Connections between Diabetes Mellitus and Metabolic Syndrome and the Outcome of Cardiac Dysfunctions Diagnosed during the Recovery from COVID-19 in Patients without a Previous History of Cardiovascular Diseases
Abstract
Simple Summary
Abstract
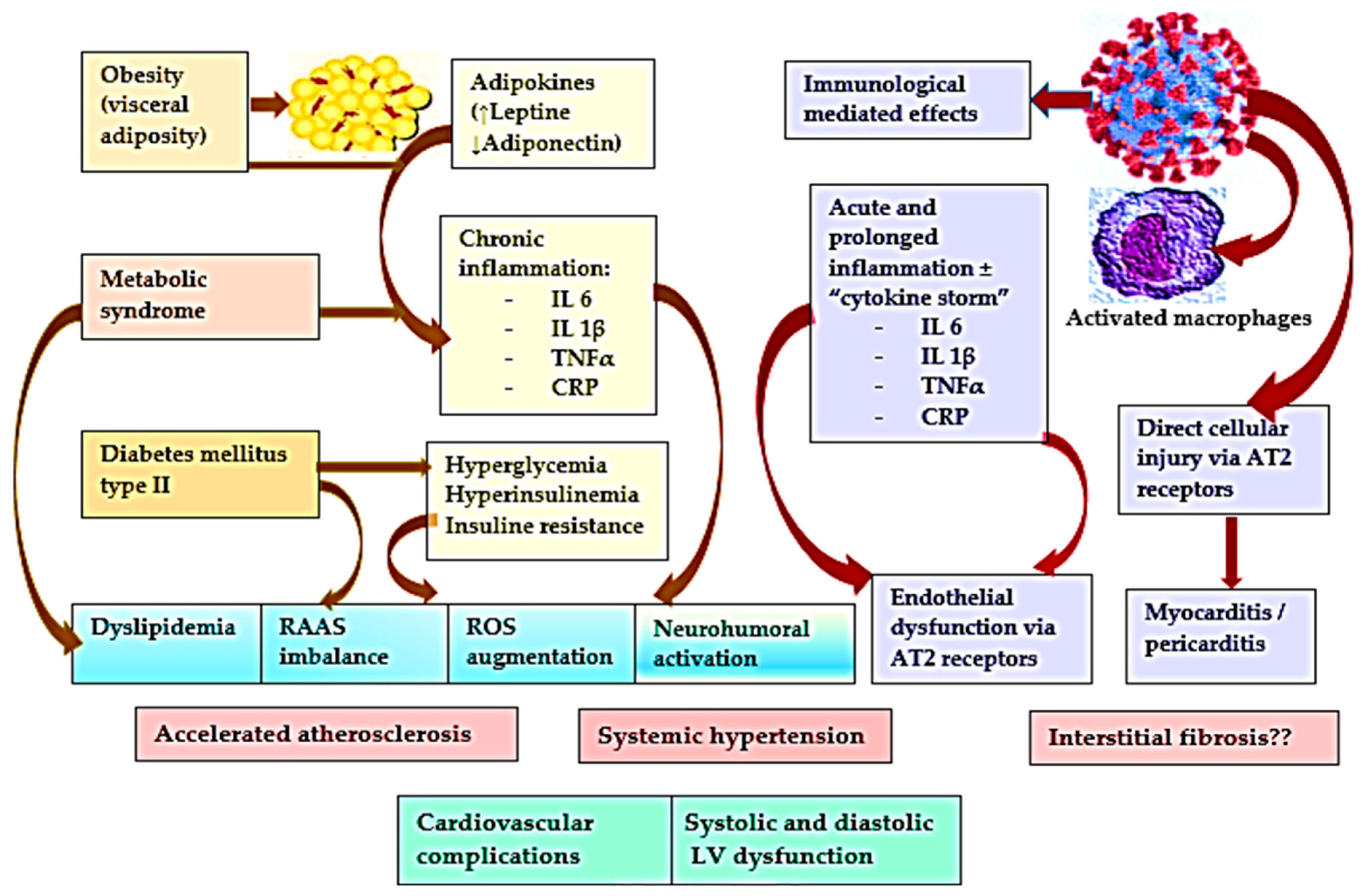
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Procedures and Clinical and Laboratory Examinations
- Patient evaluation: for the 238 participants, after they signed the individual informed consents, we gathered all available medical information regarding the course of the acute phase of the infection, along with their most recent health assessments. First, we evaluated the gravity and consequences of the infection from medical records containing results of the chest radiography or CT describing the extent of the lung injury, an ECG, and blood tests. Subsequently, we focused on the analysis of the pre-COVID-19 health status and several clinical and laboratory parameters of interest for this study, such as the presence of chronic diseases (those with current therapies for systemic hypertension or T2DM were excluded from our study [36,37,38,39,40,41], but occasionally elevated or borderline values of blood pressure or BBG were accepted), mentions regarding body weight and height, health risk, blood pressure values, and ECG and TTE results (even if considered as normal). Subsequently, all patients had an ECG and TTE to identify any significant cardiovascular alterations that could have been missed in the previous evaluations. We repeated these examinations at 3 and 6 months for all study patients.
- Echocardiographic examination: Every TTE determination was performed following guideline recommendations. After the standard measurements of all cardiac structures and the assessment of their function, from a long axis parasternal view, we determined the left ventricular (LV) mass index (LVMI), LV hypertrophy (LVH), and confirmed by LVMI over a value of 115 g/m2 (men) or 95 g/m2 (women). The following parameters, characterizing four patterns of cardiac abnormalities, were also evaluated.
- −
