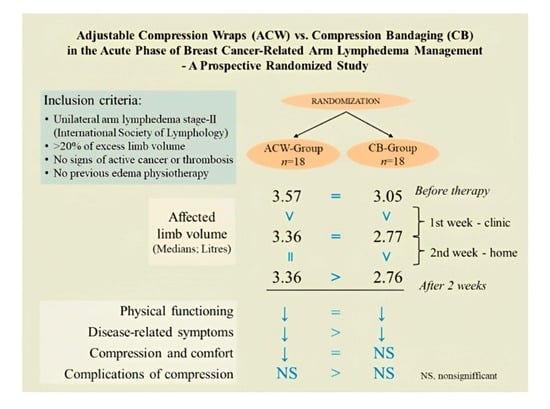
Adjustable Compression Wraps (ACW) vs. Compression Bandaging (CB) in the Acute Phase of Breast Cancer-Related Arm Lymphedema Management—A Prospective Randomized Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
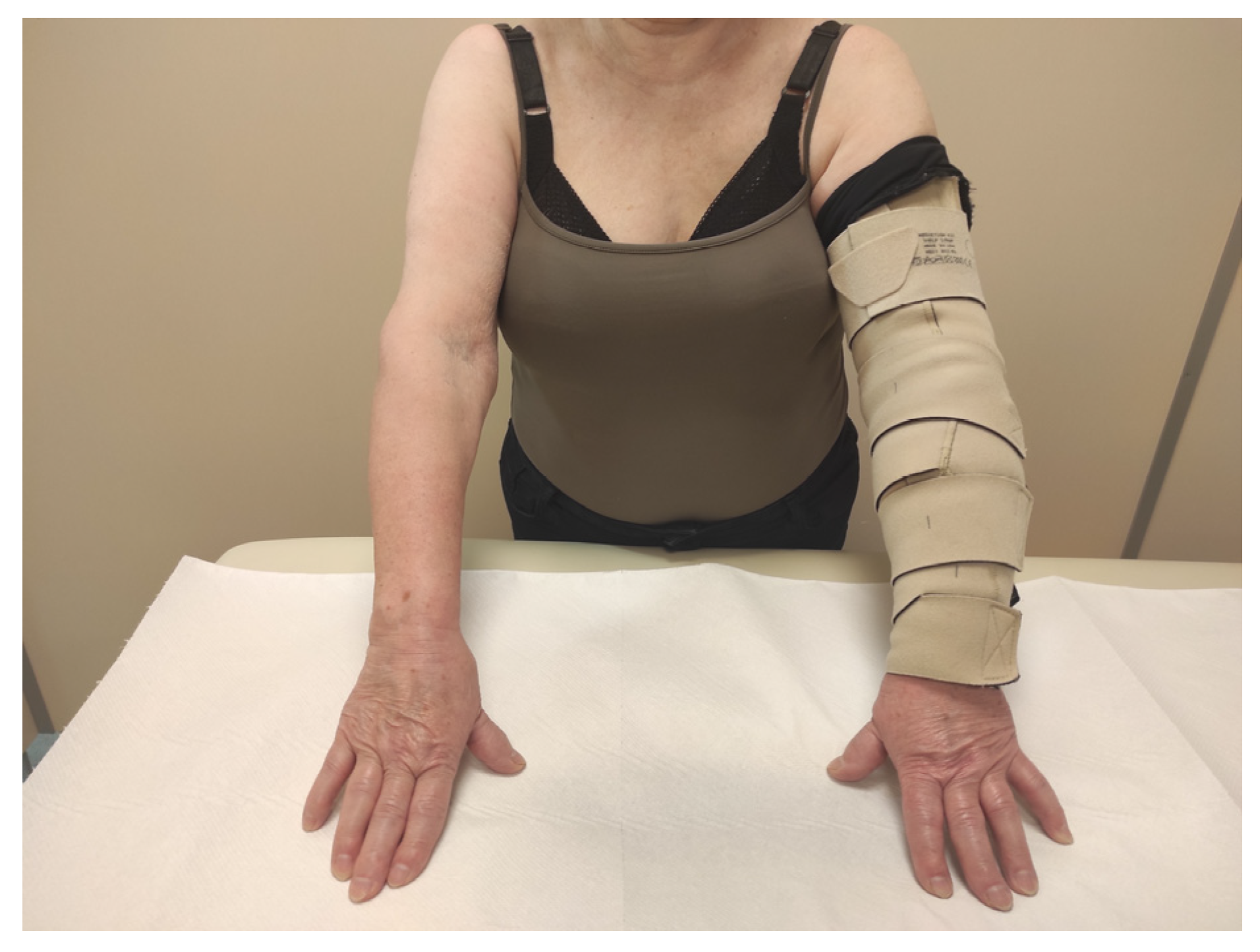
2.2. Intervention
2.3. Measurements
2.4. Statistics
2.5. Sample Size
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rockson, S.G.; Keeley, V.; Kilbreath, S.; Szuba, A.; Towers, A. Cancer-associated secondary lymphoedema. Nat. Rev. Dis. Prim. 2019, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Iyer, D.; Jannaway, M.; Yang, Y.; Scallan, J.P. Lymphatic valves and lymph flow in cancer-related lymphedema. Cancers 2020, 12, 2297. [Google Scholar] [CrossRef] [PubMed]
- Executive Committee. The diagnosis and treatment of peripheral lymphedema: 2020 consensus document of the international society of lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Ogino, R.; Yokooji, T.; Hayashida, M.; Suda, S.; Yamakawa, S. Emerging Anti-Inflammatory Pharmacotherapy and Cell-Based Therapy for Lymphedema. Int. J. Mol. Sci. 2022, 23, 7614. [Google Scholar] [CrossRef]
- Badger, C.M.; Peacock, J.L.; Mortimer, P.S. A randomized, controlled, parallel-group clinical trial comparing multilayer bandaging followed by hosiery versus hosiery alone in the treatment of patients with lymphedema of the limb. Cancer 2000, 88, 2832–2837. [Google Scholar] [CrossRef]
- King, M.; Deveaux, A.; White, H.; Rayson, D. Compression garments versus compression bandaging in decongestive lymphatic therapy for breast cancer-related lymphedema: A randomized controlled trial. Support. Care Cancer 2012, 20, 1031–1036. [Google Scholar] [CrossRef]
- Cavezzi, A.; Colucci, R.; Barsotti, N.; Di Ionna, G. Compression therapy, autonomic nervous system, and heart rate variability: A narrative review and our preliminary personal experience. Phlebology 2022, 37, 739–753. [Google Scholar] [CrossRef]
