Effects of Different Fasting Interventions on Cardiac Autonomic Modulation in Healthy Individuals: A Secondary Outcome Analysis of the EDIF Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Trial Schedule
2.3. Fasting Interventions
2.3.1. 16/8. Intermittent Fasting
2.3.2. 20/4. Intermittent Fasting
2.3.3. Alternate Day Fasting
2.4. Study Visits
2.4.1. Screening Appointment (Visit 1)
2.4.2. Trial Visits (Visits 2 and 3)
2.5. ECG Assessment
2.6. HRV Assessment
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krause, L.; Lampert, T. Relation between Overweight/Obesity and Self-Rated Health Among Adolescents in Germany. Do Socio-Economic Status and Type of School Have an Impact on That Relation? Int. J. Environ. Res. Public Health 2015, 12, 2262–2276. [Google Scholar] [CrossRef] [Green Version]
- Haftenberger, M.; Mensink, G.B.M.; Herzog, B.; Kluttig, A.; Greiser, K.H.; Merz, B.; Nöthlings, U.; Schlesinger, S.; Vogt, S.; Thorand, B.; et al. Changes in Body Weight and Obesity Status in German Adults: Results of Seven Population-Based Prospective Studies. Eur. J. Clin. Nutr. 2016, 70, 300–305. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 February 2023).
- St-Onge, M.-P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-Day Fasting in Nonobese Subjects: Effects on Body Weight, Body Composition, and Energy Metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate Day Fasting for Weight Loss in Normal Weight and Overweight Subjects: A Randomized Controlled Trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-Obese Humans. Cell Metab. 2019, 30, 462–476.e6. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.M.; Sainsbury, A.; King, N.A.; Hills, A.P.; Wood, R.E. Intermittent Energy Restriction Improves Weight Loss Efficiency in Obese Men: The MATADOR Study. Int. J. Obes. 2018, 42, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Templeman, I.; Gonzalez, J.T.; Thompson, D.; Betts, J.A. The Role of Intermittent Fasting and Meal Timing in Weight Management and Metabolic Health. Proc. Nutr. Soc. 2020, 79, 76–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klempel, M.C.; Kroeger, C.M.; Varady, K.A. Alternate Day Fasting (ADF) with a High-Fat Diet Produces Similar Weight Loss and Cardio-Protection as ADF with a Low-Fat Diet. Metabolism 2013, 62, 137–143. [Google Scholar] [CrossRef]
- Klempel, M.C.; Kroeger, C.M.; Bhutani, S.; Trepanowski, J.F.; Varady, K.A. Intermittent Fasting Combined with Calorie Restriction Is Effective for Weight Loss and Cardio-Protection in Obese Women. Nutr. J. 2012, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.-H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Tellejohan, R.; et al. Alternate Day Calorie Restriction Improves Clinical Findings and Reduces Markers of Oxidative Stress and Inflammation in Overweight Adults with Moderate Asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The Effects of Intermittent or Continuous Energy Restriction on Weight Loss and Metabolic Disease Risk Markers: A Randomized Trial in Young Overweight Women. Int. J. Obes. (Lond) 2011, 35, 714–727. [Google Scholar] [CrossRef] [Green Version]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-Term Modified Alternate-Day Fasting: A Novel Dietary Strategy for Weight Loss and Cardioprotection in Obese Adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef] [Green Version]
- Obermayer, A.; Tripolt, N.J.; Pferschy, P.N.; Kojzar, H.; Aziz, F.; Müller, A.; Schauer, M.; Oulhaj, A.; Aberer, F.; Sourij, C.; et al. Efficacy and Safety of Intermittent Fasting in People With Insulin-Treated Type 2 Diabetes (INTERFAST-2)—A Randomized Controlled Trial. Diabetes Care 2022, 46, 463–468. [Google Scholar] [CrossRef]
- Streja, D.A.; Marliss, E.B.; Steiner, G. The Effects of Prolonged Fasting on Plasma Triglyceride Kinetics in Man. Metabolism 1977, 26, 505–516. [Google Scholar] [CrossRef]
- Pinto, A.M.; Bordoli, C.; Buckner, L.P.; Kim, C.; Kaplan, P.C.; del Arenal, I.M.; Jeffcock, E.J.; Hall, W.L. Intermittent Energy Restriction Is Comparable to Continuous Energy Restriction for Cardiometabolic Health in Adults with Central Obesity: A Randomized Controlled Trial; the Met-IER Study. Clin. Nutr. 2020, 39, 1753–1763. [Google Scholar] [CrossRef]
- Fontana, L. Interventions to Promote Cardiometabolic Health and Slow Cardiovascular Ageing. Nat. Rev. Cardiol. 2018, 15, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Mouridsen, M.R.; Bendsen, N.T.; Astrup, A.; Haugaard, S.B.; Binici, Z.; Sajadieh, A. Modest Weight Loss in Moderately Overweight Postmenopausal Women Improves Heart Rate Variability. Eur. J. Prev. Cardiol. 2013, 20, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Harriss, D.J.; MacSween, A.; Atkinson, G. Ethical Standards in Sport and Exercise Science Research: 2020 Update. Int. J. Sports Med. 2019, 40, 813–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. Available online: https://Randomizer.Org/about/ (accessed on 27 September 2021).