- The Left Ventricular ejection fraction (LVEF), calculated according to the Simpson method (modified) formula (results under 50% considered as pathological).
- −
- The MAPSE (lateral mitral annular plane systolic excursion), with values lower than 10 mm appreciated as pathological.
- −
- We assessed the Left Ventricular global longitudinal strain (LV-GLS) by speckle tracking, and automatically generated the ROI (region of interest) after tracing the Left Ventricular endocardial border, with manual adjustments as needed, in order to adjust the width of the LV wall [8,19]. An impaired LVF was represented by values lower than −18%, while scores between −18 and −19 were considered as borderline values [9,11].
- −
- We measured the TAPSE (tricuspid annular plane systolic excursion), in M-Mode, at the lateral tricuspid valve annulus level, and considered values below 17 mm as abnormal.
- −
- From an apical view, we determined the FAC (fractional area change), and deemed any scores lower than 35% as representative for a Right Ventricle dysfunction (RVD).
- −
- −
- We determined the sPAP by looking at the velocity of the peak tricuspid regurgitation (TRV) assessed by a continuous Doppler, while considering the pressure in the right atrium (RAP), appreciated in terms of the diameter of the inferior vena cava (IVC) as well as its respiratory differences. For this study, any resting sPAP values above 35 mm Hg were suggestive of a PH [36], with severities in the mild (35–44 mmHg) to either a moderate (45–60 mmHg) or severe range (above 60 mmHg).
- −
- The volume index of the left atrium (LAVI) was measured from an apical 4-chamber view, with scores above 34 mL considered pathological.
- −
- At the mitral valve level, we used a pulsed Doppler with a similar interpretation for recording mitral inflow and measuring the early peak diastolic velocity (E), as well as the late diastolic velocity (A); subsequently, an E/A ratio was calculated.
- −
- We used tissue Doppler imaging (TDI) at the septal and lateral mitral annulus levels to measure early (e’) and late diastolic velocity (a’); average and E/e’ ratios were subsequently calculated.