- Sugisawa, R.; Unno, N.; Saito, T.; Yamamoto, N.; Inuzuka, K.; Tanaka, H.; Sano, M.; Katahashi, K.; Uranaka, H.; Marumo, T.; et al. Effects of compression stockings on elevation of leg lymph pumping pressure and improvement of quality of life in healthy female volunteers: A randomized controlled trial. Lymphat. Res. Biol. 2016, 14, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Yamamoto, N.; Kageyama, T.; Sakai, H.; Fuse, Y.; Tsuihiji, K.; Tsukuura, R. Technical pearls in lymphatic supermicrosurgery. Glob. Health Med. 2020, 2, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Koshima, I.; Imai, H.; Roh, S.; Mese, T.; Uchiki, T.; Sasaki, A.; Nagamatsu, S. Effect of postoperative compression therapy on the success of liposuction in patients with advanced lower limb lymphedema. J. Clin. Med. 2021, 10, 4852. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Imai, H.; Roh, S.; Mese, T.; Koshima, I. Comparison of the Effectiveness of Liposuction for Lower Limb versus Upper Limb Lymphedema. J. Clin. Med. 2023, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Niimi, K.; Iwata, H.; Sugimoto, I.; Ishibashi, H.; Ota, T.; Nakamura, H. Comparison of stiffness and interface pressure during rest and exercise among various arm sleeves. Phlebology 2010, 25, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Rabe, E.; Partsch, H.; Hafner, J.; Lattimer, C.; Mosti, G.; Neumann, M.; Urbanek, T.; Huebner, M.; Gaillard, S.; Carpentier, P. Indications for medical compression stockings in venous and lymphatic disorders: An evidence-based consensus statement. Phlebology 2018, 33, 163–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gradalski, T.; Ochalek, K.; Kurpiewska, J. Complex Decongestive Lymphatic Therapy with or Without Vodder II Manual Lymph Drainage in More Severe Chronic Postmastectomy Upper Limb Lymphedema: A Randomized Noninferiority Prospective Study. J. Pain Symptom Manag. 2015, 50, 750–757. [Google Scholar] [CrossRef]
- McNeely, M.L.; Dolgoy, N.D.; Rafn, B.S.; Ghosh, S.; Ospina, P.A.; Al Onazi, M.M.; Radke, L.; Shular, M.; Kuusk, U.; Webster, M.; et al. Nighttime compression supports improved self-management of breast cancer-related lymphedema: A multicenter randomized controlled trial. Cancer 2022, 128, 587–596. [Google Scholar] [CrossRef]
