Effects of Sea-Ice Persistence on the Diet of Adélie Penguin (Pygoscelis adeliae) Chicks and the Trophic Differences between Chicks and Adults in the Ross Sea, Antarctica
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
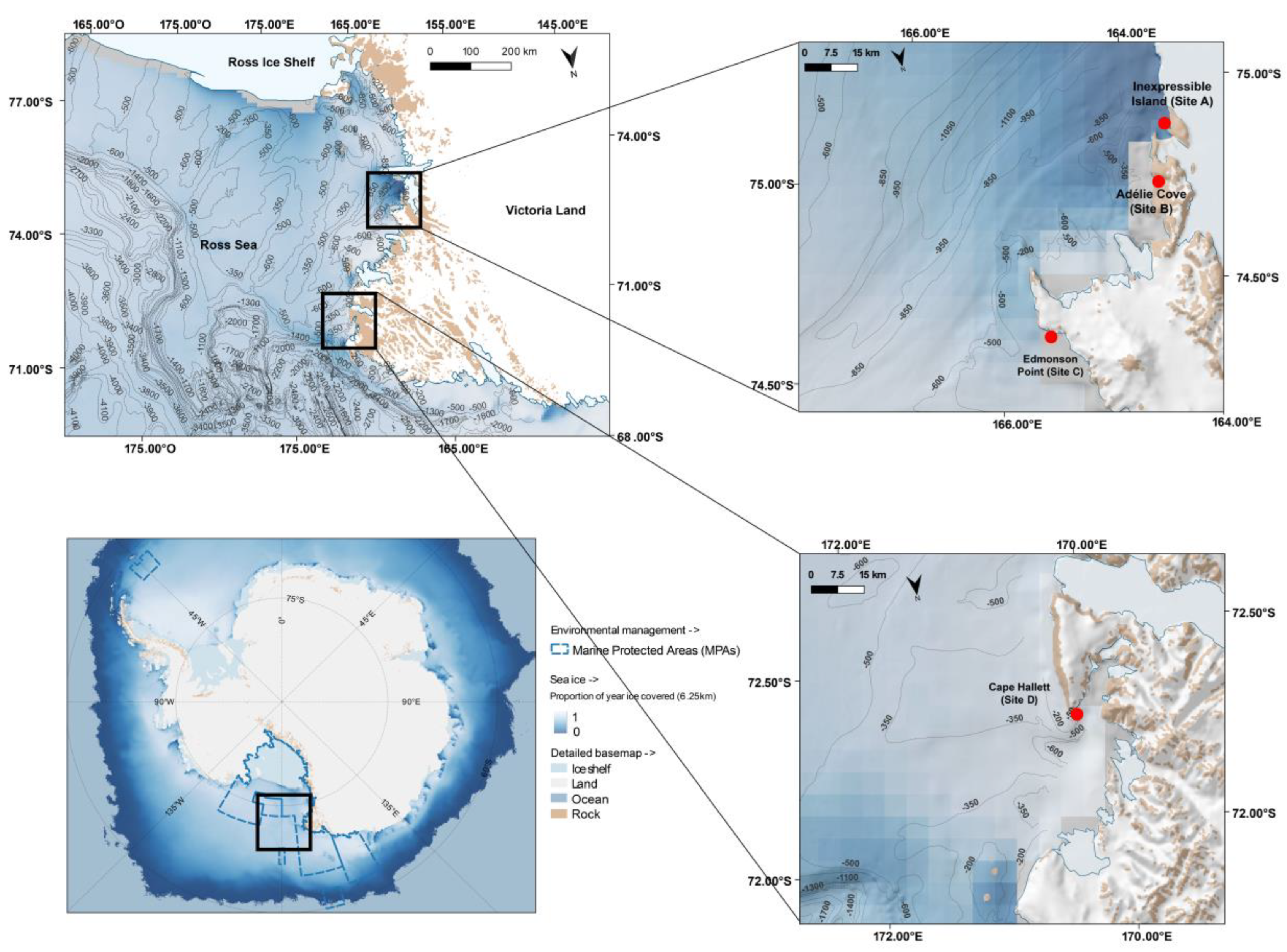
2.1. Study Area
2.2. Sampling Procedures
2.3. Laboratory Procedures and Stable Isotope Analysis
2.4. Data Analysis
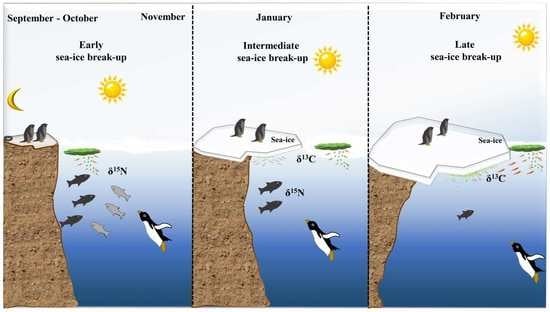
2.4.1. Sea-Ice Persistence through Satellite Image Analysis
2.4.2. Isotopic Niche Analysis and Mixing Models
2.4.3. Data and Statistical Analysis
3. Results
3.1. Sea-Ice Persistence
3.2. Comparisons of Penguin Prey
3.3. Comparisons of Chick Faeces between Years and between Colonies
3.4. Comparisons between Chicks and Adults
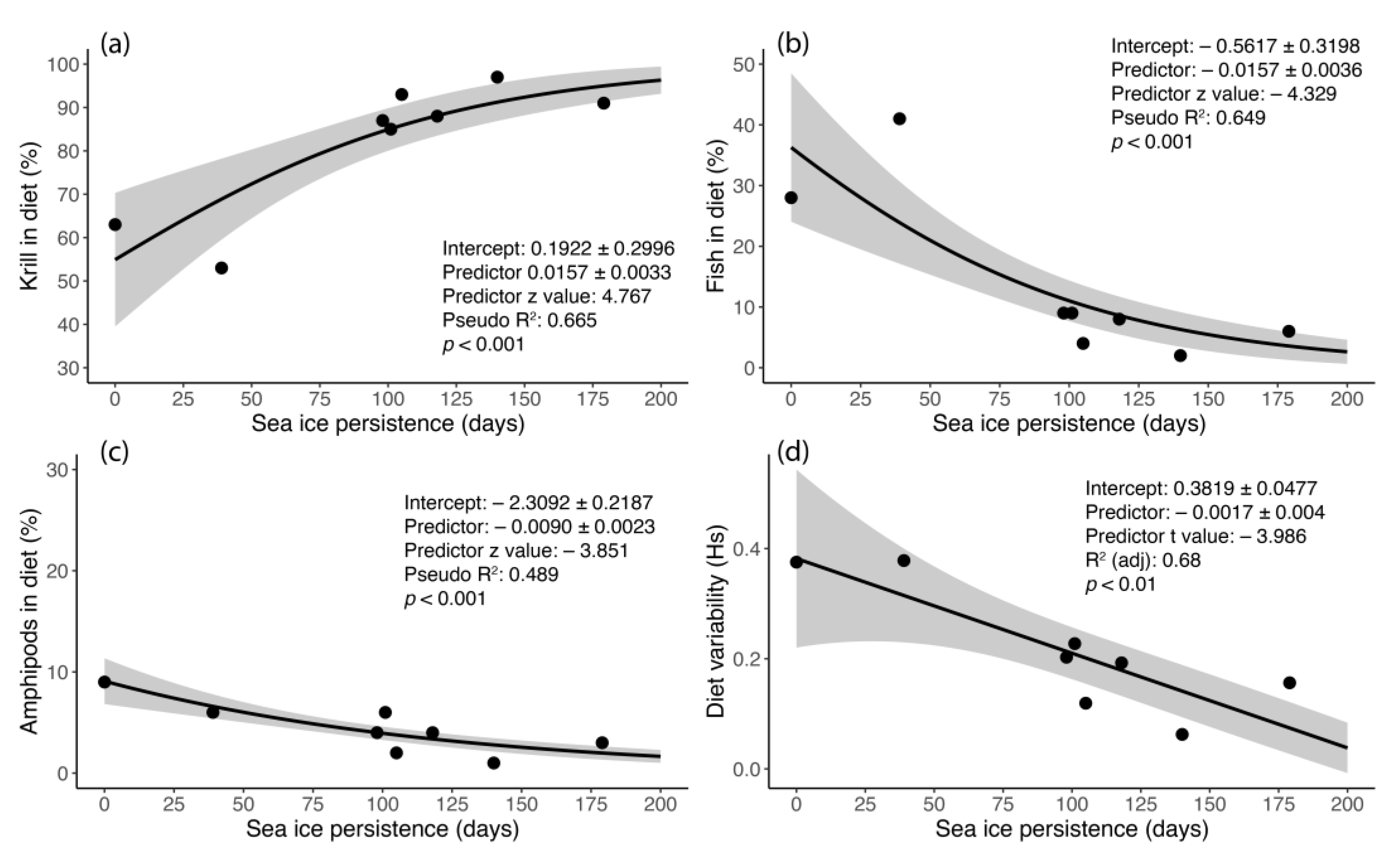
3.5. The Diet of Adélie Penguins and Its Relationship to Sea-Ice Persistence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Calizza, E.; Rossi, L.; Careddu, G.; Sporta Caputi, S.; Costantini, M.L. Species Richness and Vulnerability to Disturbance Propagation in Real Food Webs. Sci. Rep. 2019, 9, 19331. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R. Community and Ecosystem Responses to Recent Climate Change. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef]
- Constable, A.J.; Melbourne-Thomas, J.; Corney, S.P.; Arrigo, K.R.; Barbraud, C.; Barnes, D.K.A.; Bindoff, N.L.; Boyd, P.W.; Brandt, A.; Costa, D.P.; et al. Climate Change and Southern Ocean Ecosystems I: How Changes in Physical Habitats Directly Affect Marine Biota. Glob. Chang. Biol. 2014, 20, 3004–3025. [Google Scholar] [CrossRef]
- Rogers, A.D.; Frinault, B.A.V.; Barnes, D.K.A.; Bindoff, N.L.; Downie, R.; Ducklow, H.W.; Friedlaender, A.S.; Hart, T.; Hill, S.L.; Hofmann, E.E.; et al. Antarctic Futures: An Assessment of Climate-Driven Changes in Ecosystem Structure, Function, and Service Provisioning in the Southern Ocean. Annu. Rev. Mar. Sci. 2020, 12, 87–120. [Google Scholar] [CrossRef]
- Steiner, N.S.; Bowman, J.; Campbell, K.; Chierici, M.; Eronen-Rasimus, E.; Falardeau, M.; Flores, H.; Fransson, A.; Herr, H.; Insley, S.J.; et al. Climate Change Impacts on Sea-Ice Ecosystems and Associated Ecosystem Services. Elem. Sci. Anthr. 2021, 9, 00007. [Google Scholar] [CrossRef]
- Arrigo, K.R. Sea Ice Ecosystems. Annu. Rev. Mar. Sci. 2014, 6, 439–467. [Google Scholar] [CrossRef]
- Lizotte, M.P. The Contributions of Sea Ice Algae to Antarctic Marine Primary Production. Am. Zool. 2001, 41, 57–73. [Google Scholar] [CrossRef]
- Rossi, L.; Sporta Caputi, S.; Calizza, E.; Careddu, G.; Oliverio, M.; Schiaparelli, S.; Costantini, M.L. Antarctic Food Web Architecture under Varying Dynamics of Sea Ice Cover. Sci. Rep. 2019, 9, 12454. [Google Scholar] [CrossRef] [PubMed]
- Saggiomo, M.; Escalera, L.; Saggiomo, V.; Bolinesi, F.; Mangoni, O. Phytoplankton Blooms Below the Antarctic Landfast Ice During the Melt Season Between Late Spring and Early Summer. J. Phycol. 2021, 57, 541–550. [Google Scholar] [CrossRef]
- Smith, W.O.; Marra, J.; Hiscock, M.R.; Barber, R.T. The Seasonal Cycle of Phytoplankton Biomass and Primary Productivity in the Ross Sea, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 3119–3140. [Google Scholar] [CrossRef]
- Youngflesh, C.; Jenouvrier, S.; Li, Y.; Ji, R.; Ainley, D.G.; Ballard, G.; Barbraud, C.; Delord, K.; Dugger, K.M.; Emmerson, L.M.; et al. Circumpolar Analysis of the Adélie Penguin Reveals the Importance of Environmental Variability in Phenological Mismatch. Ecology 2017, 98, 940–951. [Google Scholar] [CrossRef]
- Calizza, E.; Careddu, G.; Sporta Caputi, S.; Rossi, L.; Costantini, M.L. Time- and Depth-Wise Trophic Niche Shifts in Antarctic Benthos. PLoS ONE 2018, 13, e0194796. [Google Scholar] [CrossRef] [PubMed]
- Sporta Caputi, S.; Careddu, G.; Calizza, E.; Fiorentino, F.; Maccapan, D.; Rossi, L.; Costantini, M.L. Seasonal Food Web Dynamics in the Antarctic Benthos of Tethys Bay (Ross Sea): Implications for Biodiversity Persistence under Different Seasonal Sea-Ice Coverage. Front. Mar. Sci. 2020, 7, 1046. [Google Scholar] [CrossRef]
- Ropert-Coudert, Y.; Hindell, M.A.; Phillips, R.A.; Charrassin, J.-B.; Trudelle, L.; Raymond, B. 8. Biogeographic Patterns of Birds and Mammals; SCAR-Scientific Committee on Antarctic Research: Cambridge, UK, 2014; pp. 364–387. [Google Scholar]
- Harris, C.; Lorenz, K.; van Franeker, J. Important Bird Areas in Antarctica 2015; BirdLife Int. and Env. Research & Assessment: Cambridge, UK, 2015. [Google Scholar]
- Watanuki, Y.; Miyamoto, Y.; Kato, A. Dive Bouts and Feeding Sites of Adélie Penguins Rearing Chicks in an Area with Fast Sea-Ice. Waterbirds Int. J. Waterbird Biol. 1999, 22, 120. [Google Scholar] [CrossRef]