- Physical and laboratory examination: Because of these assessments, a further 35 patients had to be excluded from our study due to previously unidentified significant health conditions. We measured the BMI (≥30 kg/m2 indicating obesity) and waist circumference (WC) for the remaining 203 subjects, followed by blood sample collection to determine the BBG, serum creatinine, and the calculation of eGFR, uric acid, total (TChol), low-density lipoprotein (LDLChol), and high-density lipoprotein (HDLChol) cholesterol levels, triglyceride (TG), and C-reactive protein (CRP). T2DM was certified by a BBG exceeding 126 mg/dL twice in non-consecutive days, and a glycated hemoglobin exceeding 6.5%; MS was defined by the presence of three or more of the following factors: IMC ≥ 30 kg/m2, WC ≥ 102 cm in men and ≥88 cm in women, impaired glucose metabolism, HDLC ˂ 40 mg/dL in men and ˂50 mg/dL in women, TG ≥ 150 mg/dL, and uric acid ˃ 6.5 mg/dL, and BP ˃ 135/85 mmHg. Several significant indexes for the evaluation of MS were calculated, as follows:
- −
- The triglyceride-glucose index (TyG) represents the logarithm of the product of BBG and fasting TG, the formula being: ln[BBG(mg/dl) × TG (mg/dl)/2]. This has been recommended as an alternative indicator for IR because it correlates to lipotoxicity and glucotoxicity [35,43]. A close relationship has been demonstrated between the TyG and cardio metabolic outcomes, T2DM, endothelial dysfunction, systemic hypertension, cardiovascular diseases, stroke, and—more recently—with patient outcome in COVID-19 [34,35,44]. In the medical literature, the normal cut-off values reported for TyG vary widely between 4 and 8 (due to the position of 2 in the TyG index formula) [34,35].
- −
- The product of lipid accumulation (LAP), which is accepted as an indicator for visceral adiposity, was calculated based on WC and fasting TG. The formulas are: LAP = (WC(cm) − 65) × TG(moll⁄) (men); LAP = (WC(cm) − 58) × TG(moll⁄) (women). Reference LAP cut-off values range between25.16 to 31.59 cm × moll/l (women), and between 20.10 and 63.89 cm × moll/l (men). LAP is largely employed as an indicator for MS, abdominal obesity, and is deemed as a health risk for cardiovascular illnesses, predicting adverse cardiovascular events [45,46].
- −
- We calculated the VAI (visceral adiposity index) with the following formulas: VAI = (WC(cm)/(39.68 + (1.88 × BMI) × (TG/1.03) × (1.31/HDL) (men) and VAI = (WC(cm)/(36.58 + (BMI × 1.89) × (TG/0.81) × (1.52/HDL) (women).
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; Fonarow, G.C.; O’Gara, P.T.; Yancy, C.W. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020, 5, 751. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and Impact of Cardiovascular Metabolic Diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef]
- Manolis, A.S.; Manolis, A.A.; Manolis, T.A.; Apostolaki, N.E.; Melita, H. COVID-19 Infection and Body Weight: A Deleterious Liaison in a J-Curve Relationship. Obes. Res. Clin. Pract. 2021, 15, 523–535. [Google Scholar] [CrossRef]
- Landstra, C.P.; de Koning, E.J.P. COVID-19 and Diabetes: Understanding the Interrelationship and Risks for a Severe Course. Front. Endocrinol. 2021, 12, 649525. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Hendren, N.S.; Drazner, M.H.; Bozkurt, B.; Cooper, L.T. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation 2020, 141, 1903–1914. [Google Scholar] [CrossRef]
