Lipid Aldehydes 4-Hydroxynonenal and 4-Hydroxyhexenal Exposure Differentially Impact Lipogenic Pathways in Human Placenta
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Placental Explant Isolation and Culture
2.3. Placental Gene Expression Analysis
2.4. Statistics
3. Results
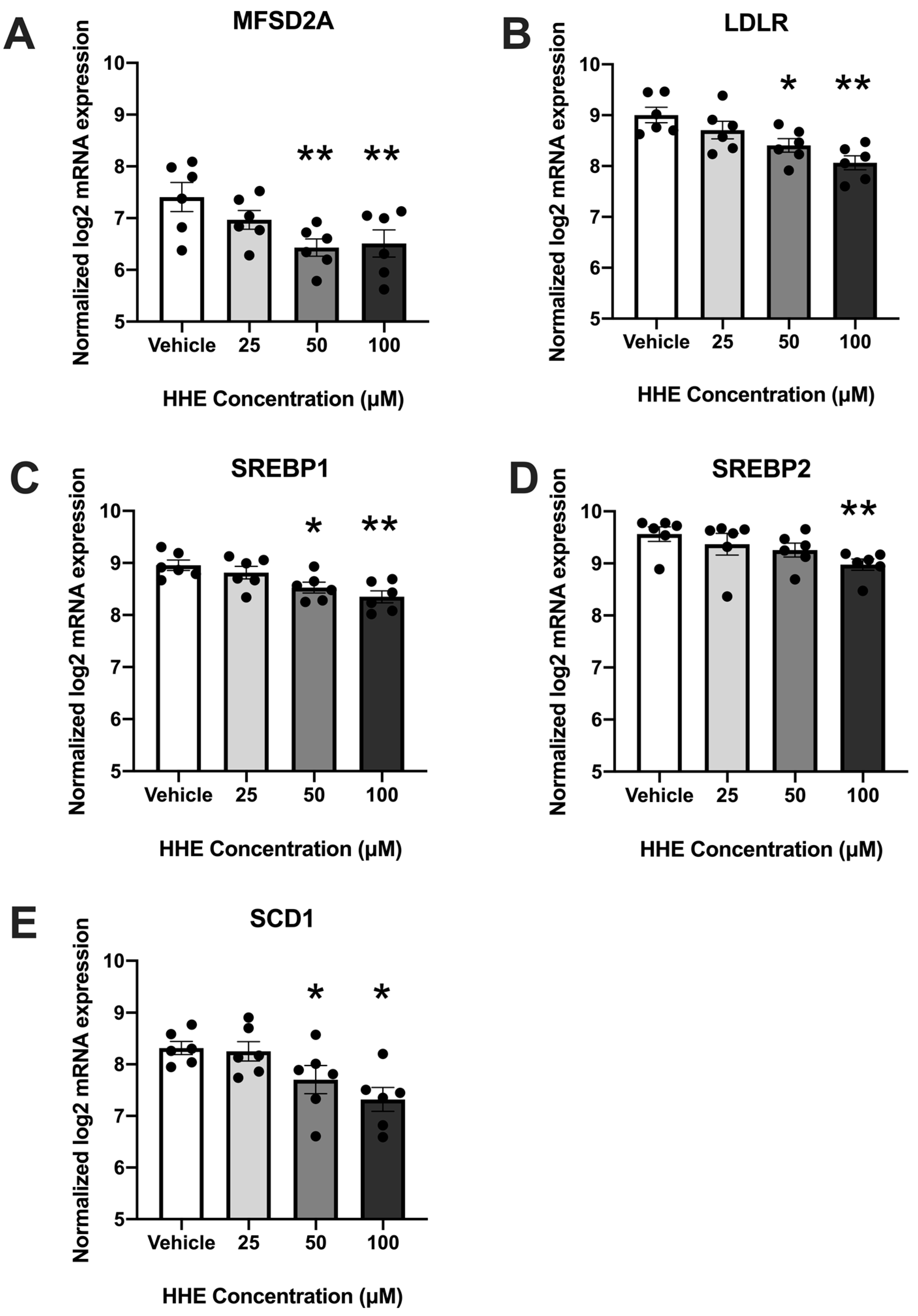
3.1. 4-HHE Exposure Decreases Fatty Acid Uptake and Synthesis-Related Genes
3.2. 4-HNE Exposure Increases Fatty Acid Synthesis and Transport-Related Genes
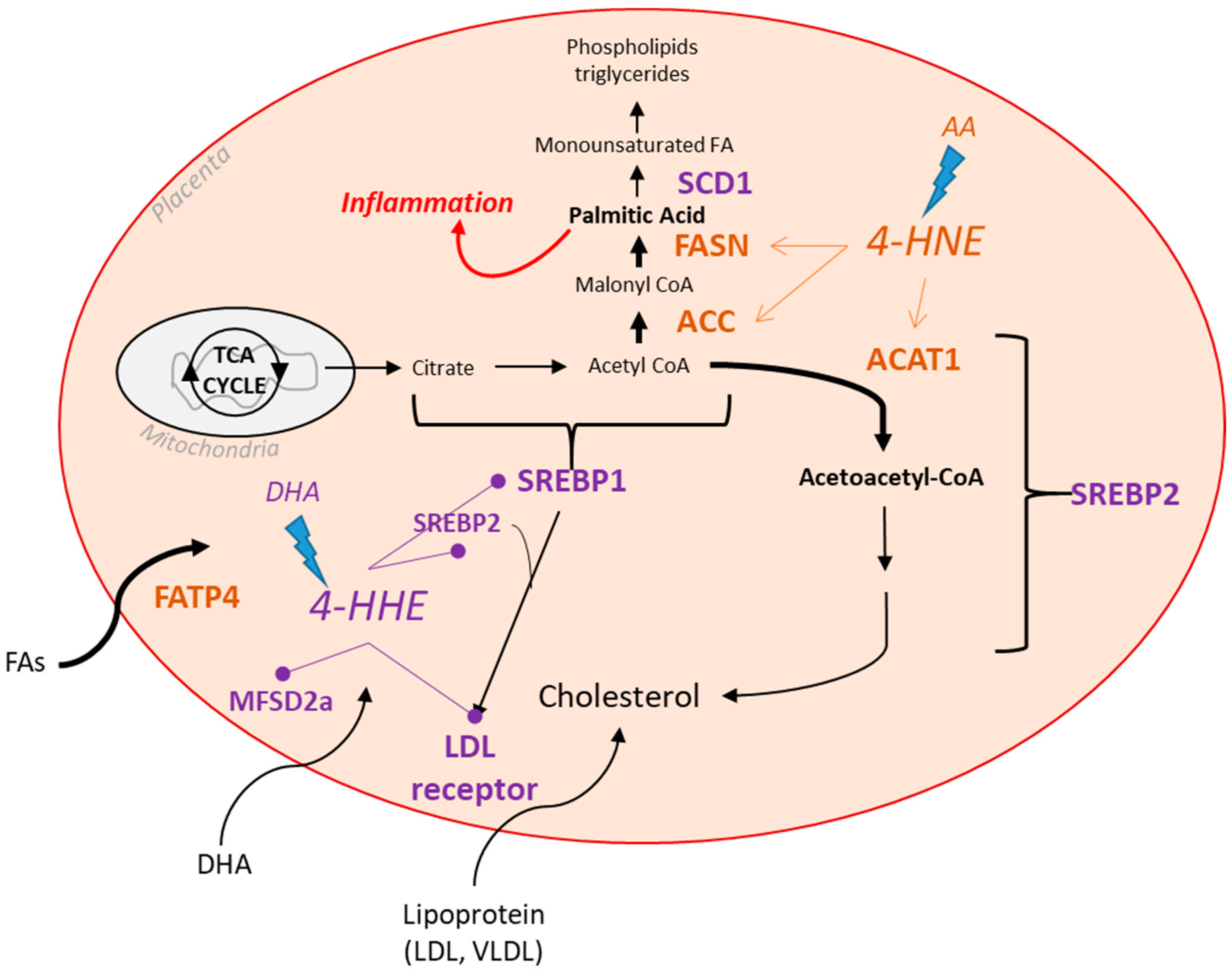
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mean ± SD | |
|---|---|
| Age (y) | 29 ± 3 |
| Early pregnancy BMI (kg/m2) | 26.3 ± 1.7 |
| Race/Ethnicity | 4 NHW 1; 2 Hispanic |
| Placenta weight (g) | 504.3 ± 33.5 |
| Gene | Gene Symbol | |
|---|---|---|
| Peroxisomal Oxidation | ACOX1 | ACOX1 |
| PEX3 | PEX3 | |
| HSD17B4 | HSD17B4 | |
| CROT | CROT | |
| Mitochondrial FA Oxidation | CPT2 | CPT2 |
| AMPKα | PRKAA1 | |
| SLC22A5 | SLC22A5 | |
| COX7B | COX7B | |
| CACT | CACT | |
| ACAT1 | ACAT1 | |
| ACAA2 | ACAA2 | |
| PPARA | PPARA | |
| CPT1B | CPT1B | |
| Lipases | LPL | LPL |
| EL | LIPG | |
| FA Uptake and Transporters | MFSD2A | MFSD2A |
| FATP4 | SLC27A4 | |
| FATP3 | SLC27A3 | |
| CD36 | CD36 | |
| FABPpm | GOT2 | |
| FABP4 | FABP4 | |
| VLDLR | VLDLR | |
| FATP6 | SLC27A6 | |
| FABP3 | FABP3 | |
| GPR120 | GPR120 | |
| Lipogenesis | DGAT1 | DGAT1 |
| DGAT2 | DGAT2 | |
| PLIN2 | PLIN2 | |
| ACC | ACACA | |
| ACSS2 | ACSS2 | |
| ACSL1 | ACSL1 | |
| ACSL5 | ACSL5 | |
| SREBP1 | SREBP1 | |
| SREBF2 | SREBF2 | |
| SCD-1 | SCD | |
| PPARƔ | PPARG | |
| PGC1A | PGC1A |
References
- Innis, S.M. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 2003, 143 (Suppl. S4), 8. [Google Scholar] [CrossRef] [Green Version]
- Cetin, I.; Alvino, G.; Cardellicchio, M. Long chain fatty acids and dietary fats in fetal nutrition. J. Physiol. 2009, 587 Pt 14, 3441–3451. [Google Scholar] [CrossRef]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar]