- Kato, A.; Watanuki, Y.; Naito, Y. Annual and Seasonal Changes in Foraging Site and Diving Behavior in Adélie Penguins. Polar Biol. 2003, 26, 389–395. [Google Scholar] [CrossRef]
- Barreau, E.; Ropert-Coudert, Y.; Delord, K.; Barbraud, C.; Kato-Ropert, A. Scale Matters: Sea Ice and Breeding Success of Adélie Penguins. Polar Biol. 2019, 42, 1405–1410. [Google Scholar] [CrossRef]
- Koubbi, P.; Hosie, G.; Constable, A.; Raymond, B.; Moteki, M.; Améziane, N.; Causse, R.; Fuentes, V.; Heerah, K.; Penot, F. Estimating the Biodiversity of the Shelf and Oceanic Zone of the d’Urville Sea (East Antarctica) for Ecoregionalisation Using the CEAMARC (Collaborative East Antarctic Marine Census) CAML Surveys. Polar Sci. 2011, 4, 115–133. [Google Scholar] [CrossRef]
- Davis, L.; Hofmann, E.; Klinck, J.; Piñones, A.; Dinniman, M. Distributions of Krill and Antarctic Silverfish and Correlations with Environmental Variables in the Western Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 2017, 584, 45–65. [Google Scholar] [CrossRef]
- Vacchi, M.; Pisano, E.; Ghigliotti, L. The Antarctic Silverfish: A Keystone Species in a Changing Ecosystem; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 3-319-55893-5. [Google Scholar]
- Michelot, C.; Kato, A.; Raclot, T.; Shiomi, K.; Goulet, P.; Bustamante, P.; Ropert-Coudert, Y. Sea-Ice Edge is more Important than Closer Open Water Access for Foraging Adélie Penguins: Evidence from Two Colonies. Mar. Ecol. Prog. Ser. 2020, 640, 215–230. [Google Scholar] [CrossRef]
- Olmastroni, S.; Fattorini, N.; Pezzo, F.; Focardi, S. Gone Fishing: Adélie Penguin Site-Specific Foraging Tactics and Breeding Performance. Antarct. Sci. 2020, 32, 199–209. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Gal, J.-K.; Lee, B.-Y.; Son, W.-J.; Jung, J.-W.; La, H.-S.; Shin, K.-H.; Kim, J.-H.; Ha, S.-Y. Regional Differences in the Diets of Adélie and Emperor Penguins in the Ross Sea, Antarctica. Animals 2021, 11, 2681. [Google Scholar] [CrossRef]
- Jafari, V.; Maccapan, D.; Careddu, G.; Sporta Caputi, S.; Calizza, E.; Rossi, L.; Costantini, M.L. Spatial and Temporal Diet Variability of Adélie (Pygoscelis Adeliae) and Emperor (Aptenodytes Forsteri) Penguin: A Multi Tissue Stable Isotope Analysis. Polar Biol. 2021, 44, 1869–1881. [Google Scholar] [CrossRef]
- Olmastroni, S.; Ferretti, F.; Burrini, L.; Ademollo, N.; Fattorini, N. Breeding Ecology of Adélie Penguins in Mid Victoria Land, Ross Sea Antarctica. Diversity 2022, 14, 429. [Google Scholar] [CrossRef]
- Tabassum, N.; Lee, J.-H.; Lee, S.-R.; Kim, J.-U.; Park, H.; Kim, H.-W.; Kim, J.-H. Molecular Diet Analysis of Adélie Penguins (Pygoscelis Adeliae) in the Ross Sea Using Fecal DNA. Biology 2022, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Jarman, S.N.; McInnes, J.C.; Faux, C.; Polanowski, A.M.; Marthick, J.; Deagle, B.E.; Southwell, C.; Emmerson, L. Adélie Penguin Population Diet Monitoring by Analysis of Food DNA in Scats. PLoS ONE 2013, 8, e82227. [Google Scholar] [CrossRef] [PubMed]
- Wing, S.R.; Wing, L.C.; O’Connell-Milne, S.A.; Barr, D.; Stokes, D.; Genovese, S.; Leichter, J.J. Penguins and Seals Transport Limiting Nutrients Between Offshore Pelagic and Coastal Regions of Antarctica Under Changing Sea Ice. Ecosystems 2021, 24, 1203–1221. [Google Scholar] [CrossRef]
- Offredo, C.; Ridoux, V.; Clarke, M.R. Cephalopods in the Diets of Emperor and Adelie Penguins in Adelie Land, Antarctica. Mar. Biol. 1985, 86, 199–202. [Google Scholar] [CrossRef]
- Ainley, D.; Dugger, K.; La Mesa, M.; Ballard, G.; Barton, K.; Jennings, S.; Karl, B.; Lescroël, A.; Lyver, P.; Schmidt, A.; et al. Post-Fledging Survival of Adélie Penguins at Multiple Colonies: Chicks Raised on Fish Do Well. Mar. Ecol. Prog. Ser. 2018, 601, 239–251. [Google Scholar] [CrossRef]
- Sladen, W.J. The Pygoscelid Penguins: I. Methods of Study. II. The Adélie Penguin; Falkland Islands Dependencies Survey Scientific Reports 17: London, UK, 1958. [Google Scholar]
- Kooyman, G.L. The Adélie Penguin: Bellwether of Climate Change. Condor 2003, 105, 835–836. [Google Scholar] [CrossRef]
- McInnes, J.C.; Emmerson, L.; Southwell, C.; Faux, C.; Jarman, S.N. Simultaneous DNA-Based Diet Analysis of Breeding, Non-Breeding and Chick Adélie Penguins. R. Soc. Open Sci. 2016, 3, 150443. [Google Scholar] [CrossRef]
- Ainley, D.G.; Ballard, G.; Barton, K.J.; Karl, B.J.; Rau, G.H.; Ribic, C.A.; Wilson, P.R. Spatial and Temporal Variation of Diet Within a Presumed Metapopulation Of Adélie Penguins. Condor 2003, 105, 95–106. [Google Scholar] [CrossRef]
