Byssus of Green-Lipped Mussel Perna viridis as a Biomonitoring Biopolymer for Zinc Pollution in Coastal Waters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
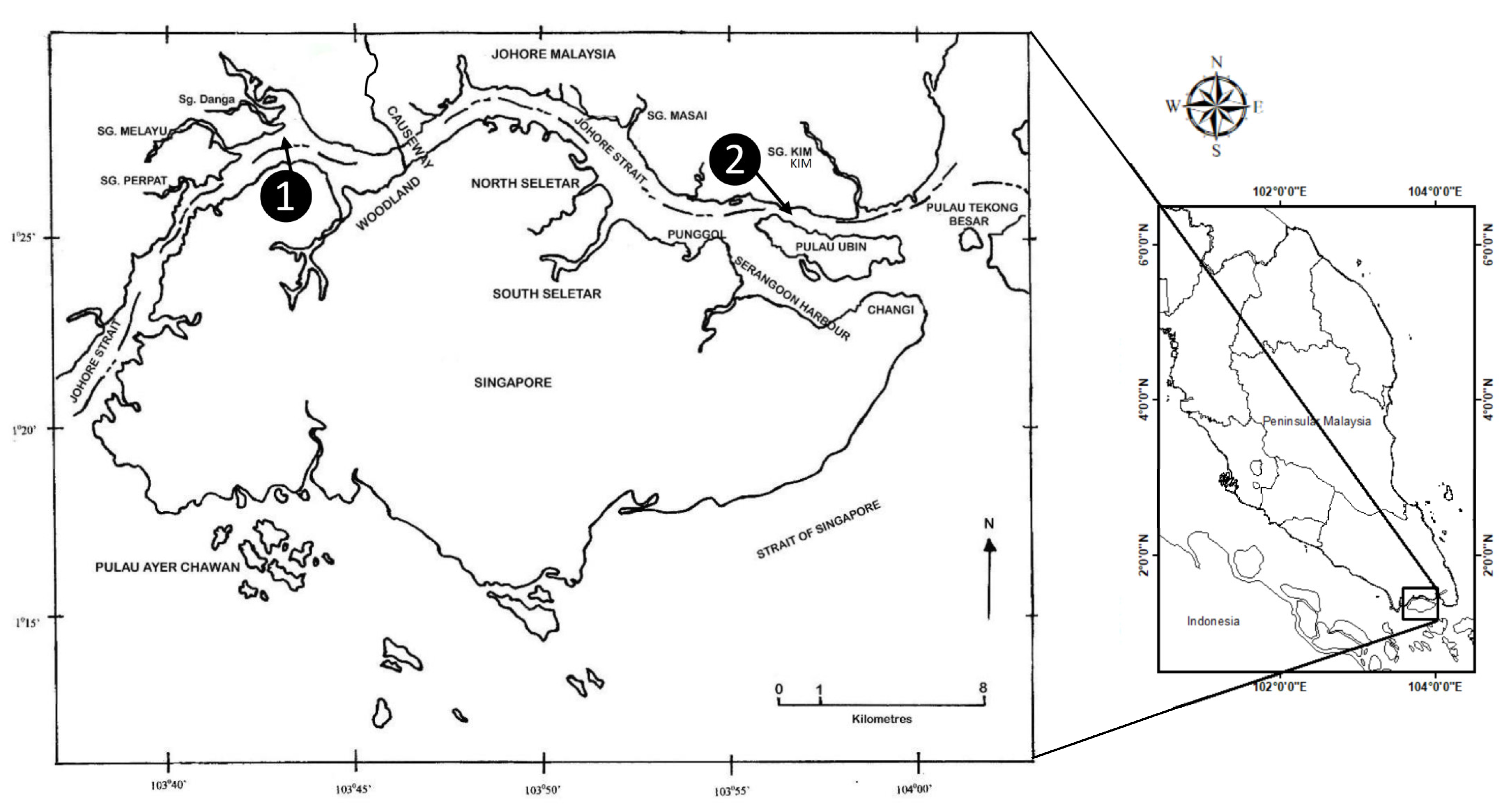
2.1. Field-Collected Samples
2.2. Experimental Field-Based Transplantation of Mussel Populations
2.3. Metal Identification
2.4. Statistical Analysis
3. Results
3.1. Mussel Transplantation in the Straits of Johore
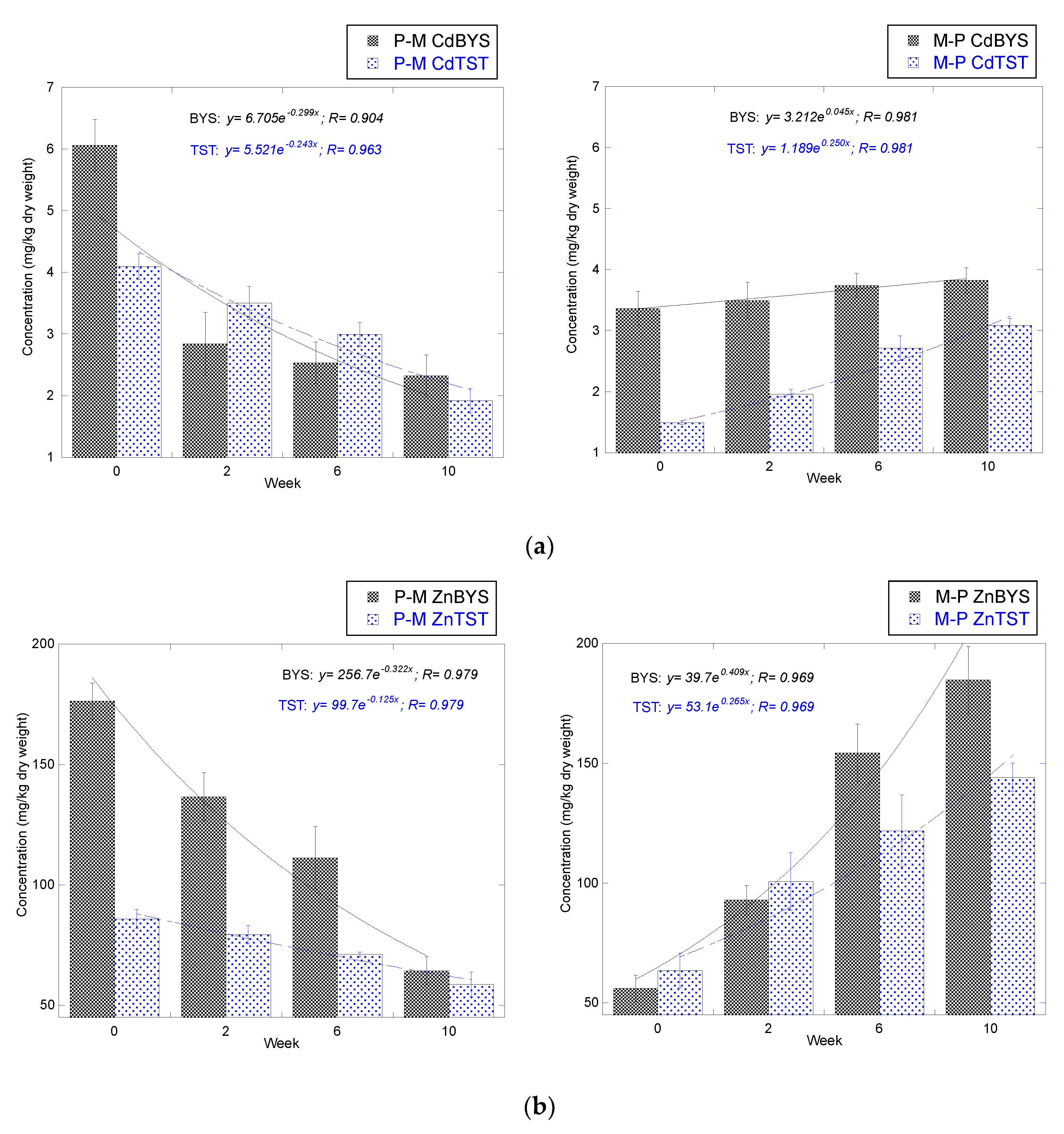
3.1.1. Cd
3.1.2. Zn
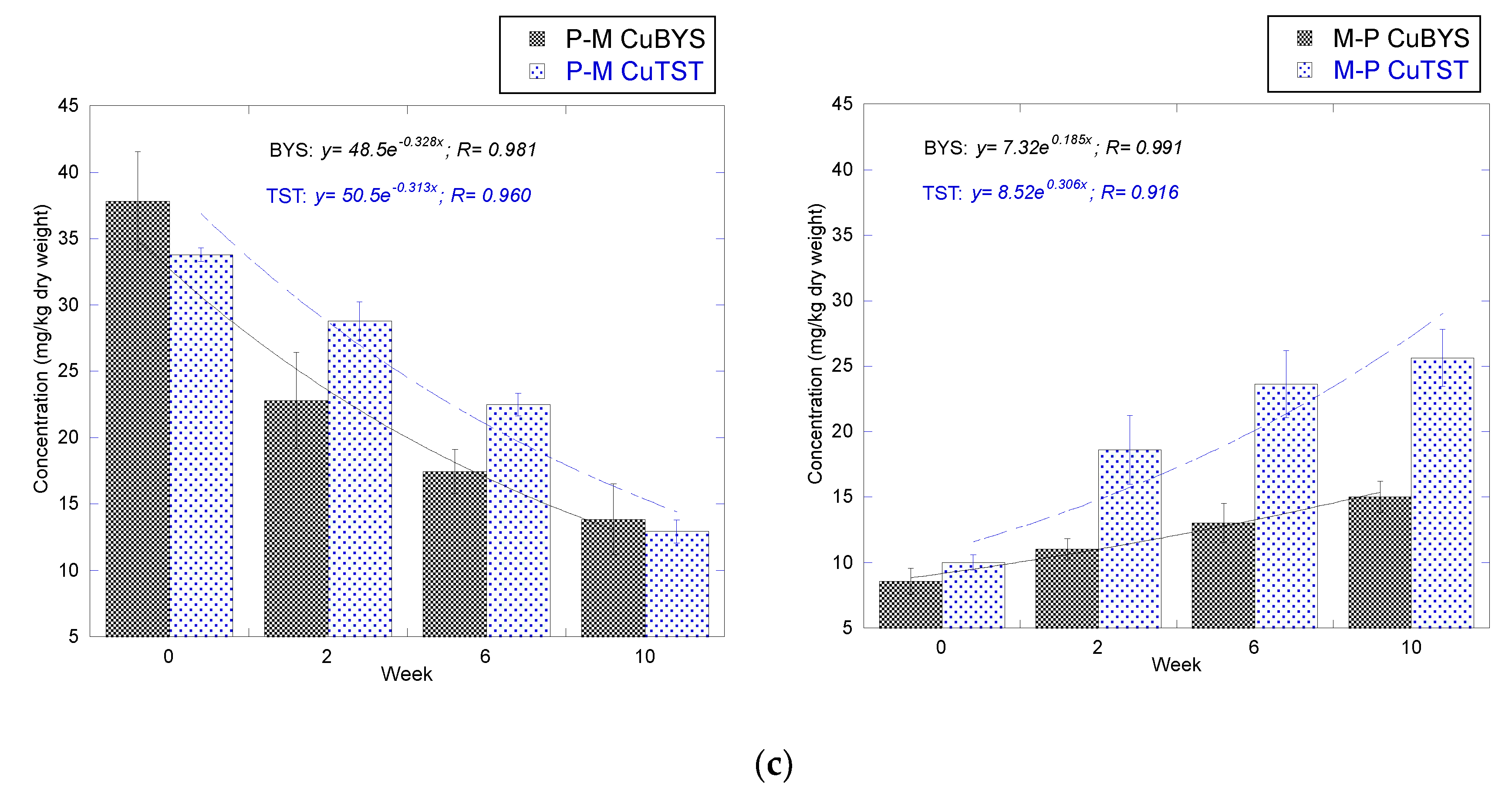
3.1.3. Cu
3.2. Ratios of Metals between Byssus and Total Soft Tissues
3.2.1. Cd
3.2.2. Zn
3.2.3. Cu
3.3. Correlation of Metals between Byssus and Habitat Sedimentary Geochemical Fractions
3.4. Multiple Linear Stepwise Regression Analytical Outputs Based on BYS and TST as Dependent Variables
4. Discussion
4.1. Higher Metal Accumulation in the Byssus Than in the Soft Tissues: A Sensitive Organ
4.2. Higher Correlation Coefficients of Metals with Environmental Sedimentary Fractions in Byssus Than Total Soft Tissues
4.3. Byssus as an Effective Biopolymer for the Monitoring of Zn Pollution
4.4. The Role of Byssal Threads in Zn, Cu, and Cd Excretion
4.5. The Prospects of Using the Byssus of Perna viridis as a Biomonitoring Biopolymer for Potentially Toxic Metals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, K.; Onitsuka, Y.; Koito, T. Mussel biology: From the byssus to ecology and physiology, including microplastic ingestion and deep-sea adaptations. Fish Sci. 2021, 87, 761–771. [Google Scholar] [CrossRef]
- Reinecke, A.; Harrington, D.M.J. The Role of Metal Ions in the Mussel Byssus. In Physiology of Molluscs, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; p. 40. ISBN 9781315207124. [Google Scholar]
- Montroni, D.; Giusti, G.; Simoni, A.; Cau, G.; Ciavatta, C.; Marzadori, C.; Falini, G. Metal ion removal using waste byssus from aquaculture. Sci. Rep. 2022, 10, 22222. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Mates, T.E.; Toprak, M.S.; Stucky, G.D.; Zok, F.W.; Waite, J.H. Metals and the integrity of a biological coating: The cuticle of mussel byssus. Langmuir 2009, 25, 3323–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coombs, T.L.; Keller, P.J. Mytilus byssal threads as an environmental marker for metals. Aquat. Toxicol. 1981, 1, 291–300. [Google Scholar] [CrossRef]
- Ikuta, K. Metal concentrations in byssuses and soft bodies of bivalves. Bull. Fac. Agric. Miyazaki Uni. 1986, 33, 255–264. [Google Scholar]
- Ikuta, K. Correlations between ratios of metal concentrations in byssuses to those in soft bodies and metal concentrations in soft bodies of bivalves. Bull. Fac. Agric. Miyazaki Uni. 1986, 33, 265–273. [Google Scholar]
- Yap, C.K.; Ismail, A.; Tan, S.G.; Rahim, I.A. Can the byssus of green-lipped mussel Perna viridis (Linnaeus) from the west coast of Peninsular Malaysia be a biomonitoring organ for Cd, Pb and Zn? Field and laboratory studies. Environ. Int. 2003, 29, 521–528. [Google Scholar] [CrossRef]
- Yap, C.K.; Tan, S.G. Iron concentrations in the byssus and soft tissues of the green-lipped mussel Perna viridis (L.): Byssus as an excretion route of Fe and Fe bioavailability in the coastal waters. Indian J. Mar. Sci. 2007, 36, 227–234. [Google Scholar]
- Yap, C.K. Byssus as a means of metal excretion route and high metal levels in fecal materials as metal retention: An experimental laboratory study using Perna viridis. Int. J. Adv. Appl. Sci. 2012, 4, 191–196. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, M.; Lin, C.-Y.; Zhao, Q.; Zhang, R.; Dong, X.; Zhang, Y.; Tian, S.; Tian, Y.; Xia, Z. Mussel byssus cuticle: Metal coordination-mediated functional grading and self-healing in mussel byssus cuticle. Adv. Sci. 2019, 23, 1902043. [Google Scholar] [CrossRef] [Green Version]
- Yap, C.K.; Ismail, A.; Omar, H.; Tan, S.G. Accumulation, depuration and distribution of cadmium and zinc in the green-lipped mussel Perna viridis (Linnaeus) under laboratory condition. Hydrobiologia 2003, 498, 151–160. [Google Scholar] [CrossRef]
- Yap, C.K.; Ismail, A.; Tan, S.G. Byssus of the green-lipped mussel Perna viridis (Linnaeus) as a biomonitoring material for Zn. Russ. J. Mar. Biol. 2005, 31, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, P.G.; Grilo, T.F.; Reis, A.T.; Coelho, J.P.; Pereira, E.; Pardal, M.A. Field transplantation of the bivalve Scrobicularia plana along a mercury gradient in Ria de Aveiro (Portugal): Uptake and depuration kinetics. Sci. Tot. Environ. 2015, 512–513, 55–61. [Google Scholar] [CrossRef]