- Ligabue, M.B.; Campanini, I.; Veroni, P.; Cepelli, A.; Lusuardi, M.; Merlo, A. Efficacy of self-administered complex decongestive therapy on breast cancer-related lymphedema: A single-blind randomized controlled trial. Breast Cancer Res. Treat. 2019, 175, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Mestre, S.; Calais, C.; Gaillard, G.; Nou, M.; Pasqualini, M.; Ben Amor, C.; Quere, I. Interest of an auto-adjustable nighttime compression sleeve (MOBIDERM® Autofit) in maintenance phase of upper limb lymphedema: The MARILYN pilot RCT. Support. Care Cancer 2017, 25, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Shallwani, S.M.; Towers, A. Self-Management Strategies for Malignant Lymphedema: A Case Report with 1-Year and 4-Year Follow-Up Data. Physiother. Can. 2018, 70, 204–211. [Google Scholar] [CrossRef]
- Hara, H.; Yoshida, M.; Ikehata, N.; Tachibana, S.; Hamanaka, N.; Nakakawaji, K.; Mihara, M. Compression Pressure Variability in Upper Limb Multilayer Bandaging Applied by Lymphedema Therapists. Lymphat. Res. Biol. 2021, 19, 378–382. [Google Scholar] [CrossRef]
- Damstra, R.J.; Partsch, H. Prospective, randomized, controlled trial comparing the effectiveness of adjustable compression Velcro wraps versus inelastic multicomponent compression bandages in the initial treatment of leg lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2013, 1, 13–19. [Google Scholar] [CrossRef]
- Stather, P.W.; Petty, C.; Howard, A.Q. Review of adjustable velcro wrap devices for venous ulceration. Int. Wound J. 2019, 16, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Williams, A. A review of the evidence for adjustable compression wrap devices. J. Wound Care 2016, 25, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borman, P.; Koyuncu, E.G.; Yaman, A.; Calp, E.; Koç, F.; Sargut, R.; Karahan, S. The Comparative Efficacy of Conventional Short-Stretch Multilayer Bandages and Velcro Adjustable Compression Wraps in Active Treatment Phase of Patients with Lower Limb Lymphedema. Lymphat. Res. Biol. 2021, 19, 286–294. [Google Scholar] [CrossRef]