- Tierney, M.; Southwell, C.; Emmerson, L.; Hindell, M. Evaluating and Using Stable-Isotope Analysis to Infer Diet Composition and Foraging Ecology of Adélie Penguins Pygoscelis Adeliae. Mar. Ecol. Prog. Ser. 2008, 355, 297–307. [Google Scholar] [CrossRef]
- Spurr, E.B. Behavior of the Adélie Penguin Chick. Condor 1975, 77, 272–280. [Google Scholar] [CrossRef]
- Ainley, D. The Adélie Penguin: Bellwether of Climate Change; Columbia University Press: New Yourk, NY, USA, 2002; ISBN 978-0-231-12306-8. [Google Scholar]
- Whitehead, A.; Lyver, P.; Ballard, G.; Barton, K.; Karl, B.; Dugger, K.; Jennings, S.; Lescroël, A.; Wilson, P.; Ainley, D. Factors Driving Adélie Penguin Chick Size, Mass and Condition at Colonies of Different Sizes in the Southern Ross Sea. Mar. Ecol. Prog. Ser. 2015, 523, 199–213. [Google Scholar] [CrossRef]
- Massaro, M.; Ainley, D.G.; Santora, J.A.; Quillfeldt, P.; Lescroël, A.; Whitehead, A.; Varsani, A.; Ballard, G.; Lyver, P.O.B. Diet Segregation in Adélie Penguins: Some Individuals Attempt to Overcome Colony-Induced and Annual Foraging Challenges. Mar. Ecol. Prog. Ser. 2020, 645, 205–218. [Google Scholar] [CrossRef]
- Watanabe, Y.Y.; Ito, K.; Kokubun, N.; Takahashi, A. Foraging Behavior Links Sea Ice to Breeding Success in Antarctic Penguins. Sci. Adv. 2020, 6, eaba4828. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, B.C.; Lawless, R.; Rodary, D.; Bost, C.-A.; Thomson, R.; Pauly, T.; Robertson, G.; Kerry, K.R.; LeMaho, Y. Adélie Penguin Foraging Behaviour and Krill Abundance along the Wilkes and Adélie Land Coasts, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 2573–2587. [Google Scholar] [CrossRef]
- Strickland, M.E.; Polito, M.; Emslie, S.D. Spatial and Seasonal Variation in Adélie Penguin Diet as Inferred from Stable Isotope Analysis of Eggshell. J. N. C. Acad. Sci. 2008, 124, 65–71. [Google Scholar]
- Ballerini, T.; Tavecchia, G.; Olmastroni, S.; Pezzo, F.; Focardi, S. Nonlinear Effects of Winter Sea Ice on the Survival Probabilities of Adélie Penguins. Oecologia 2009, 161, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, C.; Kato, A.; Raymond, B.; Barbraud, C.; Beaulieu, M.; Bost, C.; Delord, K.; MacIntosh, A.J.J.; Meyer, X.; Raclot, T.; et al. Reproductive Performance and Diving Behaviour Share a Common Sea-ice Concentration Optimum in Adélie Penguins (Pygoscelis adeliae). Glob. Chang. Biol. 2018, 24, 5304–5317. [Google Scholar] [CrossRef]
- Ballerini, T.; Tavecchia, G.; Pezzo, F.; Jenouvrier, S.; Olmastroni, S. Predicting Responses of the Adélie Penguin Population of Edmonson Point to Future Sea Ice Changes in the Ross Sea. Front. Ecol. Evol. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Kokubun, N.; Emmerson, L.; McInnes, J.; Wienecke, B.; Southwell, C. Sea-Ice and Density-Dependent Factors Affecting Foraging Habitat and Behaviour of Adélie Penguins throughout the Breeding Season. Mar. Biol. 2021, 168, 97. [Google Scholar] [CrossRef]
- Hobson, K.A.; Piatt, J.F.; Pitocchelli, J. Using Stable Isotopes to Determine Seabird Trophic Relationships. J. Anim. Ecol. 1994, 63, 786. [Google Scholar] [CrossRef]
- Thompson, D.R.; Bury, S.J.; Hobson, K.A.; Wassenaar, L.I.; Shannon, J.P. Stable Isotopes in Ecological Studies. Oecologia 2005, 144, 517–519. [Google Scholar] [CrossRef]
- Cherel, Y. Isotopic Niches of Emperor and Adélie Penguins in Adélie Land, Antarctica. Mar. Biol. 2008, 154, 813–821. [Google Scholar] [CrossRef]
- Polito, M.J.; Trivelpiece, W.Z.; Karnovsky, N.J.; Ng, E.; Patterson, W.P.; Emslie, S.D. Integrating Stomach Content and Stable Isotope Analyses to Quantify the Diets of Pygoscelid Penguins. PLoS ONE 2011, 6, e26642. [Google Scholar] [CrossRef]
- Polito, M.J.; Lynch, H.J.; Naveen, R.; Emslie, S.D. Stable Isotopes Reveal Regional Heterogeneity in the Pre-Breeding Distribution and Diets of Sympatrically Breeding Pygoscelis spp. Penguins. Mar. Ecol. Prog. Ser. 2011, 421, 265–277. [Google Scholar] [CrossRef]
- Lorenzini, S.; Baroni, C.; Fallick, A.E.; Baneschi, I.; Salvatore, M.C.; Zanchetta, G.; Dallai, L. Stable Isotopes Reveal Holocene Changes in the Diet of Adélie Penguins in Northern Victoria Land (Ross Sea, Antarctica). Oecologia 2010, 164, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Bird, M.I.; Tait, E.; Wurster, C.M.; Furness, R.W. Stable Carbon and Nitrogen Isotope Analysis of Avian Uric Acid. Rapid Commun. Mass Spectrom. 2008, 22, 3393–3400. [Google Scholar] [CrossRef]
- Tieszen, L.L.; Boutton, T.W.; Tesdahl, K.G.; Slade, N.A. Fractionation and Turnover of Stable Carbon Isotopes in Animal Tissues: Implications for δ 13 C Analysis of Diet. Oecologia 1983, 57, 32–37. [Google Scholar] [CrossRef]
