CiXTH29 and CiLEA4 Role in Water Stress Tolerance in Cichorium intybus Varieties
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Seed Germination and Seedling Drought Treatment
2.2. RNA Extraction, Cloning and Sequence Analysis
2.3. RT-qPCR Analysis for CiXTH29 and CiLEA4 Gene Expression
2.4. Leaf Sections and Xyloglucan Immunolocalization
2.5. Statistical Analysis
3. Results
3.1. Seed Germination and Chicory Seedling Growth under Drought Stress
3.2. Phenotypic Analysis of Chicory Plants under 10 Days of Dehydration
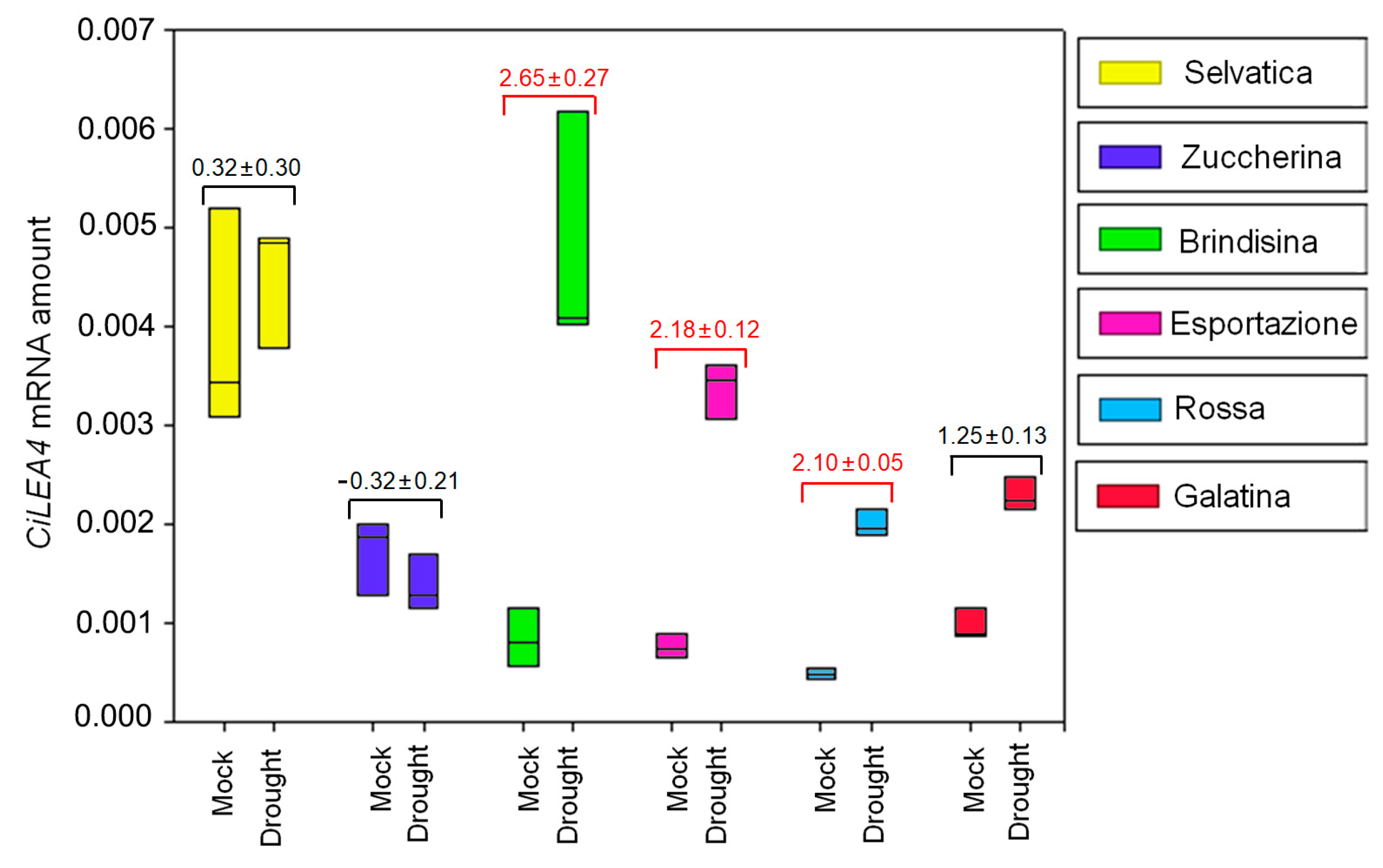
3.3. CiXTH29 and CiLEA4 Gene Expression in Chicory Varieties
3.4. Xyloglucan Quantification through Antibody Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Zlatev, Z.; Cebola Lidon, F. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. 2012, 24, 57–72. [Google Scholar] [CrossRef]
- Verslues, P.E.; Bailey-Serres, J.; Brodersen, C.; Buckley, T.N.; Conti, L.; Christmann, A.; Dinneny, J.R.; Grill, E.; Hayes, S.; Heckman, R.W.; et al. Burning questions for a warming and changing world: 15 unknowns in plant abiotic stress. Plant Cell 2023, 35, 67–108. [Google Scholar] [CrossRef] [PubMed]
- Billah, M.; Aktar, S.; Brestic, M.; Zivcak, M.; Khaldun, A.B.M.; Uddin, M.S.; Bagum, S.A.; Yang, X.; Skalicky, M.; Mehari, T.G.; et al. Progressive genomic approaches to explore drought- and salt-induced oxidative stress responses in plants under changing climate. Plants 2021, 10, 1910. [Google Scholar] [CrossRef]
- Waititu, J.K.; Zhang, X.; Chen, T.; Zhang, C.; Zhao, Y.; Wang, H. Transcriptome analysis of tolerant and susceptible maize genotypes reveals novel insights about the molecular mechanisms underlying drought responses in leaves. Int. J. Mol. Sci. 2021, 22, 6980. [Google Scholar] [CrossRef]
- Sheoran, S.; Kaur, Y.; Kumar, S.; Shukla, S.; Rakshit, S.; Kumar, R. Recent advances for drought stress tolerance in maize (Zea mays L.): Present status and future prospects. Front. Plant Sci. 2022, 13, 872566. [Google Scholar] [CrossRef]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef]
- Rui, Y.; Dinneny, J.R. A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020, 225, 1428–1439. [Google Scholar] [CrossRef]
