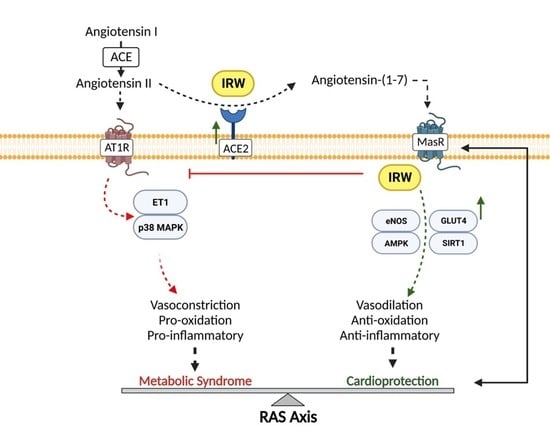
Tripeptide IRW Improves AMPK/eNOS Signaling Pathway via Activating ACE2 in the Aorta of High-Fat-Diet-Fed C57BL/6 Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Model Study
2.3. Tissue Collection
2.4. Protein Extraction and Western Blotting
2.5. Cell Culture
2.6. SiRNA Transfection
2.7. RT-PCR
2.8. Statistics
3. Results
3.1. IRW Treatment Upregulated ACE2 and Diminished ACE and AT1R Expression in the Aorta
3.2. IRW Enhanced AMPK/SIRT1/eNOS Cascade in Aorta of HFD Mice via Aortic ACE2 Activation
3.3. IRW Improved GLUT4 in Aorta of HFD Mice
3.4. IRW Downregulated ET1/MAPK Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jahandideh, F.; Wu, J. A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance. Food Sci. Hum. Wellness. 2022, 11, 1441–1454. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Shefer, G.; Stern, N. Adipose tissue renin–angiotensin–aldosterone system (RAAS) and progression of insulin resistance. Mol. Cell. Endocrinol. 2013, 378, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Chan, C.B.; Wu, J. Egg white ovotransferrin-derived ACE inhibitory peptide ameliorates angiotensin II-stimulated insulin resistance in skeletal muscle cells. Mol. Nutr. Food Res. 2018, 62, 1700602. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Fan, H.; Davidge, S.T.; Wu, J. Egg white–derived antihypertensive peptide IRW (Ile-Arg-Trp) reduces blood pressure in spontaneously hypertensive rats via the ACE2/ang (1–7)/mas receptor Axis. Mol. Nutr. Food Res. 2019, 63, 1900063. [Google Scholar] [CrossRef] [Green Version]
- Serfozo, P.; Wysocki, J.; Gulua, G.; Schulze, A.; Ye, M.; Liu, P.; Jin, J.; Bader, M.; Myöhänen, T.; García-Horsman, J.A. Ang II (angiotensin II) conversion to angiotensin-(1–7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension 2020, 75, 173–182. [Google Scholar] [CrossRef]
- Jahandideh, F.; Wu, J. Perspectives on the potential benefits of antihypertensive peptides towards metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2192. [Google Scholar] [CrossRef] [Green Version]
- Wu, J. A novel angiotensin converting enzyme 2 (ACE2) activating peptide: A reflection of 10 years of research on a small peptide Ile-Arg-Trp (IRW). J. Agric. Food Chem. 2020, 68, 14402–14408. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; He, G.; Wu, J. Molecular targets and mechanisms of bioactive peptides against metabolic syndromes. Food Funct. 2018, 9, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Bhullar, K.S.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg white-derived tripeptide IRW (Ile-Arg-Trp) is an activator of angiotensin converting enzyme 2. J. Agric. Food Chem. 2018, 66, 11330–11336. [Google Scholar] [CrossRef]
- Liao, W.; Fan, H.; Wu, J. Egg white-derived antihypertensive peptide IRW (Ile-Arg-Trp) inhibits angiotensin II-stimulated migration of vascular smooth muscle cells via angiotensin type I receptor. J. Agric. Food Chem. 2018, 66, 5133–5138. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.B.; Johns, D.; Watts, S.; Webb, R.C.; Brosius III, F.C. Decreased vascular glucose transporter expression and glucose uptake in DOCA-salt hypertension. J. Hypertens. 2001, 19, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; England, R.; Nguyen, K.; Charron, M.J.; Briggs, J.P.; Brosius, F., 3rd. Altered renal expression of the insulin-responsive glucose transporter GLUT4 in experimental diabetes mellitus. Am. J. Physiol. Renal Physiol. 1994, 267, 816–824. [Google Scholar] [CrossRef]
- Park, J.L.; Loberg, R.D.; Duquaine, D.; Zhang, H.; Deo, B.K.; Ardanaz, N.; Coyle, J.; Atkins, K.B.; Schin, M.; Charron, M.J. GLUT4 facilitative glucose transporter specifically and differentially contributes to agonist-induced vascular reactivity in mouse aorta. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1596–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Campos Zani, S.C.; Son, M.; Bhullar, K.S.; Chan, C.B.; Wu, J. IRW (Isoleucine–Arginine–Tryptophan) Improves Glucose Tolerance in High Fat Diet Fed C57BL/6 Mice via Activation of Insulin Signaling and AMPK Pathways in Skeletal Muscle. Biomedicines 2022, 10, 1235. [Google Scholar] [CrossRef]