- Steenweg-de Graaff, J.C.; Tiemeier, H.; Basten, M.G.; Rijlaarsdam, J.; Demmelmair, H.; Koletzko, B.; Hofman, A.; Jaddoe, V.W.V.; Verhulst, F.C.; Roza, S.J. Maternal LC-PUFA status during pregnancy and child problem behavior: The Generation R Study. Pediatr. Res. 2015, 77, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, R.J.; Harvey, N.C.; Robinson, S.M.; Ntani, G.; Davies, J.H.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Calder, P.C.; Cooper, C.; et al. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J. Clin. Endocrinol. Metab. 2013, 98, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Cetin, I.; Koletzko, B. Long-chain omega-3 fatty acid supply in pregnancy and lactation. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Requena, J.R.; Fu, M.X.; Ahmed, M.U.; Jenkins, A.J.; Lyons, T.J.; Thorpe, S.R. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol. Dial. Transplant. 1996, 11 (Suppl. S5), 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, H.; Johnson, D.; Gensler, C.; Decker, E.; Zhang, G. Chemistry and biology of ω-3 PUFA peroxidation-derived compounds. Prostaglandins Other Lipid Mediat. 2017, 132, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Sosinsky, A.Z.; Moustafa, D.; Viguera, A.C.; Cohen, L.S. Supplement use by women during pregnancy: Data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Arch. Women’s Ment. Health 2015, 19, 437–441. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef] [Green Version]
- Fruhwirth, G.O.; Loidl, A.; Hermetter, A. Oxidized phospholipids: From molecular properties to disease. Biochim. Biophys. Acta 2007, 1772, 718–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [PubMed] [Green Version]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Fogarty, N.M.E.; Jones, C.J.P.; Kingdom, J.; Burton, G.J. Evidence of oxidative stress-induced senescence in mature, post-mature and pathological human placentas. Placenta 2018, 68, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; González, M.C.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.K.; Powers, S.K.; Dirks, A.J.; Scarpace, P.J. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.P.; Gastaldi, G.; Bobbioni-Harsch, E.; Arboit, P.; Gobelet, C.; Dériaz, O.; Golay, A.; Witztum, J.L.; Giacobino, J.-P. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: A case of good vs. bad lipids? FEBS Lett. 2003, 551, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Frohnert, B.I.; Sinaiko, A.R.; Serrot, F.J.; Foncea, R.E.; Moran, A.; Ikramuddin, S.; Choudry, U.; Bernlohr, D.A. Increased adipose protein carbonylation in human obesity. Obesity 2011, 19, 1735–1741. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Y.; Li, J.; Wang, H.; Cheng, C.; Yang, L.; Li, Q.; Deng, J.; Liang, Z.; Yin, Y.; et al. Maternal Diet-Induced Obesity Compromises Oxidative Stress Status and Angiogenesis in the Porcine Placenta by Upregulating Nox2 Expression. Oxid. Med. Cell. Longev. 2019, 2019, 2481592. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.K.; Lechowicz, A.; Petrik, J.J.; Storozhuk, Y.; Paez-Parent, S.; Dai, Q.; Samjoo, I.A.; Mansell, M.; Gruslin, A.; Holloway, A.C.; et al. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: Role of altered development of the placental vasculature. PLoS ONE 2012, 7, e33370. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.D.; Prabhu, K.S.; Thompson, J.T.; Reddy, P.S.; Peters, J.M.; Peterson, B.R.; Reddy, C.C.; Heuvel, J.P.V. The oxidative stress mediator 4-hydroxynonenal is an intracellular agonist of the nuclear receptor peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). Free Radic. Biol. Med. 2007, 42, 1155–1164. [Google Scholar] [PubMed] [Green Version]
- Wang, Z.; Dou, X.; Gu, D.; Shen, C.; Yao, T.; Nguyen, V.; Braunschweig, C.; Song, Z. 4-Hydroxynonenal differentially regulates adiponectin gene expression and secretion via activating PPARγ and accelerating ubiquitin-proteasome degradation. Mol. Cell. Endocrinol. 2012, 349, 222–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschmugl, B.; Perazzolo, S.; Sengers, B.G.; Lewis, R.M.; Gruber, M.; Desoye, G.; Wadsack, C. Placental mobilization of free fatty acids contributes to altered materno-fetal transfer in obesity. Int. J. Obes. 2021, 45, 1114–1123. [Google Scholar]
- Calabuig-Navarro, V.; Haghiac, M.; Minium, J.; Glazebrook, P.; Ranasinghe, G.C.; Hoppel, C.; Hauguel de-Mouzon, S.; Catalano, P.; O’Tierney-Ginn, P. Effect of Maternal Obesity on Placental Lipid Metabolism. Endocrinology 2017, 158, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Schaiff, W.T.; Bildirici, I.; Cheong, M.; Chern, P.L.; Nelson, D.M.; Sadovsky, Y. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J. Clin. Endocrinol. Metab. 2005, 90, 4267–4275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daoud, G.; Simoneau, L.; Masse, A.; Rassart, E.; Lafond, J. Expression of cFABP and PPAR in trophoblast cells: Effect of PPAR ligands on linoleic acid uptake and differentiation. Biochim. Biophys. Acta 2005, 1687, 181–194. [Google Scholar] [CrossRef]