- Baez, L.A.; Tichá, T.; Hamann, T. Cell wall integrity regulation across plant species. Plant Mol. Biol. 2022, 109, 483–504. [Google Scholar] [CrossRef]
- Wolf, S. Plant cell wall signalling and receptor-like kinases. Biochem. J. 2017, 15, 471–492. [Google Scholar] [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.-K.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Patakas, A.; Kofidis, G.; Bosabalidis, A.; Nastou, A. Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Sci. Hortic. 2002, 95, 39–50. [Google Scholar] [CrossRef]
- Saito, T.; Terashima, I. Reversible decreases in the bulk elastic modulus of mature leaves of deciduous Quercus species subjected to two drought treatments. Plant Cell Environ. 2004, 27, 863–875. [Google Scholar] [CrossRef]
- De Diego, N.; Sampedro, M.C.; Barrio, R.J.; Saiz-Fernández, I.; Moncaleán, P.; Lacuesta, M. Solute accumulation and elastic modulus changes in six radiata pine breeds exposed to drought. Tree Physiol. 2013, 33, 69–80. [Google Scholar] [CrossRef]
- Martínez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Sweet, W.J.; Morrison, J.C.; Labavitch, J.M.; Matthews, M.A. Altered synthesis and composition of cell wall of grape (Vitis vinifera L.) leaves during expansion and growth-inhibiting water deficits. Plant Cell Physiol. 1990, 31, 407–414. [Google Scholar] [CrossRef]
- Muñoz, F.J.; Dopico, B.; Labrador, E. Effect of osmotic stress on the growth of epicotyls of Cicer arietinum in relation to changes in cell wall composition. Physiol. Plant. 1993, 87, 552–560. [Google Scholar] [CrossRef]
- Piro, G.; Leucci, M.R.; Waldron, K.; Dalessandro, G. Exposure to water stress causes changes in the biosynthesis of cell wall polysaccharides in roots of wheat cultivars varying in drought tolerance. Plant Sci. 2003, 165, 559–569. [Google Scholar] [CrossRef]
- Leucci, M.R.; Lenucci, M.S.; Piro, G.; Dalessandro, G. Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. J. Plant Physiol. 2008, 165, 1168–1180. [Google Scholar] [CrossRef]
- Hori, C.; Yu, X.; Mortimer, J.C.; Sano, R.; Matsumoto, T.; Kikuchi, J.; Demura, T.; Ohtani, M. Impact of abiotic stress on the regulation of cell wall biosynthesis in Populus trichocarpa. Plant Biotechnol. 2020, 37, 273–283. [Google Scholar] [CrossRef]