- Schintu, M.; Durante, L.; Maccioni, A.; Meloni, P.; Degetto, S.; Contu, A. Measurement of environmental trace-metal levels in Mediterranean coastal areas with transplanted mussels and DGT techniques. Mar. Pollut. Bull. 2008, 57, 832–837. [Google Scholar] [CrossRef]
- Andral, B.; Galgani, F.; Tomasino, C.; Bouchoucha, M.; Blottiere, C.; Scarpato, A.; Benedicto, J.; Deudero, S.; Calvo, M.; Cento, A.; et al. Chemical contamination baseline in the Western basin of the Mediterranean sea based on transplanted mussels. Arch. Environ. Contam. Toxicol. 2011, 61, 261–271. [Google Scholar] [CrossRef]
- Catharino, M.G.M.; Vasconcellos, M.B.A.; De Sousa, E.C.P.M.; Moreira, E.G.; Pereira, C.D.S. Biomonitoring of Hg, Cd, Pb and other elements in coastal regions of São Paulo State, Brazil, using the transplanted mussel Perna perna (Linnaeus, 1758). J. Radioanal. Nuclear Chem. 2008, 278, 547–551. [Google Scholar] [CrossRef]
- Giarratano, E.; Duarte, C.A.; Amin, O.A. Biomarkers and heavy metal bioaccumulation in mussels transplanted to coastal waters of the Beagle Channel. Ecotoxicol. Environ. Saf. 2010, 73, 270–279. [Google Scholar] [CrossRef]
- Haynes, D.; Toohey, D. The use of transplanted, cultured mussels (M. edulis) to monitor pollutants along the Ninety Mile Beach, Victoria, Australia. III. Heavy metals. Mar. Pollut. Bull. 1998, 36, 396–399. [Google Scholar] [CrossRef]
- Mikac, N.; Kwokal, Z.; Martinčić, D.; Branica, M. Uptake of mercury species by transplanted mussels Mytilus galloprovincialis under estuarine conditions (Krka river estuary). Sci. Tot. Environ. 1996, 184, 173–182. [Google Scholar] [CrossRef]
- Odzak, N.; Zvonarić, T.; Kljaković Gašpić, Z.; Horvat, M.; Barić, A. Biomonitoring of mercury in the Kastela Bay using transplanted mussels. Sci. Tot. Environ. 2000, 261, 61–68. [Google Scholar] [CrossRef]
- Odzak, N.; Zvonarić, T.; Kljaković Gašpić, Z.; Barić, A. Biomonitoring of copper, cadmium, lead, zinc and chromium in the Kastela Bay using transplanted mussels. Fresenius Environ. Bull. 2001, 10, 37–41. [Google Scholar]
- Ritthong, C.; Puanglarp, N.; Piyatiratitivorakul, S.; Menasvata, P. Biomonitoring of mercury contamination at petroleum production platforms in the Gulf of Thailand using transplanted green mussel, Perna viridis. Environ. Asia 2011, 4, 21–29. [Google Scholar]
- Wepener, V.; Bervoets, L.; Mubiana, V.; Blust, R. Metal exposure and biological responses in resident and transplanted blue mussels (M. edulis) from the Scheldt estuary. Mar. Pollut. Bull. 2008, 57, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Chiffoleau, J.F.; Barrah, M.; Drebika, U.; Tomasino, C.; Andral, B. Assessment of heavy metal and organic contaminants levels along the Libyan coast using transplanted mussels (Mytilus galloprovincialis). Environ. Sci Pollut. Res. 2014, 21, 11331–11339. [Google Scholar] [CrossRef] [PubMed]
- Hedouin, L.; Pringault, O.; Bustamante, P.; Fichez, R.; Warnau, M. Validation of two tropical marine bivalves as bioindicators of mining contamination in the New Caledonia lagoon: Field transplantation experiments. Wat. Res. 2011, 45, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Katsanevakis, S. Transplantation as a conservation action to protect the Mediterranean fan mussel Pinna nobilis. Mar. Ecol. Progr. Ser. 2016, 546, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-K.; Choi, M.S. Biomonitoring of trace metals using transplanted mussels, Mytilus galloprovincialis, in coastal areas around Ulsan and Onsan Bays, Korea. Ocean Sci. J. 2017, 52, 31–42. [Google Scholar] [CrossRef]
- Kucuksezgin, F.; Pazi, I.; Yucel-Gier, G.; Akcali, B.; Galgani, F. Monitoring of heavy metal and organic compound levels along the Eastern Aegean coast with transplanted mussels. Chemosphere 2013, 93, 1511–1518. [Google Scholar] [CrossRef]
- Moschino, V.; Del Negro, P.; De Vittor, C.; Da Ros, L. Biomonitoring of a polluted coastal area (Bay of Muggia, Northern Adriatic Sea): A five-year study using transplanted mussels. Ecotox. Environ. Saf. 2016, 128, 1–10. [Google Scholar] [CrossRef]
- Parolini, M.; Panseri, S.; Håland Gaeta, F.; Rossi, L.; Dell’Anno, M.; Ceriani, F.; De Felice, B.; Rafoss, T.; Arioli, F.; Pilu, S.; et al. Trends and potential human health risk of trace elements accumulated in transplanted blue mussels during restoration activities of Flekkefjord fjord (Southern Norway). Environ. Monitor. Assess. 2022, 194, 208. [Google Scholar] [CrossRef]
