Advancements in Ocular Regenerative Therapies
Abstract
:Simple Summary
Abstract
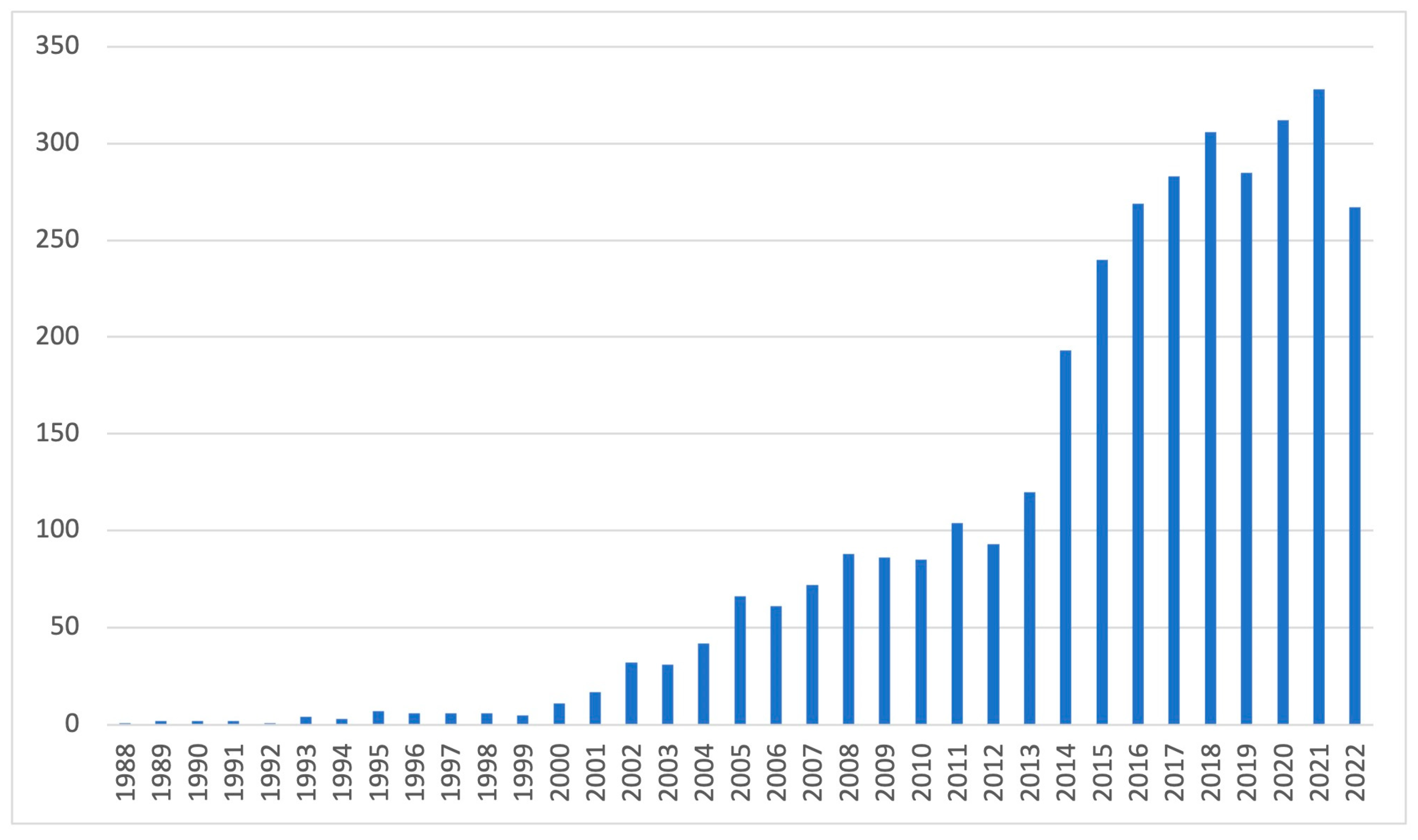
1. Introduction
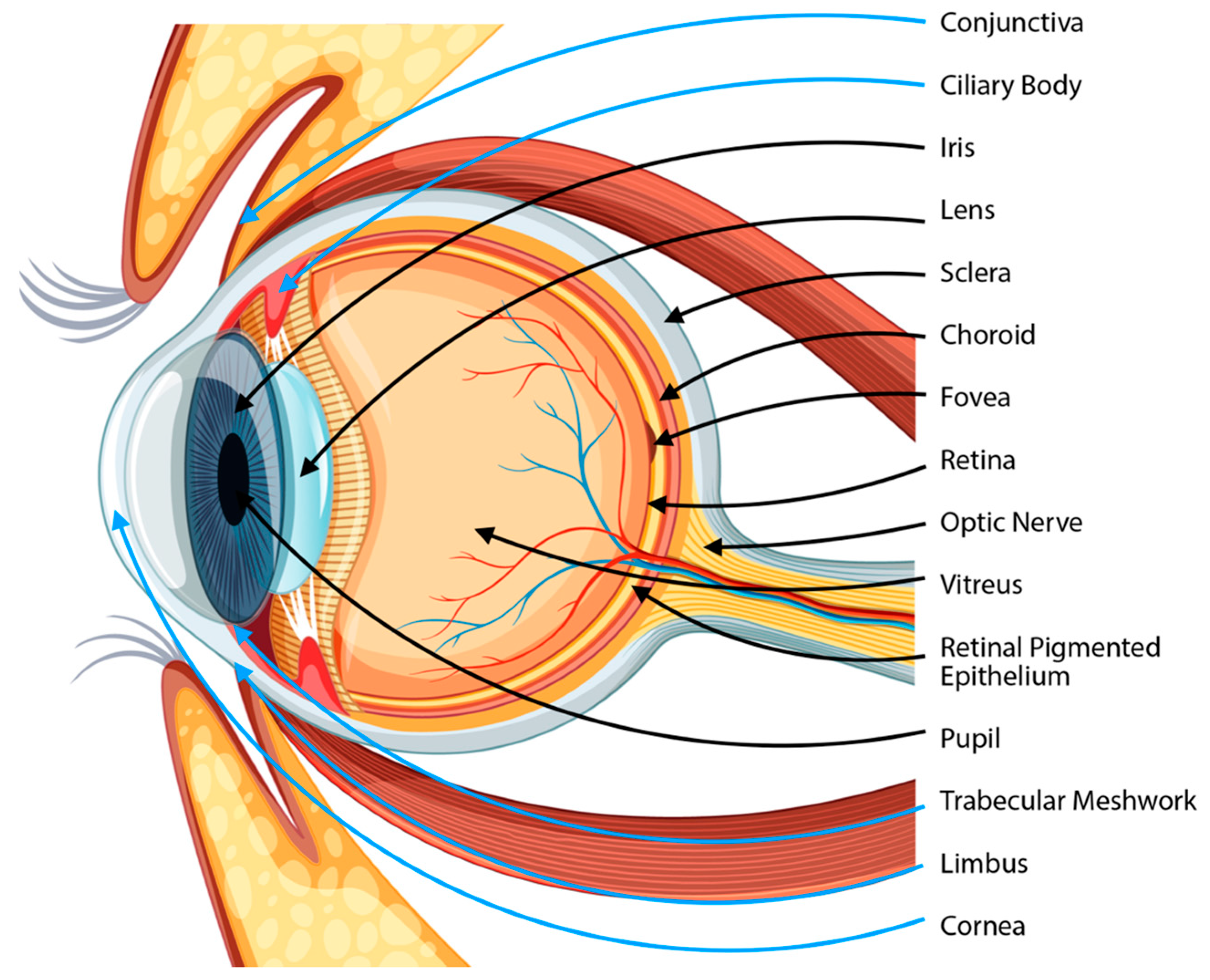
2. Cornea
2.1. Basic Knowledge
2.2. Recent Advancements
2.2.1. CLAU
2.2.2. CLET
2.2.3. SLET
2.2.4. KLAL
2.2.5. lr-CLAL
2.2.6. COMET
2.2.7. MSC
2.3. Future Directions
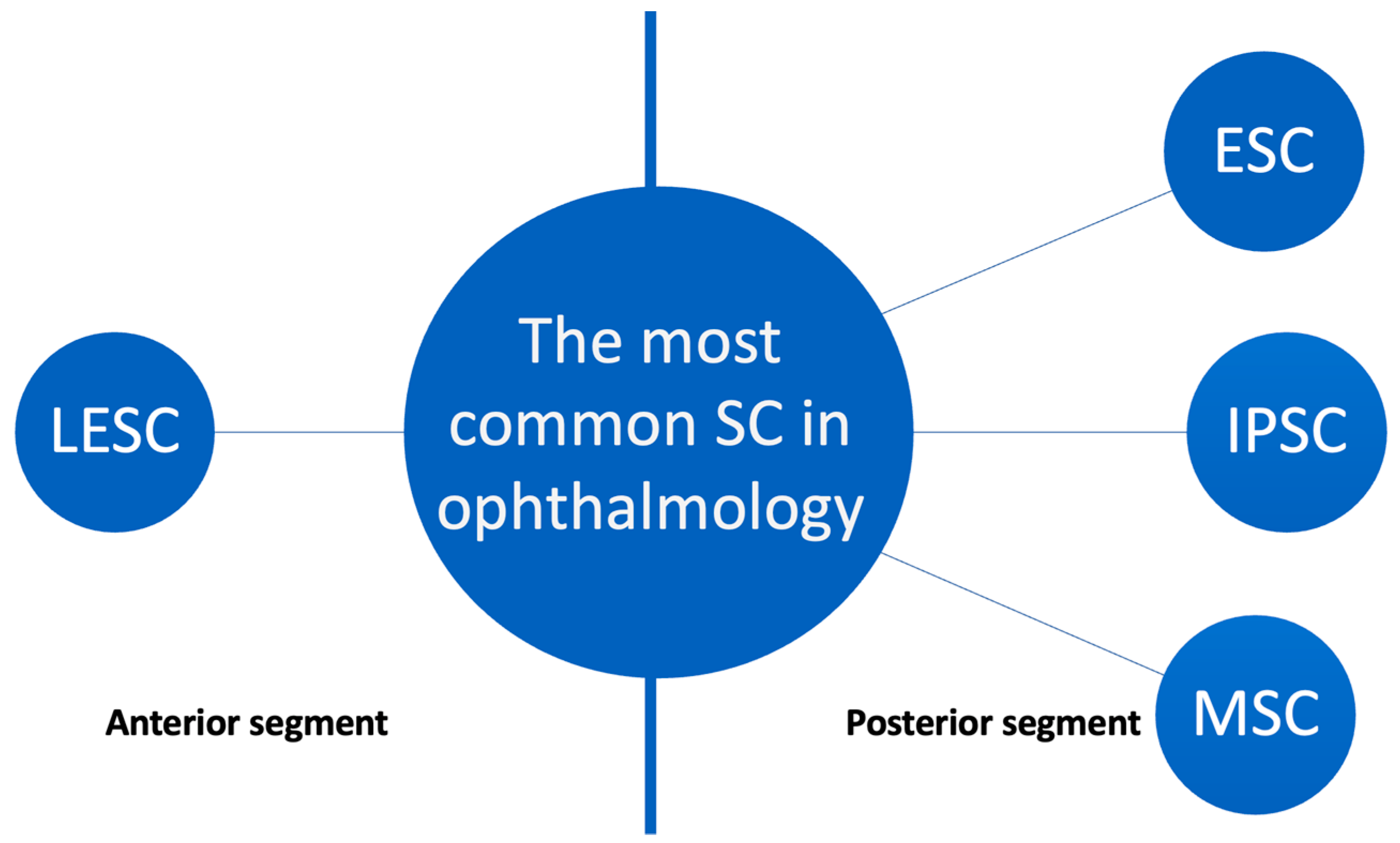
3. Retina
3.1. Basic Knowledge
3.2. Recent Advancements
3.2.1. hESC-Derived RPE Cells Replacement
3.2.2. iPSC Derived RPE Cells Replacement
3.2.3. Retinal Organoids
3.2.4. Retinal Ganglion Cells Replacement
3.2.5. Photoreceptors Replacement
3.3. Future Directions
4. Conjunctiva
4.1. Basic Knowledge
4.2. Future Directions
5. Iris
Basic Knowledge and Future Directions
6. Trabecular Meshwork
Basic Knowledge and Future Directions
7. Ciliary Body
Basic Knowledge and Future Directions
8. Lens
Basic Knowledge and Future Directions
9. Sclera
Basic Knowledge and Future Directions
10. Orbital Fat
Basic Knowledge and Future Directions
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laforge, R.G.; Spector, W.D.; Sternberg, J. The Relationship of Vision and Hearing Impairment to One-Year Mortality and Functional Decline. J. Aging Health 1992, 4, 126–148. [Google Scholar] [CrossRef]
- Haeckel, E. Natürliche Schöpfungs-Geschichte; George Reimer: Berlin, Germany, 1868. [Google Scholar]
- National Institutes of Health. Stem Cells Basics. Available online: https://stemcells.nih.gov/info/basics/stc-basics/#stc-I (accessed on 6 December 2022).
