Agouti-Signalling Protein Overexpression Reduces Aggressiveness in Zebrafish
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Housing
2.2. Behavioural Experiments
2.3. Quantification of Aggressive Behaviour
2.4. Cortisol Determination
2.5. Statistical Analysis
3. Results
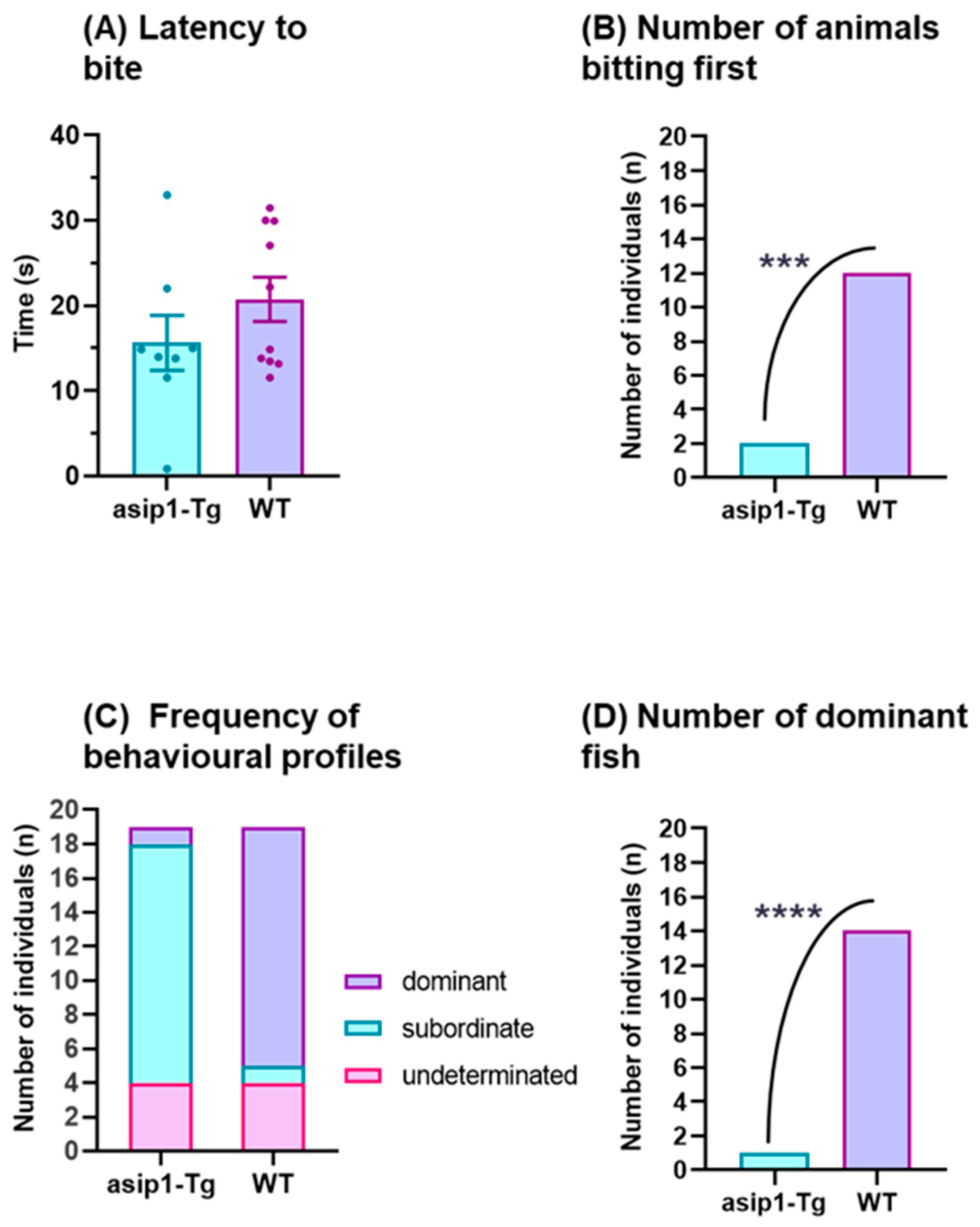
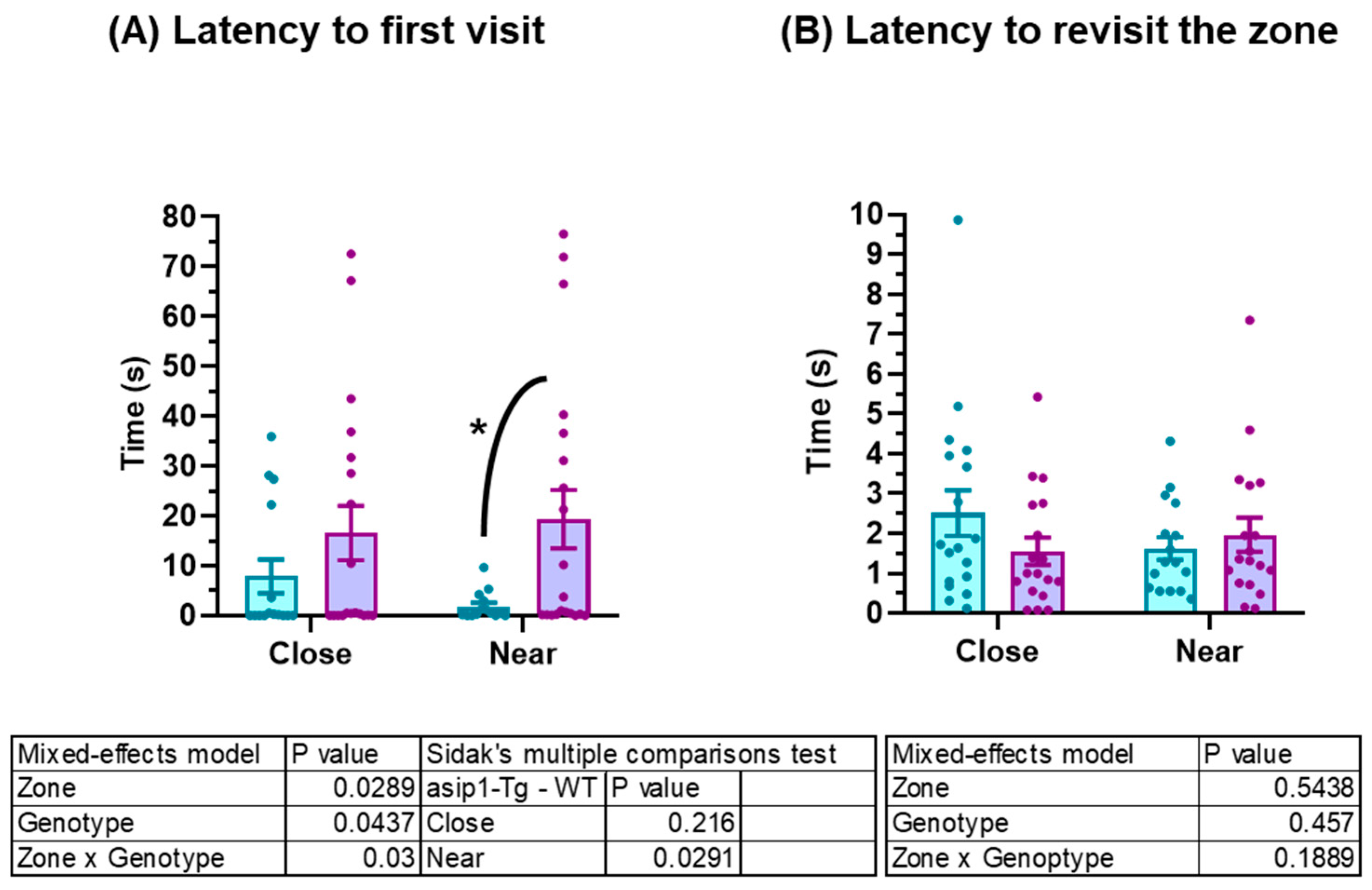
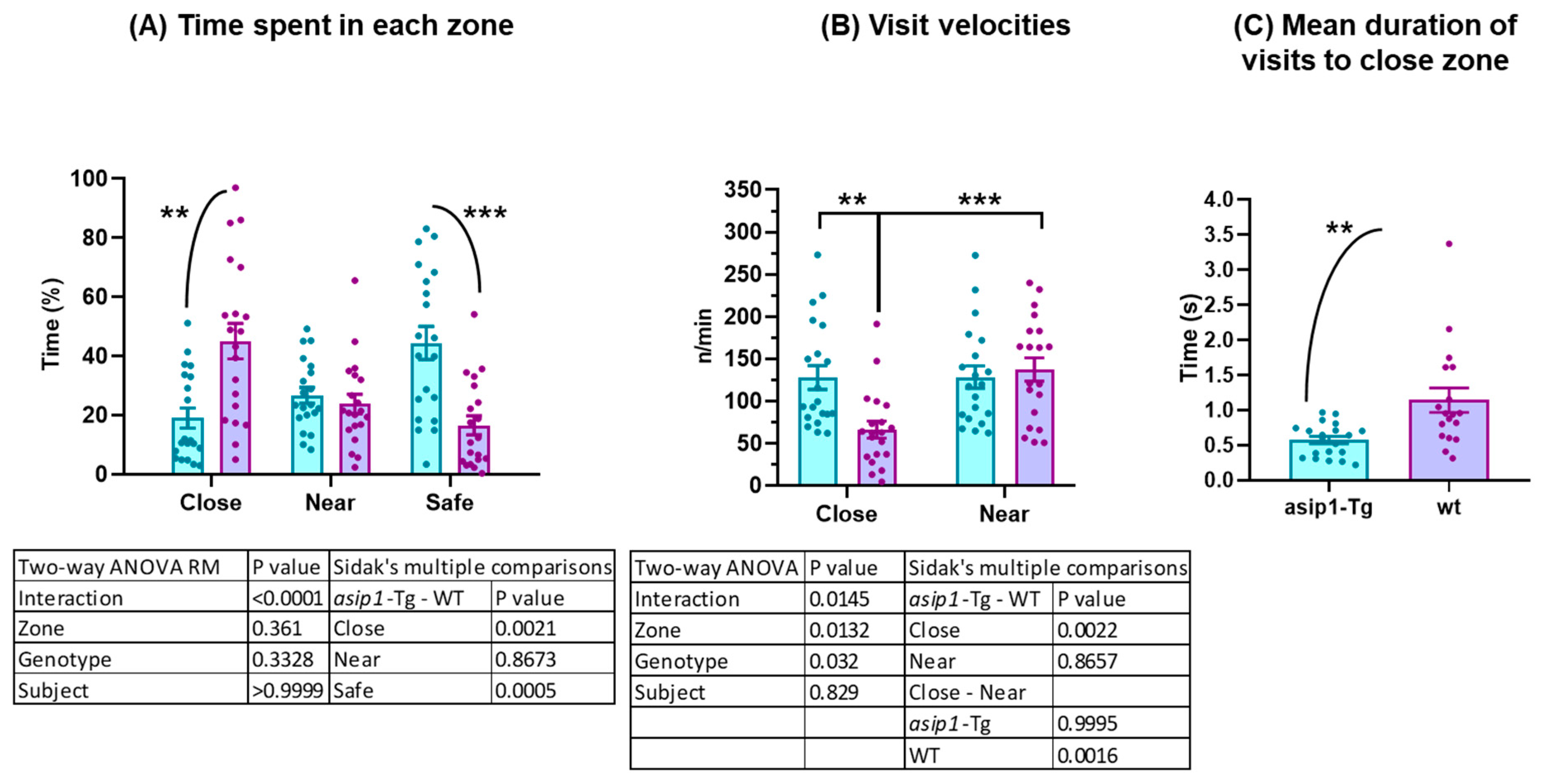
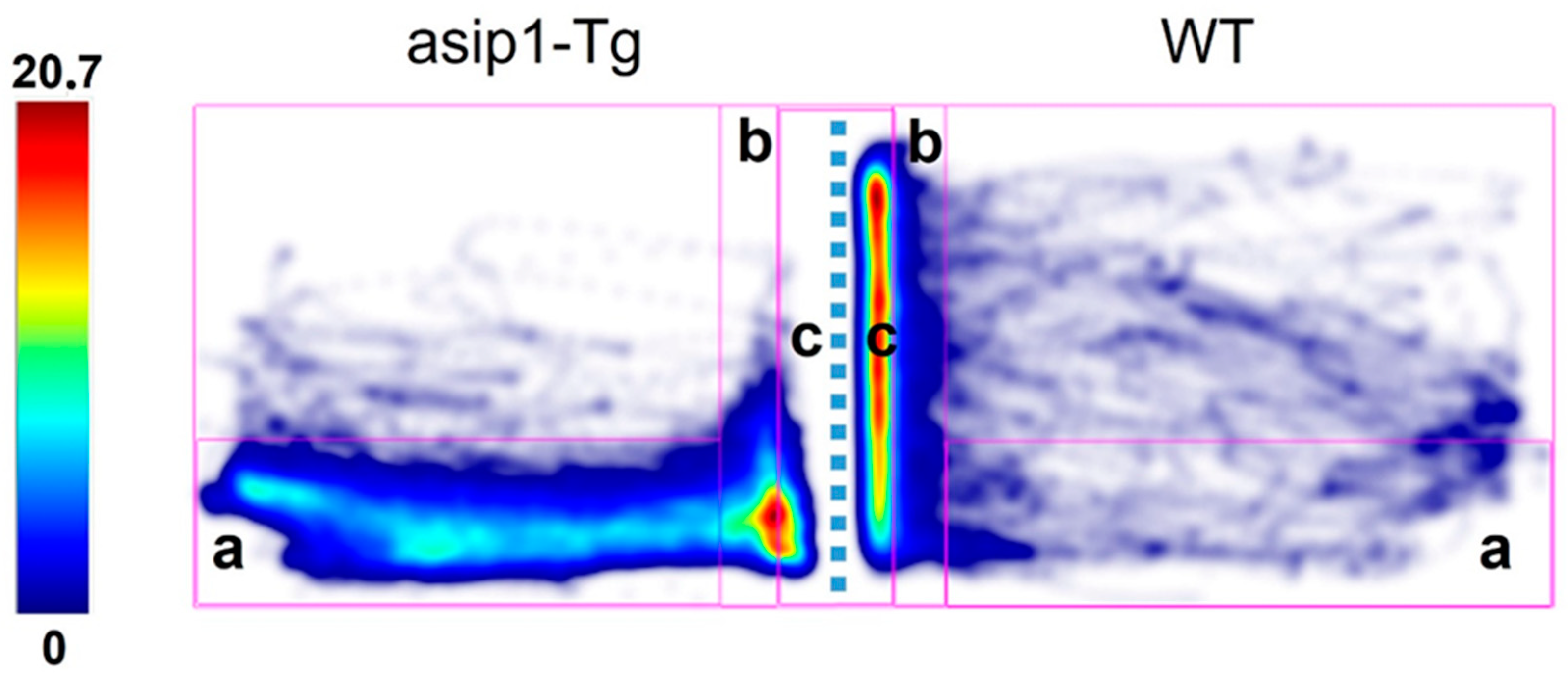
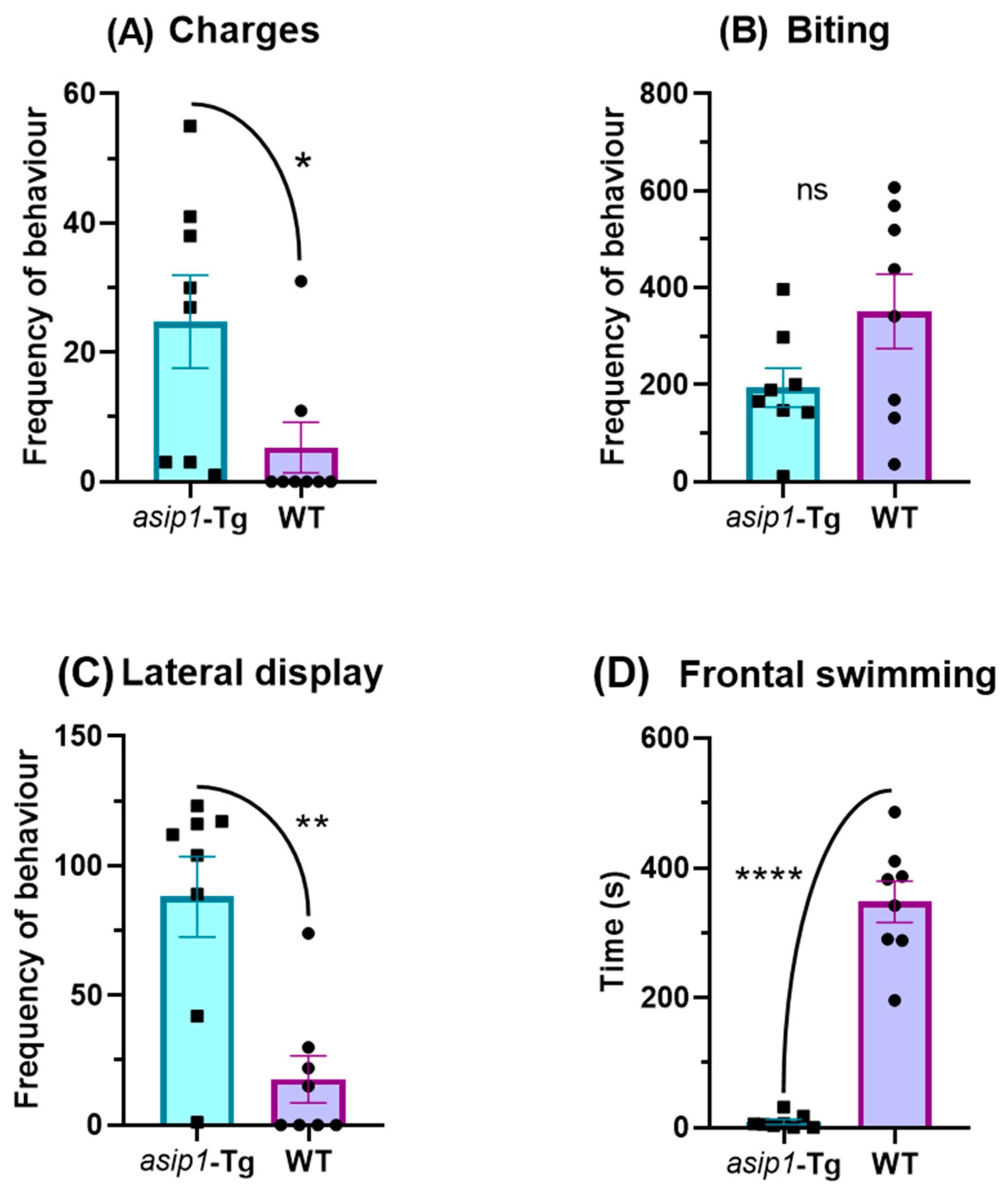
3.1. Agonistic Behaviour in Zebrafish
3.2. Whole-Body Cortisol Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerdá-Reverter, J.M.; Agulleiro, M.J.; Raúl Guillot, G.; Sánchez, E.; Ceinos, R.; Rotllant, J. Fish melanocortin system. Eur. J. Pharmacol. 2011, 660, 53–60. [Google Scholar] [CrossRef]
- Rocha, A.; Godino-Gimeno, A.; Cerdá-Reverter, J.M. Evolution of proopiomelanocortin. Vitam. Horm. 2019, 111, 1–16. [Google Scholar] [PubMed]
- Schiöth, H.B.; Haitina, T.; Ling, M.K.; Ringholm, A.; Fredriksson, R.; Cerdá-Reverter, J.M.; Klovins, J. Evolutionary conservation of the structural, pharmacological, and genomic characteristics of the melanocortin receptor subtypes. Peptides 2005, 26, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted disruption of melanocortin-4 receptor results in obesity in mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kelly, M.A.; Opitz-Araya, X.; Thomas, R.E.; Low, M.J.; Cone, R.D. Exocrine glands dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 1997, 91, 89–798. [Google Scholar] [CrossRef]
- Miller, M.W.; Duhl, D.M.J.; Vrieling, H.; Cordes, S.P.; Ollmann, M.M.; Winkes, B.M.; Barsh, G.S. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying a lethal yellow mutation. Genes Dev. 1993, 7, 454–467. [Google Scholar] [CrossRef]
- Tolle, V.; Low, M.J. In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes 2008, 57, 86–94. [Google Scholar] [CrossRef]
- Ollmann, M.M.; Wilson, B.D.; Yang, Y.K.; Kerns, J.A.; Chen, I.; Gantz, I.; Barsh, G.S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 1997, 278, 135–138. [Google Scholar] [CrossRef]
- Cerdá-Reverter, J.M.; Peter, R.E. Endogenous melanocortin antagonist in fish: Structure, brain mapping, and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology 2003, 144, 4552–4561. [Google Scholar] [CrossRef]
- Song, Y.; Cone, R.D. Creation of a genetic model of obesity in a teleost. FASEB J. 2007, 21, 2042–2049. [Google Scholar] [CrossRef]
- Zhang, C.; Forlano, P.M.; Cone, R.D. AgRP and POMC neurons are hypophysiotropic and coordinately regulate multiple endocrine axes in a larval teleost. Cell Metab. 2012, 15, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Shainer, I.; Michel, M.; Marquart, G.D.; Bhandiwad, A.A.; Zmora, N.; Ben-Moshe Livne, Z.; Zohar, Y.; Hazak, A.; Mazon, Y.; Förster, D.; et al. Agouti-Related Protein 2 Is a New Player in the Teleost Stress Response System. Curr. Biol. 2019, 29, 2009–2019.e7. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Reverter, J.M.; Haitina, T.; Schiöth, H.B.; Peter, R.E. Gene structure of the goldfish agouti-signaling protein: A putative role in the dorsal-ventral pigment pattern of fish. Endocrinology 2005, 146, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Ceinos, R.M.; Guillot, R.; Kelsh, R.N.; Cerdá-Reverter, J.M.; Rotllant, J. Pigment patterns in adult fish result from superimposition of two largely independent pigmentation mechanisms. Pigment Cell Melanoma Res. 2015, 28, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Cal, L.; Suarez-Bregua, P.; Comesaña, P.; Owen, J.; Braasch, I.; Kelsh, R.; Cerdá-Reverter, J.M.; Rotllant, J. Countershading