- Regoli, F.; Orlando, E. Accumulation and subcellular distribution of metals (Cu, Fe, Mn, Pb and Zn) in the Mediterranean mussel Mytilus galloprovincialis during a field transplant experiment. Mar. Pollut. Bull. 1994, 28, 592–600. [Google Scholar] [CrossRef]
- Riget, F.; Johansen, P.; Asmund, G. Uptake and release of lead and zinc by blue mussels. Experience from transplantation experiments in Greenland. Mar. Pollut. Bull. 1997, 34, 805–815. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; He, T.; Wang, J.; Wang, Z.; Li, P.; Lai, Y.; Sanganyado, E.; Liu, W. Integrated assessment of heavy metal pollution using transplanted mussels in eastern Guangdong, China. Environ. Pollut. 2018, 243, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Shulkin, V.M.; Presley, B.J.; Kavun, V.I. Metal concentrations in mussel Creno Mytilus grayanus and oyster Crassostrea gigas in relation to contamination of ambient sediments. Environ. Int. 2003, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Tsangaris, C.; Kaberi, H.; Catsiki, V.A. Metal levels in sediments and transplanted mussels in Pagassitikos Gulf (Aegean Sea, Eastern Mediterranean). Environ. Monitor. Assess. 2013, 185, 6077–6087. [Google Scholar] [CrossRef]
- Turja, R.; Soirinsuo, A.; Budzinski, H.; Devier, M.H.; Lehtonen, K.K. Biomarker responses and accumulation of hazardous substances in mussels (Mytilus trossulus) transplanted along a pollution gradient close to an oil terminal in the Gulf of Finland (Baltic Sea). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 80–92. [Google Scholar] [CrossRef]
- Zimmer, L.A.; Asmund, G.; Johansen, P.; Mortensen, J.; Hansen, B.W. Pollution from mining in South Greenland: Uptake and release of Pb by blue mussels (M. edulis L.) documented by transplantation experiments. Polar Biol. 2011, 34, 431–439. [Google Scholar] [CrossRef]
- Kljaković-Gašpić, Z.; Odžak, N.; Ujević, I.; Zvonarić, T.; Horvat, M.; Barić, A. Biomonitoring of mercury in polluted coastal area using transplanted mussels. Sci. Tot. Environ. 2006, 368, 199–209. [Google Scholar] [CrossRef]
- Beckvar, N.; Salazar, S.; Salazar, M.; Finkelstein, K. An in situ assessment of mercury contamination in the Sudbury River, Massachusetts, using transplanted freshwater mussels (Elliptio complanata). Can. J. Fish. Aquat. Sci. 2000, 57, 1103–1112. [Google Scholar] [CrossRef]
- Mersch, J.; Wagner, P.; Pihan, J.C. Copper in indigenous and transplanted zebra mussels in relation to changing water concentrations and body weight. Environ. Toxicol. Chem. 1996, 15, 886–893. [Google Scholar] [CrossRef]
- Bervoets, L.; Voets, J.; Covaci, A.; Chu, S.; Qadah, D.; Smolders, R.; Schepens, P.; Blust, R. Use of transplanted zebra mussels (Dreissena polymorpha) to assess the bioavailability of microcontaminants in flemish surface waters. Environ. Sci. Technol. 2005, 39, 1492–1505. [Google Scholar] [CrossRef]
- Bervoets, L.; Voets, J.; Smolders, R.; Blust, R. Metal accumulation and condition of transplanted zebra mussel (Dreissena polymorpha) in metal polluted rivers. Aquat. Ecosyst. Health Manage. 2005, 8, 451–460. [Google Scholar] [CrossRef]
- Hickey, C.W.; Roper, D.S.; Buckland, S.J. Metal concentrations of resident and transplanted freshwater mussels Hyridella menziesi (Unionacea: Hyriidae) and sediments in the Waikato River, New Zealand. Sci. Tot. Environ. 1995, 175, 163–177. [Google Scholar] [CrossRef]
- Andres, S.; Baudrimont, M.; Lapaquellerie, Y.; Ribeyre, F.; Maillet, N.; Latouche, C.; Boudou, A. Field transplantation of the freshwater bivalve Corbicula fluminea along a polymetallic contamination gradient (River Lot, France): I. Geochemical characteristics of the sampling sites and cadmium and zinc bioaccumulation kinetics. Environ. Toxicol. Chem. 1999, 18, 2462–2471. [Google Scholar] [CrossRef]
- Baudrimont, M.; Andrès, S.; Metivaud, J.; Lapaquellerie, Y.; Ribeyre, F.; Maillet, N.; Latouche, C.; Boudou, A. Field transplantation of the freshwater bivalve Corbicula fluminea along a polymetallic contamination gradient (River Lot, France): II. Metallothionein response to metal exposure. Environ. Toxicol. Chem. 1999, 18, 2472–2477. [Google Scholar] [CrossRef]
- Cooper, S.; Bonneris, E.; Michaud, A.; Pinel-Alloul, B.; Campbell, P.G.C. Influence of a step-change in metal exposure (Cd, Cu, Zn) on metal accumulation and subcellular partitioning in a freshwater bivalve, Pyganodon grandis: A long-term transplantation experiment between lakes with contrasting ambient metal levels. Aquat. Toxicol. 2013, 132–133, 73–83. [Google Scholar] [CrossRef]
