar] [CrossRef]
- Zou, M.; Zhong, Y. Ecological investigation and artificial trial culture observation of Quasipaa spinosa in northwest Fujian. Chin. J. Zool. 1986, 3, 4–8. [Google Scholar] [CrossRef]
- Fei, L.; Ye, C.; Jiang, J. Color Map of Amphibians and Their Distribution in China; Sichuan Science and Technology Press: Sichuan, Chian, 2012. [Google Scholar]
- Liang, R.; Dong, Y. Ecological survey of Rana spinosa. J. Anhui Norm. Univ. Nat. Sci. 1984, 1, 30–38. [Google Scholar]
- Zhao, J.; Yang, X.; Liu, Z.; Cheng, D.; Wang, W.; Chen, F. The possible effect of global climate changes on cropping systems boundary in China II. The characteristics of climatic variables and the possible effect on northern limits of cropping systems in south China. Sci. Agric. Sin. 2010, 43, 1860–1867. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Zhou, Y.; Huang, Q.; Yu, Z.; Gu, Z. Diurnal variation of summer precipitation and its relationship with altitude in Tianshan Mountains of Xinjiang. Arid Land Geogr. 2022, 1, 57–65. [Google Scholar]
- Miao, W.; Liu, S.; Zhu, Y.; Duan, S.; Han, F. Spatio-temporal differentiation and altitude dependence of temperature and precipitation in Meili Snow Mountains. Clim. Chang. Res. 2022, 18, 328–342. [Google Scholar]
- Yang, Q.; Ma, Z.; Chen, L. A preliminary study on the relationship between precipitation trend and altitude in China. In Proceedings of the 28th Annual Meeting of the Chinese Meteorological Society—S17 3rd Annual Meeting of Graduate, Xiamen, China, 1 November 2011; p. 138. [Google Scholar]
- Zhang, H.; Zhang, Q.; Liu, Q.; Yan, P. Analysis on variation characteristics and differences of the Climate drying degree between South and North of China. Plateau Meteorol. 2016, 35, 1339–1351. [Google Scholar] [CrossRef]
- Zhao, R.; Huang, X.; Yun, W.; Wu, K.; Chen, Y.; Wang, S.; Lu, H.; Fang, K.; Li, Y. Key issues in natural resource management under carbon emission peak and carbon neutrality targets. J. Nat. Resour. 2022, 37, 1123–1136. [Google Scholar] [CrossRef]
- Tian, S. Study on impact of “Operation Green Fence” on China’s carbon emission. Jiangxi Univ. Financ. Econ. 2021. [Google Scholar] [CrossRef]
- Yin, Y.; Chang, X. Does China’s carbon emission trading policy promote regional green total factor productivity? Financ. Econ. 2022, 3, 60–70. [Google Scholar] [CrossRef]
- Normile, D. Can China, the world’s biggest coal consumer, become carbon neutral by 2060? Science 2020. [Google Scholar] [CrossRef]
- Wang, S. Comparative study on tadpole growth and development of Quasipaa spinosa introduced from different places and local species. Mod. Agric. Sci. Technol. 2010, 278–279. [Google Scholar]



| Environmental Variables | Description | Unit | PC (%) | PI (%) |
|---|---|---|---|---|
| ele | Elevation | m | 5.5 | 8.2 |
| bio2 | Mean Diurnal Range | °C | 3.9 | 3.2 |
| bio3 | Isothermality | - | 3.4 | 20.0 |
| bio4 | Temperature Seasonality | - | 2.6 | 40.9 |
| bio5 | Max Temperature of Warmest Month | °C | 0.6 | 0 |
| bio6 | Min Temperature of Coldest Month | °C | 1.9 | 9.6 |
| bio14 | Precipitation of Driest Month | mm | 79.3 | 11.7 |
| bio15 | Precipitation Seasonality | - | 1.6 | 2.9 |
| bio18 | Precipitation of Warmest Quarter | mm | 0.7 | 1.9 |
| people | Human Foot | - | 0.5 | 1.7 |
| Type | FC | β | delta. AICc | avg. diff. AUC |
|---|---|---|---|---|
| Default | LQHPT | 1 | 101.9003 | 0.1211 |
| Optimized | LQHP | 3 | 0 | 0.1008 |
| Current | 50s-RCP2.6 | 50s-RCP8.5 | 70s-RCP2.6 | 70s-RCP8.5 | |
|---|---|---|---|---|---|
| AUC | 0.962 ± 0.0073 | 0.958 ± 0.0073 | 0.958 ± 0.0073 | 0.959 ± 0.0072 | 0.959 ± 0.008 |
| TSS | 0.817 ± 0.014 | 0.813 ± 0.009 | 0.804 ± 0.010 | 0.822 ± 0.015 | 0.818 ± 0.015 |
| Circumstances | Low Suitability | Medium Suitability | High Suitability | All | ||||
|---|---|---|---|---|---|---|---|---|
| Area (×104 km2) | Percentage (%) | Area (×104 km2) | Percentage (%) | Area (×104 km2) | Percentage/% | Area (×104 km2) | Percentage (%) | |
| Current | 53 | 5.52 | 63 | 6.56 | 49 | 5.10 | 165 | 17.19 |
| 2050s-RCP 2.6 | 67 | 6.98 | 58 | 6.04 | 47 | 4.90 | 172 | 17.92 |
| 2050s-RCP 8.5 | 66 | 6.88 | 67 | 6.98 | 43 | 4.48 | 176 | 18.33 |
| 2070s-RCP 2.6 | 61 | 6.35 | 64 | 6.67 | 41 | 4.27 | 166 | 17.29 |
| 2070s-RCP 8.5 | 64 | 6.67 | 61 | 6.35 | 45 | 4.69 | 170 | 17.71 |
| Circumstances | Area (×104 km2) | Rate of Change (%) | ||||
|---|---|---|---|---|---|---|
| Gain | Loss | Change | Gain | Loss | Change | |
| 2050s-RCP2.6 | 14 | 8 | 6 | 8.48 | 4.85 | 3.64 |
| 2050s-RCP8.5 | 9 | 7 | 2 | 5.45 | 4.24 | 1.21 |
| 2070s-RCP2.6 | 18 | 5 | 13 | 10.91 | 3.03 | 7.88 |
| 2070s-RCP8.5 | 14 | 6 | 8 | 8.48 | 3.64 | 4.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Xiang, J.; Li, D.; Liu, X. Prediction of Potential Suitable Distribution Areas of Quasipaa spinosa in China Based on MaxEnt Optimization Model. Biology 2023, 12, 366. https://doi.org/10.3390/biology12030366
Hou J, Xiang J, Li D, Liu X. Prediction of Potential Suitable Distribution Areas of Quasipaa spinosa in China Based on MaxEnt Optimization Model. Biology. 2023; 12(3):366. https://doi.org/10.3390/biology12030366
Chicago/Turabian StyleHou, Jinliang, Jianguo Xiang, Deliang Li, and Xinhua Liu. 2023. "Prediction of Potential Suitable Distribution Areas of Quasipaa spinosa in China Based on MaxEnt Optimization Model" Biology 12, no. 3: 366. https://doi.org/10.3390/biology12030366
APA StyleHou, J., Xiang, J., Li, D., & Liu, X. (2023). Prediction of Potential Suitable Distribution Areas of Quasipaa spinosa in China Based on MaxEnt Optimization Model. Biology, 12(3), 366. https://doi.org/10.3390/biology12030366