- van den Oever, I.A.; Raterman, H.G.; Nurmohamed, M.T.; Simsek, S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010, 2010, 792393. [Google Scholar] [CrossRef] [Green Version]
- van Sloten, T.T.; Henry, R.M.; Dekker, J.M.; Nijpels, G.; Unger, T.; Schram, M.T.; Stehouwer, C.D. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: The Hoorn study. Hypertension 2014, 64, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Elzinga, S.E.; Savelieff, M.G.; O’Brien, P.D.; Mendelson, F.E.; Hayes, J.M.; Feldman, E.L. Sex differences in insulin resistance, but not peripheral neuropathy, in a diet-induced prediabetes mouse model. Dis. Model. Mech. 2021, 14, dmm048909. [Google Scholar] [CrossRef]
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-derived tri-peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS ONE 2013, 8, e82829. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L. High-carbohydrate, high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Modulatory effects of egg white ovotransferrin-derived tripeptide IRW (Ile-Arg-Trp) on vascular smooth muscle cells against angiotensin II stimulation. J. Agric. Food Chem. 2016, 64, 7342–7347. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zou, M.-H. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 2009, 120, 1266–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. 2010, 298, 751–760. [Google Scholar] [CrossRef]
- Haye, A.; Ansari, M.A.; Rahman, S.O.; Shamsi, Y.; Ahmed, D.; Sharma, M. Role of AMP-activated protein kinase on cardio-metabolic abnormalities in the development of diabetic cardiomyopathy: A molecular landscape. Eur. J. Pharmacol. 2020, 888, 173376. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, X.; Zhao, M.; Zhou, B.; Han, Y. Angiotensin-(1–7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci. Rep. 2016, 6, 34621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouallegue, A.; Bou Daou, G.; Srivastava, A.K. Endothelin-1-induced signaling pathways in vascular smooth muscle cells. Curr. Vasc. Pharmacol. 2007, 5, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.D.; Oh, H.; Kim, M.-S. Higher intakes of fruits, vegetables, and multiple individual nutrients is associated with a lower risk of metabolic syndrome among adults with comorbidities. Nutr. Res. 2022, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yarizadeh, H.; Setayesh, L.; Majidi, N.; Rasaei, N.; Mehranfar, S.; Ebrahimi, R.; Casazzza, K.; Mirzaei, K. Nutrient patterns and their relation to obesity and metabolic syndrome in Iranian overweight and obese adult women. Eat Weight Disord. 2022, 27, 1327–1337. [Google Scholar] [CrossRef]
- Prasad, H.; Ryan, D.A.; Celzo, M.F.; Stapleton, D. Metabolic syndrome: Definition and therapeutic implications. Postgrad. Med. 2012, 124, 21–30. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Torres, N.; Tovar, A.R. The renin–angiotensin system in adipose tissue and its metabolic consequences during obesity. J. Nutr. Biochem. 2013, 24, 2003–2015. [Google Scholar] [CrossRef]
- Skov, J.; Persson, F.; Frøkiær, J.; Christiansen, J.S. Tissue renin–angiotensin systems: A unifying hypothesis of metabolic disease. Front. Endocrinol. 2014, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Li, L.; Yi-Ming, W.; Li, Z.-Z.; Zhao, L.; Yu, Y.-S.; Li, D.-J.; Xia, C.-Y.; Liu, J.-G.; Su, D.-F. Local RAS and inflammatory factors are involved in cardiovascular hypertrophy in spontaneously hypertensive rats. Pharmacol. Res. 2008, 58, 196–201. [Google Scholar] [CrossRef]