- Goto, K.; Iso, T.; Hanaoka, H.; Yamaguchi, A.; Suga, T.; Hattori, A.; Irie, Y.; Shinagawa, Y.; Matsui, H.; Syamsunarno, M.R.A.A.; et al. Peroxisome proliferator-activated receptor-gamma in capillary endothelia promotes fatty acid uptake by heart during long-term fasting. J. Am. Heart Assoc. 2013, 2, e004861. [Google Scholar] [PubMed] [Green Version]
- Xu, Y.; Wang, Q.; Cook, T.J.; Knipp, G.T. Effect of placental fatty acid metabolism and regulation by peroxisome proliferator activated receptor on pregnancy and fetal outcomes. J. Pharm. Sci. 2007, 96, 2582–2606. [Google Scholar]
- Yoon, M. The role of PPARalpha in lipid metabolism and obesity: Focusing on the effects of estrogen on PPARalpha actions. Pharmacol. Res. 2009, 60, 151–159. [Google Scholar] [CrossRef]
- Calabuig-Navarro, V.; Puchowicz, M.; Glazebrook, P.; Haghiac, M.; Minium, J.; Catalano, P.; Hauguel de Mouzon, S.; O’Tierney-Ginn, P. Effect of omega-3 supplementation on placental lipid metabolism in overweight and obese women. Am. J. Clin. Nutr. 2016, 103, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Siman, C.M.; Sibley, C.P.; Jones, C.J.; Turner, M.A.; Greenwood, S.L. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 280, R1116–R1122. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K. 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar]
- Chen, Z.H.; Niki, E. 4-hydroxynonenal (4-HNE) has been widely accepted as an inducer of oxidative stress. Is this the whole truth about it or can 4-HNE also exert protective effects? IUBMB Life 2006, 58, 372–373. [Google Scholar] [PubMed]
- Rasool, A.; Mahmoud, T.; Mathyk, B.; Kaneko-Tarui, T.; Roncari, D.; White, K.O.; O’Tierney-Ginn, P. Obesity downregulates lipid metabolism genes in first trimester placenta. Sci. Rep. 2022, 12, 19368. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Haghiac, M.; Yang, X.H.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Mouzon, S.H.-D. Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar]