- Xiong, T.-Y.; Redwood, S.; Prendergast, B.; Chen, M. Coronaviruses and the Cardiovascular System: Acute and Long-Term Implications. Eur. Heart J. 2020, 41, 1798–1800. [Google Scholar] [CrossRef]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol 2020, 5, 831. [Google Scholar] [CrossRef]
- Freaney, P.M.; Shah, S.J.; Khan, S.S. COVID-19 and Heart Failure With Preserved Ejection Fraction. JAMA 2020, 324, 1499. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819. [Google Scholar] [CrossRef]
- American Diabetes Association. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. 1), S111–S134. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity: Implications for Metabolic Syndrome, Diabetes, Hypertension, Dyslipidemia, Atherosclerosis, and Cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Hussain, M.E. Obesity and Diabetes: An Update. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 73–79. [Google Scholar] [CrossRef]
- Reiterer, M.; Rajan, M.; Gómez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Ma, L.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.T.; Chandar, V.; et al. Hyperglycemia in Acute COVID-19 Is Characterized by Insulin Resistance and Adipose Tissue Infectivity by SARS-CoV-2. Cell Metab. 2021, 33, 2174–2188.e5. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D.; et al. COVID-19 Induces New-Onset Insulin Resistance and Lipid Metabolic Dysregulation via Regulation of Secreted Metabolic Factors. Signal Transduct. Target. Ther. 2021, 6, 427. [Google Scholar] [CrossRef]
- Dini, F.L.; Fabiani, I.; Miccoli, M.; Galeotti, G.G.; Pugliese, N.R.; D’Agostino, A.; Scartabelli, A.; Conte, L.; Salvetti, G.; Santini, F.; et al. Prevalence and Determinants of Left Ventricular Diastolic Dysfunction in Obese Subjects and the Role of Left Ventricular Global Longitudinal Strain and Mass Normalized to Height. Echocardiography 2018, 35, 1124–1131. [Google Scholar] [CrossRef]
- Świerblewska, E.; Wolf, J.; Kunicka, K.; Graff, B.; Polonis, K.; Hoffmann, M.; Chrostowska, M.; Szyndler, A.; Bandosz, P.; Graff, B.; et al. Prevalence and Distribution of Left Ventricular Diastolic Dysfunction in Treated Patients with Long-Lasting Hypertension. Blood Press. 2018, 27, 376–384. [Google Scholar] [CrossRef]
- Kalligeros, M.; Shehadeh, F.; Mylona, E.K.; Benitez, G.; Beckwith, C.G.; Chan, P.A.; Mylonakis, E. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity 2020, 28, 1200–1204. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Rubino, F.; Ludwig, B.; Rietzsch, H.; Schwarz, P.E.H.; Rodionov, R.N.; Khunti, K.; Hopkins, D.; Birkenfeld, A.L.; Boehm, B.; et al. Consequences of the COVID-19 Pandemic for Patients with Metabolic Diseases. Nat. Metab. 2021, 3, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Bende, F.; Fofiu, R.; Enache, A.; Pescariu, S.A.; Novacescu, D. The Impact of Metabolic Syndrome and Obesity on the Evolution of Diastolic Dysfunction in Apparently Healthy Patients Suffering from Post-COVID-19 Syndrome. Biomedicines 2022, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Steenblock, C.; Schwarz, P.E.H.; Ludwig, B.; Linkermann, A.; Zimmet, P.; Kulebyakin, K.; Tkachuk, V.A.; Markov, A.G.; Lehnert, H.; de Angelis, M.H.; et al. COVID-19 and Metabolic Disease: Mechanisms and Clinical Management. Lancet Diabetes Endocrinol. 