Intergenerational Perioperative Neurocognitive Disorder
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Pathogenesis of PND
3.1. Hypothalamic-Pituitary-Adrenal Axis and PND
Stress-like Effects of GABAergic Anesthetics
3.2. Inflammation
3.2.1. Peripheral Inflammatory Response
3.2.2. Disrupting the Integrity of the BBB
3.2.3. Activation of Microglia
3.3. Interaction of Stress and Inflammation
3.4. Epigenetic Mechanisms
3.4.1. DNA Methylation
3.4.2. Histone Modifications
3.4.3. Noncoding RNA
3.5. Intergenerational PND
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, M.S.; Maruff, P.; Silbert, B.S.; Evered, L.A.; Scott, D.A. Detection of Postoperative Cognitive Decline After Coronary Artery Bypass Graft Surgery Is Affected by the Number of Neuropsychological Tests in the Assessment Battery. Ann. Thorac. Surg. 2006, 81, 2097–2104. [Google Scholar] [CrossRef]
- Rosa, G. General Anesthesia and Postoperative Cognitive Dysfunction. J. Alzheimer’s Dis. Park. 2012, 2, e113. [Google Scholar] [CrossRef]
- Bilotta, F.; Qeva, E.; Matot, I. Anesthesia and Cognitive Disorders: A Systematic Review of the Clinical Evidence. Expert Rev. Neurother. 2016, 16, 1311–1320. [Google Scholar] [CrossRef]
- Evered, L.A.; Silbert, B.S.; Scott, D.A.; Maruff, P.; Ames, D. Prevalence of Dementia 7.5 Years after Coronary Artery Bypass Graft Surgery. Anesthesiology 2016, 125, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Olotu, C. Postoperative Neurocognitive Disorders. Curr. Opin. Anaesthesiol. 2020, 33, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Vacas, S.; Canales, C.; Deiner, S.G.; Cole, D.J. Perioperative Brain Health in the Older Adult: A Patient Safety Imperative. Anesth. Analg. 2022, 135, 316–328. [Google Scholar] [CrossRef]
- Lewis, C.; Dokucu, M.E.; Brown, C.H.; Balmert, L.; Srdanovic, N.; Madhan, A.S.; Samra, S.S.; Csernansky, J.; Grafman, J.; Hogue, C.W. Postoperative but Not Preoperative Depression Is Associated with Cognitive Impairment after Cardiac Surgery: Exploratory Analysis of Data from a Randomized Trial. BMC Anesthesiol. 2022, 22, 157. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Panda, N.; Sabharwal, P.; Luthra, A.; Balu, M.; Chauhan, R.; Bhagat, H. Effect of Anesthetic Agents on Cognitive Function and Peripheral Inflammatory Biomarkers in Young Patients Undergoing Surgery for Spine Disorders. Asian J. Neurosurg. 2019, 14, 1095–1105. [Google Scholar] [CrossRef]
- Edipoglu, I.S.; Celik, F. The Associations Between Cognitive Dysfunction, Stress Biomarkers, and Administered Anesthesia Type in Total Knee Arthroplasties: Prospective, Randomized Trial. Pain Physician 2019, 22, 495–507. [Google Scholar] [CrossRef]
- Eckenhoff, R.G.; Maze, M.; Xie, Z.; Culley, D.J.; Goodlin, S.J.; Zuo, Z.; Wei, H.; Whittington, R.A.; Terrando, N.; Orser, B.A.; et al. Perioperative Neurocognitive Disorder. Anesthesiology 2020, 132, 55–68. [Google Scholar] [CrossRef]
- Hogue, C.W.; Grafman, J. Aligning Nomenclature for Cognitive Changes Associated with Anaesthesia and Surgery with Broader Diagnostic Classifications of Non-Surgical Populations: A Needed First Step. Br. J. Anaesth. 2018, 121, 991–993. [Google Scholar] [CrossRef] [Green Version]
- Evered, L.; Silbert, B.; Knopman, D.S.; Scott, D.A.; DeKosky, S.T.; Rasmussen, L.S.; Oh, E.S.; Crosby, G.; Berger, M.; Eckenhoff, R.G.; et al. Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery—2018. Anesthesiology 2018, 129, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Xu, L.; Wang, D. Perioperative Neurocognitive Disorders: A Narrative Review Focusing on Diagnosis, Prevention, and Treatment. CNS Neurosci. Ther. 2022, 28, 1147–1167. [Google Scholar] [CrossRef] [PubMed]
- Soehle, M.; Coburn, M. Risk Assessment of Perioperative Neurocognitive Disorders, Where Are We Now? Curr. Opin. Anaesthesiol. 2022, 35, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Androsova, G.; Krause, R.; Winterer, G.; Schneider, R. Biomarkers of Postoperative Delirium and Cognitive Dysfunction. Front. Aging Neurosci. 2015, 7, 112. [Google Scholar] [CrossRef]
- Chen, L.; Au, E.; Saripella, A.; Kapoor, P.; Yan, E.; Wong, J.; Tang-Wai, D.F.; Gold, D.; Riazi, S.; Suen, C.; et al. Postoperative Outcomes in Older Surgical Patients with Preoperative Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Clin. Anesth. 2022, 80, 110883. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, X.; Li, M.; Jiang, Y.; Zhang, H. Identification of Individuals at Risk for Postoperative Cognitive Dysfunction (POCD). Ther. Adv. Neurol. Disord. 2022, 15, 175628642211143. [Google Scholar] [CrossRef]
- Culley, D.J.; Baxter, M.; Yukhananov, R.; Crosby, G. The Memory Effects of General Anesthesia Persist for Weeks in Young and Aged Rats. Anesth. Analg. 2003, 1004–1009. [Google Scholar] [CrossRef]
- Crosby, C.; Culley, D.J.; Baxter, M.G.; Yukhananov, R.; Crosby, G. Spatial Memory Performance 2 Weeks After General Anesthesia in Adult Rats. Anesth. Analg. 2005, 101, 1389–1392. [Google Scholar] [CrossRef]
- Landin, J.D.; Palac, M.; Carter, J.M.; Dzumaga, Y.; Santerre-Anderson, J.L.; Fernandez, G.M.; Savage, L.M.; Varlinskaya, E.I.; Spear, L.P.; Moore, S.D.; et al. General Anesthetic Exposure in Adolescent Rats Causes Persistent Maladaptations in Cognitive and Affective Behaviors and Neuroplasticity. Neuropharmacology 2019, 150, 153–163. [Google Scholar] [CrossRef]
- Guo, S.; Liu, L.; Wang, C.; Jiang, Q.; Dong, Y.; Tian, Y. Repeated Exposure to Sevoflurane Impairs the Learning and Memory of Older Male Rats. Life Sci. 2018, 192, 75–83. [Google Scholar] [CrossRef]
- Ji, M.; Dong, L.; Jia, M.; Liu, W.; Zhang, M.; Ju, L.; Yang, J.; Xie, Z.; Yang, J. Epigenetic Enhancement of Brain-Derived Neurotrophic Factor Signaling Pathway Improves Cognitive Impairments Induced by Isoflurane Exposure in Aged Rats. Mol. Neurobiol. 2014, 50, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Jin, Q.; Song, D.; Diao, Y. Kappa Opioid Receptor Agonists Improve Postoperative Cognitive Dysfunction in Rats via the JAK2/STAT3 Signaling Pathway. Int. J. Mol. Med. 2019, 44, 1866–1876. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, X.; Ye, L.; Bai, Y.; Zhang, H.; Xuan, Z.; Feng, Y.; Zhang, P.; Chen, Y.; Yan, Y.; et al. Edaravone at High Concentrations Attenuates Cognitive Dysfunctions Induced by Abdominal Surgery under General Anesthesia in Aged Mice. Metab. Brain Dis. 2020, 35, 373–383. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, W.; Wang, T.; Wen, H.; Yao, L. Up-Regulation of MiR-106a Targets LIMK1 and Contributes to Cognitive Impairment Induced by Isoflurane Anesthesia in Mice. Genes Genom. 2020, 42, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.-L.; Pan, W.; Luo, D.; Zhang, G.-F.; Zhou, Z.-Q.; Sun, X.-Y.; Yang, J.-J.; Ji, M.-H. Dysregulation of BDNF/TrkB Signaling Mediated by NMDAR/Ca2+/Calpain Might Contribute to Postoperative Cognitive Dysfunction in Aging Mice. J. Neuroinflamm. 2020, 17, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monk, T.G.; Craig Weldon, B.; Garvan, C.W.; Dede, D.E.; van der Aa, M.T.; Heilman, K.M.; Gravenstein, J.S. Predictors of Cognitive Dysfunction after Major Noncardiac Surgery. J. Am. Soc. Anesthesiol. 2008, 108, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- le Freche, H.; Brouillette, J.; Fernandez-Gomez, F.-J.; Patin, P.; Caillierez, R.; Zommer, N.; Sergeant, N.; Buée-Scherrer, V.; Lebuffe, G.; Blum, D.; et al. Tau Phosphorylation and Sevoflurane Anesthesia. Anesthesiology 2012, 116, 779–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengel, K.F.; Pandharipande, P.P.; Hughes, C.G. Special Considerations for the Aging Brain and Perioperative Neurocognitive Dysfunction. Anesthesiol. Clin. 2019, 37, 521–536. [Google Scholar] [CrossRef]
- Moller, J.T.; Cluitmans, P.; Rasmussen, L.S.; Houx, P.; Rasmussen, H.; Canet, J.; Rabbitt, P.; Jolles, J.; Larsen, K.; Hanning, C.D.; et al. Long-Term Postoperative Cognitive Dysfunction in the Elderly ISPOCD1 Study. ISPOCD Investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998, 351, 857–861. [Google Scholar] [CrossRef]
- Newman, S.; Stygall, J.; Hirani, S.; Shaefi, S.; Maze, M. Postoperative Cognitive Dysfunction after Noncardiac Surgery: A Systematic Review. Anesthesiology 2007, 106, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Nugent, B.M.; Bale, T.L. Parental Advisory: Maternal and Paternal Stress Can Impact Offspring Neurodevelopment. Biol. Psychiatry 2018, 83, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, A.; Jehle, K.-L.; Mansuy, I.M. Impact of Parental Exposure on Offspring Health in Humans. Trends Genet. 2021, 37, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Fitz-James, M.H.; Cavalli, G. Molecular Mechanisms of Transgenerational Epigenetic Inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Bošković, A.; Rando, O.J. Transgenerational Epigenetic Inheritance. Annu. Rev. Genet. 2018, 52, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Daskalakis, N.P.; Lehrner, A.; Desarnaud, F.; Bader, H.N.; Makotkine, I.; Flory, J.D.; Bierer, L.M.; Meaney, M.J. Influences of Maternal and Paternal PTSD on Epigenetic Regulation of the Glucocorticoid Receptor Gene in Holocaust Survivor Offspring. Am. J. Psychiatry 2014, 171, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, A.B.; Morgan, C.P.; Bronson, S.L.; Revello, S.; Bale, T.L. Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation. J. Neurosci. 2013, 33, 9003–9012. [Google Scholar] [CrossRef] [Green Version]
- Rompala, G.R.; Simons, A.; Kihle, B.; Homanics, G.E. Paternal Preconception Chronic Variable Stress Confers Attenuated Ethanol Drinking Behavior Selectively to Male Offspring in a Pre-Stress Environment Dependent Manner. Front. Behav. Neurosci. 2018, 12, 257. [Google Scholar] [CrossRef]
- van Steenwyk, G.; Roszkowski, M.; Manuella, F.; Franklin, T.B.; Mansuy, I.M. Transgenerational Inheritance of Behavioral and Metabolic Effects of Paternal Exposure to Traumatic Stress in Early Postnatal Life: Evidence in the 4th Generation. Environ. Epigenet. 2018, 4, dvy023. [Google Scholar] [CrossRef]
- Dagher, J.B.; Hahn-Townsend, C.K.; Kaimal, A.; al Mansi, M.; Henriquez, J.E.; Tran, D.G.; Laurent, C.R.; Bacak, C.J.; Buechter, H.E.; Cambric, C.; et al. Independent and Combined Effects of Bisphenol A and Diethylhexyl Phthalate on Gestational Outcomes and Offspring Development in Sprague-Dawley Rats. Chemosphere 2021, 263, 128307. [Google Scholar] [CrossRef]
- Drobná, Z.; Henriksen, A.D.; Wolstenholme, J.T.; Montiel, C.; Lambeth, P.S.; Shang, S.; Harris, E.P.; Zhou, C.; Flaws, J.A.; Adli, M.; et al. Transgenerational Effects of Bisphenol A on Gene Expression and DNA Methylation of Imprinted Genes in Brain. Endocrinology 2018, 159, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Brehm, E.; Flaws, J.A. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulyas, L.; Powell, J.R. Predicting the Future: Parental Progeny Investment in Response to Environmental Stress Cues. Front. Cell Dev. Biol. 2019, 7, 115. [Google Scholar] [CrossRef]
- van Cauwenbergh, O.; di Serafino, A.; Tytgat, J.; Soubry, A. Transgenerational Epigenetic Effects from Male Exposure to Endocrine-Disrupting Compounds: A Systematic Review on Research in Mammals. Clin. Epigenetics 2020, 12, 65. [Google Scholar] [CrossRef]
- Koabel, J.; McNivens, M.; McKee, P.; Pautassi, R.; Bordner, K.; Nizhnikov, M. The Offspring of Alcohol-Exposed Sires Exhibit Heightened Ethanol Intake and Behavioral Alterations in the Elevated plus Maze. Alcohol 2021, 92, 65–72. [Google Scholar] [CrossRef]