- Bray, E.A. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yokoyama, R. Reconsidering the function of the xyloglucan endotransglucosylase/hydrolase family. J. Plant Res. 2022, 135, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Park, Y.B.; Caporini, M.A.; Rosay, M.; Zhong, L.; Cosgrove, D.J.; Hong, M. Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc. Natl. Acad. Sci. USA 2013, 110, 16444–16449. [Google Scholar] [CrossRef]
- Iurlaro, A.; De Caroli, M.; Sabella, E.; De Pascali, M.; Rampino, P.; De Bellis, L.; Perrotta, C.; Dalessandro, G.; Piro, G.; Fry, S.C.; et al. Drought and heat differentially affect XTH expression and XET activity and action in 3-day-old seedlings of durum wheat cultivars with different stress susceptibility. Front. Plant Sci. 2016, 7, 1686. [Google Scholar] [CrossRef]
- Cho, S.K.; Kim, J.E.; Park, J.; Eom, T.J.; Kim, W.T. Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef]
- Choi, J.Y.; Seo, Y.S.; Kim, S.J.; Kim, W.T.; Shin, J.S. Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/hydrolase, enhanced tolerance to salt and drought stresses without phenotypic defects in tomato plants (Solanum lycopersicum cv. Dotaerang). Plant Cell Rep. 2011, 30, 867–877. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.-Z.; Fu, J.-Y.; Du, Y.-Y.; Qu, J.; Song, Y.; Wang, P.-W. The GmXTH1 gene improves drought stress resistance of soybean seedlings. Mol. Breed. 2022, 42, 3. [Google Scholar] [CrossRef]
- De Caroli, M.; Manno, E.; Piro, G.; Lenucci, M.S. Ride to cell wall: Arabidopsis XTH11, XTH29 and XTH33 exhibit different secretion pathways and responses to heat and drought stress. Plant J. 2021, 107, 448–466. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Battaglia, M.; Covarrubias, A.A. Late Embryogenesis Abundant (LEA) proteins in legumes. Front. Plant Sci. 2013, 25, 190. [Google Scholar] [CrossRef]
- Rorat, T. Plant dehydrins-tissue location, structure and function. Cell. Mol. Biol. Lett. 2006, 11, 536–556. [Google Scholar] [CrossRef]
