Automatic Segmentation of Osteonal Microstructure in Human Cortical Bone Using Deep Learning: A Proof of Concept
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
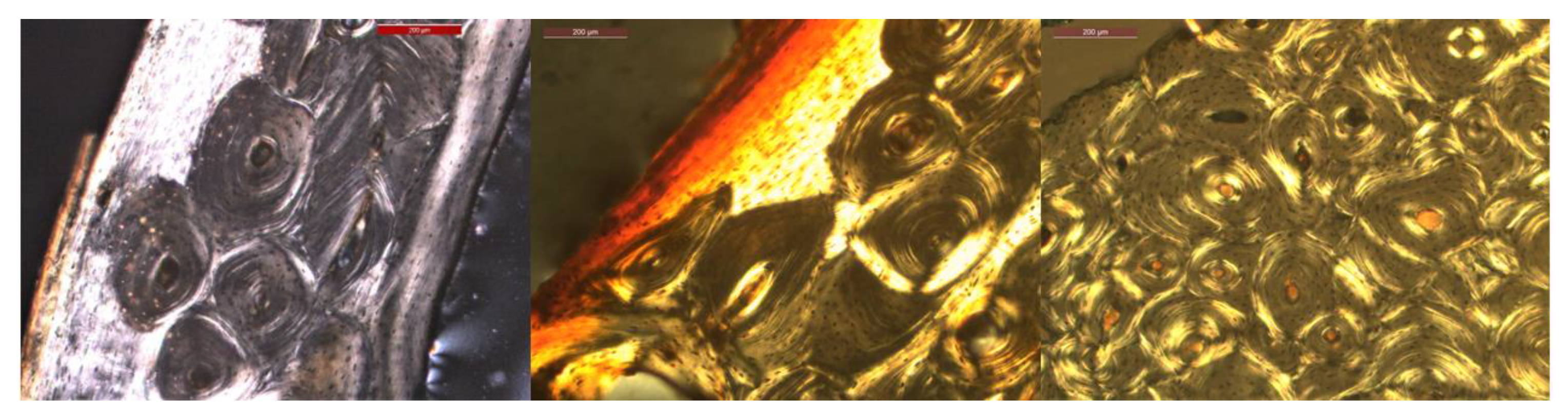
2.1. Dataset
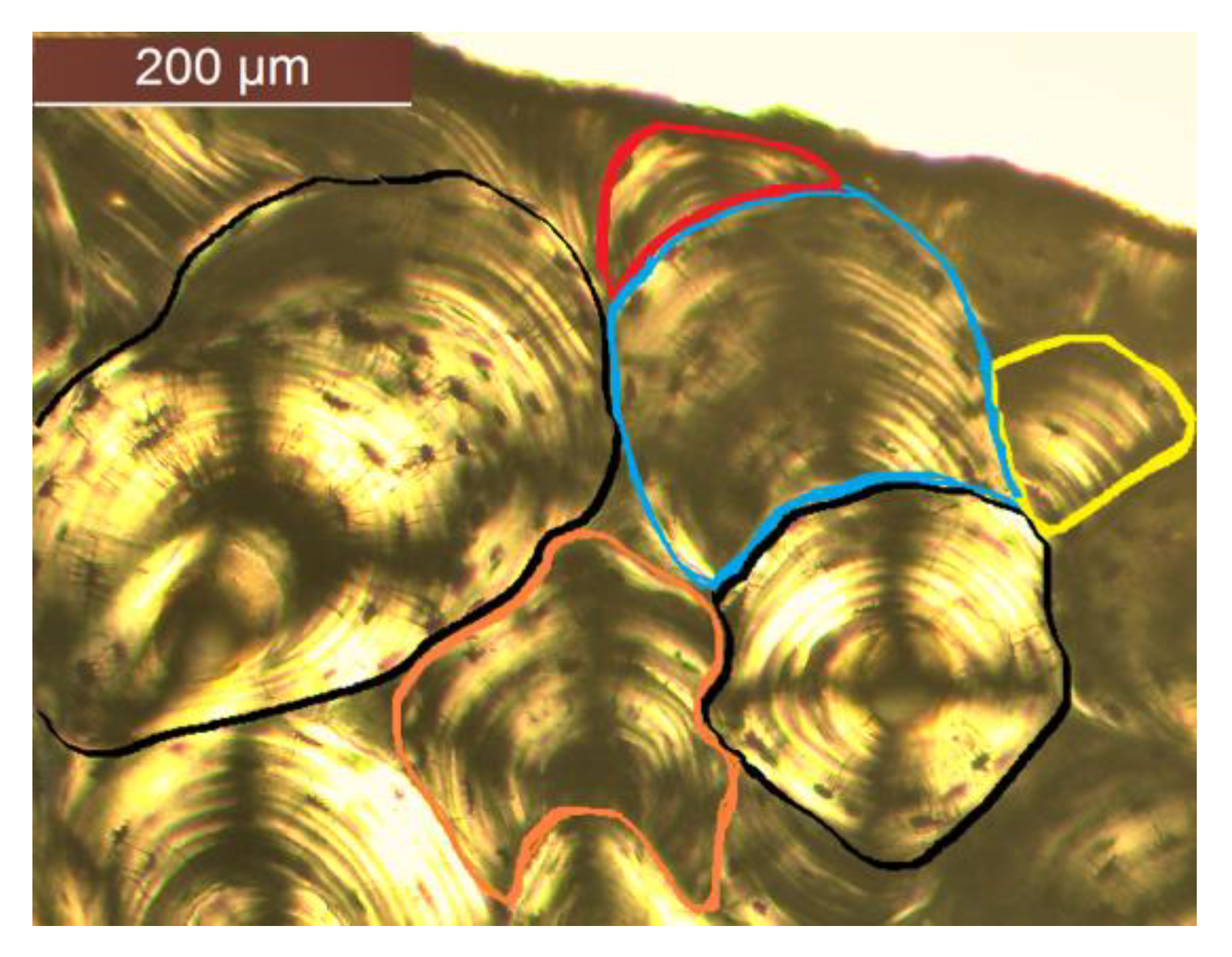
2.2. Manual Image Annotation
2.3. Deep Learning Model: Semantic Segmentation
3. Results
3.1. Qualitative Results
3.2. Quantitative Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- İşcan, M.; Steyn, M. The Human Skeleton in Forensic Medicine, 3rd ed.; Charles C Thomas: Springfield, IL, USA, 2013; ISBN 9780398088781. [Google Scholar]
- Zapico, S.C.; Ubelaker, D.H. Applications of physiological bases of ageing to forensic sciences. Estimation of age-at-death. Ageing Res. Rev. 2013, 12, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Hollund, H.I.; Jans, M.M.E.; Collins, M.J.; Kars, H.; Joosten, I.; Kars, S.M. What Happened Here? Bone Histology as a Tool in Decoding the Postmortem Histories of Archaeological Bone from Castricum, The Netherlands. Int. J. Osteoarchaeol. 2012, 22, 537–548. [Google Scholar] [CrossRef]
- De Boer, H.H.; Van der Merwe, A.E. Diagnostic dry bone histology in human paleopathology. Clin. Anat. 2016, 29, 831–843. [Google Scholar] [CrossRef]
- Hillier, M.L.; Bell, L.S. Differentiating human bone from animal bone: A review of histological methods. J. Forensic Sci. 2007, 52, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Streeter, M. Histological Age-at-death estimation. In Bone Histology: An Anthropological Perspective; Crowder, C.M., Stout, S.D., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 135–152. [Google Scholar]
- Goliath, J.; Stewart, M.; Stout, S. Variation in osteon histomorphometrics and their impact on age-at-death estimation in older individuals. Forensic Sci. Int. 2016, 262, 282.e1–282.e6. [Google Scholar] [CrossRef]
- Lee, U.Y.; Jung, G.U.; Choi, S.G.; Kim, Y.S. Anthropological age estimation with bone histomorphometry from the human clavicle. Anthropologist 2014, 17, 929–936. [Google Scholar] [CrossRef]
- Stout, S.D.; Paine, R.R. Brief Communication: Histological Age Estimation Using Rib and Clavicle. Am. J. Phys. Anthropol. 1992, 87, 111–115. [Google Scholar]
- Black, J.; Mattson, R.; Korostoff, E. Haversian osteons: Size, distribution, internal structure, and orientation. J. Biomed. Mater. Res. 1974, 8, 299–319. [Google Scholar] [CrossRef]
- Martin, R.; Burr, D. The microscopic structure of bone. In Structure, Function and Adaptation of Bone; Raven Press: New York, NY, USA, 1989; pp. 18–56. [Google Scholar]