in zebrafish results from an Asip1 controlled dorsoventral gradient of pigment cell differentiation. Sci. Rep. 2019, 9, 3449. [Google Scholar] [CrossRef]
- Cal, L.; Suarez-Bregua, P.; Braasch, I.; Irion, U.; Kelsh, R.; Cerdá-Reverter, J.M.; Rotllant, J. Loss-of-function mutations in the melanocortin 1 receptor cause disruption of dorso-ventral countershading in teleost fish. Pigment Cell Melanoma Res. 2019, 32, 817–828. [Google Scholar] [CrossRef]
- Guillot, R.; Cortés, R.; Navarro, S.; Mischitelli, M.; García-Herranz, V.; Sánchez, E.; Cal, L.; Navarro, J.C.; Míguez, J.M.; Afanasyev, S.; et al. Behind melanocortin antagonist overexpression in the zebrafish brain: A behavioral and transcriptomic approach. Horm. Behav. 2016, 82, 87–100. [Google Scholar] [CrossRef]
- Godino-Gimeno, A.; Sánchez, E.; Guillot, R.; Rocha, A.; Angotzi, A.R.; Leal, E.; Rotllant, J.; Cerdá-Reverter, J.M. Growth performance after agouti-signaling protein 1 (asip1) overexpression in transgenic zebrafish. Zebrafish 2020, 17, 373–381. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Huntingford, F.A.; Graham, W.D.; Thorpe, J.E. Early social status and the development of life-history strategies in Atlantic salmon. Proc. Royal Soc. B 1989, 236, 7–19. [Google Scholar]
- Lahti, K.; Laurila, A.; Enberg, K.; Piironen, J. Variation in aggressive behaviour and growth rate between populations and migratory forms in the brown trout, Salmo trutta. Anim. Behav. 2001, 62, 935–944. [Google Scholar] [CrossRef]
- Devlin, R.H.; D’Andrade, M.; Uh, M.; Biagi, C.A. Population effects of growth hormone transgenic coho salmon depend on food availability and genotype by environment interactions. Proc. Natl. Acad. Sci. USA 2004, 101, 9303. [Google Scholar] [CrossRef] [PubMed]
- Hallerman, E.M.; McLean, E.; Fleming, I.A. Effects of growth hormone transgenes on the behavior and welfare of aquacultured fishes: A review identifying research needs. Appl. Anim. Behav. Sci. 2007, 104, 265–294. [Google Scholar] [CrossRef]
- Johnsson, J.I.; Bjornsson, B.T. Growth-enhanced fish can be competitive in the wild. Funct. Ecol. 2001, 15, 654–659. [Google Scholar] [CrossRef]
- Jonsson E, Johnsson JI, Bjornsson BT 1989 Growth hormone increases aggressive behavior in juvenile rainbow trout. Horm. Behav. 1989, 33, 9–15.