- Couillard, Y.; Campbell, P.G.C.; Tessier, A.; Pellerin-Massicotte, J.; Auclair, J.C. Field transplantation of a freshwater bivalve, Pyganodon grandis, across a metal contamination gradient. 1. Temporal changes in metallothionein and metal (Cd, Cu, and Zn) concentrations in soft tissues. Can. J. Fish. Aquat. Sci. 1995, 52, 690–702. [Google Scholar] [CrossRef]
- Couillard, Y.; Campbell, P.G.C.; Pellerin-Massicotte, J.; Auclair, J.C. Field transplantation of a freshwater bivalve, Pyganodon grandis, across a metal contamination gradient. II. Metallothionein response to Cd and Zn exposure, evidence for cytotoxicity, and links to effects at higher levels of biological organization. Can. J. Fish. Aquat. Sci. 1995, 52, 703–715. [Google Scholar] [CrossRef]
- Faverney, C.R.; Guibbolini-Sabatier, M.E.; Francour, P. An ecotoxicological approach with transplanted mussels (Mytilus galloprovincialis) for assessing the impact of tyre reefs immersed along the NW Mediterranean Sea. Mar. Environ. Res. 2010, 70, 87–94. [Google Scholar] [CrossRef]
- Yap, C.K.; Tan, S.G.; Ismail, A.; Omar, H. Genetic variation of green-lipped mussel Perna viridis (Linnaeus) from the west coast of Peninsular Malaysia. Zool. Stud. 2002, 41, 376–387. [Google Scholar]
- Nasci, C.; Nesto, N.; Monteduro, R.A.; Da Ros, L. Field application of biochemical markers and a physiological index in the mussel, Mytilus galloprovincialis: Transplantation and biomonitoring in the Lagoon of Venice (NE Italy). Mar. Environ. Res. 2002, 54, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Romeo, M.; Hoarau, P.; Garello, G.; Gnassia-Barelli, M.; Girare, J.P. Mussel transplantation and biomarkers as useful tools for assessing water quality in the NW Mediterranean. Environ. Pollut. 2003, 122, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Frenzilli, G.; Bocchetti, R.; Annarumma, F.; Scancelli, V.; Fattorini, D.; Nigro, M. Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a field translocation experiment. Aquat. Toxicol. 2004, 68, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Nigro, M.; Falleni, A.; Barga, I.D.; Scancelli, V.; Lucchesi, P.; Regoli, F.; Frenzilli, G. Cellular biomarkers for monitoring estuarine environments: Transplanted versus native mussels. Aquat. Toxicol. 2006, 77, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Gorbi, S.; Lamberti, C.V.; Notti, A.; Benedetti, M.; Fattorini, D.; Moltedo, G.; Regoli, F. An ecotoxicological protocol with caged mussels, Mytilus galloprovincialis, for monitoring the impact of an offshore platform in the Adriatic Sea. Mar. Environ. Res. 2007, 65, 34–49. [Google Scholar] [CrossRef]
- Hunt, C.D.; Slone, E. Long-term monitoring using resident and caged mussels in Boston Harbor yield similar spatial and temporal trends in chemical contamination. Mar. Environ. Res. 2010, 70, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Alfonso, S.; Giulia, R.; Francois, G.; Bruno, A.; Marina, A.; Pierpaolo, G.; Josep, C.; Monica, C.; Juan, A.C.; Jose, B.A.; et al. Western Mediterranean coastal waters—Monitoring PCBs and pesticides accumulation in Mytilus galloprovincialis by active mussel watching: The Mytilus project. J. Environ. Monitor. 2010, 12, 924–935. [Google Scholar]
- Szefer, P.; Frelek, K.; Szefer, K.; Lee, C.-B.; Kim, B.-S.; Warzocha, J.; Zdrojewska, I.; Ciesielski, T. Distribution and relationships of trace metals in soft tissue, byssus and shells of M. edulis trossulus from southern Baltic. Environ. Pollut. 2002, 120, 423–444. [Google Scholar] [CrossRef]
- Yap, C.K.; Ismail, A.; Tan, S.G.; Rahim Ismail, A. Assessment of different soft tissues of the green-lipped mussel Perna viridis (Linnaeus) as biomonitoring agents of Pb: Field and laboratory studies. Wat. Air Soil Pollut. 2004, 153, 253–268. [Google Scholar] [CrossRef] [Green Version]
- Yap, C.K.; Ismail, A.; Edward, F.B.; Tan, S.G.; Siraj, S.S. Use of different soft tissues of Perna viridis as biomonitors of bioavailability and contamination by heavy metals (Cd, Cu, Fe, Pb, Ni, and Zn) in a semi-enclosed intertidal water, the Johore Straits. Toxicol. Environ. Chem. 2006, 88, 683–695. [Google Scholar] [CrossRef]
- Yap, C.K.; Ismail, A.; Tan, S.G. Background concentrations of Cd, Cu, Pb and Zn in the green-lipped mussel Perna viridis (Linnaeus) from Peninsular Malaysia. Mar. Pollut. Bull. 2003, 46, 1035–1048. [Google Scholar] [CrossRef]
- Yap, C.K.; Ismail, A.; Tan, S.G.; Omar, H. Correlations between speciation of Cd, Cu, Pb and Zn in sediment and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia. Environ. Int. 2002, 28, 117–126. [Google Scholar] [CrossRef]