- Kuwae, T.; Hosoya, J.; Ichimi, K.; Watanabe, K.; Drever, M.C.; Moriya, T.; Elner, R.W.; Hobson, K.A. Using Stable Isotope (Δ13C, Δ15N) Values from Feces and Breath to Infer Shorebird Diets. Oecologia 2022, 200, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Careddu, G.; Costantini, M.L.; Calizza, E.; Carlino, P.; Bentivoglio, F.; Orlandi, L.; Rossi, L. Effects of Terrestrial Input on Macrobenthic Food Webs of Coastal Sea Are Detected by Stable Isotope Analysis in Gaeta Gulf. Estuar. Coast. Shelf Sci. 2015, 154, 158–168. [Google Scholar] [CrossRef]
- Vanderklift, M.A.; Ponsard, S. Sources of Variation in Consumer-Diet δ15N Enrichment: A Meta-Analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef]
- Olmastroni, S.; Pezzo, F.; Volpi, V.; Focardi, S. Effects of Weather and Sea-Ice on the Reproductive Performance of the Adélie Penguin at Edmonson Point, Ross Sea. CCAMLR Sci. 2004, 11, 99–109. [Google Scholar]
- Hinke, J.T.; Trivelpiece, S.G.; Trivelpiece, W.Z. Adélie Penguin (Pygoscelis adeliae) Survival Rates and Their Relationship to Environmental Indices in the South Shetland Islands, Antarctica. Polar Biol. 2014, 37, 1797–1809. [Google Scholar] [CrossRef]
- Iles, D.T.; Lynch, H.; Ji, R.; Barbraud, C.; Delord, K.; Jenouvrier, S. Sea Ice Predicts Long-term Trends in Adélie Penguin Population Growth, but Not Annual Fluctuations: Results from a Range-wide Multiscale Analysis. Glob. Chang. Biol. 2020, 26, 3788–3798. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.; Phillips, R.; Takahashi, A. Antarctic Seabirds as Indicators of Climate Change. In Seabird Biodiversity and Human Activities; CRC Press: Boca Raton, FL, USA, 2022; pp. 189–210. ISBN 978-1-00-304752-0. [Google Scholar]
- Quillfeldt, P.; McGill, R.A.; Furness, R.W. Diet and Foraging Areas of Southern Ocean Seabirds and Their Prey Inferred from Stable Isotopes: Review and Case Study of Wilson’s Storm-Petrel. Mar. Ecol. Prog. Ser. 2005, 295, 295–304. [Google Scholar] [CrossRef]
- Croxall, J.P. Seabirds: Feeding Ecology and Role in Marine Ecosystems; Cambridge University Press: Cambridge, UK, 1987; ISBN 0-521-30178-5. [Google Scholar]
- Forero, M.; Hobson, K.; Bortolotti, G.; Donázar, J.; Bertellotti, M.; Blanco, G. Food Resource Utilisation by the Magellanic Penguin Evaluated through Stable-Isotope Analysis: Segregation by Sex and Age and Influence on Offspring Quality. Mar. Ecol. Prog. Ser. 2002, 234, 289–299. [Google Scholar] [CrossRef]
- Ballard, G.; Jongsomjit, D.; Veloz, S.D.; Ainley, D.G. Coexistence of Mesopredators in an Intact Polar Ocean Ecosystem: The Basis for Defining a Ross Sea Marine Protected Area. Biol. Conserv. 2012, 156, 72–82. [Google Scholar] [CrossRef]
- Brooks, C.M.; Bloom, E.; Kavanagh, A.; Nocito, E.S.; Watters, G.M.; Weller, J. The Ross Sea, Antarctica: A Highly Protected MPA in International Waters. Mar. Policy 2021, 134, 104795. [Google Scholar] [CrossRef]
- Bindschadler, R.; Vornberger, P.; Fleming, A.; Fox, A.; Mullins, J.; Binnie, D.; Paulsen, S.; Granneman, B.; Gorodetzky, D. The Landsat Image Mosaic of Antarctica. Remote Sens. Environ. 2008, 112, 4214–4226. [Google Scholar] [CrossRef]
- Spreen, G.; Kaleschke, L.; Heygster, G. Sea Ice Remote Sensing Using AMSR-E 89-GHz Channels. J. Geophys. Res. 2008, 113, C02S03. [Google Scholar] [CrossRef]
- Van Woert, M.L. Wintertime Dynamics of the Terra Nova Bay Polynya. J. Geophys. Res. Oceans 1999, 104, 7753–7769. [Google Scholar] [CrossRef]
- Fusco, G.; Flocco, D.; Budillon, G.; Spezie, G.; Zambianchi, E. Dynamics and Variability of Terra Nova Bay Polynya. Mar. Ecol. 2002, 23, 201–209. [Google Scholar] [CrossRef]
- Costantini, M.L.; Carlino, P.; Calizza, E.; Careddu, G.; Cicala, D.; Sporta Caputi, S.; Fiorentino, F.; Rossi, L. The Role of Alien Fish (the Centrarchid Micropterus salmoides) in Lake Food Webs Highlighted by Stable Isotope Analysis. Freshw. Biol. 2018, 63, 1130–1142. [Google Scholar] [CrossRef]
- Mengxi, Z.; Tiancheng, Z.; Fengming, H.; Xiao, C.; Aobo, L.; Jiawei, Y.; Yining, Y.; Yifan, D. Anomalous extensive landfast sea ice in the vicinity of Inexpressible Island, Antarctica. Adv. Polar Sci. 2019, 30, 406–411. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can Stable Isotope Ratios Provide for Community-Wide Measures of Trophic Structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing Isotopic Niche Widths among and within Communities: SIBER—Stable Isotope Bayesian Ellipses in R: Bayesian Isotopic Niche Metrics. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Smith, J.A.; Mazumder, D.; Suthers, I.M.; Taylor, M.D. To Fit or Not to Fit: Evaluating Stable Isotope Mixing Models Using Simulated Mixing Polygons. Methods Ecol. Evol. 2013, 4, 612–618. [Google Scholar] [CrossRef]