- Mosti, G.; Cavezzi, A.; Partsch, H.; Urso, S.; Campana, F. Adjustable Velcro Compression Devices are More Effective than Inelastic Bandages in Reducing Venous Edema in the Initial Treatment Phase: A Randomized Controlled Trial. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2015, 50, 368–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partsch, H. Reliable self-application of short stretch leg compression: Pressure measurements under self-applied, adjustable compression wraps. Phlebology 2019, 34, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Blaya, V.; Salinas-Huertas, S.; Catasús, M.L.; Pascual, T.; Belmonte, R. Effectiveness of a precast adjustable compression system compared to multilayered compression bandages in the treatment of breast cancer-related lymphoedema: A randomized, single-blind clinical trial. Clin. Rehabil. 2019, 33, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Devoogdt, N.; Partsch, H.; Heroes, A.K.; De Vrieze, T.; De Groef, A.; Geraerts, I.; Damstra, R.J.; Hafner, J.; Keeley, V.; Becker, A.; et al. The ICC Compression Questionnaire: A Comprehensive Tool to Evaluate Compression Materials or Devices Applied in Subjects with Lymphedema or Chronic Venous Disease. Lymphat. Res. Biol. 2022, 20, 191–202. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Gradalski, T.; Ochalek, K. Lay Caregivers Education in Multicomponent Compression Bandaging in Obese Patients with Lower Limb Edema: A Case-Control Pilot Study. Lymphat. Res. Biol. 2020, 18, 428–432. [Google Scholar] [CrossRef]
- Mosti, G.; Mancini, S.; Bruni, S.; Serantoni, S.; Gazzabin, L.; Bucalossi, M.; Polignano, R.; Mariani, F.; Luca, B.; Partsch, H. Adjustable compression wrap devices are cheaper and more effective than inelastic bandages for venous leg ulcer healing. A Multicentric Italian Randomized Clinical Experience. Phlebology 2020, 35, 124–133. [Google Scholar] [CrossRef]
- Stather, P.; Petty, C.; Langthorne, H.; Rayner, E.; Zhang, J.; Hayden, K.; Howard, A. A randomised controlled clinical trial comparing the effectiveness of bandaging compared to the JuxtaCuresTM device in the management of people with venous ulceration: Feasibility study. Phlebology 2021, 36, 505–514. [Google Scholar] [CrossRef]