- Smith, M.A.; Graether, S.P. The disordered dehydrin and its role in plant protection: A biochemical perspective. Biomolecules 2022, 12, 294. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Olvera-carrillo, Y.; Campos, F.; Reyes, L.; Garciarrubio, A.; Covarrubias, A.A. Functional analysis of the group 4 late embryogenesis abundant proteins reveals their relevance in the adaptive response during water deficit in Arabidopsis. Plant Physiol. 2010, 154, 373–390. [Google Scholar] [CrossRef]
- Zamora-Briseño, J.A.; de Jiménez, E.S. A LEA 4 protein up-regulated by ABA is involved in drought response in maize roots. Mol. Biol. Rep. 2016, 43, 221–228. [Google Scholar] [CrossRef]
- Mosaddegh, M.; Naghibi, F.; Moazzeni, H.; Pirani, A.; Esmaeili, S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012, 141, 80–95. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J. Perspectives and utilization technologies of chicory (Cichorium intybus L.): A review. Afr. J. Biotech. 2011, 10, 1966–1977. [Google Scholar] [CrossRef]
- Timmermans, J.W.; Slaghek, T.M.; Iizuka, M.; Van den Ende, W.; De Roover, J.; van Laere, A. Isolation and structural analysis of new fructans produced by chicory. J. Carbohydr. Chem. 2001, 20, 375–395. [Google Scholar] [CrossRef]
- Innocenti, M.; Gallori, S.; Giaccherini, C.; Ieri, F.; Vincieri, F.F.; Mulinacci, N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium intybus L. J. Agric. Food Chem. 2005, 53, 6497–6502. [Google Scholar] [CrossRef]
- Montefusco, A.; Semitaio, G.; Marrese, P.P.; Iurlaro, A.; De Caroli, M.; Piro, G.; Dalessandro, G.; Lenucci, M.S. Antioxidants in varieties of chicory (Cichorium intybus L.) and wild poppy (Papaver rhoeas L.) of Southern Italy. J. Chem. 2015, 10, 8. [Google Scholar] [CrossRef]
- Monti, A.; Amaducci, M.T.; Pritoni, G.; Venturi, G. Growth, fructan yield, and quality of chicory (Cichorium intybus L.) as related to photosynthetic capacity, harvest time, and water regime. J. Exp. Bot. 2005, 56, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, V.; Arjmand-Ghajur, E. Exogenous plant growth regulators/plant growth promoting bacteria roles in mitigating water-deficit stress on chicory (Cichorium pumilum Jacq.) at a physiological level. Agric. Water Manag. 2021, 245, 106439. [Google Scholar] [CrossRef]