- Curtis, S.W.; Conneely, K.N.; Marder, M.E.; Terrell, M.L.; Marcus, M.; Smith, A.K. Intergenerational Effects of Endocrine-Disrupting Compounds: A Review of the Michigan Polybrominated Biphenyl Registry. Epigenomics 2018, 10, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Hipwell, A.E.; Tung, I.; Northrup, J.; Keenan, K. Transgenerational Associations between Maternal Childhood Stress Exposure and Profiles of Infant Emotional Reactivity. Dev. Psychopathol. 2019, 31, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Lehrner, A. Intergenerational Transmission of Trauma Effects: Putative Role of Epigenetic Mechanisms. World Psychiatry 2018, 17, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Sproul Bassett, A.M.; Wood, E.K.; Lindell, S.G.; Schwandt, M.L.; Barr, C.S.; Suomi, S.J.; Higley, J.D. Intergenerational Effects of Mother’s Early Rearing Experience on Offspring Treatment and Socioemotional Development. Dev. Psychobiol. 2020, 62, 920–931. [Google Scholar] [CrossRef]
- Day, G.S.; Cruchaga, C.; Wingo, T.; Schindler, S.E.; Coble, D.; Morris, J.C. Association of Acquired and Heritable Factors With Intergenerational Differences in Age at Symptomatic Onset of Alzheimer Disease Between Offspring and Parents With Dementia. JAMA Netw. Open 2019, 2, e1913491. [Google Scholar] [CrossRef]
- Letcher, P.; Greenwood, C.J.; Romaniuk, H.; Spry, E.; Macdonald, J.A.; McAnally, H.; Thomson, K.C.; Youssef, G.; Hutchinson, D.; McIntosh, J.; et al. Adolescent and Young Adult Mental Health Problems and Infant Offspring Behavior: Findings from a Prospective Intergenerational Cohort Study. J. Affect. Disord. 2020, 272, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, C.L.; Dilks, D.D.; McKenna, B.G.; Dunlop, A.L.; Corwin, E.J.; Brennan, P.A. Maternal Childhood Adversity Associates With Frontoamygdala Connectivity in Neonates. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 470–478. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; Xu, C.; Bader, H.N.; Chatzinakos, C.; Weber, P.; Makotkine, I.; Lehrner, A.; Bierer, L.M.; Binder, E.B.; Yehuda, R. Intergenerational Trauma Is Associated with Expression Alterations in Glucocorticoid- and Immune-Related Genes. Neuropsychopharmacology 2021, 46, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Baratta, A.M.; Rathod, R.S.; Plasil, S.L.; Seth, A.; Homanics, G.E. Exposure to Drugs of Abuse Induce Effects That Persist across Generations. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2021; pp. 217–277. [Google Scholar]
- Bowers, M.E.; Yehuda, R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacology 2016, 41, 232–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-L.V.; Forestier, S.; Corces, V.G. Exposure to Sevoflurane Results in Changes of Transcription Factor Occupancy in Sperm and Inheritance of Autism†. Biol. Reprod. 2021, 105, 705–719. [Google Scholar] [CrossRef]
- Ju, L.-S.; Yang, J.-J.; Xu, N.; Li, J.; Morey, T.E.; Gravenstein, N.; Seubert, C.N.; Setlow, B.; Martynyuk, A.E. Intergenerational Effects of Sevoflurane in Young Adult Rats. Anesthesiology 2019, 131, 1092–1109. [Google Scholar] [CrossRef]
- Ju, L.-S.; Yang, J.-J.; Morey, T.E.; Gravenstein, N.; Seubert, C.N.; Resnick, J.L.; Zhang, J.-Q.; Martynyuk, A.E. Role of Epigenetic Mechanisms in Transmitting the Effects of Neonatal Sevoflurane Exposure to the next Generation of Male, but Not Female, Rats. Br. J. Anaesth. 2018, 121, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Lei, L.; Lin, Y.; Ju, L.-S.; Morey, T.E.; Gravenstein, N.; Yang, J.; Martynyuk, A.E. A Methyltransferase Inhibitor (Decitabine) Alleviates Intergenerational Effects of Paternal Neonatal Exposure to Anesthesia With Sevoflurane. Anesth. Analg. 2020, 131, 1291–1299. [Google Scholar] [CrossRef]
- Chastain-Potts, S.E.; Tesic, V.; Tat, Q.L.; Cabrera, O.H.; Quillinan, N.; Jevtovic-Todorovic, V. Sevoflurane Exposure Results in Sex-Specific Transgenerational Upregulation of Target IEGs in the Subiculum. Mol. Neurobiol. 2020, 57, 11–22. [Google Scholar] [CrossRef]
- Senaldi, L.; Smith-Raska, M. Evidence for Germline Non-Genetic Inheritance of Human Phenotypes and Diseases. Clin. Epigenetics 2020, 12, 136. [Google Scholar] [CrossRef]
- Siddeek, B.; Mauduit, C.; Simeoni, U.; Benahmed, M. Sperm Epigenome as a Marker of Environmental Exposure and Lifestyle, at the Origin of Diseases Inheritance. Mutat. Res./Rev. Mutat. Res. 2018, 778, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Beijers, R.; Scher, A.; Ohana, H.; Maayan-Metzger, A.; Leshem, M. Exposure to War Prior to Conception: Maternal Emotional Distress Forecasts Sex-Specific Child Behavior Problems. Int. J. Environ. Res. Public Health 2022, 19, 3802. [Google Scholar] [CrossRef] [PubMed]
- Bygren, L.; Tinghög, P.; Carstensen, J.; Edvinsson, S.; Kaati, G.; Pembrey, M.E.; Sjöström, M. Change in Paternal Grandmothers’ Early Food Supply Influenced Cardiovascular Mortality of the Female Grandchildren. BMC Genet. 2014, 15, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pembrey, M.E.; Bygren, L.O.; Kaati, G.; Edvinsson, S.; Northstone, K.; Sjöström, M.; Golding, J. Sex-Specific, Male-Line Transgenerational Responses in Humans. Eur. J. Hum. Genet. 2006, 14, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Yehuda, R.; Flory, J.D.; Bierer, L.M.; Henn-Haase, C.; Lehrner, A.; Desarnaud, F.; Makotkine, I.; Daskalakis, N.P.; Marmar, C.R.; Meaney, M.J. Lower Methylation of Glucocorticoid Receptor Gene Promoter 1F in Peripheral Blood of Veterans with Posttraumatic Stress Disorder. Biol. Psychiatry 2015, 77, 356–364. [Google Scholar] [CrossRef]
- Sack, W.H.; Clarke, G.N.; Seeley, J. Posttraumatic Stress Disorder across Two Generations of Cambodian Refugees. J. Am. Acad. Child Adolesc. Psychiatry 1995, 34, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Solomon, Z.; Kotler, M.; Mikulincer, M. Combat-Related Posttraumatic Stress Disorder among Second-Generation Holocaust Survivors: Preliminary Findings. Am. J. Psychiatry 1988, 145, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Lehrner, A.; Bierer, L.M.; Passarelli, V.; Pratchett, L.C.; Flory, J.D.; Bader, H.N.; Harris, I.R.; Bedi, A.; Daskalakis, N.P.; Makotkine, I.; et al. Maternal PTSD Associates with Greater Glucocorticoid Sensitivity in Offspring of Holocaust Survivors. Psychoneuroendocrinology 2014, 40, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Perroud, N.; Rutembesa, E.; Paoloni-Giacobino, A.; Mutabaruka, J.; Mutesa, L.; Stenz, L.; Malafosse, A.; Karege, F. The Tutsi Genocide and Transgenerational Transmission of Maternal Stress: Epigenetics and Biology of the HPA Axis. World J. Biol. Psychiatry 2014, 15, 334–345. [Google Scholar] [CrossRef]
- Rasmussen, L.S. Postoperative Cognitive Dysfunction: Incidence and Prevention. Best Pr. Res. Clin. Anaesthesiol. 2006, 20, 315–330. [Google Scholar] [CrossRef]
- Bedford, P.D. Adverse Cerebral Effects of Anaesthesia on Old People. Lancet 1955, 269, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.R.; Williams, M.; Scott, J.F.; Smith, A.C. The Effects of Anesthesia and Elective Surgery on Old People. Lancet 1961, 2, 887–893. [Google Scholar]
- Dijkstra, J.B.; Jolles, J. Postoperative Cognitive Dysfunction versus Complaints: A Discrepancy in Long-Term Findings. Neuropsychol. Rev. 2002, 12, 1–14. [Google Scholar] [CrossRef]
- Williams-Russo, P.; Sharrock, N.E.; Mattis, S.; Szatrowski, T.P.; Charlson, M.E. Cognitive Effects after Epidural vs General Anesthesia in Older Adults. A Randomized Trial. JAMA 1995, 274, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ancelin, M.L.; de Roquefeuil, G.; Ledésert, B.; Bonnel, F.; Cheminal, J.C.; Ritchie, K. Exposure to Anaesthetic Agents, Cognitive Functioning and Depressive Symptomatology in the Elderly. Br. J. Psychiatry 2001, 178, 360–366. [Google Scholar] [CrossRef]
- Paredes, S.; Cortínez, L.; Contreras, V.; Silbert, B. Post-Operative Cognitive Dysfunction at 3 Months in Adults after Non-Cardiac Surgery: A Qualitative Systematic Review. Acta Anaesthesiol. Scand. 2016, 60, 1043–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, J.P. The Neuroendocrinology of Stress: Glucocorticoid Signaling Mechanisms. Psychoneuroendocrinology 2022, 137, 105641. [Google Scholar] [CrossRef] [PubMed]
- Leistner, C.; Menke, A. Hypothalamic–Pituitary–Adrenal Axis and Stress. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 55–64. [Google Scholar]
- Sapolsky, R.M. Stress Hormones: Good and Bad. Neurobiol. Dis. 2000, 7, 540–542. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of Stress in the Brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of Glucocorticoid Negative Feedback in the Regulation of HPA Axis Pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Mody, I.; Maguire, J. The Reciprocal Regulation of Stress Hormones and GABAA Receptors. Front. Cell Neurosci. 2012, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the Brain: From Adaptation to Disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Viho, E.M.G.; Buurstede, J.C.; Mahfouz, A.; Koorneef, L.L.; van Weert, L.T.C.M.; Houtman, R.; Hunt, H.J.; Kroon, J.; Meijer, O.C. Corticosteroid Action in the Brain: The Potential of Selective Receptor Modulation. Neuroendocrinology 2019, 109, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Meijer, O.C.; Buurstede, J.C.; Viho, E.M.G.; Amaya, J.M.; Koning, A.C.A.M.; van der Meulen, M.; van Weert, L.T.C.M.; Paul, S.N.; Kroon, J.; Koorneef, L.L. Transcriptional Glucocorticoid Effects in the Brain: Finding the Relevant Target Genes. J. Neuroendocrinol. 2022, 35, 13213. [Google Scholar] [CrossRef]
- Koning, A.-S.C.A.M.; Buurstede, J.C.; van Weert, L.T.C.M.; Meijer, O.C. Glucocorticoid and Mineralocorticoid Receptors in the Brain: A Transcriptional Perspective. J. Endocr. Soc. 2019, 3, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M. Corticosteroids and the Brain. J. Endocrinol. 2018, 238, R121–R130. [Google Scholar] [CrossRef] [Green Version]
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The Effects of Stress and Stress Hormones on Human Cognition: Implications for the Field of Brain and Cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef] [Green Version]
- REUL, J.M.H.M.; KLOET, E.R. de Two Receptor Systems for Corticosterone in Rat Brain: Microdistribution and Differential Occupation. Endocrinology 1985, 117, 2505–2511. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oitzl, M.S.; de Kloet, E.R. Selective Corticosteroid Antagonists Modulate Specific Aspects of Spatial Orientation Learning. Behav. Neurosci. 1992, 106, 62–71. [Google Scholar] [CrossRef]
- Oitzl, M.S.; Reichardt, H.M.; Joëls, M.; de Kloet, E.R. Point Mutation in the Mouse Glucocorticoid Receptor Preventing DNA Binding Impairs Spatial Memory. Proc. Natl. Acad. Sci. USA 2001, 98, 12790–12795. [Google Scholar] [CrossRef] [Green Version]
- Sarabdjitsingh, R.A.; Jezequel, J.; Pasricha, N.; Mikasova, L.; Kerkhofs, A.; Karst, H.; Groc, L.; Joëls, M. Ultradian Corticosterone Pulses Balance Glutamatergic Transmission and Synaptic Plasticity. Proc. Natl. Acad. Sci. USA 2014, 111, 14265–14270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roozendaal, B.; Griffith, Q.K.; Buranday, J.; de Quervain, D.J.-F.; McGaugh, J.L. The Hippocampus Mediates Glucocorticoid-Induced Impairment of Spatial Memory Retrieval: Dependence on the Basolateral Amygdala. Proc. Natl. Acad. Sci. USA 2003, 100, 1328–1333. [Google Scholar] [CrossRef] [Green Version]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological Functions of Glucocorticoids in Stress and Their Relation to Pharmacological Actions*. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic Stress, Glucocorticoid Receptor Resistance, Inflammation, and Disease Risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisht, K.; Sharma, K.; Tremblay, M.-È. Chronic Stress as a Risk Factor for Alzheimer’s Disease: Roles of Microglia-Mediated Synaptic Remodeling, Inflammation, and Oxidative Stress. Neurobiol. Stress 2018, 9, 9–21. [Google Scholar] [CrossRef]