- Cho, H.; Stout, S.D.; Bishop, T.A. Cortical bone remodeling rates in a sample of African American and European American descent groups from the American midwest: Comparisons of age and sex in ribs. Am. J. Phys. Anthropol. 2006, 130, 214–226. [Google Scholar] [CrossRef]
- Raguin, E.; Drapeau, M.S.M. Relation between cross-sectional bone geometry and double zonal osteon frequency and morphology. Am. J. Phys. Anthropol. 2020, 171, 598–612. [Google Scholar] [CrossRef]
- Robling, A.G.; Stout, S.D. Morphology of the drifting osteon. Cells Tissues Organs 1999, 164, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Maat, G.; Aarents, M.J.J.O.B. Manual preparation of ground sections for the microscopy of natural bone tissue: Update and modification of Frost’s “rapid manual method”. Int. J. Osteoarchaeol. 2001, 374, 366–374. [Google Scholar] [CrossRef]
- Paine, R.R. How to equip a basic histological lab for the anthropological assessment of human bone and teeth. J. Anthropol. Sci. 2007, 85, 213–219. [Google Scholar]
- Cho, H. The histological laboratory and principles of of microscope instrumentation. In Bone Histology: An Anthropological Perspective; Crowder, C., Stout, S., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 341–359. [Google Scholar]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- García-Donas, J.; Paine, R.R.; Bonicelli, A.; Kranioti, E. Age estimation for two Mediterranean populations: Rib histomorphometry applied to forensic identification and bone remodelling research. Int. J. Legal Med. 2022, 136, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Kranioti, E.F.; Michopoulou, E.; Tsiminikaki, K.; Bonicelli, A.; Kalochristianakis, M.; Xhemali, B.; Paine, R.R.; García-Donas, J.G. Bone histomorphometry of the clavicle in a forensic sample from Albania. Forensic Sci. Int. 2020, 313, 110335. [Google Scholar] [CrossRef]
- Andronowski, J.M.; Crowder, C.; Martinez, M.S. Recent advancements in the analysis of bone microstructure: New dimensions in forensic anthropology. Forensic Sci. Res. 2018, 3, 294–309. [Google Scholar] [CrossRef]
- Lynnerup, N.; Thomsen, J.L.; Frohlich, B. Intra- and inter-observer variation in histological criteria used in age at death determination based on femoral cortical bone. Forensic Sci. Int. 1998, 91, 219–230. [Google Scholar] [CrossRef]
- Bewes, J.; Low, A.; Morphett, A.; Pate, F.D.; Henneberg, M. Artificial intelligence for sex determination of skeletal remains: Application of a deep learning artificial neural network to human skulls. J. Forensic Leg. Med. 2019, 62, 40–43. [Google Scholar] [CrossRef]