- Teles, M.C.; Oliveira, R.F. Quantifying Aggressive Behavior in Zebrafish. In Zebrafish: Methods and Protocols, 2nd ed.; Kawakami, K., Patton, E.E., Orger, M., Eds.; Springer NY: New York, NY, USA, 2016; pp. 293–305. [Google Scholar]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Meth. Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Silva, J.F.; Simoes, J.M. Fighting zebrafish: Characterization of aggressive behavior and winner-loser effects. Zebrafish 2011, 8, 73–81. [Google Scholar] [CrossRef]
- Desjardins, J.K.; Fernald, R.D. What do fish make of mirror images? Biol. Lett. 2010, 23, 744–747. [Google Scholar] [CrossRef]
- Way, G.P.; Rulh, N.; Sneker, J.L.; Kiesel, A.L.; McRobert, S.P. A Comparison of Methodologies to Test Aggression in Zebrafish. Zebrafish 2016, 2, 144–151. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Simoes, J.M.; Teles, M.C.; Oliveira, C.R.; Becker, J.D.; Lopes, J.S. Assessment of fight outcome is needed to activate socially driven transcriptional changes in the zebrafish brain. Proc. Natl. Acad. Sci. USA 2016, 113, E654–E661. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008, 83, 13–34. [Google Scholar] [CrossRef]
- Cortés, R.; Teles, M.; Oliveira, M.; Fierro-Castro, C.; Tort, L.; Cerdá-Reverter, J.M. Effects of acute handling stress on short-term central expression of orexigenic/anorexigenic genes in zebrafish. Fish Physiol. Biochem. 2018, 44, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Gallup, G.G., Jr. Mirror-image stimulation. Psychol. Bull. 1968, 70, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, V.; Taborsky, M.; Wanner, S.; Koch, F.; Frommen, J.G. Mirror, mirror on the wall: The predictive value of mirror tests for measuring aggression in fish. Behav. Ecol. Sociobiol. 2014, 68, 871–878. [Google Scholar] [CrossRef]
- Navarro, S.; Crespo, D.; Schultz, R.W.; Ge, W.; Rotllant, J.; Cerda-Reverter, J.M.; Rocha, A. Role of melanocortin system in gonadal steroidogenesis of zebrafish. Animals 2022, 12, 2737. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.F.; Carneiro, L.A.; Canario, A.V.M. No hormonal response in tied fights. Nature 2005, 437, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.C.; Dahlbom, S.J.; Winberg, S.; Oliveira, R.F. Social modulation of brain monoamine levels in zebrafish. Behav. Brain Res. 2013, 253, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.D.; Maguire, S.M.; Harris, R.M.; Rodriguez, A.A.; De Angelis, R.S.; Flores, S.A.; Hofmann, H.A. The melanocortin system regulates body pigmentation and social behaviour in a colour polymorphic cichlid fish. Proc. Biol. Sci. 2017, 284, 20162838. [Google Scholar] [CrossRef] [PubMed]
- Taborsky, B.; Oliveira, R. Social competence: An evolutionary approach. Trends Ecol. Evol. 2012, 27, 679–688. [Google Scholar] [CrossRef]
- Taborsky, B.; Arnold, C.; Junker, J.; Tschopp, A. The early social environment affects social competence in a cooperative breeder. Anim. Behav. 2012, 83, 1067–1074. [Google Scholar] [CrossRef]
- Amorim, M.C.P.; Almada, V.C. The outcome of male–male encounters affects subsequent sound production during courtship in the cichlid fish Oreochromis mossambicus. Anim. Behav. 2005, 69, 595–601. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Almada, V.C. Androgenization of Dominant Males in a Cichlid Fish: Androgens Mediate the Social Modulation of Sexually Dimorphic Traits. Ethology 2010, 104, 841–858. [Google Scholar] [CrossRef]