- Tatomir, A. The Impact of Stress and Glucocorticoids on Memory. Clujul. Med. 2014, 87, 3–6. [Google Scholar] [CrossRef] [Green Version]
- de Kloet, E.R.; Oitzl, M.S.; Joëls, M. Stress and Cognition: Are Corticosteroids Good or Bad Guys? Trends Neurosci. 1999, 22, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Ostroumov, A.; Thomas, A.M.; Kimmey, B.A.; Karsch, J.S.; Doyon, W.M.; Dani, J.A. Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area. Neuron 2016, 92, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Steptoe, A.; Hamer, M.; Chida, Y. The Effects of Acute Psychological Stress on Circulating Inflammatory Factors in Humans: A Review and Meta-Analysis. Brain Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef]
- Marques, A.H.; Silverman, M.N.; Sternberg, E.M. Glucocorticoid Dysregulations and Their Clinical Correlates. Ann. N. Y. Acad. Sci. 2009, 1179, 1–18. [Google Scholar] [CrossRef]
- Patchev, V. The Neurosteroid Tetrahydroprogesterone Attenuates the Endocrine Response to Stress and Exerts Glucocorticoid-like Effects on Vasopressin Gene Transcription in the Rat Hypothalamus. Neuropsychopharmacology 1996, 15, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Patchev, V.K.; Shoaib, M.; Holsboer, F.; Almeida, O.F.X. The Neurosteroid Tetrahydroprogesterone Counteracts Corticotropin-Releasing Hormone-Induced Anxiety and Alters the Release and Gene Expression of Corticotropin-Releasing Hormone in the Rat Hypothalamus. Neuroscience 1994, 62, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Yan, Q.; Al-Tarrah, K.; Akturk, H.K.; Prokop, L.J.; Alahdab, F.; Foster, M.A.; Lord, J.M.; Karavitaki, N.; Wass, J.A.; et al. The Cortisol Stress Response Induced by Surgery: A Systematic Review and Meta-Analysis. Clin. Endocrinol. 2018, 89, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Colkesen, Y.; Giray, S.; Ozenli, Y.; Sezgin, N.; Coskun, I. Relation of Serum Cortisol to Delirium Occurring after Acute Coronary Syndromes. Am. J. Emerg. Med. 2013, 31, 161–165. [Google Scholar] [CrossRef]
- van Munster, B.C.; Bisschop, P.H.; Zwinderman, A.H.; Korevaar, J.C.; Endert, E.; Wiersinga, W.J.; van Oosten, H.E.; Goslings, J.C.; Rooij, S.E.J.A. de Cortisol, Interleukins and S100B in Delirium in the Elderly. Brain Cogn. 2010, 74, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerejeira, J.; Batista, P.; Nogueira, V.; Vaz-Serra, A.; Mukaetova-Ladinska, E.B. The Stress Response to Surgery and Postoperative Delirium. J. Geriatr. Psychiatry Neurol. 2013, 26, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bisschop, P.H.; de Rooij, S.E.; Zwinderman, A.H.; van Oosten, H.E.; van Munster, B.C. Cortisol, Insulin, and Glucose and the Risk of Delirium in Older Adults with Hip Fracture. J. Am. Geriatr. Soc. 2011, 59, 1692–1696. [Google Scholar] [CrossRef]
- Pearson, A.; de Vries, A.; Middleton, S.D.; Gillies, F.; White, T.O.; Armstrong, I.R.; Andrew, R.; Seckl, J.R.; MacLullich, A.M. Cerebrospinal Fluid Cortisol Levels Are Higher in Patients with Delirium versus Controls. BMC Res. Notes 2010, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Jia, P.; Zhang, J.; Zhang, X.; Zhang, Y.; Jiang, H.; Jiang, W.; Guo, Y. Production of Inflammatory Cytokines, Cortisol, and Aβ1-40 in Elderly Oral Cancer Patients with Postoperative Delirium. Neuropsychiatr. Dis. Treat 2016, 12, 2789–2795. [Google Scholar] [CrossRef] [Green Version]
- Mu, D.-L.; Wang, D.-X.; Li, L.-H.; Shan, G.-J.; Li, J.; Yu, Q.-J.; Shi, C.-X. High Serum Cortisol Level Is Associated with Increased Risk of Delirium after Coronary Artery Bypass Graft Surgery: A Prospective Cohort Study. Crit. Care 2010, 14, R238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaschke, K.; Fichtenkamm, P.; Schramm, C.; Hauth, S.; Martin, E.; Verch, M.; Karck, M.; Kopitz, J. Early Postoperative Delirium after Open-Heart Cardiac Surgery Is Associated with Decreased Bispectral EEG and Increased Cortisol and Interleukin-6. Intensive Care Med. 2010, 36, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, J.; Banys, A.; Latek, J.; Bourke, J.; Jaszewski, R. Cortisol Levels and Neuropsychiatric Diagnosis as Markers of Postoperative Delirium: A Prospective Cohort Study. Crit. Care 2013, 17, R38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Q.; Qian, X.; An, J.; Wen, H.; Cope, D.K.; Williams, J.P. Higher Dose Dexamethasone Increases Early Postoperative Cognitive Dysfunction. J. Neurosurg. Anesthesiol. 2014, 26, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Manenschijn, L.; van Rossum, E.F.C.; Jetten, A.M.; de Rooij, S.E.; van Munster, B.C. Glucocorticoid Receptor Haplotype Is Associated with a Decreased Risk of Delirium in the Elderly. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2011, 156, 316–321. [Google Scholar] [CrossRef]
- Qiao, Y.; Feng, H.; Zhao, T.; Yan, H.; Zhang, H.; Zhao, X. Postoperative Cognitive Dysfunction after Inhalational Anesthesia in Elderly Patients Undergoing Major Surgery: The Influence of Anesthetic Technique, Cerebral Injury and Systemic Inflammation. BMC Anesthesiol. 2015, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Valentin, L.S.S.; Pereira, V.F.A.; Pietrobon, R.S.; Schmidt, A.P.; Oses, J.P.; Portela, L.V.; Souza, D.O.; Vissoci, J.R.N.; da Luz, V.F.; de Araujo de Souza Trintoni, L.M.; et al. Effects of Single Low Dose of Dexamethasone before Noncardiac and Nonneurologic Surgery and General Anesthesia on Postoperative Cognitive Dysfunction—A Phase III Double Blind, Randomized Clinical Trial. PLoS ONE 2016, 11, e0152308. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, L.; Hermans, E.J.; Joëls, M.; Roozendaal, B. Mechanisms of Memory under Stress. Neuron 2022, 110, 1450–1467. [Google Scholar] [CrossRef]
- Spiga, F.; Zavala, E.; Walker, J.J.; Zhao, Z.; Terry, J.R.; Lightman, S.L. Dynamic Responses of the Adrenal Steroidogenic Regulatory Network. Proc. Natl. Acad. Sci. USA 2017, 114, E6466–E6474. [Google Scholar] [CrossRef] [Green Version]
- Rains, P.C.; Rampersad, N.; de Lima, J.; Murrell, D.; Kinchington, D.; Lee, J.W.; Maguire, A.M.; Donaghue, K.C. Cortisol Response to General Anaesthesia for Medical Imaging in Children. Clin. Endocrinol. 2009, 71, 834–839. [Google Scholar] [CrossRef]
- Salerno, R.; Forti, G.; Busoni, P.; Casadio, C. Effects of Surgery and General or Epidural Anesthesia on Plasma Levels of Cortisol, Growth Hormone and Prolactin in Infants under One Year of Age. J. Endocrinol. Investig. 1989, 12, 617–621. [Google Scholar] [CrossRef]
- Hsu, A.A.; von Elten, K.; Chan, D.; Flynn, T.; Walker, K.; Barnhill, J.; Naun, C.; Pedersen, A.M.; Ponaman, M.; Fredericks, G.J.; et al. Characterization of the Cortisol Stress Response to Sedation and Anesthesia in Children. J. Clin. Endocrinol. Metab. 2012, 97, E1830–E1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yang, B.; Ju, L.; Yang, J.; Allen, A.; Zhang, J.; Martynyuk, A.E. The Estradiol Synthesis Inhibitor Formestane Diminishes the Ability of Sevoflurane to Induce Neurodevelopmental Abnormalities in Male Rats. Front. Syst. Neurosci. 2020, 14, 546531. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tan, S.; Zhang, J.; Seubert, C.N.; Gravenstein, N.; Sumners, C.; Vasilopoulos, T.; Martynyuk, A.E. Anesthesia with Sevoflurane in Neonatal Rats: Developmental Neuroendocrine Abnormalities and Alleviating Effects of the Corticosteroid and Cl− Importer Antagonists. Psychoneuroendocrinology 2015, 60, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Xu, N.; Lin, Y.; Lei, L.; Ju, L.-S.; Morey, T.E.; Gravenstein, N.; Zhang, J.; Martynyuk, A.E. Roles of Testosterone and Estradiol in Mediation of Acute Neuroendocrine and Electroencephalographic Effects of Sevoflurane During the Sensitive Period in Rats. Front. Endocrinol. 2020, 11, 545973. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.-S.; Zhu, J.; Brant, J.O.; Morey, T.E.; Gravenstein, N.; Seubert, C.N.; Vasilopoulos, T.; Setlow, B.; Martynyuk, A.E. Intergenerational Perioperative Neurocognitive Disorder in Young Adult Male Rats with Traumatic Brain Injury. Anesthesiology 2023, 138, 388–402. [Google Scholar] [CrossRef]
- Xu, C.; Seubert, C.N.; Gravenstein, N.; Martynyuk, A.E. Propofol, but Not Etomidate, Increases Corticosterone Levels and Induces Long-Term Alteration in Hippocampal Synaptic Activity in Neonatal Rats. Neurosci. Lett. 2016, 618, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Xu, C.; Zhu, W.; Willis, J.; Seubert, C.N.; Gravenstein, N.; Sumners, C.; Martynyuk, A.E. Endocrine and Neurobehavioral Abnormalities Induced by Propofol Administered to Neonatal Rats. Anesthesiology 2014, 121, 1010–1017. [Google Scholar] [CrossRef] [Green Version]
- Willis, J.; Zhu, W.; Perez-Downes, J.; Tan, S.; Xu, C.; Seubert, C.; Gravenstein, N.; Martynyuk, A. Propofol-Induced Electroencephalographic Seizures in Neonatal Rats. Anesth. Analg. 2015, 120, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Lei, L.; Ju, L.-S.; Xu, N.; Morey, T.E.; Gravenstein, N.; Yang, J.; Martynyuk, A.E. Neonatal Exposure to Sevoflurane Expands the Window of Vulnerability to Adverse Effects of Subsequent Exposure to Sevoflurane and Alters Hippocampal Morphology via Decitabine-Sensitive Mechanisms. Neurosci. Lett. 2020, 735, 135240. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA Receptors: Structure, Function, Pharmacology, and Related Disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Khalilov, I.; Kahle, K.T.; Cherubini, E. The GABA Excitatory/Inhibitory Shift in Brain Maturation and Neurological Disorders. Neurosci. 2012, 18, 467–486. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Cherubini, E. The GABA Polarity Shift and Bumetanide Treatment: Making Sense Requires Unbiased and Undogmatic Analysis. Cells 2022, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Hyde, T.M.; Lipska, B.K.; Ali, T.; Mathew, S.V.; Law, A.J.; Metitiri, O.E.; Straub, R.E.; Ye, T.; Colantuoni, C.; Herman, M.M.; et al. Expression of GABA Signaling Molecules KCC2, NKCC1, and GAD1 in Cortical Development and Schizophrenia. J. Neurosci. 2011, 31, 11088–11095. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.-M.; Nothwang, H.G. NKCC1 and KCC2: Structural Insights into Phospho-Regulation. Front. Mol. Neurosci. 2022, 15, 964488. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Liang, S.; Zhang, G.; Yang, X. Role of NKCC1 and KCC2 in Epilepsy: From Expression to Function. Front. Neurol. 2019, 10, 1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Zhu, T.; Zhang, A.; Li, F.; Qian, W.; Qian, B. Heightened Stress Response and Cognitive Impairment after Repeated Neonatal Sevoflurane Exposures Might Be Linked to Excessive GABAAR-Mediated Depolarization. J. Anesth. 2016, 30, 834–841. [Google Scholar] [CrossRef]
- Yang, J.; Ju, L.; Jia, M.; Zhang, H.; Sun, X.; Ji, M.; Yang, J.; Martynyuk, A.E. Subsequent Maternal Separation Exacerbates Neurobehavioral Abnormalities in Rats Neonatally Exposed to Sevoflurane Anesthesia. Neurosci. Lett. 2017, 661, 137–142. [Google Scholar] [CrossRef]
- Sidhu, G.; Puckett, Y. Bumetanide; Statpearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Ben-Ari, Y. NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. 2017, 40, 536–554. [Google Scholar] [CrossRef]