- Mackenzie, R.W.; Elliott, B.T. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasnezhad, A.; Niazmand, S.; Mahmoudabady, M.; Rezaee, S.A.; Soukhtanloo, M.; Mosallanejad, R.; Hayatdavoudi, P. Nigella sativa L. seed regulated eNOS, VCAM-1 and LOX-1 genes expression and improved vasoreactivity in aorta of diabetic rat. J. Ethnopharmacol. 2019, 228, 142–147. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, C.F.; Hernández-Nuño, F.; Rio, D.D.; Ruiz-Hurtado, G.; Aránguez, I.; Ruiz-Gayo, M.; Somoza, B.; Fernández-Alfonso, M.S. High-fat diet induces endothelial dysfunction through a down-regulation of the endothelial AMPK–PI3K–Akt–eNOS pathway. Mol. Nutr. Food Res. 2015, 59, 520–532. [Google Scholar] [CrossRef]
- Ewart, M.-A.; Kennedy, S. AMPK and vasculoprotection. Pharmacol. Ther. 2011, 131, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Machha, A.; Schechter, A.N. Inorganic nitrate: A major player in the cardiovascular health benefits of vegetables? Nutr. Rev. 2012, 70, 367–372. [Google Scholar] [CrossRef]
- Triggle, C.R.; Ding, H. A review of endothelial dysfunction in diabetes: A focus on the contribution of a dysfunctional eNOS. J. Am. Soc. Hypertens. 2010, 4, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Harris, R.C. Role of endothelial nitric oxide synthase in diabetic nephropathy: Lessons from diabetic eNOS knockout mice. J. Diabetes Res. 2014, 2014, 590541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakumar, P.; Kathuria, S. Submaximal PPARγ activation and endothelial dysfunction: New perspectives for the management of cardiovascular disorders. Br. J. Pharmacol. 2012, 166, 1981–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-Z.; Cheng, Y.-W.; Jin, H.-Y.; Chang, Q.; Shang, Q.-H.; Xu, Y.-L.; Chen, L.-X.; Xu, R.; Song, B.; Zhong, J.-C. The sirtuin 6 prevents angiotensin II-mediated myocardial fibrosis and injury by targeting AMPK-ACE2 signaling. Oncotarget 2017, 8, 72302. [Google Scholar] [CrossRef] [Green Version]
- Ota, H.; Akishita, M.; Eto, M.; Iijima, K.; Kaneki, M.; Ouchi, Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell. Cardiol. 2007, 43, 571–579. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.-S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.-B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [Green Version]
- Karpe, P.A.; Tikoo, K. Heat shock prevents insulin resistance–induced vascular complications by augmenting angiotensin-(1-7) signaling. Diabetes 2014, 63, 1124–1139. [Google Scholar] [CrossRef] [Green Version]
- Jansson, P.A. Endothelial dysfunction in insulin resistance and type 2 diabetes. J. Intern. Med. 2007, 262, 173–183. [Google Scholar] [CrossRef]
- Ye, Y.; Zhong, X.; Li, N.; Pan, T. Protective effects of liraglutide on glomerular podocytes in obese mice by inhibiting the inflammatory factor TNF-α-mediated NF-κB and MAPK pathway. Obes. Res. Clin. Pract. 2019, 13, 385–390. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashkar, F.; Bhullar, K.S.; Jiang, X.; Wu, J. Tripeptide IRW Improves AMPK/eNOS Signaling Pathway via Activating ACE2 in the Aorta of High-Fat-Diet-Fed C57BL/6 Mice. Biology 2023, 12, 556. https://doi.org/10.3390/biology12040556
Ashkar F, Bhullar KS, Jiang X, Wu J. Tripeptide IRW Improves AMPK/eNOS Signaling Pathway via Activating ACE2 in the Aorta of High-Fat-Diet-Fed C57BL/6 Mice. Biology. 2023; 12(4):556. https://doi.org/10.3390/biology12040556
Chicago/Turabian StyleAshkar, Fatemeh, Khushwant S. Bhullar, Xu Jiang, and Jianping Wu. 2023. "Tripeptide IRW Improves AMPK/eNOS Signaling Pathway via Activating ACE2 in the Aorta of High-Fat-Diet-Fed C57BL/6 Mice" Biology 12, no. 4: 556. https://doi.org/10.3390/biology12040556
APA StyleAshkar, F., Bhullar, K. S., Jiang, X., & Wu, J. (2023). Tripeptide IRW Improves AMPK/eNOS Signaling Pathway via Activating ACE2 in the Aorta of High-Fat-Diet-Fed C57BL/6 Mice. Biology, 12(4), 556. https://doi.org/10.3390/biology12040556