- Sørensen, C.; Johansen, I.B.; Øverli, Ø. Chapter 9. In The Physiology of Fishes, 2nd ed.; Evans, D.H., Clairborne, J.B., Currie, S., Eds.; Editorial Taylor and Francis Group: Washington, DC, USA, 2013; p. 289. [Google Scholar]
- Maruska, K.P.; Anselmo, C.M.; King, T.; Mobley, R.B.; Ray, E.J.; Wayne, R. Endocrine and neuroendocrine regulation of social status in cichlid fishes. Horm. Behav. 2022, 139, 105110. [Google Scholar] [CrossRef] [PubMed]
- Kohda, M.; Hotta, T.; Takeyama, T.; Awata, S.; Tanaka, H.; Asai, J.Y.; Jordan, A.L. If a fish can pass the mark test, what are the implications for consciousness and self-awareness testing in animals? PloS Biol. 2019, 17, e3000021. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J. A fish eye view of the mirror test. Learn. Behav. 2020, 48, 193–194. [Google Scholar] [CrossRef]
- Kohda, M.; Sogawa, S.; Jordan, A.L.; Kubo, N.; Awata, S.; Satoh, S.; Kobayashi, T.; Fujita, A.; Bshary, R. Further evidence for the capacity of mirror self-recognition in cleaner fish and the significance of ecologically relevant marks. PloS Biol. 2022, 20, e3001529. [Google Scholar] [CrossRef]
- Goodson, J.L. Nonapeptides and the evolutionary patterning of sociality. In Progress in Brain Research; Neumann, I.D., Landgraf, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 170, pp. 3–15. [Google Scholar]
- Godwin, J.; Thompson, R. Nonapeptides and Social Behavior in Fishes. Horm. Behav. 2012, 61, 230–238. [Google Scholar] [CrossRef]
- Quintana, L.; Jalabert, C.; Fokidis, H.B.; Soma, K.K.; Zubizarreta, L. Neuroendocrine mechanisms underlying non-breeding aggression: Common strategies between birds and fish. Front. Neural Circuits 2021, 15, 716605. [Google Scholar] [CrossRef]
- Mastinu, A.; Premoli, M.; Maccarinelli, G.; Grilli, M.; Memo, M.; Bonini, S.A. Melanocortin 4 receptor stimulation improves social deficits in mice through oxytocin pathway. Neuropharmacology 2018, 133, 366–374. [Google Scholar] [CrossRef]
- Grieb, Z.A.; Cross, E.A.; Albers, H.E. Alpha-melanocyte-stimulating hormone (αMSH) modulates the rewarding properties of social interactions in an oxytocin receptor-dependent manner in Syrian hamsters (Mesocricetus auratus). Physiol. Behav. 2022, 252, 113828. [Google Scholar] [CrossRef]
- Cerdá-Reverter, J.M.; Ringholm, A.; Schiöth, H.B.; Peter, R.E. Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: Involvement in the control of food intake. Endocrinology 2003, 144, 2336–2349. [Google Scholar] [CrossRef]
- Sánchez, E.; Rubio, V.C.; Thompson, D.; Metz, J.; Flik, G.; Millhauser, G.L.; Cerdá-Reverter, J.M. Phosphodiesterase inhibitor-dependent inverse agonism of agouti-related protein on melanocortin 4 receptor in sea bass (Dicentrarchus labrax). Am. J. Physiol. 2009, 296, R1293–R1306. [Google Scholar] [CrossRef] [PubMed]
- Josep Agulleiro, M.; Cortés, R.; Fernández-Durán, B.; Navarro, S.; Guillot, R.; Meimaridou, E.; Clark, A.J.; Cerdá-Reverter, J.M. Melanocortin 4 receptor becomes an ACTH receptor by coexpression of melanocortin receptor accessory protein 2. Mol. Endocrinol. 2013, 27, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Filby, A.L.; Paull, G.C.; Bartlett, E.J.; Van Look, K.J.; Tyler, C.R. Physiological and health consequences of social status in zebrafish (Danio rerio). Physiol. Behav. 2010, 101, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.T.; O’Malley, D.M.; Melloni, R.H., Jr. Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav. Brain Res. 2006, 167, 94–102. [Google Scholar] [CrossRef]