- Bjork, R.; Ehmann, S. STRIDE Professional guide to compression garment selection for the lower extremities. J. Wound Care 2019, 28, S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidhar, D.; Armer, J.M.; Stewart, B.R. What is clinically important in lymphedema management? A systematic review. Rehabil. Oncol. 2018, 36, 13–27. [Google Scholar] [CrossRef]

| Parameter | ACW Group (n = 18) | CB Group (n = 18) | p |
|---|---|---|---|
| Age (years) | 62.3 (9.4) | 69.6 (9.5) | 0.03 * |
| Body mass index (kg/m2) | 32.9 (4.1) | 28.2 (5.6) | 0.67 * |
| Dominant limb affected (patients no) | 10 | 12 | |
| Months since surgery | 55.0 (27.5–122.0) | 30.5 (14.0–126.3) | 0.45 ** |
| Type of surgery (patients no) | |||
| breast conserving | 7 | 3 | |
| mastectomy | 11 | 15 | 0.48 *** |
| axillary nodes dissection | 18 | 18 | |
| Adjuvant therapy (patients no) | |||
| chemotherapy | 18 | 13 | |
| axillary irradiation | 16 | 14 | |
| immunotherapy | 4 | 1 | |
| Affected limb volume (L) | 3.57 (3.08–4.22) | 3.05 (2.80–3.49) | 0.08 ** |
| Opposite limb volume (L) | 2.57 (2.19–3.18) | 2.3 (2.14–2.50) | 0.14 ** |
| Excess volume (%) | 39.3 (27.6–51.9) | 33.3 (25.3–39.7) | 0.41 ** |
| Volume | ACW Group (Median, IQR) | CB Group (Median, IQR) | p |
|---|---|---|---|
| Affected limb volume (L) | |||
| A before therapy | 3.57 (3.08–4.22) | 3.05 (2.80–3.49) | 0.08 ** |
| B after 1 week | 3.36 (2.84–3.96) | 2.77 (2.56–3.17) | 0.08 ** |
| C after 2 weeks | 3.36 (2.84–3.85) | 2.76 (2.50–3.12) | 0.02 ** |
| A > B,C | A > B> C | ||
| p < 0.001 * | p < 0.001 * | ||
| Excess volume reduction (%) | |||
| after 1 week | −8.3 (−14.38–−5.23) | −12.6 (−14.6–−10.9) | 0.12 ** |
| after 2 weeks | −10.4 (−15.4–−5.05) | −15.2 (−17.8–−10.6) | 0.17 ** |
| p = 0.42 *** | p = 0.3 *** | ||
| WAC (%) | −7.7 (−8.5–−5.1) | −10.1 (−12.3–−6.36) | 0.12 ** |
| Domain (NRS) | ACW Group (Median, IQR) | CB Group (Median, IQR) | p ** |
|---|---|---|---|
| Physical functioning in relation to compression a | |||
| A before therapy | 54 (48.0–65.3) | 51.5(50.0–61.0) | 0.77 |