- Lozovaya, N.; Eftekhari, S.; Cloarec, R.; Gouty-Colomer, L.A.; Dufour, A.; Riffault, B.; Billon-Grand, M.; Pons-Bennaceur, A.; Oumar, N.; Burnashev, N.; et al. GABAergic Inhibition in Dual-Transmission Cholinergic and GABAergic Striatal Interneurons Is Abolished in Parkinson Disease. Nat. Commun. 2018, 9, 1422. [Google Scholar] [CrossRef]
- Delpire, E.; Ben-Ari, Y. A Wholistic View of How Bumetanide Attenuates Autism Spectrum Disorders. Cells 2022, 11, 2419. [Google Scholar] [CrossRef]
- Taubes, A.; Nova, P.; Zalocusky, K.A.; Kosti, I.; Bicak, M.; Zilberter, M.Y.; Hao, Y.; Yoon, S.Y.; Oskotsky, T.; Pineda, S.; et al. Experimental and Real-World Evidence Supporting the Computational Repurposing of Bumetanide for APOE4-Related Alzheimer’s Disease. Nat. Aging 2021, 1, 932–947. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, S.; Arisi, I.; Malerba, F.; D’Onofrio, M.; Cattaneo, A.; Cherubini, E. Targeting the Cation-Chloride Co-Transporter NKCC1 to Re-Establish GABAergic Inhibition and an Appropriate Excitatory/Inhibitory Balance in Selective Neuronal Circuits: A Novel Approach for the Treatment of Alzheimer’s Disease. Brain Sci. 2022, 12, 783. [Google Scholar] [CrossRef]
- Hewitt, S.A.; Wamsteeker, J.I.; Kurz, E.U.; Bains, J.S. Altered Chloride Homeostasis Removes Synaptic Inhibitory Constraint of the Stress Axis. Nat. Neurosci. 2009, 12, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Wakefield, S.; MacKenzie, G.; Moss, S.J.; Maguire, J. Neurosteroidogenesis Is Required for the Physiological Response to Stress: Role of Neurosteroid-Sensitive GABAA Receptors. J. Neurosci. 2011, 31, 18198–18210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wang, X.; Shapiro, L.A.; Cotrina, M.L.; Liu, W.; Wang, E.W.; Gu, S.; Wang, W.; He, X.; Nedergaard, M.; et al. NKCC1 Up-Regulation Contributes to Early Post-Traumatic Seizures and Increased Post-Traumatic Seizure Susceptibility. Brain Struct. Funct. 2017, 222, 1543–1556. [Google Scholar] [CrossRef] [Green Version]
- Lizhnyak, P.N.; Muldoon, P.P.; Pilaka, P.P.; Povlishock, J.T.; Ottens, A.K. Traumatic Brain Injury Temporal Proteome Guides KCC2-Targeted Therapy. J. Neurotrauma 2019, 36, 3092–3102. [Google Scholar] [CrossRef]
- Tsukahara, T.; Masuhara, M.; Iwai, H.; Sonomura, T.; Sato, T. The Effect of Repeated Stress on KCC2 and NKCC1 Immunoreactivity in the Hippocampus of Female Mice. Data Brief 2016, 6, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.M.; Ostroumov, A.; Kimmey, B.A.; Taormina, M.B.; Holden, W.M.; Kim, K.; Brown-Mangum, T.; Dani, J.A. Adolescent Nicotine Exposure Alters GABAA Receptor Signaling in the Ventral Tegmental Area and Increases Adult Ethanol Self-Administration. Cell Rep 2018, 23, 68–77. [Google Scholar] [CrossRef]
- Miller, S.; Maguire, J. Deficits in KCC2 and Activation of the HPA Axis Lead to Depressionlike Behavior Following Social Defeat. Horm. Stud. 2014, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Genç, F.; Kara, M.; Ünal, Y.; Uygur Küçükseymen, E.; Biçer Gömceli, Y.; Kaynar, T.; Tosun, K.; Kutlu, G. Methylation of Cation–Chloride Cotransporters NKCC1 and KCC2 in Patients with Juvenile Myoclonic Epilepsy. Neurol. Sci. 2019, 40, 1007–1013. [Google Scholar] [CrossRef]
- Maguire, J. Stress-Induced Plasticity of GABAergic Inhibition. Front. Cell Neurosci. 2014, 8, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzi, D.; Rasile, M.; Corradini, I.; Matteoli, M. Environmental Regulation of the Chloride Transporter KCC2: Switching Inflammation off to Switch the GABA On? Transl. Psychiatry 2020, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Masuhara, M.; Iwai, H.; Sonomura, T.; Sato, T. Repeated Stress-Induced Expression Pattern Alterations of the Hippocampal Chloride Transporters KCC2 and NKCC1 Associated with Behavioral Abnormalities in Female Mice. Biochem. Biophys. Res. Commun. 2015, 465, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ju, L.; Yang, C.; Xue, J.; Setlow, B.; Morey, T.E.; Gravenstein, N.; Seubert, C.N.; Vasilopoulos, T.; Martynyuk, A.E. Effects of Combined Brief Etomidate Anesthesia and Postnatal Stress on Amygdala Expression of Cl−Cotransporters and Corticotropin-Releasing Hormone and Alcohol Intake in Adult Rats. Neurosci. Lett. 2018, 685, 83–89. [Google Scholar] [CrossRef]
- Ju, L.-S.; Yang, J.-J.; Gravenstein, N.; Seubert, C.N.; Morey, T.E.; Sumners, C.; Vasilopoulos, T.; Yang, J.-J.; Martynyuk, A.E. Role of Environmental Stressors in Determining the Developmental Outcome of Neonatal Anesthesia. Psychoneuroendocrinology 2017, 81, 96–104. [Google Scholar] [CrossRef]
- Seubert, C.N.; Zhu, W.; Pavlinec, C.; Gravenstein, N.; Martynyuk, A.E. Developmental Effects of Neonatal Isoflurane and Sevoflurane Exposure in Rats. Anesthesiology 2013, 119, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, C.; Puentes, D.L.; Seubert, C.N.; Gravenstein, N.; Martynyuk, A.E. Role of Steroids in Hyperexcitatory Adverse and Anesthetic Effects of Sevoflurane in Neonatal Rats. Neuroendocrinology 2016, 103, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.A.; Shah, H.P.; Cao, W.; Gravenstein, N.; Seubert, C.N.; Martynyuk, A.E. Bumetanide Alleviates Epileptogenic and Neurotoxic Effects of Sevoflurane in Neonatal Rat Brain. Anesthesiology 2010, 112, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Valk, B.I.; Struys, M.M.R.F. Etomidate and Its Analogs: A Review of Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2021, 60, 1253–1269. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Kang, Y.; Qin, S.; Chai, J. Neuroinflammation as the Underlying Mechanism of Postoperative Cognitive Dysfunction and Therapeutic Strategies. Front. Cell Neurosci. 2022, 16, 843069. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, H.; Wang, T. Neuroinflammation in Perioperative Neurocognitive Disorders: From Bench to the Bedside. CNS Neurosci. Ther. 2022, 28, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.; Terrando, N. Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth. Analg. 2019, 128, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Skvarc, D.R.; Berk, M.; Byrne, L.K.; Dean, O.M.; Dodd, S.; Lewis, M.; Marriott, A.; Moore, E.M.; Morris, G.; Page, R.S.; et al. Post-Operative Cognitive Dysfunction: An Exploration of the Inflammatory Hypothesis and Novel Therapies. Neurosci. Biobehav. Rev. 2018, 84, 116–133. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in Sepsis: A Novel Understanding of the Disorder and a New Therapeutic Approach. Lancet. Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Vénéreau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef] [Green Version]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Kierdorf, K.; Fritz, G. RAGE Regulation and Signaling in Inflammation and Beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate Immune Responses to Trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Lin, G.-X.; Wang, T.; Chen, M.-H.; Hu, Z.-H.; Ouyang, W. Serum High-Mobility Group Box 1 Protein Correlates with Cognitive Decline after Gastrointestinal Surgery. Acta Anaesthesiol. Scand. 2014, 58, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dong, R.; Lu, Y.; Yang, X.; Chen, C.; Zhang, Z.; Peng, M. Short-Term Postoperative Cognitive Dysfunction and Inflammatory Response in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Pilot Study. Mediat. Inflamm. 2017, 2017, 3605350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrando, N.; Monaco, C.; Ma, D.; Foxwell, B.M.J.; Feldmann, M.; Maze, M. Tumor Necrosis Factor-Alpha Triggers a Cytokine Cascade Yielding Postoperative Cognitive Decline. Proc. Natl. Acad. Sci. USA 2010, 107, 20518–20522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacas, S.; Degos, V.; Tracey, K.J.; Maze, M. High-Mobility Group Box 1 Protein Initiates Postoperative Cognitive Decline by Engaging Bone Marrow-Derived Macrophages. Anesthesiology 2014, 120, 1160–1167. [Google Scholar] [CrossRef] [Green Version]
- Li, R.L.; Zhang, Z.Z.; Peng, M.; Wu, Y.; Zhang, J.J.; Wang, C.Y.; Wang, Y.L. Postoperative Impairment of Cognitive Function in Old Mice: A Possible Role for Neuroinflammation Mediated by HMGB1, S100B, and RAGE. J. Surg. Res. 2013, 185, 815–824. [Google Scholar] [CrossRef]
- Terrando, N.; Yang, T.; Wang, X.; Fang, J.; Cao, M.; Andersson, U.; Harris, H.E.; Ouyang, W.; Tong, J. Systemic HMGB1 Neutralization Prevents Postoperative Neurocognitive Dysfunction in Aged Rats. Front. Immunol. 2016, 7, 441. [Google Scholar] [CrossRef] [Green Version]
- He, H.J.; Wang, Y.; Le, Y.; Duan, K.M.; Yan, X.B.; Liao, Q.; Liao, Y.; Tong, J.B.; Terrando, N.; Ouyang, W. Surgery Upregulates High Mobility Group Box-1 and Disrupts the Blood-Brain Barrier Causing Cognitive Dysfunction in Aged Rats. CNS Neurosci. Ther. 2012, 18, 994–1002. [Google Scholar] [CrossRef]
- Hu, J.; Feng, X.; Valdearcos, M.; Lutrin, D.; Uchida, Y.; Koliwad, S.K.; Maze, M. Interleukin-6 Is Both Necessary and Sufficient to Produce Perioperative Neurocognitive Disorder in Mice. Br. J. Anaesth. 2018, 120, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Cibelli, M.; Fidalgo, A.R.; Terrando, N.; Ma, D.; Monaco, C.; Feldmann, M.; Takata, M.; Lever, I.J.; Nanchahal, J.; Fanselow, M.S.; et al. Role of Interleukin-1β in Postoperative Cognitive Dysfunction. Ann. Neurol. 2010, 68, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Degos, V.; Vacas, S.; Han, Z.; van Rooijen, N.; Gressens, P.; Su, H.; Young, W.L.; Maze, M. Depletion of Bone Marrow–Derived Macrophages Perturbs the Innate Immune Response to Surgery and Reduces Postoperative Memory Dysfunction. Anesthesiology 2013, 118, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, J.; Gao, J.; Zheng, X. Comparison of the Effects of Sevoflurane and Propofol Anesthesia on Pulmonary Function, MMP-9 and Postoperative Cognition in Patients Receiving Lung Cancer Resection. Oncol. Lett. 2019, 17, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shan, G.-J.; Zhang, Y.-X.; Cao, S.-J.; Zhu, S.-N.; Li, H.-J.; Ma, D.; Wang, D.-X. Propofol Compared with Sevoflurane General Anaesthesia Is Associated with Decreased Delayed Neurocognitive Recovery in Older Adults. Br. J. Anaesth. 2018, 121, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Bryan, L.J.; Atkins, K.J.; Lipszyc, A.; Scott, D.A.; Silbert, B.S.; Evered, L.A. Inflammatory Biomarker Levels After Propofol or Sevoflurane Anesthesia: A Meta-Analysis. Anesth. Analg. 2022, 134, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.-J.; Wu, Q.-H.; Zhang, R.-Q. Effect of Propofol, Sevoflurane, and Isoflurane on Postoperative Cognitive Dysfunction Following Laparoscopic Cholecystectomy in Elderly Patients: A Randomized Controlled Trial. J. Clin. Anesth. 2017, 38, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Y.; Zhu, S. Inflammatory Markers in Postoperative Delirium (POD) and Cognitive Dysfunction (POCD): A Meta-Analysis of Observational Studies. PLoS ONE 2018, 13, e0195659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.-H.; Yuan, H.-M.; Zhang, G.-F.; Li, X.-M.; Dong, L.; Li, W.-Y.; Zhou, Z.-Q.; Yang, J.-J. Changes in Plasma and Cerebrospinal Fluid Biomarkers in Aged Patients with Early Postoperative Cognitive Dysfunction Following Total Hip-Replacement Surgery. J. Anesth. 2013, 27, 236–242. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood–Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and Functional Features of Central Nervous System Lymphatic Vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Engblom, D.; Ek, M.; Saha, S.; Ericsson-Dahlstrand, A.; Jakobsson, P.-J.; Blomqvist, A. Prostaglandins as Inflammatory Messengers across the Blood-Brain Barrier. J. Mol. Med. 2002, 80, 5–15. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.; Bauer, B. Matrix Metalloproteinases in the Brain and Blood–Brain Barrier: Versatile Breakers and Makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Mo, N.; Li, L.; Cao, Y.; Wang, W.; Liang, Y.; Deng, H.; Xing, R.; Yang, L.; Ni, C.; et al. Surgery-Induced Hippocampal Angiotensin II Elevation Causes Blood-Brain Barrier Disruption via MMP/TIMP in Aged Rats. Front. Cell Neurosci. 2016, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Terrando, N.; Eriksson, L.I.; Kyu Ryu, J.; Yang, T.; Monaco, C.; Feldmann, M.; Jonsson Fagerlund, M.; Charo, I.F.; Akassoglou, K.; Maze, M. Resolving Postoperative Neuroinflammation and Cognitive Decline. Ann. Neurol. 2011, 70, 986–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Li, Z.; Li, H.; Ni, C.; Li, L.; Yang, N.; Shi, C.; Zhong, Y.; Cui, D.; Guo, X. Hypoxia-Inducible Factor-1α Is Involved in Isoflurane-Induced Blood-Brain Barrier Disruption in Aged Rats Model of POCD. Behav. Brain Res. 2018, 339, 39–46. [Google Scholar] [CrossRef]