- Careddu, G.; Ciucci, P.; Mondovì, S.; Calizza, E.; Rossi, L.; Costantini, M.L. Gaining Insight into the Assimilated Diet of Small Bear Populations by Stable Isotope Analysis. Sci. Rep. 2021, 11, 14118. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Stock, B.C.; Jackson, A.L.; Ward, E.J.; Parnell, A.C.; Phillips, D.L.; Semmens, B.X. Analyzing Mixing Systems Using a New Generation of Bayesian Tracer Mixing Models. PeerJ 2018, 6, e5096. [Google Scholar] [CrossRef] [PubMed]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source Partitioning Using Stable Isotopes: Coping with Too Much Variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed]
- Vacchi, M.; La Mesa, M.; Dalu, M.; MacDonald, J. Early Life Stages in the Life Cycle of Antarctic Silverfish, Pleuragramma Antarcticum in Terra Nova Bay, Ross Sea. Antarct. Sci. 2004, 16, 299–305. [Google Scholar] [CrossRef]
- Cherel, Y.; Koubbi, P.; Giraldo, C.; Penot, F.; Tavernier, E.; Moteki, M.; Ozouf-Costaz, C.; Causse, R.; Chartier, A.; Hosie, G. Isotopic Niches of Fishes in Coastal, Neritic and Oceanic Waters off Adélie Land, Antarctica. Polar Sci. 2011, 5, 286–297. [Google Scholar] [CrossRef]
- Giraldo, C.; Cherel, Y.; Vallet, C.; Mayzaud, P.; Tavernier, E.; Moteki, M.; Hosie, G.; Koubbi, P. Ontogenic Changes in the Feeding Ecology of the Early Life Stages of the Antarctic Silverfish (Pleuragramma antarcticum) Documented by Stable Isotopes and Diet Analysis in the Dumont d’Urville Sea (East Antarctica). Polar Sci. 2011, 5, 252–263. [Google Scholar] [CrossRef]
- Goetz, K.T.; Burns, J.M.; Hückstädt, L.A.; Shero, M.R.; Costa, D.P. Temporal Variation in Isotopic Composition and Diet of Weddell Seals in the Western Ross Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 140, 36–44. [Google Scholar] [CrossRef]
- Mizutani, H.; McFarlane, D.A.; Kabaya, Y. Carbon and Nitrogen Isotopic Signatures of Bat Guanos as Record of Past Environments. J. Mass Spectrom. Soc. Jpn. 1992, 40, 67–82. [Google Scholar] [CrossRef]
- Stowasser, G.; Atkinson, A.; McGill, R.; Phillips, R.; Collins, M.A.; Pond, D. Food Web Dynamics in the Scotia Sea in Summer: A Stable Isotope Study. Deep Sea Res. Part II Top. Stud. Oceanogr. 2012, 59, 208–221. [Google Scholar] [CrossRef]
- Polito, M.J.; Trivelpiece, W.Z.; Reiss, C.S.; Trivelpiece, S.G.; Hinke, J.T.; Patterson, W.P.; Emslie, S.D. Intraspecific Variation in a Dominant Prey Species Can Bias Marine Predator Dietary Estimates Derived from Stable Isotope Analysis. Limnol. Oceanogr. Methods 2019, 17, 292–303. [Google Scholar] [CrossRef]
- Cribari-Neto, F.; Zeileis, A. Beta Regression in R. J. Stat. Softw. 2010, 34, 74–88. [Google Scholar] [CrossRef]
- Polito, M.; Emslie, S.D.; Walker, W. A 1000-Year Record of Adélie Penguin Diets in the Southern Ross Sea. Antarct. Sci. 2002, 14, 327–332. [Google Scholar] [CrossRef]
- Bromwich, D.H.; Kurtz, D.D. Katabatic Wind Forcing of the Terra Nova Bay Polynya. J. Geophys. Res. 1984, 89, 3561. [Google Scholar] [CrossRef]
- Petrelli, P.; Bindoff, N.L.; Bergamasco, A. The Sea Ice Dynamics of Terra Nova Bay and Ross Ice Shelf Polynyas during a Spring and Winter Simulation. J. Geophys. Res. 2008, 113, C09003. [Google Scholar] [CrossRef]
- Lauriano, G.; Pirotta, E.; Joyce, T.; Pitman, R.L.; Borrell, A.; Panigada, S. Movements, Diving Behaviour and Diet of Type-C Killer Whales (Orcinus orca) in the Ross Sea, Antarctica. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 2428–2440. [Google Scholar] [CrossRef]
- Croxall, J.P.; Trathan, P.N.; Murphy, E.J. Environmental Change and Antarctic Seabird Populations. Science 2002, 297, 1510–1514. [Google Scholar] [CrossRef]
- Nicol, S.; Clarke, J.; Romaine, S.; Kawaguchi, S.; Williams, G.; Hosie, G. Krill (Euphausia superba) Abundance and Adélie Penguin (Pygoscelis adeliae) Breeding Performance in the Waters off the Béchervaise Island Colony, East Antarctica in 2 Years with Contrasting Ecological Conditions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 540–557. [Google Scholar] [CrossRef]
- Forcada, J.; Trathan, P.; Reid, K.; Murphy, E.; Croxall, J. Contrasting Population Changes in Sympatric Penguin Species in Association with Climate Warming. Glob. Chang. Biol. 2006, 12, 411–423. [Google Scholar] [CrossRef]
- McBride, M.M.; Dalpadado, P.; Drinkwater, K.F.; Godø, O.R.; Hobday, A.J.; Hollowed, A.B.; Kristiansen, T.; Murphy, E.J.; Ressler, P.H.; Subbey, S. Krill, Climate, and Contrasting Future Scenarios for Arctic and Antarctic Fisheries. ICES J. Mar. Sci. 2014, 71, 1934–1955. [Google Scholar] [CrossRef]
- Nicol, S. Krill, Currents, and Sea Ice: Euphausia Superba and Its Changing Environment. BioScience 2006, 56, 111. [Google Scholar] [CrossRef]