- Delfine, S.; Fratianni, A.; D’Agostino, A.; Panfili, G. Influence of drought stress on physiological responses and bioactive compounds in chicory (Cichorium intybus L.): Opportunity for a sustainable agriculture. Foods 2022, 11, 3725. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- De Paolis, A.; De Caroli, M.; Rojas, M.; Curci, L.M.; Piro, G.; Di Sansebastiano, G.P. Evaluation of Dittrichia viscosa aquaporin Nip1.1 gene as marker for arsenic-tolerant plant selection. Plants 2022, 11, 1968. [Google Scholar] [CrossRef]
- Maroufi, A.; Van Bockstaele, E.; De Loose, M. Validation of reference genes for gene expression analysis in chicory (Cichorium intybus) using quantitative real-time PCR. BMC Mol. Biol. 2010, 11, 15. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; Martins, C.d.P.S.; Gonçalves, L.P.; Costa, M.G.C. Late embryogenesis abundant (LEA) constitutes a large and diverse family of proteins involved in development and abiotic stress responses in sweet orange (Citrus sinensis L. Osb.). PLoS ONE 2015, 10, e0145785. [Google Scholar] [CrossRef]
- Chen, L.-R.; Chao, C.-H.; Chen, C.-F.; Lee, Y.-P.; Chen, Y.-L.; Shiue, Y.-L. Expression of 25 high egg production related transcripts that identified from hypothalamus and pituitary gland in red-feather Taiwan country chickens. Anim. Reprod. Sci. 2007, 100, 172–185. [Google Scholar] [CrossRef]
- De Caroli, M.; Rampino, P.; Pecatelli, G.; Girelli, C.R.; Fanizzi, F.P.; Piro, G.; Lenucci, M.S. Expression of exogenous GFP-CesA6 in tobacco enhances cell wall biosynthesis and biomass production. Biology 2022, 11, 1139. [Google Scholar] [CrossRef]
- Nishitani, K. The role of endoxyloglucan transferase in the organization of plant cell walls. Int. Rev. Cytol. 1997, 173, 157–206. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Yao, W.; Gao, Y.; Zhao, K.; Guo, Q.; Zhou, B.; Jang, T. Genome-wide identification and expression analysis of the xyloglucan endotransglucosylase/hydrolase gene family in poplar. BMC Genom. 2021, 22, 804. [Google Scholar] [CrossRef]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.