2021, 9, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R.; Kalita, P.; Pathak, M.P. Diabetes Mellitus and COVID-19: Understanding the Association in Light of Current Evidence. WJCC 2021, 9, 8327–8339. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-Acute Sequelae of COVID-19 with a Cardiovascular Focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID Syndrome in Non-Hospitalised Patients with COVID-19: A Longitudinal Prospective Cohort Study. Lancet Reg. Health-Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the Cardiovascular System: Implications for Risk Assessment, Diagnosis, and Treatment Options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef]
- Ogata, H.; Mori, M.; Jingushi, Y.; Matsuzaki, H.; Katahira, K.; Ishimatsu, A.; Enokizu-Ogawa, A.; Taguchi, K.; Moriwaki, A.; Yoshida, M. Impact of Visceral Fat on the Prognosis of Coronavirus Disease 2019: An Observational Cohort Study. BMC Infect. Dis. 2021, 21, 1240. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, Y.; Huang, Y.-M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.-L. Obesity in Patients with COVID-19: A Systematic Review and Meta-Analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef] [PubMed]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef]
- Pranata, R.; Lim, M.A.; Huang, I.; Yonas, E.; Henrina, J.; Vania, R.; Lukito, A.A.; Nasution, S.A.; Alwi, I.; Siswanto, B.B. Visceral Adiposity, Subcutaneous Adiposity, and Severe Coronavirus Disease-2019 (COVID-19): Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2021, 43, 163–168. [Google Scholar] [CrossRef]
- Franco, B.N.; Asano, S. COVID-19-Related Trends and Characteristics of Type 2 Diabetes Mellitus and Metabolic Syndrome. Cureus 2022, 14, e21483. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, G.; Cheng, T.; Liu, J.; Song, G.; Ma, H. Association between Triglyceride-Glucose Index and Risk of Incident Diabetes: A Secondary Analysis Based on a Chinese Cohort Study. Lipids. Health Dis. 2020, 19, 236. [Google Scholar] [CrossRef]
- Liu, X.; He, G.; Lo, K.; Huang, Y.; Feng, Y. The Triglyceride-Glucose Index, an Insulin Resistance Marker, Was Non-Linear Associated With All-Cause and Cardiovascular Mortality in the General Population. Front. Cardiovasc. Med. 2021, 7, 628109. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, B.; Chen, D.; Hu, X.; Xu, X.; Shen, W.-J.; Hu, C.; Li, J.; Qu, S. COVID-19 May Increase the Risk of Insulin Resistance in Adult Patients Without Diabetes: A 6-Month Prospective Study. Endocr. Pract. 2021, 27, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Ding, X.; Chen, S.; Li, J.; Shen, B. The Correlation Between Triglyceride-Glucose Index and SARS-CoV-2 RNA Re-Positive in Discharged COVID-19 Patients. Infect. Drug. Resist. 2022, 15, 3815–3828. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jeon, J.; Song, T.-J.; Kim, J. Association of Triglyceride-Glucose Index with Prognosis of COVID-19: A Population-Based Study. J. Infect. Public Health 2022, 15, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Malavazos, A.E.; Secchi, F.; Basilico, S.; Capitanio, G.; Boveri, S.; Milani, V.; Dubini, C.; Schiaffino, S.; Morricone, L.; Foschini, C.; et al. Abdominal Obesity Phenotype Is Associated with COVID-19 Chest X-Ray Severity Score Better than BMI-Based Obesity. Eat. Weight. Disord. 2022, 27, 345–359. [Google Scholar] [CrossRef]