- Badri, M.A.; Aston, S.R. Observation on heavy metal geochemical associations in polluted and non-polluted estuarine sediments. Environ. Pollut. Ser. B Chem. Phys. 1983, 6, 181–193. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1996; p. 662. [Google Scholar]
- Manly, B.F.J. Multivariate Statistical Methods: A Primer, 2nd ed.; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Yap, C.K.; Noorhaidah, A.; Tan, S.G. Zn Concentrations in the Different Soft Tissues of Telescopium telescopium and Their Relationships with Zn Speciation by Sequential Extraction in Surface Sediments: A Statistical Multiple Linear Stepwise Regression Analysis. In Gastropods: Diversity, Habitat and Genetics; Bianchi, A.M., Fields, J.N., Eds.; Nova Science Publishers: New York, NY, USA, 2011; pp. 127–148. [Google Scholar]
- Yap, C.K.; Rahim Ismail, A. Relationships of distribution of macrobenthic invertebrates and the physico-chemical parameters from Semenyih River by using correlation and multiple linear stepwise regression analyses. Pertanika J. Trop. Agric. Sci. 2011, 34, 229–245. [Google Scholar]
- Yap, C.K.; Rahim Ismail, A.; Ismail, A.; Tan, S.G. Studies on heavy metal accumulations in green-lipped mussel Perna viridis by using multiple linear stepwise regression analysis. Pertanika J. Sci. Technol. 2003, 11, 43–55. [Google Scholar]
- Yap, C.K.; Rahim Ismail, A.; Ismail, A.; Tan, S.G. Analysis of heavy metal level data (Cd, Cu, Pb and Zn) in different geochemical fractions of the surface sediments in the Straits of Malacca by the use of correlation and multiple linear stepwise regression analyses. Malays. Appl. Biol. 2005, 34, 51–59. [Google Scholar]
- Yap, C.K.; Edward, F.B.; Tan, S.G. Similarities and differences of metal distributions in the tissues of molluscs by using multivariate analyses. Environ. Monitor. Assess. 2010, 165, 39–53. [Google Scholar] [CrossRef]
- Peng, S.H.T.; Chee, K.H.; Saud, H.M.; Yusop, M.R.; Tan, G.H. A Cost-Effective Novel Biochemical Fertilizer for Better Managing Nutrient Levels and Vegetative Growth in the Immature Oil Palm (Elaeis guineensis Jacq.). Horticulturae 2022, 8, 758. [Google Scholar] [CrossRef]
- Bayen, S.; Thomas, G.O.; Lee, H.K.; Jeffrey, P.O. Organochlorine, pesticides and heavy metals in green-lipped mussel, Perna viridis in Singapore. Wat. Air Soil Pollut. 2003, 155, 103–116. [Google Scholar] [CrossRef]
- Shazili, N.A.M.; Yunus, K.; Ahmad, A.S.; Abdullah, N.; Rashid, M.K.A. Heavy metal pollution status in the Malaysian aquatic environment. Aquat. Ecosyst. Health 2006, 9, 137–145. [Google Scholar] [CrossRef]
- Wood, A.K.; Ahmad, Z.; Shazili, N.A.M.; Yaakob, R.; Carpenter, R. Geochemistry of sediments in Johor Strait between Malaysia and Singapore. Continent. Shelf Res. 1997, 17, 1207–1228. [Google Scholar] [CrossRef]
- Zulkifli, S.Z.; Ismail, A.; Mohamat-Yusuff, F.; Arai, T.; Miyazaki, N. Johor Strait as a hotspot for trace elements contamination in Peninsular Malaysia. Bull. Environ. Contam. Toxicol. 2010, 84, 568–573. [Google Scholar] [CrossRef]
- Nicholson, S.; Szefer, P. Accumulation of metals in the soft tissues, byssus and shell of the mytilid mussel Perna viridis (Bivalvia: Mytilidae) from polluted and uncontaminated locations in Hong Kong coastal waters. Mar. Pollut. Bull. 2003, 46, 1040–1043. [Google Scholar] [CrossRef]
- Szefer, P.; Kim, B.-S.; Kim, C.-K.; Kim, E.H.; Lee, C.-B. Trace metals in Mytilus edulis galloprovincialis and the associated water and suspended matter of the southern part of Korea Peninsula. Environ. Pollut. 2004, 129, 209–228. [Google Scholar] [CrossRef]
- Szefer, P.; Ikuta, K.; Frelek, K.; Zdrojewska, I.; Nabrzyski, M. Mercury and other trace metals (Ag, Cr, Co, and Ni) in the soft tissue and byssus of M. edulis from the East Coast of Kyushu Island, Japan. Sci. Tot. Environ. 1999, 229, 227–234. [Google Scholar] [CrossRef]
- Phillips, D.J.H.; Rainbow, P.S. Strategies of trace metals sequestration in aquatic organisms. Mar. Environ. Res. 1989, 28, 207–210. [Google Scholar] [CrossRef]
- Phillips, D.J.H. The chemistries and environmental fates of trace metals and organchlorines in aquatic ecosystems. Mar. Pollut. Bull. 1995, 31, 193–200. [Google Scholar] [CrossRef]
- Blackmore, G.; Wang, W.X. Comparison of metal accumulation in mussels at different local and global scales. Environ. Toxicol. Chem. 2003, 22, 388–395. [Google Scholar] [CrossRef]