- Eckstein, M.L.; Zimmermann, P.; Erlmann, M.P.; Wachsmuth, N.B.; Haupt, S.; Zimmer, R.T.; Schierbauer, J.; Herz, D.; Aberer, F.; Sourij, H.; et al. Glucose and Fructose Supplementation and Their Acute Effects on Electrocardiographic Time Intervals during Anaerobic Cycling Exercise in Healthy Individuals: A Secondary Outcome Analysis of a Double-Blind Randomized Crossover-Controlled Trial. Nutrients 2022, 14, 3257. [Google Scholar] [CrossRef] [PubMed]
- Stys, A.; Stys, T. Current Clinical Applications of Heart Rate Variability. Clin. Cardiol. 1998, 21, 719–724. [Google Scholar] [CrossRef]
- Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Reginato, E.; Azzolina, D.; Folino, F.; Valentini, R.; Bendinelli, C.; Gafare, C.E.; Cainelli, E.; Vedovelli, L.; Iliceto, S.; Gregori, D.; et al. Dietary and Lifestyle Patterns Are Associated with Heart Rate Variability. J. Clin. Med. 2020, 9, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perpiñan, G.; Severeyn, E.; Wong, S.; Altuve, M. Cardiac Autonomic Modulation in Response to a Glucose Stimulus. Med. Biol. Eng. Comput. 2019, 57, 667–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutfi, M.F.; Elhakeem, R.F. Effect of Fasting Blood Glucose Level on Heart Rate Variability of Healthy Young Adults. PLoS ONE 2016, 11, e0159820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebermeister, H.; Bleckmann, A. Cardiac Effects of a Reducing Diet. Versicherungsmedizin 1991, 43, 71–75. [Google Scholar]
- Widerlöv, E.; Jostell, K.-G.; Claesson, L.; Odlind, B.; Keisu, M.; Freyschuss, U. Influence of Food Intake on Electrocardiograms of Healthy Male Volunteers. Eur. J. Clin. Pharmacol. 1999, 55, 619–624. [Google Scholar] [CrossRef]
- van der Stuijt, W.; Gal, P.; Kemme, M.J.B.; Burggraaf, J. Effect of Short-Term Fasting on Electrocardiographic Parameters. Ann. Noninvasive Electrocardiol. 2019, 24, e12643. [Google Scholar] [CrossRef] [Green Version]