- Navega, D.; Coelho, C.; Vicente, R.; Ferreira, M.T.; Wasterlain, S.; Cunha, E. AncesTrees: Ancestry estimation with randomized decision trees. Int. J. Leg. Med. 2015, 129, 1145–1153. [Google Scholar] [CrossRef]
- Cole, M.E.; Stout, S.D.; Agnew, A.M. Pore Extractor 2D: An ImageJ plugin for identification, classification, and regional characterization of cortical pores on histological bone images. Am. J. Phys. Anthropol. 2021, 174, 20–21. [Google Scholar]
- Navega, D.; Coelho, J.D.O.; Cunha, E.; Curate, F. DXAGE: A New Method for Age at Death Estimation Based on Femoral Bone Mineral Density and Artificial Neural Networks. J. Forensic Sci. 2018, 63, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Qin, F.; Li, Y.; Yu, C. Intelligent Objective Osteon Segmentation Based on Deep Learning. Front. Earth Res. 2022, 10, 783481. [Google Scholar] [CrossRef]
- Fernandes, L.C.C.; Bento, M.I.C.; Rabello, P.M.; Soriano, E.P.; Navega, D.; Júnior, E.D.; Cunha, E. Analysis of the Accuracy of AncesTrees Software in Ancestry Estimation in Brazilian Identified Sample. Adv. Anthropol. 2021, 11, 163–178. [Google Scholar] [CrossRef]
- Sieber, K.S.; García-Donas, J.G. Population affinity estimation on a Spanish sample: Testing the validity and accuracy of cranium and mandible online software methods. Leg. Med. 2023, 60, 102180. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Granados, J.C.; Irurita, J.; Pérez, R.; González, A.; Damas, S.; Alemán, I.; Cordón, O. Automating the decision making process of Todd’s age estimation method from the pubic symphysis with explainable machine learning. Inf. Sci. 2022, 612, 514–535. [Google Scholar] [CrossRef]
- García-Donas, J.G.; Dalton, A.; Chaplin, I.; Kranioti, E.F. A revised method for the preparation of dry bone samples used in histological examination: Five simple steps. J. Comp. Hum. Biol. 2017, 68, 283–288. [Google Scholar] [CrossRef]
- Cho, H.; Stout, S.D.; Madsen, R.W.; Streeter, M. a Population-specific histological age-estimating method: A model for known African-American and European-American skeletal remains. J. Forensic Sci. 2002, 47, 12–18. [Google Scholar] [CrossRef]
- Keenan, K.E.; Mears, C.S.; Skedros, J.G. Utility of osteon circularity for determining species and interpreting load history in primates and nonprimates. Am. J. Phys. Anthropol. 2017, 162, 657–681. [Google Scholar] [CrossRef]