- Prieto-Sánchez, M.T.; Ruiz-Palacios, M.; Blanco-Carnero, J.E.; Pagan, A.; Hellmuth, C.; Uhl, O.; Peissner, W.; Ruiz-Alcaraz, A.J.; Parrilla, J.J.; Koletzko, B.; et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin. Nutr. 2017, 36, 513–521. [Google Scholar]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar] [PubMed]
- Ou, J.; Tu, H.; Shan, B.; Luk, A.; DeBose-Boyd, R.A.; Bashmakov, Y.; Goldstein, J.L.; Brown, M.S. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA 2001, 98, 6027–6032. [Google Scholar]
- Miyazaki, M.; Kim, Y.C.; Ntambi, J.M. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J. Lipid Res. 2001, 42, 1018–1024. [Google Scholar] [PubMed]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Najima, Y.; Nakakuki, M.; Nagai, R.; Ishibashi, S.; Osuga, J.-I.; Yamada, N.; Shimano, H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 2003, 38, 1529–1539. [Google Scholar]
- Huang, L.L.; Wan, J.B.; Wang, B.; He, C.W.; Ma, H.; Li, T.W.; Kang, J.X. Suppression of acute ethanol-induced hepatic steatosis by docosahexaenoic acid is associated with downregulation of stearoyl-CoA desaturase 1 and inflammatory cytokines. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 347–353. [Google Scholar] [CrossRef]
- Lassance, L.; Haghiac, M.; Minium, J.; Catalano, P.; Hauguel-de Mouzon, S. Obesity-induced down-regulation of the mitochondrial translocator protein (TSPO) impairs placental steroid production. J. Clin. Endocrinol. Metab. 2015, 100, E11–E18. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, A.; Korac, A.; Srdic-Galic, B.; Buzadzic, B.; Otasevic, V.; Stancic, A.; Vucetic, M.; Markelic, M.; Velickovic, K.; Golic, I.; et al. Differences in the redox status of human visceral and subcutaneous adipose tissues--relationships to obesity and metabolic risk. Metabolism 2014, 63, 661–671. [Google Scholar] [CrossRef]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef]
- Nakazawa, T.; Nagatsuka, S.; Yukawa, O. Effects of membrane stabilizing agents and radiation on liposomal membranes. Drugs Exp. Clin. Res. 1986, 12, 831–835. [Google Scholar] [PubMed]
- Jacob, R.F.; Mason, R.P. Lipid peroxidation induces cholesterol domain formation in model membranes. J. Biol. Chem. 2005, 280, 39380–39387. [Google Scholar] [PubMed] [Green Version]