-R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pedersen, H.L.; Fangel, J.U.; McCleary, B.; Ruzanski, C.; Rydahl, M.G.; Ralet, M.-C.; Farkas, V.; von Schantz, L.; Marcus, S.E.; Andersen, M.C.; et al. Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 2012, 287, 39429–39438. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Zhang, D.; Liu, H.; Guan, K. Effects of drought stress on the seed germination and early seedling growth of the endemic desert plant Eremosparton songoricum (Fabaceae). EXCLI J. 2013, 12, 89–101. [Google Scholar]
- Makbul, S.; Güler, N.S.; Durmus, N.; Güven, S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Bot. 2011, 35, 7. [Google Scholar] [CrossRef]
- Gonçalves, C.G.; da Silva, A.C., Jr.; Pereira, M.R.R.; Gasparino, E.C.; Martins, D. Morphological modifications in soybean in response to soil water management. Plant Growth Regul. 2017, 83, 105–117. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Mao, S.L.; Li, S.F.; Ni, X.L.; Li, B.; Liu, W.Z. Programmed cell death: A mechanism for the lysigenous formation of secretory cavities in leaves of Dictammnus dasycarpus. Plant Sci. 2014, 225, 147–160. [Google Scholar] [CrossRef]
- Moore, J.P.; Vicré-Gibouin, M.; Farrant, J.M.; Driouich, A. Adaptations of higher plant cell walls to water loss: Drought vs. desiccation. Physiol. Plant 2008, 134, 237–245. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, J.M.; Brumer, H. The XTH gene family: An update on enzyme structure, function, and phylogeny in xyloglucan remodeling. Plant Physiol. 2010, 153, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-J.; Seo, Y.-S. Heat Shock Proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Nishitani, K. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol. 2001, 42, 1025–1033. [Google Scholar] [CrossRef]
- Wu, D.; Liu, A.; Qu, X.; Liang, J.; Song, M. Genome-wide identification, and phylogenetic and expression profiling analyses, of XTH gene families in Brassica rapa L. and Brassica oleracea L. BMC Genom. 2020, 21, 782. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, R.; Zhou, Z. The XTH gene family in Schima superba: Genome-wide identification, expression profiles, and functional interaction network analysis. Front. Plant Sci. 2022, 13, 911761. [Google Scholar] [CrossRef]