- Taubel, J.; Wong, A.H.; Naseem, A.; Ferber, G.; Camm, A.J. Shortening of the QT Interval After Food Can Be Used to Demonstrate Assay Sensitivity in Thorough QT Studies. J. Clin. Pharmacol. 2012, 52, 1558–1565. [Google Scholar] [CrossRef] [Green Version]
- Hobikoğlu, G.F.; Urumdaş, M.; Özkurt, Y.; Zehir, R.; Güner, A. Effect of Hunger Strike on Electrocardiographic Parameters. Turk Kardiyol Dern Ars 2019, 47, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Theorell, T.; Kjellberg, J.; Palmblad, J. Electrocardiographic Changes during Total Energy Deprivation (Fasting). Acta Med. Scand 1978, 203, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.; DeMeersman, R.; Gallagher, D.; Pietrobelli, A.; Zion, A.S.; Daly, D.; Heymsfield, S.B. QTc Interval (Cardiac Repolarization): Lengthening after Meals. Obes. Res 1997, 5, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Hnatkova, K.; Kowalski, D.; Keirns, J.J.; van Gelderen, E.M.; Malik, M. QTc Changes after Meal Intake: Sex Differences and Correlates. J. Electrocardiol. 2014, 47, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, B.C.; Bose, M. Very Low Calorie Diets and Pre-Fasting Prolonged QT Interval. A Hidden Potential Danger. West. Indian Med. J. 1992, 41, 169–171. [Google Scholar] [PubMed]
- Petrov, D.B. QT Interval Lengthening after Fasting Complicated by a Sudden Attack of Torsades de Pointes. Tex. Heart Inst. J. 2003, 30, 86–87. [Google Scholar] [PubMed]
- Kahraman, S.; Dogan, A. Ventricular Arrhythmia Linked to Long Intermittent Fasting. J. Electrocardiol. 2020, 58, 125–127. [Google Scholar] [CrossRef]

| Parameter | Overall (n = 27) | 16/8 TRF (n = 11) | 20/4 TRF (n = 8) | ADF (n = 8) | p-Value |
|---|---|---|---|---|---|
| Age (y) | 26.3 ± 3.8 | 26.3 ± 4.1 | 25.8 ± 2.4 | 25.3 ± 2.1 | 0.8386 |
| Body mass (kg) | 77.8 ± 13.9 | 80.3 ± 18.3 | 74.4 ± 12.7 | 78.1 ± 9.1 | 0.7615 |
| Height (cm) | 176.9 ± 9.2 | 177.8 ± 11.1 | 176.6 ± 7.0 | 176.4 ± 6.4 | 0.9386 |