- Skedros, J.G.; Holmes, J.L.; Vajda, E.G.; Bloebaum, R.D. Cement lines of secondary osteons in human bone are not mineral-deficient: New data in a historical perspective. Anat. Rec. 2005, 286, 781–803. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, D.I.; Park, D.K.; Lee, J.H.; Chung, N.E.; Lee, W.T.; Han, S.H. Assessment of histomorphological features of the sternal end of the fourth rib for age estimation in Koreans. J. Forensic Sci. 2007, 52, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Cooke, K.M.; Mahoney, P.; Miszkiewicz, J. Secondary osteon variants and remodeling in human bone. Anat. Rec. 2022, 305, 1299–1315. [Google Scholar] [CrossRef]
- Raguin, E.; Streeter, M.A. Brief communication: Test of a method to identify double-zonal osteon in polarized light microscopy. Am. J. Phys. Anthropol. 2018, 167, 407–415. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI; Navab, N., Hornegger, J., Wells, W., Frangi, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Kingma, D.; Ba, J. Adam: A Method for Stochastic Optimization. In Proceedings of the International Conference on Learning Representations (ICLR), San Diego, CA, USA, 7–9 May 2015; pp. 1–15. [Google Scholar]
- Miszkiewicz, J.J.; Mahoney, P. Histomorphometry and cortical robusticity of the adult human femur. J. Bone Miner. Metab. 2019, 37, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S. Variability in osteon size in recent human populations. Am. J. Phys. Anthropol. 1998, 106, 219–227. [Google Scholar] [CrossRef]
- Martiniaková, M.; Grosskopf, B.; Omelka, R.; Vondráková, M.; Bauerová, M. Differences among species in compact bone tissue microstructure of mammalian skeleton: Use of a discriminant function analysis for species identification. J. Forensic Sci. 2006, 51, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Lagacé, F.; Verna, E.; Adalian, P.; Baccino, E.; Martrille, L. Testing the accuracy of a new histomorphometric method for age-at-death estimation. Forensic Sci. Int. 2019, 296, 48–52. [Google Scholar] [CrossRef]
- Crescimanno, A.; Stout, S.D. Differentiating fragmented human and nonhuman long Bone using osteon circularity. J. Forensic Sci. 2012, 57, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Absolonova, K.; Veleminsky, P.; Dobisikova, M.; Beran, M.; Zocova, J. Histological Estimation of Age at Death from the Compact Bone of Burned and Unburned Human Ribs. J. Forensic Sci. 2013, 58 (Suppl. 1), 135–145. [Google Scholar] [CrossRef] [PubMed]
- Beauchesne, P.; Saunders, S. A test of the revised Frost’s “rapid manual method” for the preparation of bone thin sections. Int. J. Osteoarchaeol. 2006, 16, 82–87. [Google Scholar] [CrossRef]