- Quetin, L.B.; Ross, R.M.; Fritsen, C.H.; Vernet, M. Ecological Responses of Antarctic Krill to Environmental Variability: Can We Predict the Future? Antarct. Sci. 2007, 19, 253–266. [Google Scholar] [CrossRef]
- LaRue, M.A.; Ainley, D.G.; Swanson, M.; Dugger, K.M.; Lyver, P.O.; Barton, K.; Ballard, G. Climate Change Winners: Receding Ice Fields Facilitate Colony Expansion and Altered Dynamics in an Adélie Penguin Metapopulation. PLoS ONE 2013, 8, e60568. [Google Scholar] [CrossRef] [PubMed]
- Ainley, D.G.; Wilson, P.R.; Barton, K.J.; Ballard, G.; Nur, N.; Karl, B. Diet and Foraging Effort of Adélie Penguins in Relation to Pack-Ice Conditions in the Southern Ross Sea. Polar Biol. 1998, 20, 311–319. [Google Scholar] [CrossRef]
- O’Driscoll, R.L.; Ladroit, Y.; Parker, S.J.; Vacchi, M.; Canese, S.; Ghigliotti, L.; Dunford, A.J.; Mormede, S. Acoustic Deployments Reveal Antarctic Silverfish under Ice in the Ross Sea. Antarct. Sci. 2018, 30, 345–353. [Google Scholar] [CrossRef]
- Clarke, J.; Manly, B.; Kerry, K.; Gardner, H.; Franchi, E.; Corsolini, S.; Focardi, S. Sex Differences in Adélie Penguin Foraging Strategies. Polar Biol. 1998, 20, 248–258. [Google Scholar] [CrossRef]
- Kerry, K.R.; Clarke, J.R.; Brown, S.; Lawless, R.; Young, K.; Johanson, J. AdeÂlie Penguins as Consumers of Fish and Zooplankton Communities. Doc. WG− CEMP 1994, 94, 33. [Google Scholar]
- Sala, A.; Azzali, M.; Russo, A. Krill of the Ross Sea: Distribution, Abundance and Demography of Euphausia superba and Euphausia crystallorophias during the Italian Antarctic Expedition (January–February 2000). Sci. Mar. 2002, 66, 123–133. [Google Scholar] [CrossRef]
- Azzali, M.; Leonori, I.; De Felice, A.; Russo, A. Spatial–Temporal Relationships between Two Euphausiid Species in the Ross Sea. Chem. Ecol. 2006, 22, S219–S233. [Google Scholar] [CrossRef]
- Riaz, J.; Bestley, S.; Wotherspoon, S.; Cox, M.J.; Emmerson, L. Spatial Link between Adélie Penguin Foraging Effort and Krill Swarm Abundance and Distribution. Front. Mar. Sci. 2023, 10, 1060984. [Google Scholar] [CrossRef]
- Azzali, M.; Kalinowski, J.; Lanciani, G.; Cosimi, G. Characteristic Properties and Dynamic Aspects of Krill Swarms from the Ross Sea. In Ross Sea Ecology: Italiantartide Expeditions (1987–1995); Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 413–431. ISBN 978-3-642-59607-0. [Google Scholar]
- Salihoglu, B.; Fraser, W.; Hofmann, E. Factors Affecting Fledging Weight of Adélie Penguin (Pygoscelis adeliae) Chicks: A Modeling Study. Polar Biol. 2001, 24, 328–337. [Google Scholar] [CrossRef]
- Lyver, P.O.; MacLeod, C.J.; Ballard, G.; Karl, B.J.; Barton, K.J.; Adams, J.; Ainley, D.G.; Wilson, P.R. Intra-Seasonal Variation in Foraging Behavior among Adélie Penguins (Pygocelis adeliae) Breeding at Cape Hallett, Ross Sea, Antarctica. Polar Biol. 2011, 34, 49–67. [Google Scholar] [CrossRef]
- Watanuki, Y.; Mori, Y.; Naito, Y. Adélie Penguin Parental Activities and Reproduction: Effects of Device Size and Timing of Its Attachment during Chick Rearing Period. Polar Biol. 1992, 12, 539–544. [Google Scholar] [CrossRef]
- Jennings, S.; Varsani, A.; Dugger, K.M.; Ballard, G.; Ainley, D.G. Sex-Based Differences in Adélie Penguin (Pygoscelis Adeliae) Chick Growth Rates and Diet. PLoS ONE 2016, 11, e0149090. [Google Scholar] [CrossRef]
- Clucas, G.V.; Dunn, M.J.; Dyke, G.; Emslie, S.D.; Levy, H.; Naveen, R.; Polito, M.J.; Pybus, O.G.; Rogers, A.D.; Hart, T. A Reversal of Fortunes: Climate Change ‘Winners’ and ‘Losers’ in Antarctic Peninsula Penguins. Sci. Rep. 2014, 4, 5024. [Google Scholar] [CrossRef]
- de Mendonça Dantas, G.P.; de Oliveira, L.R.; Marasco, A.C.M.; de Araujo, J.; Hurtado, R.; Durigon, E.L.; Fillipo, L.F.S.; Morgante, J.S. Demographic History of the Gentoo Penguin (Pygoscelis papua) and the Adélie Penguin (Pygoscelis adeliae) on Admiralty Bay, King George Island, Antarctica. Waterbirds 2014, 37, 410–418. [Google Scholar] [CrossRef]
- Forcada, J.; Trathan, P.N. Penguin Responses to Climate Change in the Southern Ocean. Glob. Chang. Biol. 2009, 15, 1618–1630. [Google Scholar] [CrossRef]
- Emslie, S.D.; Patterson, W.P. Abrupt Recent Shift in δ13C and δ 15 N Values in Adélie Penguin Eggshell in Antarctica. Proc. Natl. Acad. Sci. USA 2007, 104, 11666–11669. [Google Scholar] [CrossRef] [PubMed]
- Trivelpiece, W.Z.; Hinke, J.T.; Miller, A.K.; Reiss, C.S.; Trivelpiece, S.G.; Watters, G.M. Variability in Krill Biomass Links Harvesting and Climate Warming to Penguin Population Changes in Antarctica. Proc. Natl. Acad. Sci. USA 2011, 108, 7625–7628. [Google Scholar] [CrossRef] [PubMed]
- CCAMLR. Krill Fisheries. 2018. Available online: https://www.ccamlr.org/en/document/publications/krill-fishery-report-2018 (accessed on 23 January 2023).