| BMI (kg/m2) | 24.7 ± 3.4 | 25.2 ± 4.4 | 23.8 ± 2.8 | 25.0 ± 2.3 | 0.7388 |
| Parameter | Cohort | Visit 1 | Visit 2 | Visit 3 | F-Statistics | p-Value |
|---|---|---|---|---|---|---|
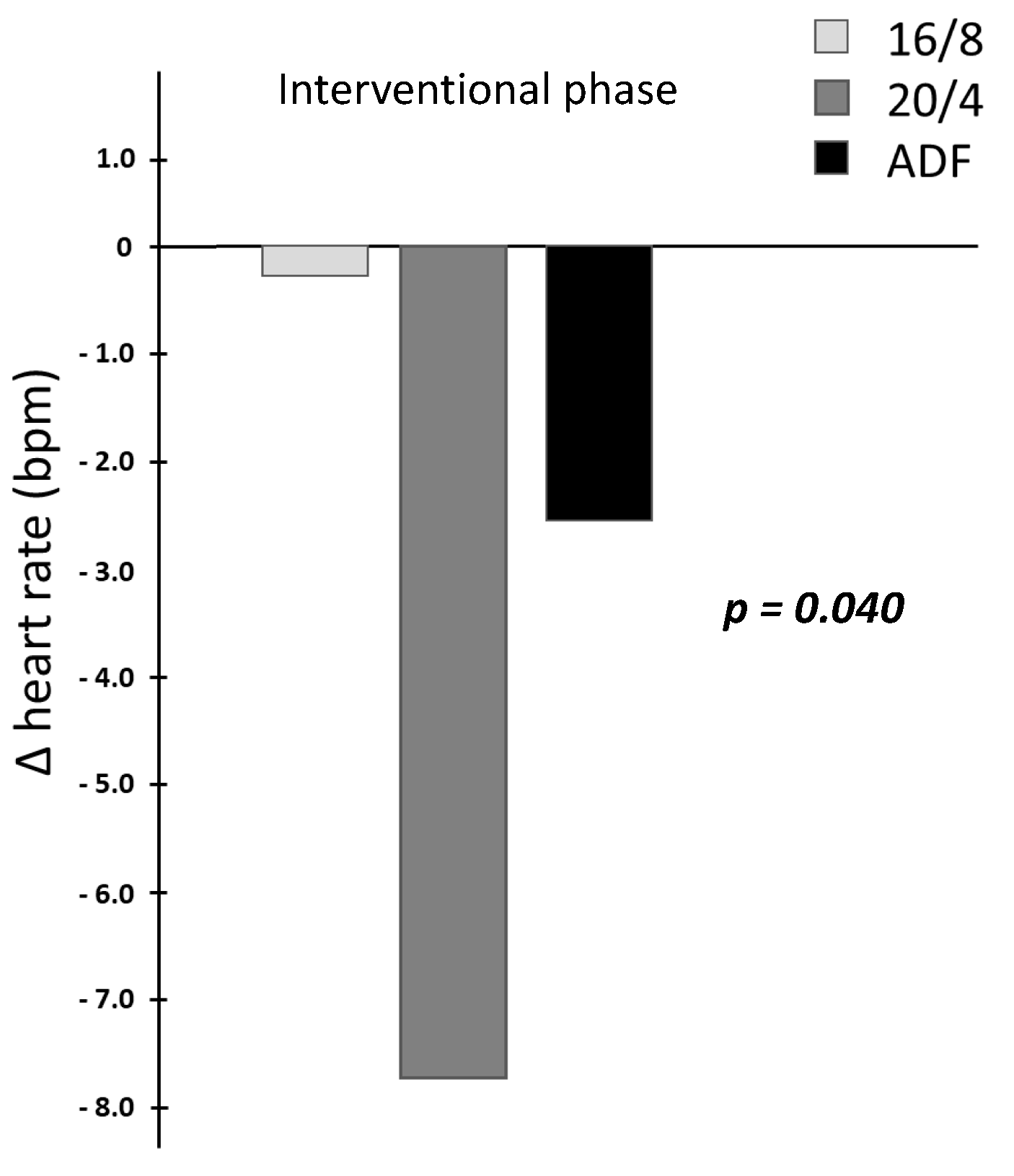
| HR (bpm) | 16/8 | 60.2 ± 8.5 | 56.1 ± 7.7 | 55.8 ± 7.1 | time: F(1,24) = 0.66 | 0.426 |
| 20/4 | 58.4 ± 4.3 | 58.9 ± 9.2 | 50.7 ± 7.5 | group: F(2,24) = 0.60 | 0.556 | |
| ADF | 66.4 ± 12.0 | 63.5 ± 12.5 | 60.8 ± 10.2 | time × group: F(2,24) = 3.68 | 0.040 * | |
| PQ interval (ms) | 16/8 | 194.8 ± 36.6 | 198.0 ± 44.4 | 190.5 ± 39.7 | time: F(1,24) = 3.30 | 0.082 |
| 20/4 | 176.1 ± 27.0 | 178.6 ± 25.5 | 172.8 ±22.1 | group: F(2,24) = 0.34 | 0.719 | |
| ADF | 176.6 ± 29.2 | 177.6 ± 26.9 | 178.4 ± 42.0 | time × group: F(2,24) = 0.84 | 0.444 | |
| QRS interval (ms) | 16/8 | 96.9 ± 9.2 | 100.9 ± 12.1 | 100.9 ± 12.3 | time: F(1,24) = 0.383 | 0.542 |
| 20/4 | 91.5 ± 6.9 | 91.3 ±6.3 | 92.2 ±6.4 | group: F(2,24) = 1.00 | 0.383 | |
| ADF | 91.0 ±4.3 | 87.9 ±5.6 | 90.3 ± 4.1 | time × group: F(2,24) = 3.97 | 0.032 * | |
| QTc interval (ms) | 16/8 | 376.8 ± 15.7 | 373.6 ± 25.8 | 373.3 ± 25.4 | time: F(1,24) = 0.05 | 0.821 |
| 20/4 | 389.3 ± 20.9 | 388.1 ± 28.0 | 384.1 ± 28.0 | group: F(2,24) = 0.26 | 0.772 | |
| ADF | 390.7 ± 14.2 | 389.9 ± 12.8 | 391.2 ± 16.0 | time × group: F(2,24) = 0.27 | 0.766 |
| Parameter | Cohort | Visit 1 | Visit 2 | Visit 3 | F-Statistics | p-Value |
|---|---|---|---|---|---|---|
| SDNN (ms) | 16/8 | 99.2 ± 61.1 | 71.9 ± 27.9 | 71.3 ± 32.5 | time: F(1,24) = 5.27 | 0.031 * |
| 20/4 | 73.6 ± 38.5 | 60.3 ± 29.3 | 74.9 ± 38.1 | group: F(2,24) = 1.03 | 0.371 | |
| ADF | 82.8 ± 35.7 | 72.9 ± 44.2 | 82.9 ± 45.4 | time × group: F(2,24) = 0.05 | 0.953 | |
| RMSSD (ms) | 16/8 | 84.0 ± 52.8 | 75.3 ± 35.4 | 74.9 ± 24.9 | time: F(1,24) = 0.44 | 0.513 |
| 20/4 | 72.7 ± 38.8 | 72.8 ± 48.1 | 78.9 ±41.0 | group: F(2,24) = 0.51 | 0.607 | |
| ADF | 77.8 ± 44.3 | 78.8 ±53.8 | 88.4 ±45.4 | time × group: F(2,24) = 0.01 | 0.995 | |