- Haas, K.; Storå, J. Different Preparation Techniques–Similar Results? On the Quality of Thin-Ground Sections of Archaeological Bone. Int. J. Osteoarchaeol. 2015, 25, 935–945. [Google Scholar] [CrossRef]
- Crowder, C.M. Evaluating the Use of Quantitative Bone Histology to Estimate Adult Age at Death. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2005. [Google Scholar]
- Wu, K.; Schubeck, K.E.; Frost, H.M.; Villanueva, A. Haversian bone formation rates determined by a new method in a mastodon, and in human diabetes mellitus and osteoporosis. Calcif. Tissue Res. 1970, 6, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, M.; Skedros, J.; Polk, J.; Drapeau, M.S.M.; Streeter, M.A. Modeling and remodeling responses to normal loading in the human lower limb. Am. J. Phys. Anthropol. 2006, 129, 403–409. [Google Scholar] [CrossRef]
- Maggio, A.; Franklin, D. Histomorphometric age estimation from the femoral cortex: A test of three methods in an Australian population. Forensic Sci. Int. 2019, 303, 109950. [Google Scholar] [CrossRef]
- Kerley, E.R. The microscopic determination of age in human bone. Am. J. Phys. Anthropol. 1965, 23, 149–164. [Google Scholar] [CrossRef]


| Predicted Label | |||
|---|---|---|---|
| Background | Fragment | Intact | |
| Background | 0.406 | 0.090 | 0.050 |
| Fragment | 0.025 | 0.066 | 0.044 |
| Intact | 0.028 | 0.054 | 0.236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Littek, A.; McKenna, S.J.; Chiam, W.X.; Kranioti, E.F.; Trucco, E.; García-Donas, J.G. Automatic Segmentation of Osteonal Microstructure in Human Cortical Bone Using Deep Learning: A Proof of Concept. Biology 2023, 12, 619. https://doi.org/10.3390/biology12040619
Littek A, McKenna SJ, Chiam WX, Kranioti EF, Trucco E, García-Donas JG. Automatic Segmentation of Osteonal Microstructure in Human Cortical Bone Using Deep Learning: A Proof of Concept. Biology. 2023; 12(4):619. https://doi.org/10.3390/biology12040619
Chicago/Turabian StyleLittek, Alina, Stephen J. McKenna, Wei Xiong Chiam, Elena F. Kranioti, Emanuele Trucco, and Julieta G. García-Donas. 2023. "Automatic Segmentation of Osteonal Microstructure in Human Cortical Bone Using Deep Learning: A Proof of Concept" Biology 12, no. 4: 619. https://doi.org/10.3390/biology12040619
APA StyleLittek, A., McKenna, S. J., Chiam, W. X., Kranioti, E. F., Trucco, E., & García-Donas, J. G. (2023). Automatic Segmentation of Osteonal Microstructure in Human Cortical Bone Using Deep Learning: A Proof of Concept. Biology, 12(4), 619. https://doi.org/10.3390/biology12040619