- Rohani-Rasaf, M.; Mirjalili, K.; Vatannejad, A.; Teimouri, M. Are Lipid Ratios and Triglyceride-Glucose Index Associated with Critical Care Outcomes in COVID-19 Patients? PLoS ONE 2022, 17, e0272000. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef]
- Baycan, O.F.; Barman, H.A.; Atici, A.; Tatlisu, A.; Bolen, F.; Ergen, P.; Icten, S.; Gungor, B.; Caliskan, M. Evaluation of Biventricular Function in Patients with COVID-19 Using Speckle Tracking Echocardiography. Int. J. Cardiovasc. Imaging. 2020, 37, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Oancea, C.; Marinescu, A.R.; Pescariu, S.A.; Pop, G.N.; Bende, F. Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19. J. Pers. Med. 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Fayol, A.; Livrozet, M.; Boutouyrie, P.; Khettab, H.; Betton, M.; Tea, V.; Blanchard, A.; Bruno, R.; Hulot, J. French COVID cohort study group Cardiac Performance in Patients Hospitalized with COVID-19: A 6 Month Follow-up Study. ESC Heart Fail. 2021, 8, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.A.M.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status (PCFS) Scale: A Tool to Measure Functional Status over Time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]

| Age | BMI | WC | SBP | DBP | HR | No. of Symptoms | PCFS | Days Since First PCR | Initial Lung Injury on CCT Scan | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | W | ||||||||||
Group I—46 patients with T2DM and MS
| 52 ± 3.54 | 30.96 [29.22–32.86] | 108 [103.25–112] | 94 [90–97.5] | 130 [120–131] | 80 [70–85] | 75 [75–80] | 6 [3.75–7] | 2 [1–3] | 56 [56–64.75] | 15 [15–30] |
Group II—66 patients with MS, but without T2DM
| 51.07 ± 4.77 | 29.48 [27.49–31.32] | 105 [103–109.5] | 89 [88–93.5] | 130 [120–140] | 80 [70–90] | 75 [75–80] | 5 [3–7] | 2 [1–3] | 63 [56–70] | 10.5 [0–30] |
Group III—91 controls without T2DM and MS
| 41.67 ± 7.44 | 24.38 [22.56–26.8] | 98 [94–100] | 79 [70–85] | 120 [100–120] | 70 [60–70] | 80 [75–85] | 0 [0–3] | 1 [1–3] | 63 [63–70] | 0 [0–6] |
| Statistical significance | |||||||||||
| I/II | 0.2636 | 0.018 | 0.176 | 0.537 | 0.647 | 0.256 | 0.947 | 0.2018 | 0.064 | 0.8349 | |
| I/III | <0.00001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.014 | 0.005 | <0.0001 | 0.0001 | 0.0002 | |
| II/III | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | 0.001 | <0.0001 | 0.0001 | 0.0001 | |
| BBG (mg/dL) | Uric Acid (mg/dL) | LDL-Cholesterol (mg/dL) | HDL-Cholest. (mg/dL) | Triglycerides (mg/dL) | CRP (mg/dL) | eGRF (mL/min) | TyG Index | VAI | LAP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Group I—46 patients with T2DM and MS | 114.5 [110–120] | 7.4 [7.2–8] | 140 [130–150] | 32.5 [30–40] | 170 [160–190] | 30.4 [26.28–38.9] | 100 [98–105] | 4.94 [4.88–5] | 3.47 [2.53–4.42] | 75.02 [61.4 5–87.35] |