| Ln(LF/HF) (-) | 16/8 | 0.41 ± 1.58 | 0.10 ± 1.20 | −0.17 ± 1.51 | time: F(1,24) = 3.02 | 0.095 |
| 20/4 | 0.22 ± 0.82 | −0.30 ± 0.81 | −0.15 ±0.79 | group: F(2,24) = 0.14 | 0.875 | |
| ADF | 0.73 ± 1.41 | −0.04 ± 0.87 | 0.09 ± 1.50 | time × group: F(2,24) = 0.74 | 0.490 |
| Parameter | Cohort | Visit 1 | Visit 2 | Visit 3 | F-Statistics | p-Value |
|---|---|---|---|---|---|---|
| ΔHR (bpm) | 16/8 | 16.4 ± 12.0 | 16.4 ± 6.0 | 15.8 ± 6.7 | time: F(1,24) = 0.34 | 0.567 |
| 20/4 | 16.5 ± 4.8 | 14.8 ± 9.2 | 20.1 ± 8.6 | group: F(2,24) = 1.77 | 0.193 | |
| ADF | 11.8 ± 5.7 | 15.7 ± 8.5 | 17.5 ± 3.8 | time × group: F(2,24) = 1.19 | 0.321 | |
| ΔPQ interval (ms) | 16/8 | −7.0 ± 14.8 | −13.4 ± 23.5 | −8.6 ± 19.2 | time: F(1,24) = 1.27 | 0.272 |
| 20/4 | −6.5 ± 12.6 | −8.4 ± 6.9 | −5.2 ± 11.6 | group: F(2,24) = 0.12 | 0.885 | |
| ADF | −9.9 ± 7.5 | −8.8 ± 10.2 | −10.9 ± 15.6 | time × group: F(2,24) = 1.20 | 0.320 | |
| ΔQRS interval (ms) | 16/8 | 1.1 ± 10.8 | −2.4 ± 2.2 | −2.9 ± 1.8 | time: F(1,24) = 0.04 | 0.838 |
| 20/4 | 3.0 ± 9.7 | −0.1 ± 3.5 | −1.0 ± 6.1 | group: F(2,24) = 0.20 | 0.819 | |
| ADF | −3.7 ± 0.8 | −2.9 ± 1.2 | −5.5 ± 4.6 | time × group: F(2,24) = 0.73 | 0.494 | |
| ΔQTc interval (ms) | 16/8 | 0.1 ± 9.0 | 7.4 ± 8.3 | 9.1 ± 7.0 | time: F(1,24) = 0.62 | 0.440 |
| 20/4 | 2.4 ± 7.8 | 3.3 ± 11.7 | 14.9 ± 14.7 | group: F(2,24) = 0.35 | 0.706 | |
| ADF | −10.6 ± 13.5 | −0.5 ± 13.7 | −5.9 ± 29.9 | time × group: F(2,24) = 2.85 | 0.077 |
| Parameter | Cohort | Visit 1 | Visit 2 | Visit 3 | F-Statistics | p-Value |
|---|---|---|---|---|---|---|
| ΔSDNN (ms) | 16/8 | −0.5 ± 44.7 | 36.4 ± 59.1 | 31.7 ± 48.5 | time: F(1,24) = 3.78 | 0.064 |
| 20/4 | 0.4 ± 45.8 | 34.3 ± 41.9 | 39.6 ± 59.7 | group: F(2,24) = 0.24 | 0.788 | |
| ADF | −6.2 ± 35.4 | 16.8 ± 49.9 | 12.0 ± 48.9 | time × group: F(2,24) = 0.08 | 0.925 | |
| ΔRMSSD (ms) | 16/8 | −32.4 ± 38.3 | −29.4 ± 38.6 | −27.4 ± 27.0 | time: F(1,24) = 0.55 | 0.464 |