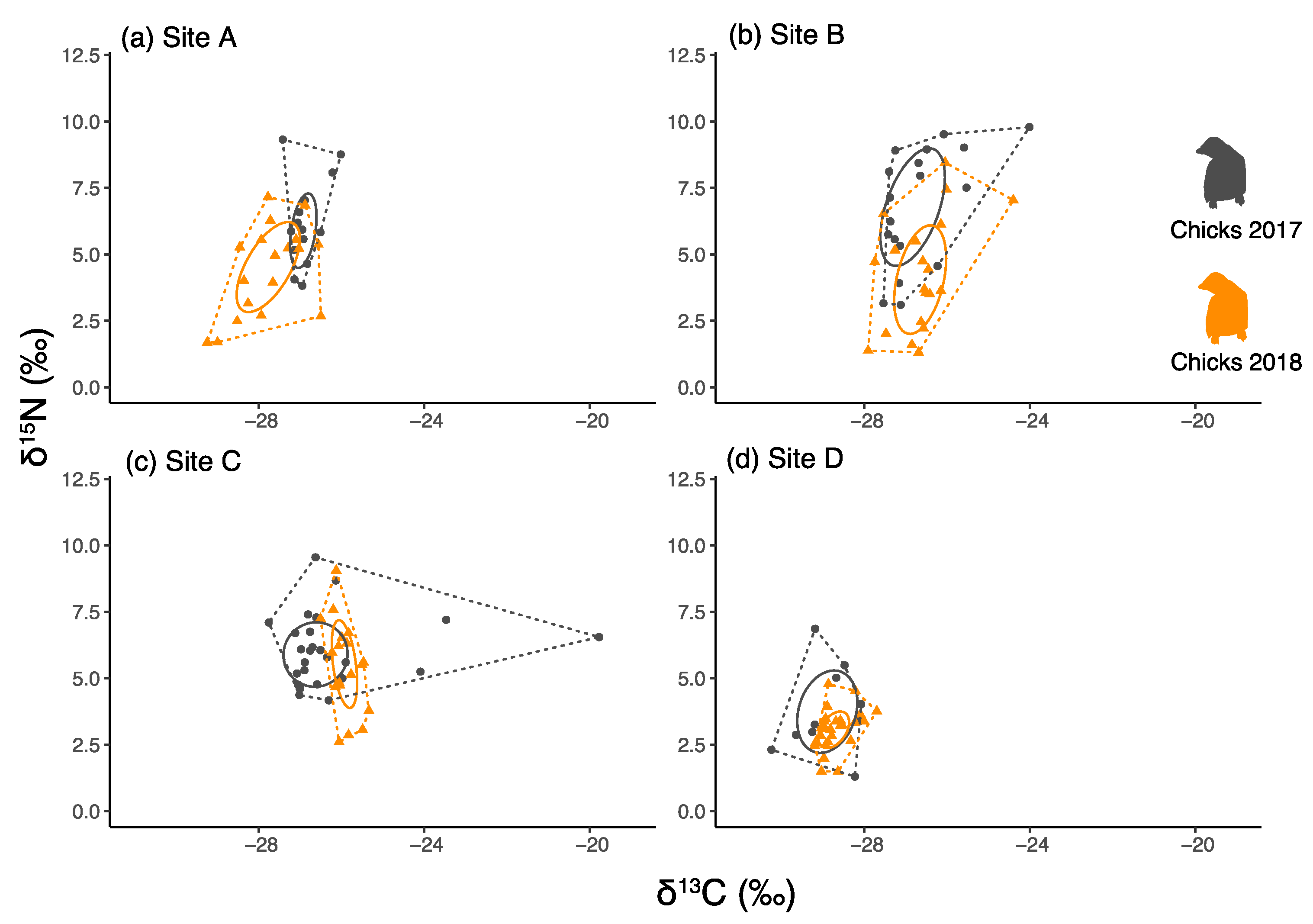
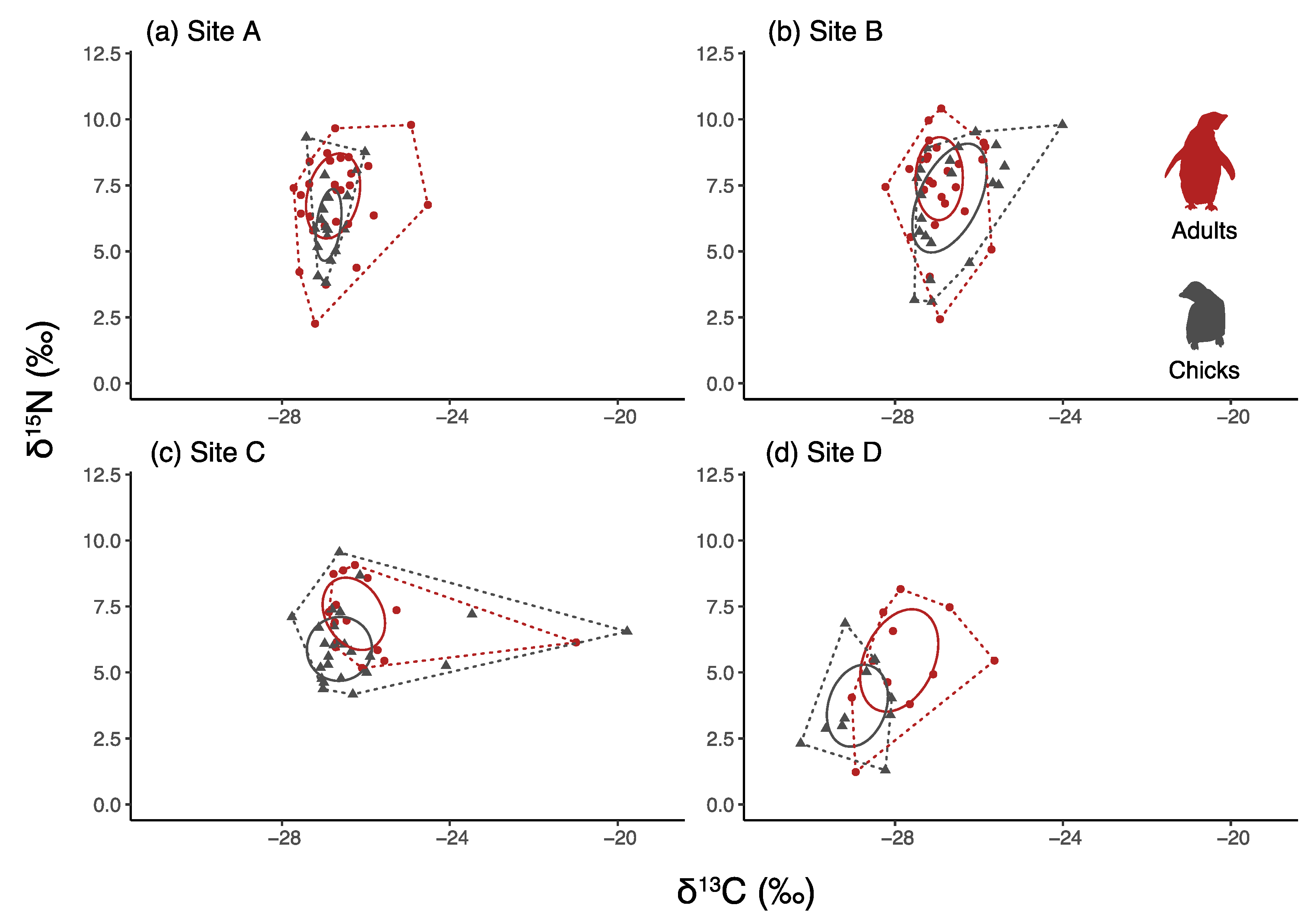

| Site | Year | Date of Sea-Ice Break-Up | Sea-Ice Persistence | Sea-Ice Break-Up Delay | Age Class | Sample Size N° | δ13C | δ15N | CR | NR | TA | SEAc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 2017 | 27 September 2016 | 0 | Adults | 28 | −26.7 ± 0.1 | 6.9 ± 0.3 | 5.5 | 9.7 | 15 | 4.1 | |
| 118 | Chicks | 18 | −26.8 ± 0.1 | 6.2 ± 0.3 | 1.4 | 5.5 | 4.3 | 1.7 | ||||
| 2018 | 23 January 2018 | 118 | Chicks | 18 | −27.8 ± 0.2 | 4.4 ± 0.2 | 2.7 | 5.4 | 9.5 | 3.7 | ||
| B | 2017 | 5 November 2016 | 39 | Adults | 26 | −26.9 ± 0.1 | 7.5± 0.3 | 7.5 | 12 | 12 | 3.54 | |
| 71 | Chicks | 21 | −26.6 ± 0.2 | 6.9 ± 0.4 | 3.5 | 6.6 | 12 | 5.4 | ||||
| 2018 | 6 January 2018 | 110 | Chicks | 20 | −26.6 ± 0.2 | 4.2 ± 0.5 | 3.5 | 7.1 | 14 | 4.8 | ||
| C | 2017 | 3 January 2017 | 98 | Adults | 15 | −25.3 ± 0.4 | 7.1 ± 0.3 | 9.1 | 3.9 | 20 | 10.8 | |
| 81 | Chicks | 25 | −26.2 ± 0.2 | 6.0 ± 0.3 | 7.9 | 5.3 | 22 | 6.9 | ||||
| 2018 | 25 March 2018 | 179 | Chicks | 18 | −25.9 ± 0.0 | 5.4 ± 0.4 | 1.1 | 6.4 | 4.2 | 1.5 | ||
| D | 2017 | 10 January 2017 | 105 | Adults | 11 | −27.8 ± 0.3 | 5.3 ± 0.4 | 3.4 | 6.9 | 12 | 6.5 | |
| 35 | Chicks | 10 | −28.9 ± 0.2 | 3.7 ± 0.4 | 2.1 | 5.5 | 7.1 | 4.1 | ||||
| 2018 | 14 February 2018 | 140 | Chicks | 27 | −29.7 ± 0.0 | 2.9 ± 0.1 | 1.8 | 4.5 | 3.6 | 1 |
| Prey | Site | Year | δ13C (‰) | δ15N (‰) | Sample Size |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | N° | |||
| Krill | A | 2017 | −27.99 ± 0.43 | 5.65 ± 1.25 | 3 |
| 2018 | −28.64 ± 1.17 | 4.48 ± 0.67 | 3 | ||
| B | 2017 | −28.03 ± 1.48 | 6.78 ± 0.49 | 3 | |
| 2018 | −27.67 ± 0.50 | 5.11 ± 0.60 | 3 | ||
| C | 2017 | −26.31 ± 0.43 | 7.10 ± 0.88 | 3 | |
| 2018 | −25.70 ± 0.50 | 7.14 ± 0.53 | 3 | ||
| D | 2017 | −28.08 ± 0.95 | 5.56 ± 0.32 | 3 | |
| 2018 | NA | NA | |||
| Amphipods | TNB | 2017−2018 | −17.34 ± 0.89 | 6.13 ± 0.77 | 14 |
| Pelagic fish (P. antarctica) | TNB | 2017−2018 | −25.54 ± 0.27 | 10.23 ± 0.29 | 4 |
| Cryopelagic fish (P. borchgrevinki) | TNB | −24.20 ± 0.85 | 11.50 ± 0.55 * | - | |
| Benthic fish (T. bernacchii) | TNB | 2017−2018 | −22.00 ± 1.08 | 12.98 ± 0.66 | 15 |
| Benthic-pelagic fish (C. hamatus) | TNB | 2017−2018 | −24.83 ± 0.98 | 13.53 ± 1.36 | 16 |
| δ13C (‰) | δ15N (‰) | ||||
|---|---|---|---|---|---|
| Factor | F | p Value | F | p Value | |
| Krill | Site | 4.05 | 0.02 | 10.47 | 3.89 × 10−4 |