- Almeida, O.; Oliveira, R.F. Social Status and Arginine Vasotocin Neuronal Phenotypes in a Cichlid Fish. Brain Behav. Evol. 2015, 85, 203–213. [Google Scholar] [CrossRef]
- Greenwood, A.K.; Wark, A.R.; Fernald, R.D.; Hofmann, H.A. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc. R. Soc. B. 2008, 275, 2393–2402. [Google Scholar] [CrossRef]
- Cerdá-Reverter, J.M.; Canosa, L.F. Neuroendocrine Systems of the Fish Brain. In Fish Neuroendocrinology. Fish Physiology; Bernier, N.J., Van Der Kraak, G., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: London, UK, 2009; Volume 28, pp. 3–74. [Google Scholar]
- Pei, H.; Sutton, A.K.; Burnett, K.H.; Fuller, P.M.; Olson, D.P. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol. Metab. 2014, 3, 209–215. [Google Scholar] [CrossRef]
- Araishi, K.; Watanabe, K.; Yamazaki, T.; Nakamachim, T.; Matsuda, K. Intracerebroventricular administration of arginine vasotocin (AVT) induces anorexigenesis and anxiety-like behavior in goldfish. Peptides 2019, 119, 170118. [Google Scholar] [CrossRef]
- Gesto, M.; Soengas, J.L.; Rodriguez-Illamola, A.; Miguez, J.M. Arginine Vasotocin Treatment Induces a Stress Response and Exerts a Potent Anorexigenic Effect in Rainbow Trout, Oncorhynchus mykiss. J. Endocrinol. 2014, 26, 89–99. [Google Scholar] [CrossRef]
- Sapolsky, R.M. The influence of social hierarchy on primate health. Science 2005, 308, 5722. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Carrick, T.R. A comparison of plasma glucose and plasma cortisol as selection markers for high and low stress-responsiveness in female rainbow trout. Aquaculture 1999, 175, 351–363. [Google Scholar] [CrossRef]
- Backström, T.; Schjolden, J.; Overli, O.; Thornqvist, P.O.; Winberg, S. Stress effects on AVT and CRF systems in two strains of rainbow trout (Oncorhynchus mykiss) divergent in stress responsiveness. Horm. Behav. 2011, 59, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Schjolden, J.; Backström, T.; Pulman, K.G.T.; Pottinger, T.G.; Winberg, W. Divergence in behavioural responses to stress in two strains of rainbow trout (Oncorhynchus mykiss) with contrasting stress responsiveness. Horm. Behav. 2005, 48, 537–544. [Google Scholar] [CrossRef] [PubMed]
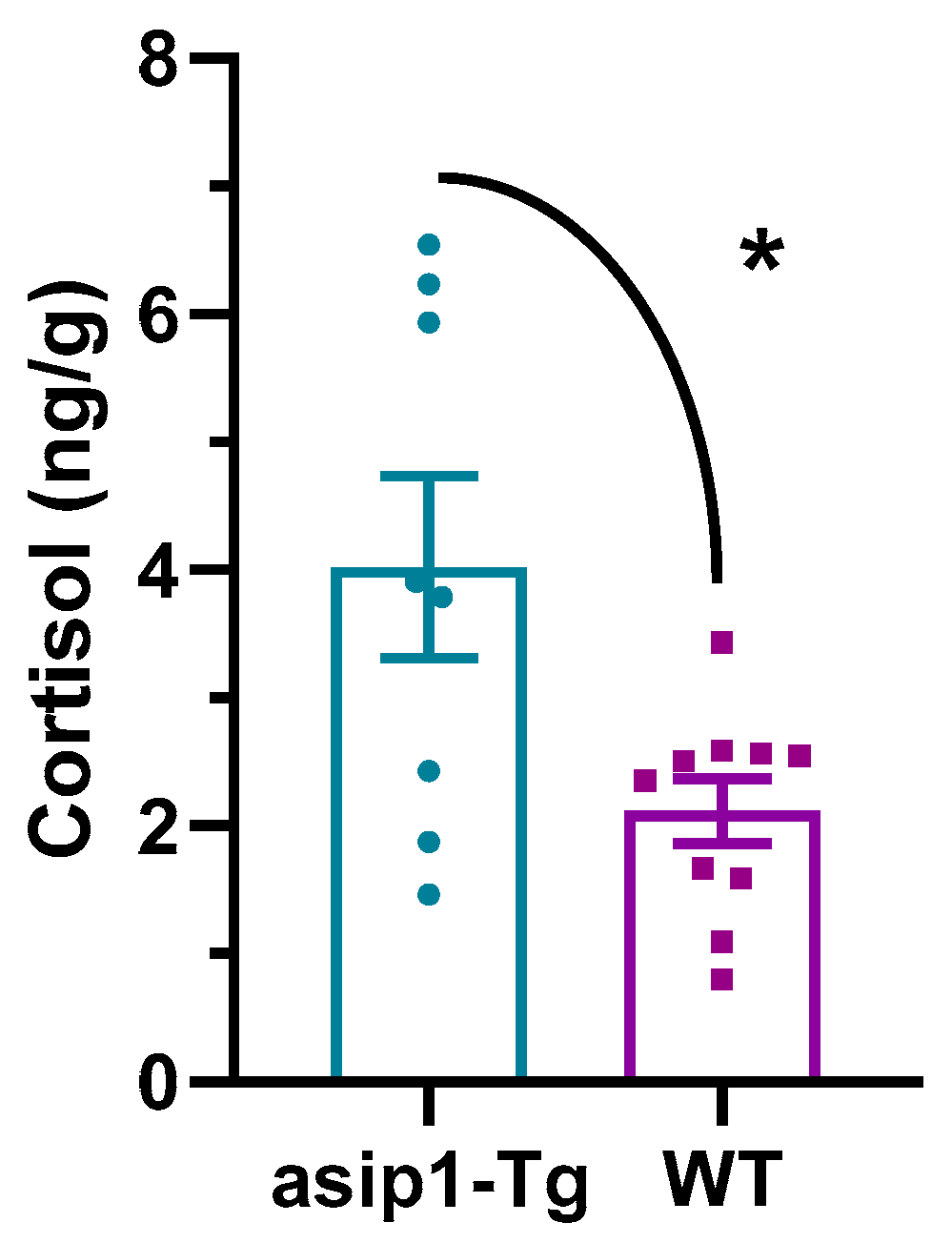
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, A.; Godino-Gimeno, A.; Rotllant, J.; Cerdá-Reverter, J.M. Agouti-Signalling Protein Overexpression Reduces Aggressiveness in Zebrafish. Biology 2023, 12, 712. https://doi.org/10.3390/biology12050712
Rocha A, Godino-Gimeno A, Rotllant J, Cerdá-Reverter JM. Agouti-Signalling Protein Overexpression Reduces Aggressiveness in Zebrafish. Biology. 2023; 12(5):712. https://doi.org/10.3390/biology12050712
Chicago/Turabian StyleRocha, Ana, Alejandra Godino-Gimeno, Josep Rotllant, and José Miguel Cerdá-Reverter. 2023. "Agouti-Signalling Protein Overexpression Reduces Aggressiveness in Zebrafish" Biology 12, no. 5: 712. https://doi.org/10.3390/biology12050712
APA StyleRocha, A., Godino-Gimeno, A., Rotllant, J., & Cerdá-Reverter, J. M. (2023). Agouti-Signalling Protein Overexpression Reduces Aggressiveness in Zebrafish. Biology, 12(5), 712. https://doi.org/10.3390/biology12050712