- Ni, P.; Dong, H.; Wang, Y.; Zhou, Q.; Xu, M.; Qian, Y.; Sun, J. IL-17A Contributes to Perioperative Neurocognitive Disorders through Blood-Brain Barrier Disruption in Aged Mice. J. Neuroinflamm. 2018, 15, 332. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Guo, D.; Wang, H.; Xie, K.; Wang, C.; Li, Y.; Wang, C.; Wang, C.; Yu, Y.; Wang, G. Involvement of the Blood–Brain Barrier Opening in Cognitive Decline in Aged Rats Following Orthopedic Surgery and High Concentration of Sevoflurane Inhalation. Brain Res. 2014, 1551, 13–24. [Google Scholar] [CrossRef]
- Bi, J.; Shan, W.; Luo, A.; Zuo, Z. Critical Role of Matrix Metallopeptidase 9 in Postoperative Cognitive Dysfunction and Age-Dependent Cognitive Decline. Oncotarget 2017, 8, 51817–51829. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Gu, C.; Mandeville, E.T.; Dong, Y.; Esposito, E.; Zhang, Y.; Yang, G.; Shen, Y.; Fu, X.; Lo, E.H.; et al. Anesthesia and Surgery Impair Blood–Brain Barrier and Cognitive Function in Mice. Front. Immunol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Xu, G.; Newton, P.T.; Chagin, A.S.; Mkrtchian, S.; Carlström, M.; Zhang, X.-M.; Harris, R.A.; Cooter, M.; Berger, M.; et al. Maresin 1 Attenuates Neuroinflammation in a Mouse Model of Perioperative Neurocognitive Disorders. Br. J. Anaesth. 2019, 122, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayram, H.; Hidiroglu, M.; Cetin, L.; Kucuker, A.; Iriz, E.; Uguz, E.; Saglam, F.; Sener, E. Comparing S-100 Beta Protein Levels and Neurocognitive Functions between Patients Undergoing on-Pump and off-Pump Coronary Artery Bypass Grafting. J. Surg. Res. 2013, 182, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.P.; Schmidt, A.P.; Valentin, L.S.; Pinto, K.O.; Zeferino, S.P.; Oses, J.P.; Wiener, C.D.; Otsuki, D.A.; Tort, A.B.L.; Portela, L.V.; et al. S100B Protein and Neuron-Specific Enolase as Predictors of Cognitive Dysfunction after Coronary Artery Bypass Graft Surgery. Eur. J. Anaesthesiol. 2016, 33, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.S.; Christiansen, M.; Eliasen, K.; Sander-Jensen, K.; Moller, J.T. Biochemical Markers for Brain Damage after Cardiac Surgery-Time Profile and Correlation with Cognitive Dysfunction. Acta Anaesthesiol. Scand. 2002, 46, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Abrahamov, D.; Levran, O.; Naparstek, S.; Refaeli, Y.; Kaptson, S.; Abu Salah, M.; Ishai, Y.; Sahar, G. Blood–Brain Barrier Disruption After Cardiopulmonary Bypass: Diagnosis and Correlation to Cognition. Ann. Thorac. Surg. 2017, 104, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and Non-Immune Functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia Development and Function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S.; Kruys, V.; Vamecq, J.; Maze, M. The Role of Microglia in Perioperative Neuroinflammation and Neurocognitive Disorders. Front. Aging Neurosci. 2021, 13, 671499. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune Cell Regulation of Glia during CNS Injury and Disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Miller-Rhodes, P.; Kong, C.; Baht, G.S.; Saminathan, P.; Rodriguiz, R.M.; Wetsel, W.C.; Gelbard, H.A.; Terrando, N. The Broad Spectrum Mixed-Lineage Kinase 3 Inhibitor URMC-099 Prevents Acute Microgliosis and Cognitive Decline in a Mouse Model of Perioperative Neurocognitive Disorders. J. Neuroinflamm. 2019, 16, 193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Li, N.; Wang, Y.; Lu, W.; Zhang, Y.; Chen, Y.; Deng, X.; Yu, X. Methane Ameliorates Post-Operative Cognitive Dysfunction by Inhibiting Microglia NF-ΚB/MAPKs Pathway and Promoting IL-10 Expression in Aged Mice. Int. Immunopharmacol. 2019, 71, 52–60. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Li, N.; Zhang, S.; Sun, J.; Zhang, S.; Qian, Y. Activated Brain Mast Cells Contribute to Postoperative Cognitive Dysfunction by Evoking Microglia Activation and Neuronal Apoptosis. J. Neuroinflamm. 2016, 13, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hierro-Bujalance, C.; Bacskai, B.J.; Garcia-Alloza, M. In Vivo Imaging of Microglia With Multiphoton Microscopy. Front. Aging Neurosci. 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Tronel, C.; Largeau, B.; Santiago Ribeiro, M.; Guilloteau, D.; Dupont, A.-C.; Arlicot, N. Molecular Targets for PET Imaging of Activated Microglia: The Current Situation and Future Expectations. Int. J. Mol. Sci. 2017, 18, 802. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Valdearcos, M.; Uchida, Y.; Lutrin, D.; Maze, M.; Koliwad, S.K. Microglia Mediate Postoperative Hippocampal Inflammation and Cognitive Decline in Mice. JCI Insight 2017, 2, e91229. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Lian, Q.; Eckenhoff, M.F.; Eckenhoff, R.G.; Pan, J.Z. Differential General Anesthetic Effects on Microglial Cytokine Expression. PLoS ONE 2013, 8, e52887. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Meng, S.; Cao, L.; Chen, Y.; Zuo, Z.; Peng, S. Critical Role of NLRP3-Caspase-1 Pathway in Age-Dependent Isoflurane-Induced Microglial Inflammatory Response and Cognitive Impairment. J. Neuroinflamm. 2018, 15, 109. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Liu, Y.; Liu, S.; Cao, S.; Wang, F.; Wang, Z.; Xi, S. Fluoride-Induced Neuron Apoptosis and Expressions of Inflammatory Factors by Activating Microglia in Rat Brain. Mol. Neurobiol. 2016, 53, 4449–4460. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, M.; Meng, T.; Fei, J. Hippocampal Microglial Activation Triggers a Neurotoxic-Specific Astrocyte Response and Mediates Etomidate-Induced Long-Term Synaptic Inhibition. J. Neuroinflamm. 2020, 17, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Suzuki, K.; Toptunov, D.; Stoyanov, S.; Yuzaki, M.; Khiroug, L.; Dityatev, A. In Vivo Two-Photon Imaging of Anesthesia-Specific Alterations in Microglial Surveillance and Photodamage-Directed Motility in Mouse Cortex. Front. Neurosci. 2019, 13, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsberg, A.; Cervenka, S.; Jonsson Fagerlund, M.; Rasmussen, L.S.; Zetterberg, H.; Erlandsson Harris, H.; Stridh, P.; Christensson, E.; Granström, A.; Schening, A.; et al. The Immune Response of the Human Brain to Abdominal Surgery. Ann. Neurol. 2017, 81, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.R. Specification and Morphogenesis of Astrocytes. Science 2010, 330, 774–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular Basis of Astrocyte Diversity and Morphology across the CNS in Health and Disease. Science 2022, 378, eaay8477. [Google Scholar] [CrossRef]
- Araki, T.; Ikegaya, Y.; Koyama, R. The Effects of Microglia- and Astrocyte-derived Factors on Neurogenesis in Health and Disease. Eur. J. Neurosci. 2021, 54, 5880–5901. [Google Scholar] [CrossRef]
- Han, R.T.; Kim, R.D.; Molofsky, A.V.; Liddelow, S.A. Astrocyte-Immune Cell Interactions in Physiology and Pathology. Immunity 2021, 54, 211–224. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neurosci. 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte Contribution to Dysfunction, Risk and Progression in Neurodegenerative Disorders. Nat. Rev. Neurosci. 2023, 24, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.; Anderson, H.D.; Anderson, C.M. Astrocyte Dysfunction in Alzheimer Disease. J. Neurosci. Res. 2017, 95, 2430–2447. [Google Scholar] [CrossRef]
- Zhou, B.; Zuo, Y.; Jiang, R. Astrocyte Morphology: Diversity, Plasticity, and Role in Neurological Diseases. CNS Neurosci. Ther. 2019, 25, 665–673. [Google Scholar] [CrossRef]
- Carter, S.F.; Herholz, K.; Rosa-Neto, P.; Pellerin, L.; Nordberg, A.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol. Med. 2019, 25, 77–95. [Google Scholar] [CrossRef]
- Xu, J.; Dong, H.; Qian, Q.; Zhang, X.; Wang, Y.; Jin, W.; Qian, Y. Astrocyte-Derived CCL2 Participates in Surgery-Induced Cognitive Dysfunction and Neuroinflammation via Evoking Microglia Activation. Behav. Brain Res. 2017, 332, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Rappold, T.; Laflam, A.; Hori, D.; Brown, C.; Brandt, J.; Mintz, C.D.; Sieber, F.; Gottschalk, A.; Yenokyan, G.; Everett, A.; et al. Evidence of an Association between Brain Cellular Injury and Cognitive Decline after Non-Cardiac Surgery. Br. J. Anaesth. 2016, 116, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Tesco, G.; Lomoio, S. Pathophysiology of Neurodegenerative Diseases: An Interplay among Axonal Transport Failure, Oxidative Stress, and Inflammation? Semin. Immunol. 2022, 59, 101628. [Google Scholar] [CrossRef]
- Tapp, Z.M.; Godbout, J.P.; Kokiko-Cochran, O.N. A Tilted Axis: Maladaptive Inflammation and HPA Axis Dysfunction Contribute to Consequences of TBI. Front. Neurol. 2019, 10, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cain, D.W.; Cidlowski, J.A. Immune Regulation by Glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Gądek-Michalska, A.; Tadeusz, J.; Rachwalska, P.; Bugajski, J. Cytokines, Prostaglandins and Nitric Oxide in the Regulation of Stress-Response Systems. Pharmacol. Rep. 2013, 65, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines Sing the Blues: Inflammation and the Pathogenesis of Depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Kiecolt-Glaser, J.K.; McGuire, L.; Robles, T.F.; Glaser, R. Psychoneuroimmunology: Psychological Influences on Immune Function and Health. J. Consult. Clin. Psychol. 2002, 70, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.D.; Agarwal, S.K.; Lloyd, C.; Cohen, L.; Henninger, E.M.; Morris, G.J. Cytokine Dysregulation Associated with Exam Stress in Healthy Medical Students. Brain Behav. Immun. 1998, 12, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The Effects of Acute Psychological Stress on Circulating and Stimulated Inflammatory Markers: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Marsland, A.L.; Bachen, E.A.; Cohen, S.; Rabin, B.; Manuck, S.B. Stress, Immune Reactivity and Susceptibility to Infectious Disease. Physiol. Behav. 2002, 77, 711–716. [Google Scholar] [CrossRef]
- Black, P.H. The Inflammatory Response Is an Integral Part of the Stress Response: Implications for Atherosclerosis, Insulin Resistance, Type II Diabetes and Metabolic Syndrome X. Brain Behav. Immun. 2003, 17, 350–364. [Google Scholar] [CrossRef]
- Manuck, S.B.; Cohen, S.; Rabin, B.S.; Muldoon, M.F.; Bachen, E.A. Individual Differences in Cellular Immune Response to Stress. Psychol. Sci. 1991, 2, 111–115. [Google Scholar] [CrossRef]