| 20/4 | −42.0 ± 32.6 | −33.3 ± 38.3 | −39.4 ± 34.6 | group: F(2,24) = 0.68 | 0.515 | |
| ADF | −34.7 ± 35.9 | −38.1 ± 48.1 | −50.7 ± 41.3 | time × group: F(2,24) = 0.14 | 0.873 | |
| ΔLn(LF/HF) (-) | 16/8 | 0.91 ± 1.21 | 1.68 ± 1.06 | 1.94 ± 1.25 | time: F(1,24) = 2.77 | 0.109 |
| 20/4 | 1.40 ± 1.20 | 1.99 ± 1.30 | 2.09 ± 1.00 | group: F(2,24) = 0.78 | 0.468 | |
| ADF | 0.63 ± 0.81 | 1.83 ± 1.00 | 2.06 ± 1.03 | time × group: F(2,24) = 0.15 | 0.866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, P.; Herz, D.; Karl, S.; Weiß, J.W.; Lackner, H.K.; Erlmann, M.P.; Sourij, H.; Schierbauer, J.; Haupt, S.; Aberer, F.; et al. Effects of Different Fasting Interventions on Cardiac Autonomic Modulation in Healthy Individuals: A Secondary Outcome Analysis of the EDIF Trial. Biology 2023, 12, 372. https://doi.org/10.3390/biology12030372
Zimmermann P, Herz D, Karl S, Weiß JW, Lackner HK, Erlmann MP, Sourij H, Schierbauer J, Haupt S, Aberer F, et al. Effects of Different Fasting Interventions on Cardiac Autonomic Modulation in Healthy Individuals: A Secondary Outcome Analysis of the EDIF Trial. Biology. 2023; 12(3):372. https://doi.org/10.3390/biology12030372
Chicago/Turabian StyleZimmermann, Paul, Daniel Herz, Sebastian Karl, Johannes W. Weiß, Helmut K. Lackner, Maximilian P. Erlmann, Harald Sourij, Janis Schierbauer, Sandra Haupt, Felix Aberer, and et al. 2023. "Effects of Different Fasting Interventions on Cardiac Autonomic Modulation in Healthy Individuals: A Secondary Outcome Analysis of the EDIF Trial" Biology 12, no. 3: 372. https://doi.org/10.3390/biology12030372
APA StyleZimmermann, P., Herz, D., Karl, S., Weiß, J. W., Lackner, H. K., Erlmann, M. P., Sourij, H., Schierbauer, J., Haupt, S., Aberer, F., Wachsmuth, N. B., & Moser, O. (2023). Effects of Different Fasting Interventions on Cardiac Autonomic Modulation in Healthy Individuals: A Secondary Outcome Analysis of the EDIF Trial. Biology, 12(3), 372. https://doi.org/10.3390/biology12030372