- Szefer, P.; Ikuta, K.; Kushiyama, S.; Szefer, K.; Frelek, K.; Geldon, J. Distribution and association of trace metals in soft tissues and byssus of M. edulis from the East Coast of Kyushu Island, Japan. Arch. Environ. Contam. Toxicol. 1997, 32, 184–190. [Google Scholar] [CrossRef]
- George, S.G. Correlation of metal accumulation in mussels with the mechanism of uptake, metabolism and detoxication: A review. Thalass. Jugosl. 1980, 16, 347–365. [Google Scholar]
- Yap, C.K.; Tan, S.G. A study on the potential of the periostracum of Perna viridis as a biomonitoring material for Pb in tropical coastal waters based on correlation analysis. Sains Malays. 2011, 40, 809–819. [Google Scholar]
- Roesijadi, G.; Robinson, W.E. Metal Regulation in Aquatic Animals: Mechanisms of Uptake, Accumulation and Release. In Aquatic Toxicity, Molecular, Biochemical and Cellular Perspectives; Malius, D.C., Ostrander, G.K., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 387–420. [Google Scholar]
- George, S.G.; Pirie, B.J.S.; Coombs, T.L. The kinetics of accumulation and excretion of ferric hydroxide in M. edulis (L.) and its distribution in the tissues. J. Exp. Mar. Biol. Ecol. 1976, 23, 71–84. [Google Scholar] [CrossRef]
- Gabr, H.R.; Gab-Alla, A.A.-F.A. Effect of transplantation on heavy metal concentrations in commercial clams of Lake Timsah, Suez Canal, Egypt. Oceanologia 2008, 50, 83–93. [Google Scholar]
- Martincic, D.; Kwokal, Z.; Peharec, Z.; Margus, D.; Branica, M. Distribution of Zn, Pb, Cd and Cu between seawater and transplanted mussels (Mytilus galloprovicialis). Sci. Tot. Environ. 1992, 119, 211–230. [Google Scholar] [CrossRef]
- Wallner-Kersanach, M.; Theede, H.; Eversberg, U.; Lobo, S. Accumulation and Elimination of trace metals in a transplantation experiment with Crassostrea rhizophorae. Arch. Environ. Contam. Toxicol. 2000, 38, 40–45. [Google Scholar] [CrossRef]
- Burt, A.; Maher, W.; Roach, A.; Krikowa, F.; Honkoop, P.; Bayne, B. The accumulation of Zn, Se, Cd, and Pb and physiological condition of Anadara trapezia transplanted to a contamination gradient in Lake Macquarie, New South Wales, Australia. Mar. Environ. Res. 2007, 64, 54–78. [Google Scholar] [CrossRef] [Green Version]
- Lobel, P.B. Intersite, intrasite and variability of the whole soft tissue zinc concentration of individual mussels, M. edulis: Importance of the kidney. Mar. Environ. Res. 1987, 21, 59–71. [Google Scholar] [CrossRef]
- Lobel, P.B. Short-term and long-term uptake of zinc by the mussel, M. edulis: A study in individual variability. Arch. Environ. Contam. Toxicol. 1987, 16, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.J.H.; Rainbow, P.S. Biomonitoring of Trace Aquatic Contaminants; Elsevier Science Publishers: London, UK, 1993. [Google Scholar]
- Mackay, E.A.; Overnell, J.; Dunbar, B. Complete amino acid sequences of five dimeric and four monomeric forms of metallothionein from the edible mussel, M. edulis. Euro. J. Biochem. 1993, 218, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Unlu, M.Y.; Fowler, S.W. Factors affecting the flux of arsenic through the mussel, Mytilus galloprovincialis. Mar. Biol. 1979, 51, 209–219. [Google Scholar] [CrossRef]
- Szefer, P.; Geldon, J.; Ali, A.A.; Bawazir, A.; Sad, M. Distribution and association of trace metals in soft tissue and byssus of molluscs Perna perna from the Gulf of Aden, Yemen. Environ. Int. 1997, 23, 53–61. [Google Scholar] [CrossRef]
- Szefer, P.; Geldon, J.; Ali, A.A.; Paez-Osuna, F.; Ruiz-Fernandez, A.C.; Guerrero-Galvanc, S.R. Distribution and association of trace metals in soft tissue and byssus of Mytella strigata and other benthic organisms from Mazatlan Harbour, mangrove lagoon of the northwest coast of Mexico. Environ. Int. 1998, 24, 359–374. [Google Scholar] [CrossRef]
- Szefer, P.; Fowler, S.W.; Ikuta, K.; Paez Osuna, F.; Ali, A.; Kim, B.-S.; Fernandes, H.M.; Belzunce, M.J.; Guterstam, B.; Kunzendorf, H.; et al. A comparative assessment of heavy metal accumulation in soft parts and byssus of mussels from subarctic, temperate, subtropical and tropical marine environments. Environ. Pollut. 2006, 139, 70–78. [Google Scholar] [CrossRef]
- Benson, D.; Gain, A.K.; Rouillard, J.J. Water governance in a comparative perspective: From IWRM to a ’nexus’ approach? Water Altern. 2015, 8, 756–773. [Google Scholar]
- Reynolds, J.; Cranston, G. Nexus Thinking: Can It Slow the Great Acceleration. Nexus Network Think Piece (November 2014). Available online: https://www.cisl.cam.ac.uk/business-action/business-nature/natural-capital-impact-group/pdfs/nexus-thinking-can-it-slow-the-great-acceleration/view (accessed on 19 February 2023).