- Brydon, L.; Steptoe, A. Stress-Induced Increases in Interleukin-6 and Fibrinogen Predict Ambulatory Blood Pressure at 3-Year Follow-Up. J. Hypertens. 2005, 23, 1001–1007. [Google Scholar] [CrossRef]
- Johnson, J.D.; O’Connor, K.A.; Deak, T.; Stark, M.; Watkins, L.R.; Maier, S.F. Prior Stressor Exposure Sensitizes LPS-Induced Cytokine Production. Brain Behav. Immun. 2002, 16, 461–476. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.D.; O’Connor, K.A.; Hansen, M.K.; Watkins, L.R.; Maier, S.F. Effects of Prior Stress on LPS-Induced Cytokine and Sickness Responses. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 284, R422–R432. [Google Scholar] [CrossRef]
- Frank, M.G.; Miguel, Z.D.; Watkins, L.R.; Maier, S.F. Prior Exposure to Glucocorticoids Sensitizes the Neuroinflammatory and Peripheral Inflammatory Responses to E. Coli Lipopolysaccharide. Brain Behav. Immun. 2010, 24, 19–30. [Google Scholar] [CrossRef]
- Frank, M.G.; Thompson, B.M.; Watkins, L.R.; Maier, S.F. Glucocorticoids Mediate Stress-Induced Priming of Microglial pro-Inflammatory Responses. Brain Behav. Immun. 2012, 26, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular Inflammation: Underpinnings of Aging and Age-Related Diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, Y.; Yao, R.; Hao, T.; Cao, J.; Huang, H.; Wang, L.; Wu, Y. Enhanced Neuroinflammation Mediated by DNA Methylation of the Glucocorticoid Receptor Triggers Cognitive Dysfunction after Sevoflurane Anesthesia in Adult Rats Subjected to Maternal Separation during the Neonatal Period. J. Neuroinflamm. 2017, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.Q.; Ji, M.H.; Zhao, Q.S.; Jia, M.; Qiu, L.L.; Yang, J.J.; Peng, Y.G.; Yang, J.J.; Martynyuk, A.E. Neurobehavioural Abnormalities Induced by Repeated Exposure of Neonatal Rats to Sevoflurane Can Be Aggravated by Social Isolation and Enrichment Deprivation Initiated after Exposure to the Anaesthetic. Br. J. Anaesth. 2015, 115, 752–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Dong, Y.; Xu, Z.; Crosby, G.; Culley, D.J.; Zhang, Y.; Xie, Z. Sevoflurane Anesthesia in Pregnant Mice Induces Neurotoxicity in Fetal and Offspring Mice. Anesthesiology 2013, 118, 516–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, J.; May, L.D.V.; Gonzalez, H.E.; Lee, E.W.; Alvi, R.S.; Sall, J.W.; Rau, V.; Bickler, P.E.; Lalchandani, G.R.; Yusupova, M.; et al. Delayed Environmental Enrichment Reverses Sevoflurane-Induced Memory Impairment in Rats. Anesthesiology 2012, 116, 586–602. [Google Scholar] [CrossRef] [Green Version]
- Warner, D.O.; Zaccariello, M.J.; Katusic, S.K.; Schroeder, D.R.; Hanson, A.C.; Schulte, P.J.; Buenvenida, S.L.; Gleich, S.J.; Wilder, R.T.; Sprung, J.; et al. Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia. Anesthesiology 2018, 129, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.S.; Li, G.; Miller, T.L.K.; Salorio, C.; Byrne, M.W.; Bellinger, D.C.; Ing, C.; Park, R.; Radcliffe, J.; Hays, S.R.; et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016, 315, 2312. [Google Scholar] [CrossRef]
- Davidson, A.J.; Disma, N.; de Graaff, J.C.; Withington, D.E.; Dorris, L.; Bell, G.; Stargatt, R.; Bellinger, D.C.; Schuster, T.; Arnup, S.J.; et al. Neurodevelopmental Outcome at 2 Years of Age after General Anaesthesia and Awake-Regional Anaesthesia in Infancy (GAS): An International Multicentre, Randomised Controlled Trial. Lancet 2016, 387, 239–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCann, M.E.; de Graaff, J.C.; Dorris, L.; Disma, N.; Withington, D.; Bell, G.; Grobler, A.; Stargatt, R.; Hunt, R.W.; Sheppard, S.J.; et al. Neurodevelopmental Outcome at 5 Years of Age after General Anaesthesia or Awake-Regional Anaesthesia in Infancy (GAS): An International, Multicentre, Randomised, Controlled Equivalence Trial. Lancet 2019, 393, 664–677. [Google Scholar] [CrossRef]
- Walsh, B.H.; Paul, R.A.; Inder, T.E.; Shimony, J.S.; Smyser, C.D.; Rogers, C.E. Surgery Requiring General Anesthesia in Preterm Infants Is Associated with Altered Brain Volumes at Term Equivalent Age and Neurodevelopmental Impairment. Pediatr. Res. 2021, 89, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Rossi, M.G.; Anghelescu, D.L.; Liu, W.; Breazeale, A.M.; Reddick, W.E.; Glass, J.O.; Phillips, N.S.; Jacola, L.M.; Sabin, N.D.; et al. Association Between Anesthesia Exposure and Neurocognitive and Neuroimaging Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. JAMA Oncol. 2019, 5, 1456. [Google Scholar] [CrossRef]
- Yang, L.; Hao, J.-R.; Gao, Y.; Yang, X.; Shen, X.-R.; Wang, H.-Y.; Sun, N.; Gao, C. HDAC3 of Dorsal Hippocampus Induces Postoperative Cognitive Dysfunction in Aged Mice. Behav. Brain Res. 2022, 433, 114002. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Zhao, Y.; Shi, R.; Wang, Y.; Xu, J.; Wu, A.; Johns, R.A.; Yue, Y. Epigenetics as a New Therapeutic Target for Postoperative Cognitive Dysfunction. Med. Hypotheses 2013, 80, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Day, J.J.; Sweatt, J.D. Epigenetic Mechanisms in Cognition. Neuron 2011, 70, 813–829. [Google Scholar] [CrossRef] [Green Version]
- Lemche, E. Early Life Stress and Epigenetics in Late-Onset Alzheimer’s Dementia: A Systematic Review. Curr. Genom. 2018, 19, 522–602. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.E.; Greer, C.B.; Coleman, B.C.; Sweatt, J.D. Histone H3 Lysine K4 Methylation and Its Role in Learning and Memory. Epigenetics Chromatin 2019, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niklison-Chirou, M.V.; Agostini, M.; Amelio, I.; Melino, G. Regulation of Adult Neurogenesis in Mammalian Brain. Int. J. Mol. Sci. 2020, 21, 4869. [Google Scholar] [CrossRef]
- Maity, S.; Farrell, K.; Navabpour, S.; Narayanan, S.N.; Jarome, T.J. Epigenetic Mechanisms in Memory and Cognitive Decline Associated with Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12280. [Google Scholar] [CrossRef]
- Srivastava, A.; Dada, O.; Qian, J.; Al-Chalabi, N.; Fatemi, A.B.; Gerretsen, P.; Graff, A.; de Luca, V. Epigenetics of Schizophrenia. Psychiatry Res. 2021, 305, 114218. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, P. Epigenetic Alterations in Anesthesia-Induced Neurotoxicity in the Developing Brain. Front. Physiol. 2018, 9, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, O.H.; Useinovic, N.; Jevtovic-Todorovic, V. Neonatal Anesthesia and Dysregulation of the Epigenome. Biol. Reprod. 2021, 105, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Rump, K.; Adamzik, M. Epigenetic Mechanisms of Postoperative Cognitive Impairment Induced by Anesthesia and Neuroinflammation. Cells 2022, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Dobs, Y.E.; Ali, M.M. The Epigenetic Modulation of Alcohol/Ethanol and Cannabis Exposure/Co-Exposure during Different Stages. Open Biol. 2019, 9, 180115. [Google Scholar] [CrossRef] [Green Version]
- Ghantous, Y.; Schussel, J.L.; Brait, M. Tobacco and Alcohol-Induced Epigenetic Changes in Oral Carcinoma. Curr. Opin. Oncol. 2018, 30, 152–158. [Google Scholar] [CrossRef]
- Malherbe, D.C.; Messaoudi, I. Transcriptional and Epigenetic Regulation of Monocyte and Macrophage Dysfunction by Chronic Alcohol Consumption. Front. Immunol. 2022, 13, 3156. [Google Scholar] [CrossRef]
- Streifer, M.; Gore, A.C. Epigenetics, Estrogenic Endocrine-Disrupting Chemicals (EDCs), and the Brain. Adv. Pharmacol. 2021, 92, 73–99. [Google Scholar]
- Nettore, I.C.; Franchini, F.; Palatucci, G.; Macchia, P.E.; Ungaro, P. Epigenetic Mechanisms of Endocrine-Disrupting Chemicals in Obesity. Biomedicines 2021, 9, 1716. [Google Scholar] [CrossRef]
- Lite, C.; Raja, G.L.; Juliet, M.; Sridhar, V.V.; Subhashree, K.D.; Kumar, P.; Chakraborty, P.; Arockiaraj, J. In Utero Exposure to Endocrine-Disrupting Chemicals, Maternal Factors and Alterations in the Epigenetic Landscape Underlying Later-Life Health Effects. Environ. Toxicol. Pharmacol. 2022, 89, 103779. [Google Scholar] [CrossRef]
- Chen, N.; Peng, C.; Li, D. Epigenetic Underpinnings of Inflammation: A Key to Unlock the Tumor Microenvironment in Glioblastoma. Front. Immunol. 2022, 13, 869307. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Gao, Z.; Chen, K.; Zhang, Q.; Hu, S.; Zhao, L. Inflammation-Related Epigenetic Modification: The Bridge Between Immune and Metabolism in Type 2 Diabetes. Front. Immunol. 2022, 13, 883410. [Google Scholar] [CrossRef]
- Alam, R.; Abdolmaleky, H.M.; Zhou, J.-R. Microbiome, Inflammation, Epigenetic Alterations, and Mental Diseases. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in Epigenetics Link Genetics to the Environment and Disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, S.; Chappell, G.; Guyton, K.Z.; Pogribny, I.P.; Rusyn, I. Epigenetic Alterations Induced by Genotoxic Occupational and Environmental Human Chemical Carcinogens: An Update of a Systematic Literature Review. Mutat. Res. /Rev. Mutat. Res. 2022, 789, 108408. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Roth, T.L. Epigenetic Pathways through Which Experiences Become Linked with Biology. Dev. Psychopathol. 2015, 27, 637–648. [Google Scholar] [CrossRef] [Green Version]
- Mather, K.A.; Kwok, J.B.; Armstrong, N.; Sachdev, P.S. The Role of Epigenetics in Cognitive Ageing. Int. J. Geriatr. Psychiatry 2014, 29, 1162–1171. [Google Scholar] [CrossRef]
- Bomans, K.; Schenz, J.; Tamulyte, S.; Schaack, D.; Weigand, M.A.; Uhle, F. Paternal Sepsis Induces Alterations of the Sperm Methylome and Dampens Offspring Immune Responses—An Animal Study. Clin. Epigenetics 2018, 10, 89. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Zhang, Y.; Zhao, R.; He, B. Paternal Systemic Inflammation Induces Offspring Programming of Growth and Liver Regeneration in Association with Igf2 Upregulation. Mol. Cell. Endocrinol. 2020, 518, 111001. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, L.; Sun, X.; Zhang, Z.; Liu, J.; Xin, Y.; Yu, J.; Jia, Y.; Sheng, J.; Hu, G.; et al. Angiogenin Mediates Paternal Inflammation-Induced Metabolic Disorders in Offspring through Sperm TsRNAs. Nat. Commun. 2021, 12, 6673. [Google Scholar] [CrossRef] [PubMed]
- Constantinof, A.; Boureau, L.; Moisiadis, V.G.; Kostaki, A.; Szyf, M.; Matthews, S.G. Prenatal Glucocorticoid Exposure Results in Changes in Gene Transcription and DNA Methylation in the Female Juvenile Guinea Pig Hippocampus Across Three Generations. Sci. Rep. 2019, 9, 18211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, S.; Matthews, S.G.; Szyf, M. Adult Glucocorticoid Exposure Leads to Transcriptional and DNA Methylation Changes in Nuclear Steroid Receptors in the Hippocampus and Kidney of Mouse Male Offspring1. Biol. Reprod. 2014, 90, 43. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Parada, G.E.; Gross, F.; Corcoba, A.; Kaur, J.; Grau, E.; Hemberg, M.; Bohacek, J.; Miska, E.A. Single Paternal Dexamethasone Challenge Programs Offspring Metabolism and Reveals Multiple Candidates in RNA-Mediated Inheritance. iScience 2021, 24, 102870. [Google Scholar] [CrossRef]
- Hjort, L.; Rushiti, F.; Wang, S.-J.; Fransquet, P.; P Krasniqi, S.; I Çarkaxhiu, S.; Arifaj, D.; Xhemaili, V.D.; Salihu, M.; A Leku, N.; et al. Intergenerational Effects of Maternal Post-Traumatic Stress Disorder on Offspring Epigenetic Patterns and Cortisol Levels. Epigenomics 2021, 13, 967–980. [Google Scholar] [CrossRef]
- Rakyan, V.K.; Chong, S.; Champ, M.E.; Cuthbert, P.C.; Morgan, H.D.; Luu, K.V.K.; Whitelaw, E. Transgenerational Inheritance of Epigenetic States at the Murine Axin Fu Allele Occurs after Maternal and Paternal Transmission. Proc. Natl. Acad. Sci. USA 2003, 100, 2538–2543. [Google Scholar] [CrossRef] [Green Version]