| Year | 0.18 | 0.68 | 17.23 | 6.70 × 10−4 | |
| Interaction | 0.46 | 0.72 | 6.70 | 3.45 × 10−3 | |
| Chicks | Site | 36.94 | 7.04 × 10−18 | 11.6 | 7.13 × 10−7 |
| Year | 4.70 | 0.41 | 8.80 | 0.003 | |
| Interaction | 1.26 | 0.29 | 1.33 | 0.27 | |
| Prey | Site | Adults 2017 | Chicks 2017 | Chicks 2018 |
|---|---|---|---|---|
| Mean% (2.5–97.5% C.I.) | Mean (2.5–97.5% C.I.) | Mean (2.5–97.5% C.I.) | ||
| Krill | A | 60% (55–74) | 63% (54–79) | 88% (80–94) |
| B | 57% (49–65) | 53% (41–64) | 85% (76–90) | |
| C | 78% (67–93) | 87% (81–98) | 91% (88–99) | |
| D | 89% (77–97) | 93% (82–99) | 97% (94–99) | |
| Pelagic/cryopelagic fish | A | 31% (26–48) | 28% (21–40) | 8% (1–16) |
| B | 40% (30–58) | 41% (26–56) | 9% (5–17) | |
| C | 14% (2–32) | 9% (1–13) | 6% (2–18) | |
| D | 7% (1–21) | 4% (1–12) | 2% (0–5) | |
| Amphipods | A | 9% (5–13) | 9% (5–12) | 4% (2–11) |
| B | 3% (1–7) | 6% (2–13) | 6% (1–13) | |
| C | 8% (3–17) | 4% (1–10) | 3% (0–13) | |
| D | 4% (1–9) | 2% (0–8) | 1% (0–2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maccapan, D.; Careddu, G.; Calizza, E.; Sporta Caputi, S.; Rossi, L.; Costantini, M.L. Effects of Sea-Ice Persistence on the Diet of Adélie Penguin (Pygoscelis adeliae) Chicks and the Trophic Differences between Chicks and Adults in the Ross Sea, Antarctica. Biology 2023, 12, 708. https://doi.org/10.3390/biology12050708
Maccapan D, Careddu G, Calizza E, Sporta Caputi S, Rossi L, Costantini ML. Effects of Sea-Ice Persistence on the Diet of Adélie Penguin (Pygoscelis adeliae) Chicks and the Trophic Differences between Chicks and Adults in the Ross Sea, Antarctica. Biology. 2023; 12(5):708. https://doi.org/10.3390/biology12050708
Chicago/Turabian StyleMaccapan, Deborah, Giulio Careddu, Edoardo Calizza, Simona Sporta Caputi, Loreto Rossi, and Maria Letizia Costantini. 2023. "Effects of Sea-Ice Persistence on the Diet of Adélie Penguin (Pygoscelis adeliae) Chicks and the Trophic Differences between Chicks and Adults in the Ross Sea, Antarctica" Biology 12, no. 5: 708. https://doi.org/10.3390/biology12050708
APA StyleMaccapan, D., Careddu, G., Calizza, E., Sporta Caputi, S., Rossi, L., & Costantini, M. L. (2023). Effects of Sea-Ice Persistence on the Diet of Adélie Penguin (Pygoscelis adeliae) Chicks and the Trophic Differences between Chicks and Adults in the Ross Sea, Antarctica. Biology, 12(5), 708. https://doi.org/10.3390/biology12050708