- Allouche, J.; Middletone, C.; Gyawali, D. The Water-Food-Energy Nexus: Power, Politics and Justice; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Goldberg, E.D. The Mussel Watch—A first step in global marine monitoring. Mar. Pollut. Bull. 1975, 6, 111. [Google Scholar] [CrossRef]
- Yap, C.K. Mussel Watch in Malaysia Past, Present and Future; UPM Press: Serdang, Malaysia, 2012. [Google Scholar]
- Yap, C.K.; Sharifinia, M.; Cheng, W.H.; Al-Shami, S.A.; Wong, K.W.; Al-Mutairi, K.A. A Commentary on the Use of Bivalve Mollusks in Monitoring Metal Pollution Levels. Int. J. Environ. Res. Public Health 2021, 18, 3386. [Google Scholar] [CrossRef]
- Yap, C.K.; Bakhtiari, A.R.; Cheng, W.H. Impacts of Marine Pollution and Toxicology: A Mussel Watch Experience in Peninsular Malaysia. J. Aquat. Pollut. Toxicol. 2017, 1, 1–4. [Google Scholar]
- Yap, C.K. From Mussel Watch monitoring to health risk assessment: A public health concern. GSL J. Public Health Epidemiol. 2017, 1, 103. [Google Scholar]
- Yap, C.K.; Mohd Syazwan, W. Mussel Watch monitoring program: A practical monitoring tool of potentially toxic metals pollution from 1970 to beyond 2070. Int. J. Hydro. 2022, 6, 20–21. [Google Scholar] [CrossRef]
| SOJ (PV; N = 8) | GG (MET; N = 6) | PB (MET; N = 12) | HK (PV; N = 7) | |||||||||
| TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | |
| Minimum | 1.49 | 2.32 | 0.81 | 1.23 | 0.30 | 0.19 | 1.74 | 0.28 | 0.06 | 1.02 | 0.77 | 0.28 |
| Maximum | 4.09 | 6.06 | 2.25 | 3.12 | 1.20 | 0.63 | 6.07 | 3.25 | 0.74 | 5.40 | 1.83 | 1.16 |
| Mean | 2.72 | 3.52 | 1.38 | 1.92 | 0.81 | 0.42 | 3.09 | 1.00 | 0.32 | 3.01 | 1.34 | 0.60 |
| Median | 2.85 | 3.43 | 1.31 | 1.81 | 0.81 | 0.43 | 2.46 | 0.83 | 0.28 | 3.13 | 1.42 | 0.49 |
| SD | 0.88 | 1.16 | 0.48 | 0.65 | 0.35 | 0.15 | 1.34 | 0.82 | 0.18 | 1.87 | 0.36 | 0.34 |
| SE | 0.31 | 0.41 | 0.17 | 0.26 | 0.14 | 0.06 | 0.39 | 0.24 | 0.05 | 0.71 | 0.14 | 0.13 |
| Skewness | 0.08 | 1.30 | 0.58 | 1.08 | −0.21 | −0.21 | 1.03 | 1.90 | 0.98 | 0.04 | −0.36 | 0.75 |
| Kurtosis | −1.11 | 0.99 | −0.46 | 0.16 | −1.25 | −0.81 | −0.07 | 2.85 | 0.74 | −1.71 | −0.91 | −0.93 |
| PM (PV; N = 34) | JP (ME; N = 4) | SB (MET; N = 5) | Korea (MG; N = 7) | |||||||||
| TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | |
| Minimum | 0.35 | 0.48 | 0.07 | 0.92 | 0.16 | 0.03 | 2.71 | 0.62 | 0.20 | 0.63 | 0.19 | 0.23 |
| Maximum | 7.04 | 6.06 | 4.55 | 18.4 | 1.05 | 0.88 | 5.57 | 3.65 | 1.07 | 9.98 | 5.40 | 1.89 |
| Mean | 2.04 | 2.35 | 1.81 | 5.51 | 0.56 | 0.36 | 3.52 | 1.92 | 0.55 | 3.17 | 1.69 | 0.71 |
| Median | 1.51 | 2.14 | 1.68 | 1.36 | 0.52 | 0.27 | 3.17 | 2.00 | 0.45 | 1.29 | 1.19 | 0.52 |
| SD | 1.88 | 1.13 | 1.11 | 8.60 | 0.38 | 0.39 | 1.18 | 1.25 | 0.35 | 3.49 | 1.73 | 0.57 |
| SE | 0.32 | 0.19 | 0.19 | 4.30 | 0.19 | 0.19 | 0.53 | 0.56 | 0.16 | 1.32 | 0.65 | 0.22 |
| Skewness | 1.96 | 0.96 | 0.66 | 1.15 | 0.33 | 0.58 | 1.28 | 0.26 | 0.55 | 1.23 | 1.60 | 1.42 |
| Kurtosis | 2.54 | 1.87 | 0.03 | −0.67 | −1.21 | −1.24 | −0.04 | −1.29 | −1.12 | −0.01 | 1.24 | 0.67 |
| SOJ (PV; N = 8) | GG (MET; N = 6) | PB (MET; N = 12) | HK (PV; N = 7) | |||||||||
| TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | |
| Minimum | 58.7 | 56.0 | 0.88 | 100 | 121 | 0.94 | 102 | 87.9 | 0.62 | 104 | 103 | 0.90 |
| Maximum | 144 | 185 | 2.05 | 151 | 226 | 1.77 | 193 | 396 | 2.37 | 152 | 342 | 2.71 |
| Mean | 90.7 | 122 | 1.35 | 126 | 163 | 1.30 | 159 | 227 | 1.40 | 119 | 202 | 1.65 |
| Median | 82.6 | 124 | 1.28 | 123 | 144 | 1.22 | 162 | 215 | 1.36 | 115 | 139 | 1.28 |
| SD | 29.8 | 48.9 | 0.41 | 17.7 | 43.8 | 0.30 | 23.6 | 87.8 | 0.43 | 16.4 | 110 | 0.80 |
| SE | 10.6 | 17.3 | 0.14 | 7.21 | 17.9 | 0.12 | 6.80 | 25.4 | 0.12 | 6.19 | 41.6 | 0.30 |
| Skewness | 0.71 | −0.08 | 0.49 | 0.03 | 0.62 | 0.52 | −0.99 | 0.36 | 0.54 | 1.34 | 0.30 | 0.48 |
| Kurtosis | −0.75 | −1.41 | −0.89 | −0.82 | −1.38 | −0.92 | 1.05 | −0.54 | 0.76 | 0.57 | −1.81 | −1.50 |
| PM (PV; N = 34) | JP (ME; N = 4) | SB (MET; N = 5) | Korea (MG; N = 7) | |||||||||
| TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | |
| Minimum | 50.9 | 34.9 | 0.36 | 127 | 98.4 | 0.77 | 124 | 120 | 0.88 | 107 | 81.1 | 0.68 |
| Maximum | 130 | 256 | 2.91 | 360 | 297 | 1.00 | 157 | 264 | 2.12 | 279 | 536 | 3.00 |
| Mean | 95.0 | 126 | 1.35 | 241 | 211 | 0.87 | 140 | 170 | 1.23 | 165 | 331 | 2.01 |
| Median | 97.6 | 114 | 1.30 | 239 | 224 | 0.86 | 135 | 139 | 0.97 | 141 | 387 | 1.92 |
| SD | 23.0 | 61.8 | 0.62 | 95.3 | 83.9 | 0.10 | 16.8 | 61.1 | 0.52 | 65.3 | 157 | 0.83 |
| SE | 3.95 | 10.6 | 0.11 | 47.6 | 41.9 | 0.05 | 7.50 | 27.3 | 0.23 | 24.7 | 59.3 | 0.31 |
| Skewness | −0.18 | 0.44 | 0.53 | 0.09 | −0.48 | 0.47 | 0.21 | 0.78 | 1.23 | 0.93 | −0.54 | −0.31 |
| Kurtosis | −1.17 | −0.65 | −0.14 | −1.01 | −1.07 | −1.10 | −1.77 | −0.97 | −0.17 | −0.75 | −0.86 | −1.07 |