| Group II—66 patients without T2DM, but with MS | 100 [99–100.25] | 7.35 [7.2–7.6] | 130 [120–141.25] | 30 [30–35.75] | 162.5 [160–171.25] | 30.11 [24–32.61] | 104 [100–110] | 4.86 [4.83 –4.89] | 3.6 [3.05–4.42] | 75 [56–191.11] |
| Group III—91 controls without T2DM and MS | 90 [89–95] | 6.4 [6–6.8] | 100 [90–120] | 45 [40–50] | 140 [130–145] | 26.23 [5.67–40.67] | 120 [110–125] | 4.72 [4.68–4.75] | 2.12 [1.81 –2.6] | 41.1 [28.45–51.42] |
| Statistical significance | ||||||||||
| I/II | p < 0.0001 | p = 0.237 | p = 0.016 | p = 0.8 | p = 0.064 | p = 0.151 | p = 0.001 | p < 0.0001 | p = 0.574 | p = 0.039 |
| I/III | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| II/III | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.0021 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Parameter | Group I—Patients with DM and MS n = 46 | Group II—Patients with MS, without DM n = 66 | Group III—Controls without DM and MS n = 91 | I/II | I/III | II/III |
|---|---|---|---|---|---|---|
| LVMI | 98.78 [93.6–110.6] | 98.5 [90.3–111.15] | 87.74 [70.45–97.45] | 0.797 | <0.0001 | <0.0001 |
| LAVI | 28.42 [21.36–34] | 23.40 [18.47–31.81] | 15.76 [13.32–21.34] | 0.059 | <0.0001 | <0.0001 |
| PT | 2.2 [1.5–3.14] | 2.2 [1.5–2.8] | 1.9 [1.5–2.5] | 0.699 | 0.053 | 0.104 |
| LVEF | 55 [50–60] | 55 [50–58] | 60 [55–65] | 0.995 | <0.0001 | <0.0001 |
| MAPSE | 14 [11–15] | 14 [12–16] | 17 [15–18] | 0.181 | <0.0001 | <0.0001 |
| LV-GLS | −19 [−21–−18] | −19.5 [−20–−19] | −21 [−22–−20] | 0.524 | <0.0001 | <0.0001 |
| TAPSE | 20 [18–22] | 20 [18–22] | 24 [20–26] | 0.726 | <0.0001 | <0.0001 |
| FAC | 35.86 [35–37.89] | 36.57 [35.46–37.69] | 37.87 [35.8–39] | 0.222 | 0.0004 | 0.0016 |
| RV-GLS | −28 [−30–−27] | −29 [−30–−28] | −31 [−33–−29] | 0.278 | <0.0001 | <0.0001 |
| TRV | 2.7 [2.5–2.73] | 2.67 [2.49–2.7] | 2.51 [2–2.7] | 0.291 | <0.0001 | <0.0001 |
| PAPs | 34.16 [31–34.8] | 33.51 [29.85–34.59] | 30.20 [21–34.16] | 0.291 | <0.0001 | 0.0002 |
| E/A | 0.97 [0.8–1.29] | 1.02 [0.76–1.26] | 1.11 [0.92–1.34] | 0.095 | 0.206 | 0.1612 |
| E/e’ | 14.08 [11.55–14.32] | 13.50 [11.67–14.24] | 11.92 [9.87–13] | 0.503 | <0.0001 | <0.0001 |
| Parameter | LV-GLS | RV-GLS | E/e’ | PT |
|---|---|---|---|---|
| Age | r = 0.45, p < 0.0001 95%CI [0.334–0.554] | r = 0.62, p < 0.0001 95%CI [0.538–0.706] | r = 0.45, p < 0.0001 95%CI [0.339–0.558] | r = 0.15, p = 0.032 95%CI [0.0126–0.281] |
| BMI | r = 0.36, p < 0.0001 95%CI [0.236–0.476] | r = 0.33, p < 0.0001 95%CI [0.202–0.448]] | r = 0.36, p < 0.0001 95%CI [0.242–0.480] | r = 0.054, p = 0.438 95%CI [−0.083–0.19] |
| Lung injury | r = 0.35, p < 0.0001 95%CI [0.231–0.472] | r = 0.71, p < 0.0001 95%CI [0.644–0.778] | r = 0.62, p < 0.0001 95%CI [0.530–0.700] | r = 0.51, p < 0.0001 95%CI [0.410–0.613] |
| Days since dg. | r = −0.28, p < 0.0001 95%CI [−0.408–−0.155] | r = −0.61, p < 0.0001 95%CI [−0.691–−0.517] | r = −0.48, p < 0.0001 95%CI [−0.584–−0.372] | r = −0.61, p < 0.0001 95%CI [−0.691–−0.518] |
| PCFS | r = 0.51, p < 0.0001 95%CI [0.409–0.611] | r = 0.68, p < 0.0001 95%CI [0.599–0.748] | r = 0.63, p < 0.0001 95%CI [0.544–0.710]] | r = 0.44, p < 0.001 95%CI [0.332–0.553] |
| No. of MS elements | r = 0.42, p < 0.0001 95%CI [0.307–0.533] | r = 0.59, p < 0.0001 95%CI [0.493–0.674] | r = 0.47, p < 0.0001 95%CI [0.355–0.571] | r = 0.22, p = 001 95%CI [0.094–0.355] |