- Maier, T.; Jenni, S.; Ban, N. Architecture of mammalian fatty acid synthase at 4.5 A resolution. Science 2006, 311, 1258–1262. [Google Scholar] [CrossRef]
- Qiu, T.; Yang, X.; Wang, J.; Pan, C.; Chu, X.; Xiong, J.; Xie, J.; Chang, Y.; Wang, C.; Zhang, J. Obesity-induced elevated palmitic acid promotes inflammation and glucose metabolism disorders through GPRs/NF-κB/KLF7 pathway. Nutr. Diabetes 2022, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedusi, V.; Martorana, F.; Brambilla, L.; Maggi, A.; Rossi, D. The peroxisome proliferator-activated receptor gamma (PPARgamma) controls natural protective mechanisms against lipid peroxidation in amyotrophic lateral sclerosis. J. Biol. Chem. 2012, 287, 35899–35911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schild, R.L.; Schaiff, W.T.; Carlson, M.G.; Cronbach, E.J.; Nelson, D.M.; Sadovsky, Y. The activity of PPAR gamma in primary human trophoblasts is enhanced by oxidized lipids. J. Clin. Endocrinol. Metab. 2002, 87, 1105–1110. [Google Scholar]
- Natarajan, S.K.; Bruett, T.; Muthuraj, P.G.; Sahoo, P.K.; Power, J.; Mott, J.L.; Hanson, C.; Anderson-Berry, A. Saturated free fatty acids induce placental trophoblast lipoapoptosis. PLoS ONE 2021, 16, e0249907. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasool, A.; Mahmoud, T.; O’Tierney-Ginn, P. Lipid Aldehydes 4-Hydroxynonenal and 4-Hydroxyhexenal Exposure Differentially Impact Lipogenic Pathways in Human Placenta. Biology 2023, 12, 527. https://doi.org/10.3390/biology12040527
Rasool A, Mahmoud T, O’Tierney-Ginn P. Lipid Aldehydes 4-Hydroxynonenal and 4-Hydroxyhexenal Exposure Differentially Impact Lipogenic Pathways in Human Placenta. Biology. 2023; 12(4):527. https://doi.org/10.3390/biology12040527
Chicago/Turabian StyleRasool, Aisha, Taysir Mahmoud, and Perrie O’Tierney-Ginn. 2023. "Lipid Aldehydes 4-Hydroxynonenal and 4-Hydroxyhexenal Exposure Differentially Impact Lipogenic Pathways in Human Placenta" Biology 12, no. 4: 527. https://doi.org/10.3390/biology12040527
APA StyleRasool, A., Mahmoud, T., & O’Tierney-Ginn, P. (2023). Lipid Aldehydes 4-Hydroxynonenal and 4-Hydroxyhexenal Exposure Differentially Impact Lipogenic Pathways in Human Placenta. Biology, 12(4), 527. https://doi.org/10.3390/biology12040527