- Weaver, I.C.G. Reversal of Maternal Programming of Stress Responses in Adult Offspring through Methyl Supplementation: Altering Epigenetic Marking Later in Life. J. Neurosci. 2005, 25, 11045–11054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA Methylation: A Historical Perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Day, J.J.; Sweatt, J.D. DNA Methylation and Memory Formation. Nat. Neurosci. 2010, 13, 1319–1323. [Google Scholar] [CrossRef]
- Światowy, W.J.; Drzewiecka, H.; Kliber, M.; Sąsiadek, M.; Karpiński, P.; Pławski, A.; Jagodziński, P.P. Physical Activity and DNA Methylation in Humans. Int. J. Mol. Sci. 2021, 22, 12989. [Google Scholar] [CrossRef]
- del Castillo Falconi, V.M.; Torres-Arciga, K.; Matus-Ortega, G.; Díaz-Chávez, J.; Herrera, L.A. DNA Methyltransferases: From Evolution to Clinical Applications. Int. J. Mol. Sci. 2022, 23, 8994. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.J.; Lewis, Z.A.; Goll, M.G. DNA Methylation: Shared and Divergent Features across Eukaryotes. Trends Genet. 2019, 35, 818–827. [Google Scholar] [CrossRef]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.C.; Qin, J.; Zoghbi, H.Y. MeCP2, a Key Contributor to Neurological Disease, Activates and Represses Transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.U.; Ma, D.K.; Mo, H.; Ball, M.P.; Jang, M.-H.; Bonaguidi, M.A.; Balazer, J.A.; Eaves, H.L.; Xie, B.; Ford, E.; et al. Neuronal Activity Modifies the DNA Methylation Landscape in the Adult Brain. Nat. Neurosci. 2011, 14, 1345–1351. [Google Scholar] [CrossRef]
- Miller, C.A.; Gavin, C.F.; White, J.A.; Parrish, R.R.; Honasoge, A.; Yancey, C.R.; Rivera, I.M.; Rubio, M.D.; Rumbaugh, G.; Sweatt, J.D. Cortical DNA Methylation Maintains Remote Memory. Nat. Neurosci. 2010, 13, 664–666. [Google Scholar] [CrossRef]
- Miller, C.A.; Sweatt, J.D. Covalent Modification of DNA Regulates Memory Formation. Neuron 2007, 53, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wu, T.-T.; Tang, L.; Liu, Q.; Mao, X.-Z.; Xu, J.-M.; Dai, R.-P. Association of Global DNA Hypomethylation with Post-Operative Cognitive Dysfunction in Elderly Patients Undergoing Hip Surgery. Acta Anaesthesiol. Scand. 2020, 64, 354–360. [Google Scholar] [CrossRef]
- Sadahiro, R.; Knight, B.; James, F.; Hannon, E.; Charity, J.; Daniels, I.R.; Burrage, J.; Knox, O.; Crawford, B.; Smart, N.J.; et al. Major Surgery Induces Acute Changes in Measured DNA Methylation Associated with Immune Response Pathways. Sci. Rep. 2020, 10, 5743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Xu, W. Characterization of DNA Hydroxymethylation in the Hypothalamus of Elderly Mice with Post-operative Cognitive Dysfunction. Exp. Ther. Med. 2019, 18, 4002–4010. [Google Scholar] [CrossRef] [Green Version]
- Ju, L.; Jia, M.; Sun, J.; Sun, X.; Zhang, H.; Ji, M.; Yang, J.; Wang, Z. Hypermethylation of Hippocampal Synaptic Plasticity-Related Genes Is Involved in Neonatal Sevoflurane Exposure-Induced Cognitive Impairments in Rats. Neurotox. Res. 2016, 29, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bie, B.; Naguib, M. Epigenetic Manipulation of Brain-Derived Neurotrophic Factor Improves Memory Deficiency Induced by Neonatal Anesthesia in Rats. Anesthesiology 2016, 124, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Y.; Shi, G.; Zhao, P. Neonatal Sevoflurane Exposure Impairs Learning and Memory by the Hypermethylation of Hippocampal Synaptic Genes. Mol. Neurobiol. 2021, 58, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tong, Y.; Brant, J.O.; Li, N.; Ju, L.-S.; Morey, T.E.; Gravenstein, N.; Setlow, B.; Zhang, J.; Martynyuk, A.E. Dexmedetomidine Diminishes, but Does Not Prevent, Developmental Effects of Sevoflurane in Neonatal Rats. Anesth. Analg. 2022, 135, 877–887. [Google Scholar] [CrossRef]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone Post-Translational Modifications—Cause and Consequence of Genome Function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef]
- Ramazi, S.; Allahverdi, A.; Zahiri, J. Evaluation of Post-Translational Modifications in Histone Proteins: A Review on Histone Modification Defects in Developmental and Neurological Disorders. J Biosci 2020, 45. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of Histone Modification. In Histone Mutations and Cancer; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–16. [Google Scholar]
- Min, J.; Lai, Z.; Wang, H.; Zuo, Z. Preoperative Environment Enrichment Preserved Neuroligin 1 Expression Possibly via Epigenetic Regulation to Reduce Postoperative Cognitive Dysfunction in Mice. CNS Neurosci. Ther. 2022, 28, 619–629. [Google Scholar] [CrossRef]
- Luo, F.; Min, J.; Wu, J.; Zuo, Z. Histone Deacetylases May Mediate Surgery-Induced Impairment of Learning, Memory, and Dendritic Development. Mol. Neurobiol. 2020, 57, 3702–3711. [Google Scholar] [CrossRef]
- Jia, M.; Liu, W.-X.; Sun, H.-L.; Chang, Y.-Q.; Yang, J.-J.; Ji, M.-H.; Yang, J.-J.; Feng, C.-Z. Suberoylanilide Hydroxamic Acid, a Histone Deacetylase Inhibitor, Attenuates Postoperative Cognitive Dysfunction in Aging Mice. Front. Mol. Neurosci. 2015, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xie, W.; Xie, W.; Zhuang, W.; Jiang, C.; Liu, N. Apigenin Attenuates Isoflurane-Induced Cognitive Dysfunction via Epigenetic Regulation and Neuroinflammation in Aged Rats. Arch. Gerontol. Geriatr. 2017, 73, 29–36. [Google Scholar] [CrossRef]
- Chai, G.; Wu, J.; Fang, R.; Liu, Y.; Wang, X.; Wang, X.; Zhang, J.; Zhou, J.; Jiang, Z.; Yi, H.; et al. Sevoflurane Inhibits Histone Acetylation and Contributes to Cognitive Dysfunction by Enhancing the Expression of ANP32A in Aging Mice. Behav. Brain Res. 2022, 431, 113949. [Google Scholar] [CrossRef]
- Jambhekar, A.; Dhall, A.; Shi, Y. Roles and Regulation of Histone Methylation in Animal Development. Nat. Rev. Mol. Cell Biol. 2019, 20, 625–641. [Google Scholar] [CrossRef]
- Snigdha, S.; Prieto, G.A.; Petrosyan, A.; Loertscher, B.M.; Dieskau, A.P.; Overman, L.E.; Cotman, C.W. H3K9me3 Inhibition Improves Memory, Promotes Spine Formation, and Increases BDNF Levels in the Aged Hippocampus. J. Neurosci. 2016, 36, 3611–3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Liu, S.; Tian, M.; Zhang, W.; Zhu, T.; Li, D.; Wu, J.; Deng, H.; Jia, Y.; Xie, W.; et al. Activity-Induced Histone Modifications Govern Neurexin-1 MRNA Splicing and Memory Preservation. Nat. Neurosci. 2017, 20, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sun, X.-Y.; Yang, X.; Liu, L.; Tong, K.; Gao, Y.; Hao, J.-R.; Cao, J.; Gao, C. Histone H3K9 Trimethylation Downregulates the Expression of Brain-Derived Neurotrophic Factor in the Dorsal Hippocampus and Impairs Memory Formation During Anaesthesia and Surgery. Front. Mol. Neurosci. 2019, 12, 246. [Google Scholar] [CrossRef]
- Virciglio, C.; Abel, Y.; Rederstorff, M. Regulatory Non-Coding RNAs: An Overview. In Small Non-Coding RNAs: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–9. [Google Scholar]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform. 2019, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, X.; Li, H.; Hui, S.; Zhang, Z.; Xiao, Y.; Peng, W. Non-Coding RNAs as Novel Regulators of Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2022, 13, 908076. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Kuo, H.-C. Functional Roles and Networks of Non-Coding RNAs in the Pathogenesis of Neurodegenerative Diseases. J. Biomed. Sci. 2020, 27, 49. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression Profiling of Mammalian MicroRNAs Uncovers a Subset of Brain-Expressed MicroRNAs with Possible Roles in Murine and Human Neuronal Differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Krichevsky, A.; Grad, Y.; Hayes, G.D.; Kosik, K.S.; Church, G.M.; Ruvkun, G. Identification of Many MicroRNAs That Copurify with Polyribosomes in Mammalian Neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 360–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blount, G.S.; Coursey, L.; Kocerha, J. MicroRNA Networks in Cognition and Dementia. Cells 2022, 11, 1882. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I.; Bartel, D.P. LincRNAs: Genomics, Evolution, and Mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Zhong, Y.; Zhang, Y.; Yang, L.; Wu, P.; Hou, X.; Xiong, F.; Li, X.; Zhang, S.; Gong, Z.; et al. Long Non-Coding RNAs Are Involved in Alternative Splicing and Promote Cancer Progression. Br. J. Cancer 2022, 126, 1113–1124. [Google Scholar] [CrossRef]
- Vangoor, V.R.; Gomes-Duarte, A.; Pasterkamp, R.J. Long Non-coding RNAs in Motor Neuron Development and Disease. J. Neurochem. 2021, 156, 777–801. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, X.; Xu, J. The New Function of CircRNA: Translation. Clin. Transl. Oncol. 2020, 22, 2162–2169. [Google Scholar] [CrossRef]
- Han, B.; Chao, J.; Yao, H. Circular RNA and Its Mechanisms in Disease: From the Bench to the Clinic. Pharmacol. Ther. 2018, 187, 31–44. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal CircRNAs: Biogenesis, Effect and Application in Human Diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Liu, C.-X.; Chen, L.-L. Circular RNAs: Characterization, Cellular Roles, and Applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef]
- Gao, R.; Chen, C.; Zhao, Q.; Li, M.; Wang, Q.; Zhou, L.; Chen, E.; Chen, H.; Zhang, Y.; Cai, X.; et al. Identification of the Potential Key Circular RNAs in Elderly Patients With Postoperative Cognitive Dysfunction. Front. Aging Neurosci. 2020, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Yazit, N.A.A.; Juliana, N.; Das, S.; Teng, N.I.M.F.; Fahmy, N.M.; Azmani, S.; Kadiman, S. Association of Micro RNA and Postoperative Cognitive Dysfunction: A Review. Mini Rev. Med. Chem. 2020, 20, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; He, S.-L.; Chen, W.-C.; Wang, C.-M.; Huang, Q.-M.; Shi, Y.-C.; Lin, S.; He, H. Recent Progress on the Role of Non-Coding RNA in Postoperative Cognitive Dysfunction. Front. Cell Neurosci. 2022, 16, 1024475. [Google Scholar] [CrossRef]
- Radtke, K.M.; Ruf, M.; Gunter, H.M.; Dohrmann, K.; Schauer, M.; Meyer, A.; Elbert, T. Transgenerational Impact of Intimate Partner Violence on Methylation in the Promoter of the Glucocorticoid Receptor. Transl. Psychiatry 2011, 1, e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perroud, N.; Paoloni-Giacobino, A.; Prada, P.; Olié, E.; Salzmann, A.; Nicastro, R.; Guillaume, S.; Mouthon, D.; Stouder, C.; Dieben, K.; et al. Increased Methylation of Glucocorticoid Receptor Gene (NR3C1) in Adults with a History of Childhood Maltreatment: A Link with the Severity and Type of Trauma. Transl. Psychiatry 2011, 1, e59. [Google Scholar] [CrossRef] [Green Version]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal Exposure to Maternal Depression, Neonatal Methylation of Human Glucocorticoid Receptor Gene (NR3C1) and Infant Cortisol Stress Responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Bierer, L.M.; Bader, H.N.; Daskalakis, N.P.; Lehrner, A.; Provençal, N.; Wiechmann, T.; Klengel, T.; Makotkine, I.; Binder, E.B.; Yehuda, R. Intergenerational Effects of Maternal Holocaust Exposure on FKBP5 Methylation. Am. J. Psychiatry 2020, 177, 744–753. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Binder, E.B. The Role of FKBP5, a Co-Chaperone of the Glucocorticoid Receptor in the Pathogenesis and Therapy of Affective and Anxiety Disorders. Psychoneuroendocrinology 2009, 34, S186–S195. [Google Scholar] [CrossRef]
- Jääskeläinen, T.; Makkonen, H.; Palvimo, J.J. Steroid Up-Regulation of FKBP51 and Its Role in Hormone Signaling. Curr. Opin. Pharmacol. 2011, 11, 326–331. [Google Scholar] [CrossRef]