- Xuan, Y.; Zhou, Z.S.; Li, H.B.; Yang, Z.M. Identification of a group of XTHs genes responding to heavy metal mercury, salinity and drought stresses in Medicago truncatula. Ecotoxicol. Environ. Saf. 2016, 132, 153–163. [Google Scholar] [CrossRef]
- Qiao, T.; Zhang, L.; Yu, Y.; Pang, Y.; Tang, X.; Wang, X.; Li, L.; Li, B.; Sun, Q. Identification and expression analysis of xyloglucan endotransglucosylase/hydrolase (XTH) family in grapevine (Vitis vinifera L.). PeerJ 2022, 10, e13546. [Google Scholar] [CrossRef]
- Tiika, R.J.; Wei, J.; Cui, G.; Ma, Y.; Yang, H.; Duan, H. Transcriptome-wide characterization and functional analysis of Xyloglucan endo-transglycosylase/hydrolase (XTH) gene family of Salicornia europaea L. under salinity and drought stress. BMC Plant Biol. 2021, 21, 491. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef]
- Kamarudin, Z.S.; Yusop, M.R.; Ismail, M.R.; Tengku Muda Mohamed, M.; Harun, A.R.; Yusuff, O.; Magaji, U.; Fatai, A. LEA Gene Expression Assessment in Advanced Mutant Rice Genotypes under Drought Stress. Int. J. Genom. 2019, 2019, 8406036. [Google Scholar] [CrossRef]
- Bourquin, V.; Nishikubo, N.; Abe, H.; Brumer, H.; Denman, S.; Eklund, M.; Christiernin, M.; Teeri, T.T.; Sundberg, B.; Mellerowicz, E.J. Xyloglucan endotransglycosylases have a function during the formation of secondary cell walls of vascular tissues. Plant Cell 2002, 14, 3073–3088. [Google Scholar] [CrossRef]
- Kushwah, S.; Banasiak, A.; Nishikubo, N.; Derba-Maceluch, M.; Majda, M.; Endo, S.; Kumar, V.; Gomez, L.; Gorzsas, A.; McQueen-Mason, S.; et al. Arabidopsis XTH4 and XTH9 Contribute to Wood Cell Expansion and Secondary Wall Formation. Plant Physiol. 2020, 182, 1946–1965. [Google Scholar] [CrossRef]
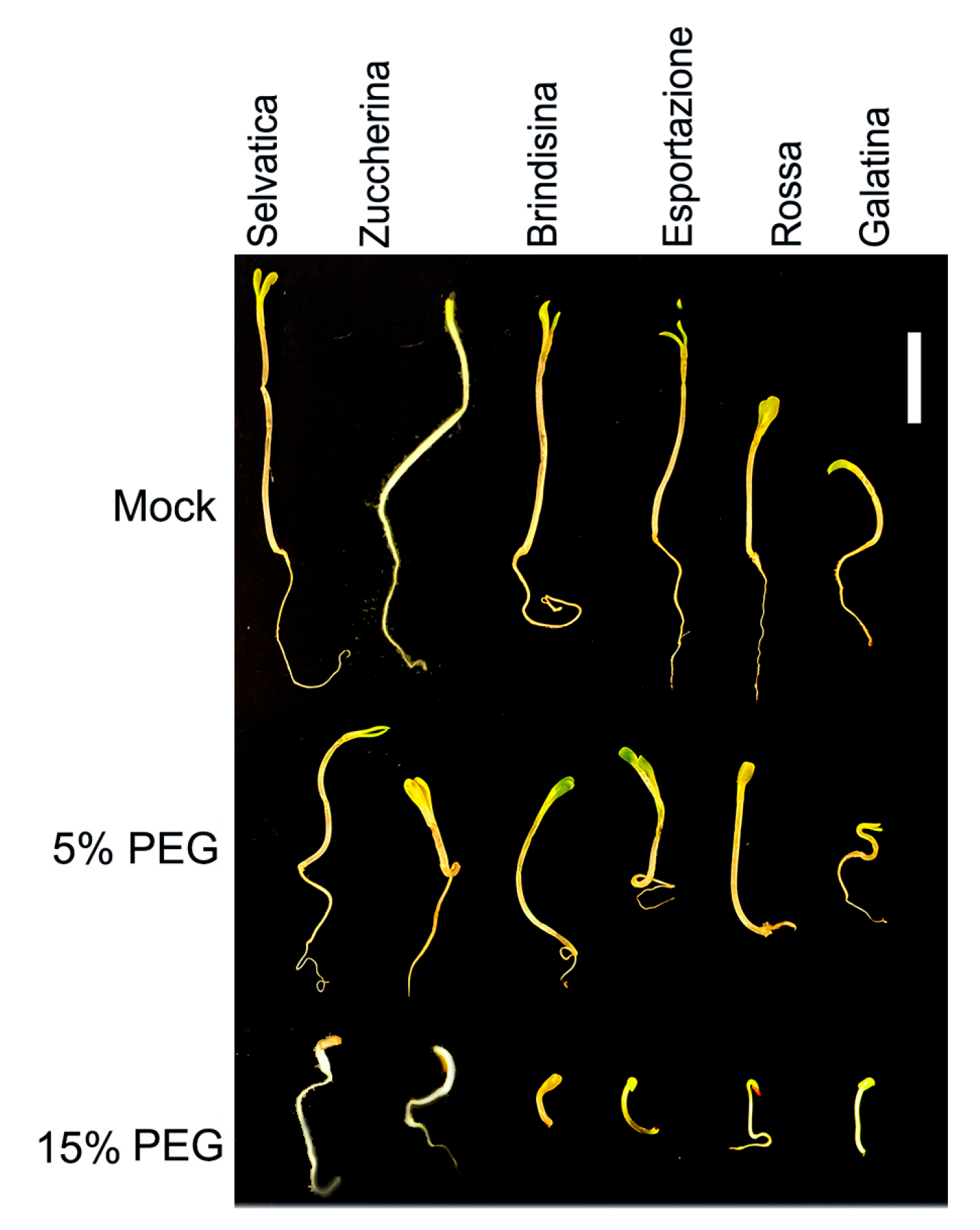

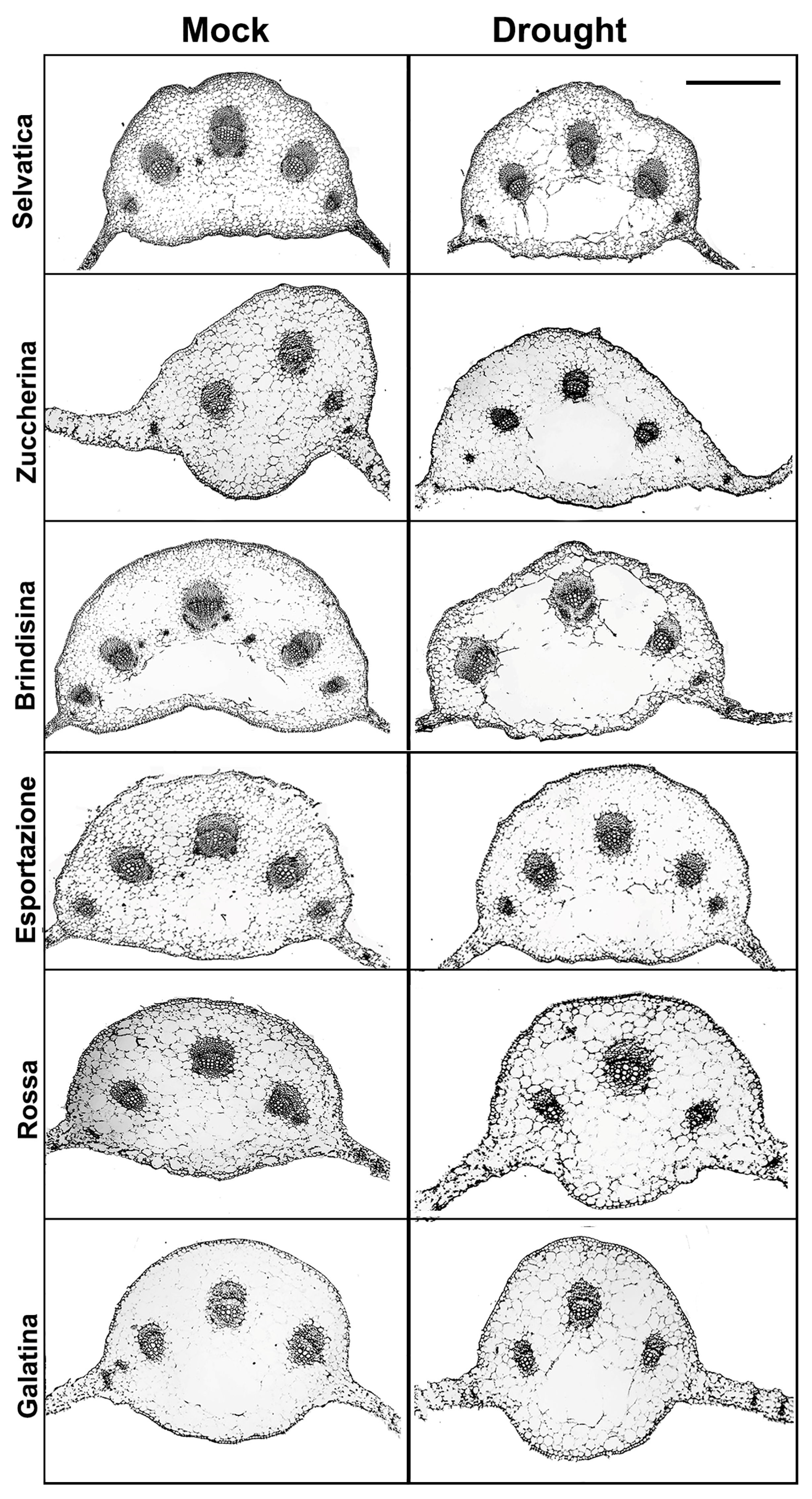

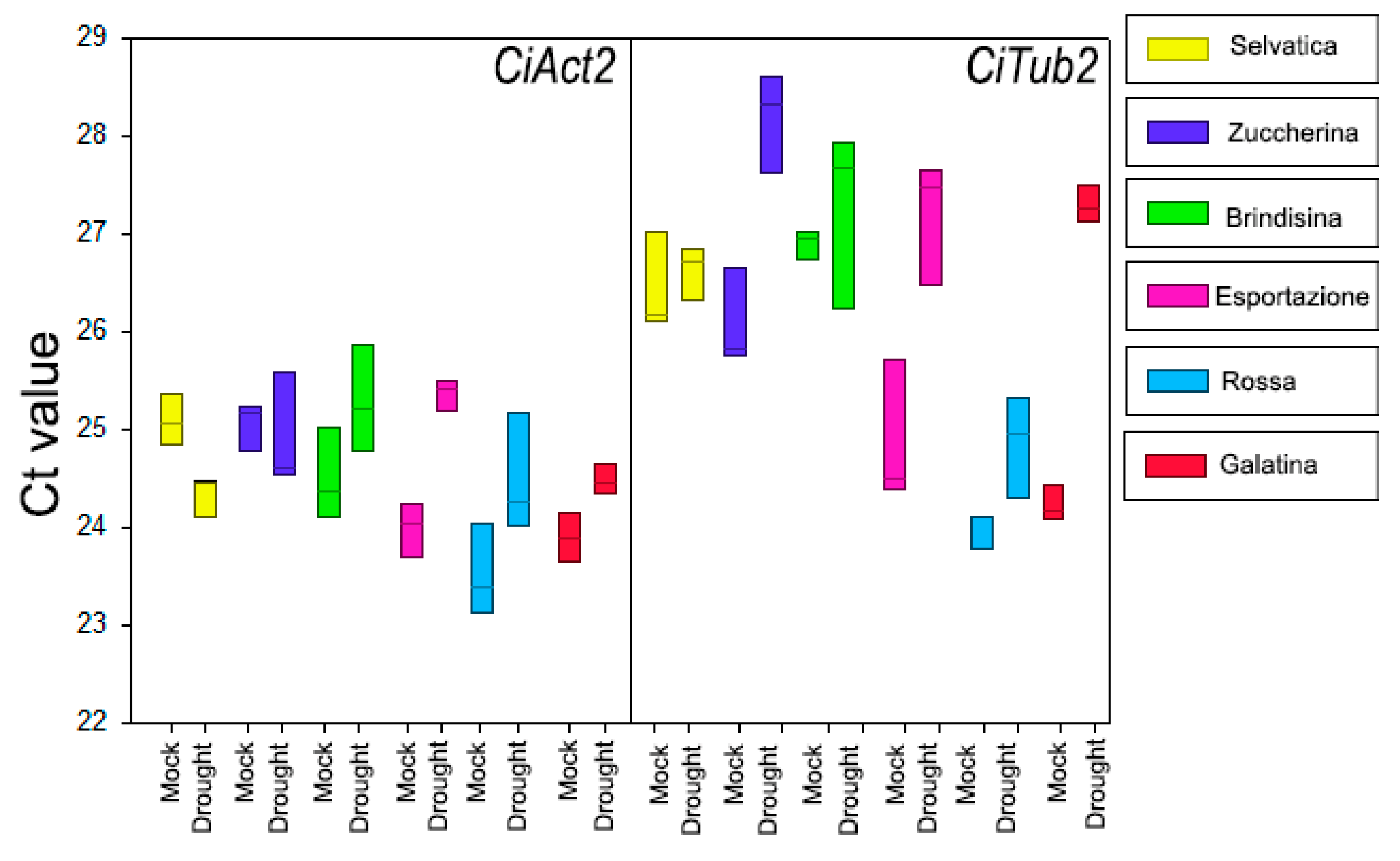
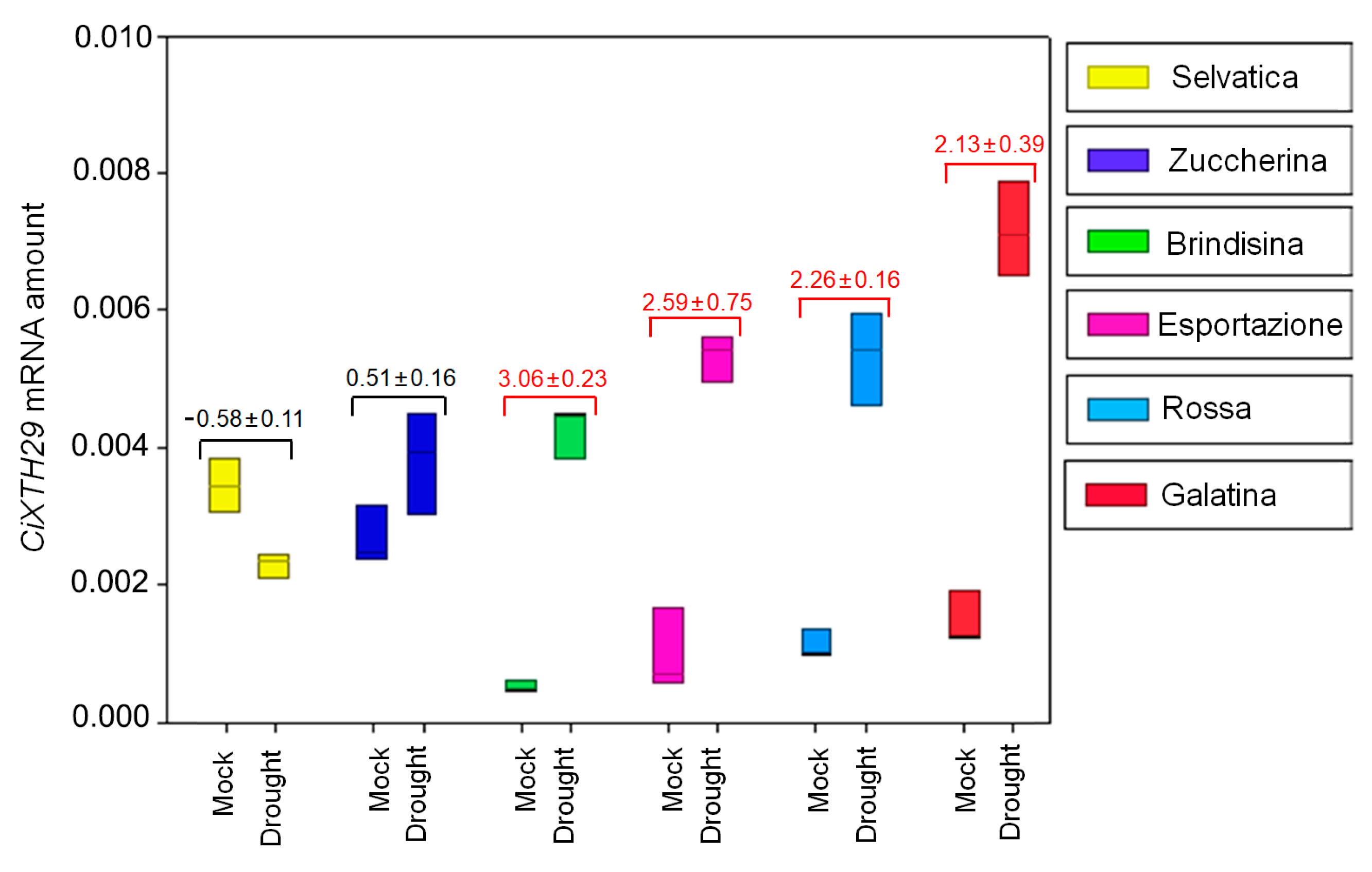

| Germination Index of Chicory Varieties (%) | ||||||
|---|---|---|---|---|---|---|
| Selvatica | Zuccherina | Brindisina | Esportazione | Rossa | Galatina | |