| B after 1 week | 29 (22.3–41.8) | 39.0 (24.8–46.5) | 0.84 |
| C after 2 weeks | 25 (16.0–32.0) | 25.5 (15.5–34.0) | 0.69 |
| A > B,C | A > B,C | ||
| p < 0.001 * | p < 0.001 * | ||
| Disease-related symptoms b | |||
| before therapy | 23.0 (14.3–36.0) | 22.0 (17.3–27.3) | 0.49 |
| after 1 week | 15.0 (12–21.5) | 10.0 (3.3–13.8) | 0.02 |
| after 2 weeks | 13.5 (8.0–27.8) | 4.0 (0.3–10.8 | 0.001 |
| A > C | A > B> C | ||
| p = 0.005 * | p < 0.001 * | ||
| Compression and comfort c | |||
| after 1 week | 27.0 (22.5–29.8) | 26.0 (19.5–39.0) | 0.73 |
| after 2 weeks | 37.5 (22.5–43.5) | 31.5 (26.5–38.5) | 0.40 |
| p = 0.049 *** | p = 0.53 *** | ||
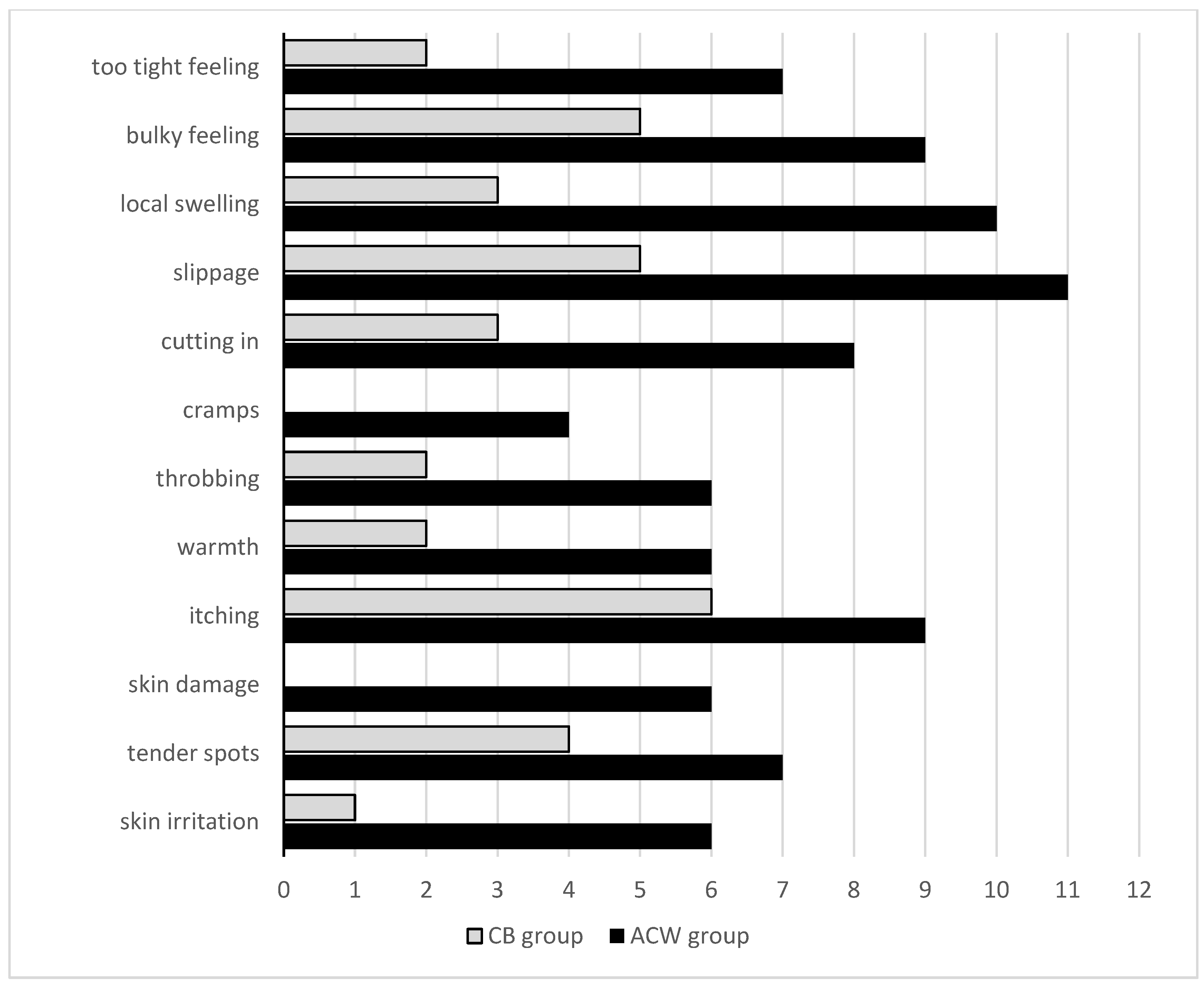
| Complications of compression d | |||
| after 1 week | 25.5 (18.0–35.5) | 7.0 (4.0–14.8) | <0.001 |
| after 2 weeks | 23.5 (14.3–40.3) | 7.0 (3.3–16.8) | 0.002 |
| p = 0.98 *** | p = 0.91 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochalek, K.; Kurpiewska, J.; Gradalski, T. Adjustable Compression Wraps (ACW) vs. Compression Bandaging (CB) in the Acute Phase of Breast Cancer-Related Arm Lymphedema Management—A Prospective Randomized Study. Biology 2023, 12, 534. https://doi.org/10.3390/biology12040534
Ochalek K, Kurpiewska J, Gradalski T. Adjustable Compression Wraps (ACW) vs. Compression Bandaging (CB) in the Acute Phase of Breast Cancer-Related Arm Lymphedema Management—A Prospective Randomized Study. Biology. 2023; 12(4):534. https://doi.org/10.3390/biology12040534
Chicago/Turabian StyleOchalek, Katarzyna, Joanna Kurpiewska, and Tomasz Gradalski. 2023. "Adjustable Compression Wraps (ACW) vs. Compression Bandaging (CB) in the Acute Phase of Breast Cancer-Related Arm Lymphedema Management—A Prospective Randomized Study" Biology 12, no. 4: 534. https://doi.org/10.3390/biology12040534
APA StyleOchalek, K., Kurpiewska, J., & Gradalski, T. (2023). Adjustable Compression Wraps (ACW) vs. Compression Bandaging (CB) in the Acute Phase of Breast Cancer-Related Arm Lymphedema Management—A Prospective Randomized Study. Biology, 12(4), 534. https://doi.org/10.3390/biology12040534