- Chan, J.C.; Morgan, C.P.; Adrian Leu, N.; Shetty, A.; Cisse, Y.M.; Nugent, B.M.; Morrison, K.E.; Jašarević, E.; Huang, W.; Kanyuch, N.; et al. Reproductive Tract Extracellular Vesicles Are Sufficient to Transmit Intergenerational Stress and Program Neurodevelopment. Nat. Commun. 2020, 11, 1499. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Lu, Y.; Jiao, Y.; Liu, B.; Li, S.; Li, Y.; Xing, F.; Chen, D.; Liu, X.; Zhao, J.; et al. Paternal Psychological Stress Reprograms Hepatic Gluconeogenesis in Offspring. Cell Metab. 2016, 23, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Short, A.K.; Fennell, K.A.; Perreau, V.M.; Fox, A.; O’Bryan, M.K.; Kim, J.H.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Elevated Paternal Glucocorticoid Exposure Alters the Small Noncoding RNA Profile in Sperm and Modifies Anxiety and Depressive Phenotypes in the Offspring. Transl. Psychiatry 2016, 6, e837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crudo, A.; Petropoulos, S.; Suderman, M.; Moisiadis, V.G.; Kostaki, A.; Hallett, M.; Szyf, M.; Matthews, S.G. Effects of Antenatal Synthetic Glucocorticoid on Glucocorticoid Receptor Binding, DNA Methylation, and Genome-Wide MRNA Levels in the Fetal Male Hippocampus. Endocrinology 2013, 154, 4170–4181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, E.J.R.; Queiroz, D.B.C.; Rodrigues, A.; Honda, L.; Avellar, M.C.W. Innate Immunity and Glucocorticoids: Potential Regulatory Mechanisms in Epididymal Biology. J. Androl. 2011, 32, 614–624. [Google Scholar] [CrossRef]
- Haeussler, S.; Claus, R. Expression of the Glucocorticoid Receptor and 11β-Hydroxysteroid Dehydrogenase 2 in Pig Testes Cells along Fetal Development. Reprod. Fertil. Dev. 2007, 19, 664. [Google Scholar] [CrossRef] [PubMed]
- Čikoš, Š.; Babeľová, J.; Špirková, A.; Burkuš, J.; Kovaříková, V.; Šefčíková, Z.; Fabian, D.; Koppel, J. Glucocorticoid Receptor Isoforms and Effects of Glucocorticoids in Ovulated Mouse Oocytes and Preimplantation Embryos†. Biol. Reprod. 2019, 100, 351–364. [Google Scholar] [CrossRef]
- Bygren, L.O.; Kaati, G.; Edvinsson, S. Longevity Determined by Paternal Ancestors’ Nutrition during Their Slow Growth Period. Acta Biotheor. 2001, 49, 53–59. [Google Scholar] [CrossRef]
- Kaati, G.; Bygren, L.; Edvinsson, S. Cardiovascular and Diabetes Mortality Determined by Nutrition during Parents’ and Grandparents’ Slow Growth Period. Eur. J. Hum. Genet. 2002, 10, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.F. What Is the Relationship of Traumatic Brain Injury to Dementia? J. Alzheimer’s Dis. 2017, 57, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Zamani, A.; Rayner, G.; Shultz, S.R.; Jones, N.C. Affective, Neurocognitive and Psychosocial Disorders Associated with Traumatic Brain Injury and Post-Traumatic Epilepsy. Neurobiol. Dis. 2019, 123, 27–41. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and Neuroprotection in Traumatic Brain Injury. JAMA Neurol. 2015, 72, 355. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.K.; Ortiz, J.B.; Thomas, T.C. Mild and Moderate Traumatic Brain Injury and Repeated Stress Affect Corticosterone in the Rat. Neurotrauma Rep. 2020, 1, 113–124. [Google Scholar] [CrossRef] [PubMed]
- al-Dahhak, R.; Khoury, R.; Qazi, E.; Grossberg, G.T. Traumatic Brain Injury, Chronic Traumatic Encephalopathy, and Alzheimer Disease. Clin. Geriatr. Med. 2018, 34, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic Brain Injury: Progress and Challenges in Prevention, Clinical Care, and Research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic Brain Injury: Integrated Approaches to Improve Prevention, Clinical Care, and Research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Aldossary, N.M.; Kotb, M.A.; Kamal, A.M. Predictive Value of Early MRI Findings on Neurocognitive and Psychiatric Outcomes in Patients with Severe Traumatic Brain Injury. J. Affect. Disord. 2019, 243, 1–7. [Google Scholar] [CrossRef]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic Brain Injury: Neuropathological, Neurocognitive and Neurobehavioral Sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef]
- Jassam, Y.N.; Izzy, S.; Whalen, M.; McGavern, D.B.; el Khoury, J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron 2017, 95, 1246–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The Far-Reaching Scope of Neuroinflammation after Traumatic Brain Injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, S.Z.Y.; Spencer, R.J.; Hodgson, M.; Mehta, S.; Phillips, N.L.; Preest, G.; Manivannan, S.; Wise, M.P.; Galea, J.; Zaben, M. Interleukin-6 as a Prognostic Biomarker of Clinical Outcomes after Traumatic Brain Injury: A Systematic Review. Neurosurg. Rev. 2022, 45, 3035–3054. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; McAllister, T.W. Exploring the Convergence of Posttraumatic Stress Disorder and Mild Traumatic Brain Injury. Am. J. Psychiatry 2009, 166, 768–776. [Google Scholar] [CrossRef]
- McGeary, D.D.; Resick, P.A.; Penzien, D.B.; McGeary, C.A.; Houle, T.T.; Eapen, B.C.; Jaramillo, C.A.; Nabity, P.S.; Reed, D.E.; Moring, J.C.; et al. Cognitive Behavioral Therapy for Veterans With Comorbid Posttraumatic Headache and Posttraumatic Stress Disorder Symptoms. JAMA Neurol. 2022, 79, 746. [Google Scholar] [CrossRef]
- Weiner, M.W.; Harvey, D.; Landau, S.M.; Veitch, D.P.; Neylan, T.C.; Grafman, J.H.; Aisen, P.S.; Petersen, R.C.; Jack Jr, C.R.; Tosun, D.; et al. Traumatic Brain Injury and Post-traumatic Stress Disorder Are Not Associated with Alzheimer’s Disease Pathology Measured with Biomarkers. Alzheimer’s Dement. 2022, 19, 884–895. [Google Scholar] [CrossRef]
- Zheng, B.; Lai, R.; Li, J.; Zuo, Z. Critical Role of P2X7 Receptors in the Neuroinflammation and Cognitive Dysfunction after Surgery. Brain Behav. Immun. 2017, 61, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Safavynia, S.A.; Goldstein, P.A. The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Front. Psychiatry 2019, 9, 752. [Google Scholar] [CrossRef]
- Powell, N.D.; Sloan, E.K.; Bailey, M.T.; Arevalo, J.M.G.; Miller, G.E.; Chen, E.; Kobor, M.S.; Reader, B.F.; Sheridan, J.F.; Cole, S.W. Social Stress Up-Regulates Inflammatory Gene Expression in the Leukocyte Transcriptome via β-Adrenergic Induction of Myelopoiesis. Proc. Natl. Acad. Sci. USA 2013, 110, 16574–16579. [Google Scholar] [CrossRef] [Green Version]
- Polsky, L.R.; Rentscher, K.E.; Carroll, J.E. Stress-Induced Biological Aging: A Review and Guide for Research Priorities. Brain Behav. Immun. 2022, 104, 97–109. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Lew, J.; Albaugh, M.D.; Botteron, K.N.; Hudziak, J.J.; Fonov, V.S.; Collins, D.L.; Ducharme, S.; McCracken, J.T. Sex-Specific Associations of Testosterone with Prefrontal-Hippocampal Development and Executive Function. Psychoneuroendocrinology 2017, 76, 206–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forgie, M.L.; Kolb, B. Manipulation of Gonadal Hormones in Neonatal Rats Alters the Morphological Response of Cortical Neurons to Brain Injury in Adulthood. Behav. Neurosci. 2003, 117, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Isgor, C.; Sengelaub, D.R. Effects of Neonatal Gonadal Steroids on Adult CA3 Pyramidal Neuron Dendritic Morphology and Spatial Memory in Rats. J. Neurobiol. 2003, 55, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Pilgrim, C.; Hutchison, J.B. Developmental Regulation of Sex Differences in the Brain: Can the Role of Gonadal Steroids Be Redefined? Neuroscience 1994, 60, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.N.; Jacome, L.F.; MacLusky, N.J. Rapid Enhancement of Visual and Place Memory by Estrogens in Rats. Endocrinology 2003, 144, 2836–2844. [Google Scholar] [CrossRef]
- Cooke, B.M.; Tabibnia, G.; Breedlove, S.M. A Brain Sexual Dimorphism Controlled by Adult Circulating Androgens. Proc. Natl. Acad. Sci. USA 1999, 96, 7538–7540. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Matsumoto, T.; Kawano, H.; Watanabe, T.; Uematsu, Y.; Sekine, K.; Fukuda, T.; Aihara, K.; Krust, A.; Yamada, T.; et al. Brain Masculinization Requires Androgen Receptor Function. Proc. Natl. Acad. Sci. USA 2004, 101, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Oti, T.; Takanami, K.; Katayama, N.; Edey, T.; Satoh, K.; Sakamoto, T.; Sakamoto, H. Perinatal Testosterone Exposure Is Critical for the Development of the Male-Specific Sexually Dimorphic Gastrin-Releasing Peptide System in the Lumbosacral Spinal Cord That Mediates Erection and Ejaculation. Biol. Sex Differ. 2016, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Tonelli, L.; Regenold, W.T.; McCarthy, M.M. Effects of Neonatal Flutamide Treatment on Hippocampal Neurogenesis and Synaptogenesis Correlate with Depression-like Behaviors in Preadolescent Male Rats. Neuroscience 2010, 169, 544–554. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, M.M.; Nugent, B.M.; Lenz, K.M. Neuroimmunology and Neuroepigenetics in the Establishment of Sex Differences in the Brain. Nat. Rev. Neurosci. 2017, 18, 471–484. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, M.M.; Arnold, A.P. Reframing Sexual Differentiation of the Brain. Nat. Neurosci. 2011, 14, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Späni, C.B.; Braun, D.J.; van Eldik, L.J. Sex-Related Responses after Traumatic Brain Injury: Considerations for Preclinical Modeling. Front. Neuroendocrinol. 2018, 50, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Waltzman, D.; Haarbauer-Krupa, J.; Daugherty, J.; Thomas, K.; Sarmiento, K. State-Level Numbers and Rates of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths by Sex, 2014. J. Head Trauma Rehabil. 2020, 35, E481–E489. [Google Scholar] [CrossRef]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR. Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rentscher, K.E.; Carroll, J.E.; Repetti, R.L.; Cole, S.W.; Reynolds, B.M.; Robles, T.F. Chronic Stress Exposure and Daily Stress Appraisals Relate to Biological Aging Marker P16INK4a. Psychoneuroendocrinology 2019, 102, 139–148. [Google Scholar] [CrossRef]
- Kahle, K.T.; Staley, K.J. The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg. Focus 2008, 25, E22. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, M.; Bergeron, M.J.; Lavertu, G.; Castonguay, A.; Tripathy, S.; Bonin, R.P.; Perez-Sanchez, J.; Boudreau, D.; Wang, B.; Dumas, L.; et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 2013, 19, 1524–1528. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.L. The Expanding Therapeutic Potential of Neuronal KCC2. Cells 2020, 9, 240. [Google Scholar] [CrossRef] [Green Version]
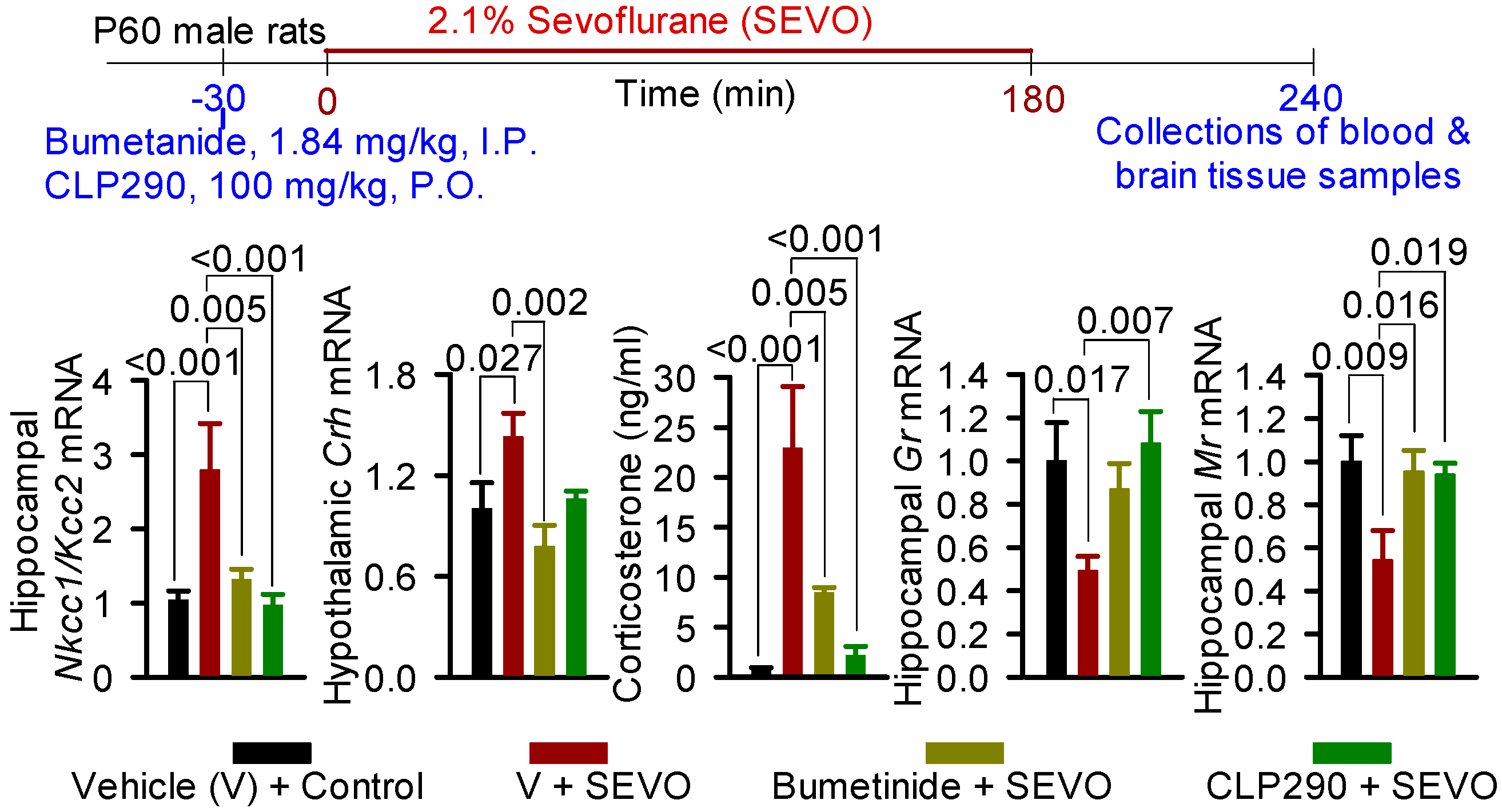
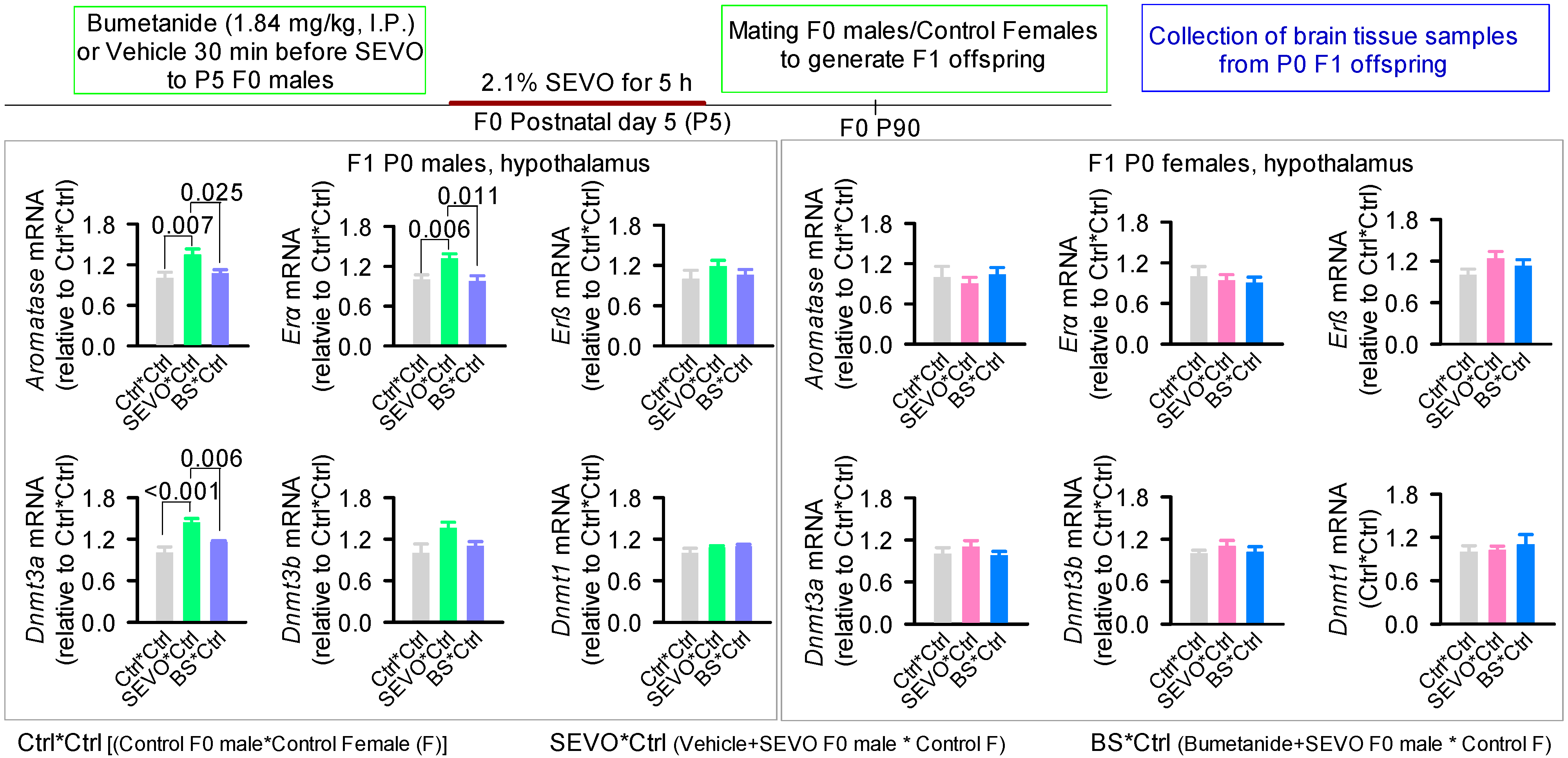
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, L.-S.; Morey, T.E.; Seubert, C.N.; Martynyuk, A.E. Intergenerational Perioperative Neurocognitive Disorder. Biology 2023, 12, 567. https://doi.org/10.3390/biology12040567
Ju L-S, Morey TE, Seubert CN, Martynyuk AE. Intergenerational Perioperative Neurocognitive Disorder. Biology. 2023; 12(4):567. https://doi.org/10.3390/biology12040567
Chicago/Turabian StyleJu, Ling-Sha, Timothy E. Morey, Christoph N. Seubert, and Anatoly E. Martynyuk. 2023. "Intergenerational Perioperative Neurocognitive Disorder" Biology 12, no. 4: 567. https://doi.org/10.3390/biology12040567
APA StyleJu, L.-S., Morey, T. E., Seubert, C. N., & Martynyuk, A. E. (2023). Intergenerational Perioperative Neurocognitive Disorder. Biology, 12(4), 567. https://doi.org/10.3390/biology12040567