| SOJ (PV; N = 8) | GG (MET; N = 6) | PB (MET; N = 12) | HK (PV; N = 7) | |||||||||
| TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | |
| Minimum | 10.0 | 8.55 | 0.55 | 5.70 | 17.3 | 2.40 | 6.63 | 17.9 | 2.37 | 10.1 | 32.6 | 2.38 |
| Maximum | 33.8 | 37.8 | 1.12 | 8.93 | 33.6 | 4.56 | 23.9 | 58.1 | 2.96 | 18.0 | 111 | 6.17 |
| Mean | 21.9 | 17.4 | 0.79 | 7.14 | 24.5 | 3.45 | 10.0 | 25.9 | 2.61 | 14.7 | 60.1 | 3.98 |
| Median | 23.1 | 14.4 | 0.79 | 7.26 | 24.0 | 3.42 | 8.83 | 23.5 | 2.57 | 15.8 | 44.8 | 3.44 |
| SD | 7.92 | 9.28 | 0.22 | 1.14 | 5.96 | 0.74 | 4.55 | 10.7 | 0.19 | 3.23 | 31.3 | 1.49 |
| SE | 2.80 | 3.28 | 0.08 | 0.47 | 2.43 | 0.30 | 1.31 | 3.08 | 0.05 | 1.22 | 11.8 | 0.56 |
| Skewness | −0.15 | 1.43 | 0.37 | 0.28 | 0.35 | 0.12 | 2.67 | 2.49 | 0.58 | −0.63 | 0.77 | 0.61 |
| Kurtosis | −0.96 | 1.00 | −1.22 | −0.77 | −1.04 | −0.72 | 5.85 | 5.28 | −0.69 | −1.22 | −1.07 | −1.21 |
| PM (PV; N = 34) | JP (ME; N = 4) | SB (MET; N = 5) | Korea (MG; N = 7) | |||||||||
| TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | TST | BYS | BYS/TST | |
| Minimum | 2.82 | 8.55 | 0.83 | 3.76 | 18.8 | 3.52 | 7.62 | 11.0 | 1.40 | 6.99 | 21.9 | 2.74 |
| Maximum | 20.1 | 135.3 | 6.73 | 385 | 1870 | 16.9 | 10.3 | 32.6 | 3.17 | 58.8 | 211 | 6.31 |
| Mean | 10.4 | 34.8 | 3.26 | 112 | 697 | 7.57 | 8.38 | 21.3 | 2.50 | 17.2 | 73.0 | 4.51 |
| Median | 10.8 | 27.4 | 2.99 | 29.2 | 449 | 4.93 | 7.87 | 19.7 | 2.52 | 8.76 | 49.6 | 4.72 |
| SD | 3.64 | 24.7 | 1.45 | 183 | 879 | 6.26 | 1.09 | 7.94 | 0.68 | 18.8 | 66.8 | 1.35 |
| SE | 0.62 | 4.24 | 0.25 | 91.7 | 440 | 3.13 | 0.49 | 3.55 | 0.30 | 7.09 | 25.3 | 0.51 |
| Skewness | 0.46 | 2.09 | 0.43 | 1.11 | 0.57 | 1.11 | 1.29 | 0.20 | −0.84 | 1.87 | 1.42 | −0.05 |
| Kurtosis | 0.90 | 6.01 | −0.47 | −0.71 | −1.28 | −0.69 | −0.06 | −0.82 | −0.51 | 1.74 | 0.68 | −1.48 |
| ZnBYS | ZnTST | ZnF1 | ZnF2 | ZnF3 | ZnF4 | ZnSUM | |
| ZnBYS | 1.00 | 0.39 | 0.28 | 0.15 | 0.18 | 0.01 | 0.22 |
| ZnTST | 0.39 | 1.00 | 0.29 | 0.10 | 0.03 | 0.12 | 0.05 |
| CuBYS | 0.66 | 0.37 | 0.53 | 0.47 | 0.42 | 0.06 | 0.44 |
| CuTST | 0.36 | 0.45 | 0.63 | 0.48 | 0.42 | 0.07 | 0.43 |
| CdBYS | 0.13 | 0.05 | 0.20 | 0.09 | −0.06 | −0.15 | −0.01 |
| CdTST | −0.43 | 0.20 | 0.29 | 0.24 | 0.26 | 0.08 | 0.18 |
| CuBYS | CuTST | CuF1 | CuF2 | CuF3 | CuF4 | CuSUM | |
| ZnBYS | 0.66 | 0.36 | 0.21 | −0.15 | 0.37 | 0.24 | 0.32 |
| ZnTST | 0.37 | 0.45 | 0.01 | −0.09 | 0.29 | 0.11 | 0.19 |
| CuBYS | 1.00 | 0.69 | 0.49 | −0.03 | 0.56 | 0.48 | 0.58 |
| CuTST | 0.69 | 1.00 | 0.57 | 0.08 | 0.51 | 0.34 | 0.50 |
| CdBYS | 0.03 | 0.18 | 0.10 | 0.08 | 0.04 | −0.08 | 0.03 |
| CdTST | 0.07 | 0.17 | 0.07 | 0.12 | 0.14 | 0.02 | 0.10 |
| CdBYS | CdTST | CdF1 | CdF2 | CdF3 | CdF4 | CdSUM | |
| ZnBYS | 0.13 | −0.43 | 0.29 | 0.49 | 0.34 | 0.15 | 0.43 |
| ZnTST | 0.05 | 0.20 | 0.17 | 0.01 | 0.13 | −0.16 | −0.03 |
| CuBYS | 0.03 | 0.07 | 0.28 | 0.30 | 0.30 | 0.19 | 0.34 |
| CuTST | 0.18 | 0.17 | 0.30 | 0.12 | 0.24 | −0.02 | 0.15 |
| CdBYS | 1.00 | −0.16 | 0.28 | 0.15 | 0.25 | −0.19 | 0.07 |
| CdTST | −0.16 | 1.00 | 0.08 | −0.01 | 0.05 | −0.16 | −0.09 |
| ZnBYS | Intercept | CuBYS | CdTST | CdF2 | ZnTST | CuF2 | ZnF2 | CdF3 | CuF1 | R | F | df | ||
| −33.74 | 0.286 | −0.56 | 0.75 | 0.359 | −0.26 | 0.439 | −0.24 | −0.17 | 0.958 | 34.9 | 8.25 | |||
| ZnTST | Intercept | CuTST | CuF1 | CuF3 | ZnSUM | ZnF4 | CdF4 | CdF3 | CuF2 | R | F | df | ||
| 205.8 | 0.537 | −0.6 | 1.04 | −0.91 | 0.653 | −0.22 | 0.322 | −0.18 | 0.814 | 6.12 | 8.25 | |||
| CuBYS | Intercept | CuTST | ZnBYS | CdTST | CuF4 | ZnTST | CdF2 | R | F | df | ||||
| 0.575 | 0.396 | 0.8 | 0.384 | 0.201 | −0.22 | −0.17 | 0.888 | 16.9 | 6.27 | |||||
| CuTST | Intercept | CuBYS | ZnF1 | ZnTST | CdF3 | CuF1 | CuF3 | R | F | df | ||||
| −3.3 | 0.415 | 0.368 | 0.359 | −0.15 | 0.593 | −0.53 | 0.845 | 11.25 | 6.27 | |||||
| CdBYS | Intercept | CdF1 | CdF4 | CdTST | CuF2 | R | F | df | ||||||
| 0.522 | 0.375 | −0.32 | −0.27 | 0.201 | 0.473 | 2.09 | 4.29 | |||||||
| CdTST | Intercept | ZnBYS | CuBYS | ZnTST | CdF2 | CuF2 | ZnF2 | CdF3 | CuF1 | ZnF1 | CdBYS | R | F | df |
| 0.086 | −1.3 | 0.301 | 0.461 | 1.05 | −0.21 | 0.628 | −0.49 | −0.47 | 0.376 | −0.14 | 0.914 | 11.64 | 10.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, C.K.; Al-Mutairi, K.A. Byssus of Green-Lipped Mussel Perna viridis as a Biomonitoring Biopolymer for Zinc Pollution in Coastal Waters. Biology 2023, 12, 523. https://doi.org/10.3390/biology12040523
Yap CK, Al-Mutairi KA. Byssus of Green-Lipped Mussel Perna viridis as a Biomonitoring Biopolymer for Zinc Pollution in Coastal Waters. Biology. 2023; 12(4):523. https://doi.org/10.3390/biology12040523
Chicago/Turabian StyleYap, Chee Kong, and Khalid Awadh Al-Mutairi. 2023. "Byssus of Green-Lipped Mussel Perna viridis as a Biomonitoring Biopolymer for Zinc Pollution in Coastal Waters" Biology 12, no. 4: 523. https://doi.org/10.3390/biology12040523