| CRP | r = 0.53, p < 0.0001 95%CI [0.431–0.628] | r = 0.74, p < 0.0001 95%CI [0.671–0.797] | r = 0.74, p < 0.0001 95%CI [0.679–0.802] | r = 0.50, p < 0.0001 95%CI [0.391–0.598] |
| TyG index | r = 0.43, p < 0.0001 95%CI [0.391–0.542] | r = 0.53, p < 0.0001 95%CI [0.432–0.629] | r = 0.43, p < 0.0001 95%CI [0.319–0.542] | r = 0.23, p = 0.0008 95%CI [0.099–0.359] |
| VAI | r = 0.28, p < 0.0001 95%CI [0.148–0.403] | r = 0.31, p < 0.0001 95%CI [0.192–0.440] | r = 0.39, p < 0.0001 95%CI [0.275–0.507] | r = 0.13, p = 0.051 95%CI [−0.00082–0.269] |
| LAP | r = 0.38, p < 0.0001 95%CI [0.265–0.499] | r = 0.45, p < 0.0001 95%CI [0.343–0.561] | r = 0.44, p < 0.0001 95%CI [0.327–0.549] | r = 0.19, p = 0.005 95%CI [0.058–0.323] |
| Predictors | Baseline | End of Follow Up (6 Months) | ||||
|---|---|---|---|---|---|---|
| β | ±SE | p | β | ±SE | p | |
| Multivariate linear regression analysis of LVF (LV-GLS) | ||||||
| CRP (mg/dL) | β = 0.064 | ±0.009 | p < 0.0001 | NS | - | - |
| TyG index | β = 3.12 | ±0.75 | p = 0.0001 | β = 4.25 | ±0.73 | p < 0.001 |
| Multivariate linear regression analysis of RVF and PH (LV-GLS, respectively PAPs) | ||||||
| CRP (mg/dL) | β = 0.136 β = 0.044 | ±0.010 ±0.005 | p < 0.0001 p < 0.0001 | β = 0.039 β = 0.24 | ±0.009 ±0.092 | p = 0.0001 p = 0.011 |
| TyG index | β = 6.96 β = 3.30 | ±2.53 ±0.82 | p = 0.0065 p = 0.0001 | NS β = 0.018 | - ±0.0071 | - p = 0.009 |
| Multivariate linear regression analysis of DD frequency | ||||||
| CRP (mg/dL) | β = 0.567 | ±0.036 | p < 0.0001 | β = 0.023 | ±0.0044 | p < 0.0001 |
| TyG index | β = 14.89 | ±2.97 | p < 0.0001 | β = 1.332 | ±0.362 | p = 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudoran, C.; Bende, R.; Bende, F.; Giurgi-Oncu, C.; Enache, A.; Dumache, R.; Tudoran, M. Connections between Diabetes Mellitus and Metabolic Syndrome and the Outcome of Cardiac Dysfunctions Diagnosed during the Recovery from COVID-19 in Patients without a Previous History of Cardiovascular Diseases. Biology 2023, 12, 370. https://doi.org/10.3390/biology12030370
Tudoran C, Bende R, Bende F, Giurgi-Oncu C, Enache A, Dumache R, Tudoran M. Connections between Diabetes Mellitus and Metabolic Syndrome and the Outcome of Cardiac Dysfunctions Diagnosed during the Recovery from COVID-19 in Patients without a Previous History of Cardiovascular Diseases. Biology. 2023; 12(3):370. https://doi.org/10.3390/biology12030370
Chicago/Turabian StyleTudoran, Cristina, Renata Bende, Felix Bende, Catalina Giurgi-Oncu, Alexandra Enache, Raluca Dumache, and Mariana Tudoran. 2023. "Connections between Diabetes Mellitus and Metabolic Syndrome and the Outcome of Cardiac Dysfunctions Diagnosed during the Recovery from COVID-19 in Patients without a Previous History of Cardiovascular Diseases" Biology 12, no. 3: 370. https://doi.org/10.3390/biology12030370
APA StyleTudoran, C., Bende, R., Bende, F., Giurgi-Oncu, C., Enache, A., Dumache, R., & Tudoran, M. (2023). Connections between Diabetes Mellitus and Metabolic Syndrome and the Outcome of Cardiac Dysfunctions Diagnosed during the Recovery from COVID-19 in Patients without a Previous History of Cardiovascular Diseases. Biology, 12(3), 370. https://doi.org/10.3390/biology12030370