| Mock | 96.7 ± 2.2 | 90.0 ± 1.9 | 83.7 ± 3.3 | 80.0 ± 2.0 | 73.3 ± 2.4 | 60.1 ± 3.3 |
| 5% PEG-6000 | 96.7 ± 3.2 | 88.3 ± 2.2 | 61.7 ± 2.5 * | 67.6 ± 3.2 * | 68.1 ± 2.2 * | 56.2 ± 2.1 * |
| 15% PEG-6000 | 84.2 ± 3.2 * | 76.7 ± 3.4 * | 45.6 ± 2.4 * | 59.8 ± 2.1 * | 36.7 ± 1.7 * | 49.7 ± 2.6 * |
| Aerial Part Length (mm) | ||||||
|---|---|---|---|---|---|---|
| Selvatica | Zuccherina | Brindisina | Esportazione | Rossa | Galatina | |
| Mock | 28.95 ± 2.91 | 26.07 ± 5.32 | 20.59 ± 2.39 | 19.73 ± 1.74 | 16.80 ± 2.45 | 10.67 ± 1.63 |
| 5% PEG-6000 | 15.68 ± 3.90 * | 11.00 ± 3.31 * | 7.75 ± 1.06 * | 7.10 ± 1.20 * | 5.85 ± 2.03 * | 5.00 ± 1.73 * |
| 15% PEG-6000 | 5.71 ± 2.73 * | 4.64 ± 1.28 * | 2.93 ± 1.33 * | 2.50 ± 1.17 * | 1.71 ± 0.49 * | 2.00 ± 0.71 * |
| Root length (mm) | ||||||
| Selvatica | Zuccherina | Brindisina | Esportazione | Rossa | Galatina | |
| Mock | 22.79 ± 2.00 | 15.27 ± 2.22 | 20.55 ± 3.45 | 18.19 ± 2.76 | 11.53 ± 2.80 | 8.33 ± 1.37 |
| 5% PEG-6000 | 14.84 ± 2.63 * | 7.94 ± 1.47 * | 10.19 ± 2.07 * | 8.06 ± 1.57 * | 4.46 ± 2.73 * | 4.54 ± 1.63 * |
| 15% PEG-6000 | 5.71 ± 2.28 * | 3.28 ± 1.14 * | 2.20 ± 0.56 * | 2.28 ± 2.13 * | 1.34 ± 0.52 * | 1.60 ± 0.55 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Caroli, M.; Rampino, P.; Curci, L.M.; Pecatelli, G.; Carrozzo, S.; Piro, G. CiXTH29 and CiLEA4 Role in Water Stress Tolerance in Cichorium intybus Varieties. Biology 2023, 12, 444. https://doi.org/10.3390/biology12030444
De Caroli M, Rampino P, Curci LM, Pecatelli G, Carrozzo S, Piro G. CiXTH29 and CiLEA4 Role in Water Stress Tolerance in Cichorium intybus Varieties. Biology. 2023; 12(3):444. https://doi.org/10.3390/biology12030444
Chicago/Turabian StyleDe Caroli, Monica, Patrizia Rampino, Lorenzo M. Curci, Gabriele Pecatelli, Sara Carrozzo, and Gabriella Piro. 2023. "CiXTH29 and CiLEA4 Role in Water Stress Tolerance in Cichorium intybus Varieties" Biology 12, no. 3: 444. https://doi.org/10.3390/biology12030444
APA StyleDe Caroli, M., Rampino, P., Curci, L. M., Pecatelli, G., Carrozzo, S., & Piro, G. (2023). CiXTH29 and CiLEA4 Role in Water Stress Tolerance in Cichorium intybus Varieties. Biology, 12(3), 444. https://doi.org/10.3390/biology12030444