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.-M.; Böttcher-Friebertshäuser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies—New Perspectives for the Injured Lung. J. Clin. Med. 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Rocco, P.R.M. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev. Respir. Med. 2020, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Q.; Qi, L.; Dai, X.; Liu, H.; Wang, Y. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol. Med. Rep. 2016, 14, 1681–1692. [Google Scholar] [CrossRef]
- Iyer, S.S.; Co, C.; Rojas, M. Mesenchymal Stem Cells and Inflammatory Lung Diseases. Panminerva Med. 2009, 51, 5–16. Available online: https://pubmed.ncbi.nlm.nih.gov/19352305/ (accessed on 6 December 2022).
- Abraham, A.; Krasnodembskaya, A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl. Med. 2020, 9, 28–38. [Google Scholar] [CrossRef]
- Liang, P.; Ye, F.; Hou, C.-C.; Pi, L.; Chen, F. Mesenchymal Stem Cell Therapy for Patients with Ischemic Heart Failure- Past, Present, and Future. Curr. Stem Cell Res. Ther. 2021, 16, 608–621. [Google Scholar] [CrossRef]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef]
- Shafei, A.E.-S.; Ali, M.A.; Ghanem, H.G.; Shehata, A.I.; AbdelGawad, A.A.; Handal, H.R.; Talaat, K.A.; Ashaal, A.E.; El-Shal, A.S. Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. J. Gene Med. 2017, 19, e2995. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Wu, Z.; Li, L. Transplantation of mesenchymal stem cells and their derivatives effectively promotes liver regeneration to attenuate acetaminophen-induced liver injury. Stem Cell Res. Ther. 2020, 11, 88. [Google Scholar] [CrossRef]
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R.; et al. Tissue Engineering in Liver Regenerative Medicine: Insights into Novel Translational Technologies. Cells 2020, 9, 304. [Google Scholar] [CrossRef]
- Missoum, A. Recent Updates on Mesenchymal Stem Cell Based Therapy for Acute Renal Failure. Curr. Urol. 2020, 13, 189–199. [Google Scholar] [CrossRef]
- Qian, X.; Xu, C.; Fang, S.; Zhao, P.; Wang, Y.; Liu, H.; Yuan, W.; Qi, Z. Exosomal MicroRNAs Derived From Umbilical Mesenchymal Stem Cells Inhibit Hepatitis C Virus Infection. Stem Cells Transl. Med. 2016, 5, 1190–1203. [Google Scholar] [CrossRef]
- Du, J.; Li, H.; Lian, J.; Zhu, X.; Qiao, L.; Lin, J. Stem cell therapy: A potential approach for treatment of influenza virus and coronavirus-induced acute lung injury. Stem Cell Res. Ther. 2020, 11, 192. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; Zhao, M.; Liu, W.; Wang, L.; Bin Zhu, B.; Xie, W.; Zhao, C.; Zhou, Y.; Ren, C.; et al. When stem cells meet COVID-19: Recent advances, challenges and future perspectives. Stem Cell Res. Ther. 2022, 13, 9. [Google Scholar] [CrossRef]
- Kang, J.M.; Kil Yeon, B.; Cho, S.-J.; Suh, Y.-H. Stem Cell Therapy for Alzheimer’s Disease: A Review of Recent Clinical Trials. J. Alzheimer’s Dis. 2016, 54, 879–889. [Google Scholar] [CrossRef]
- Xuan, A.; Luo, M.; Ji, W.; Long, D. Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci. Lett. 2009, 450, 167–171. [Google Scholar] [CrossRef]
- Fleifel, D.; Rahmoon, M.A.; AlOkda, A.; Nasr, M.; Elserafy, M.; El-Khamisy, S.F. Recent advances in stem cells therapy: A focus on cancer, Parkinson’s and Alzheimer’s. J. Genet. Eng. Biotechnol. 2018, 16, 427–432. [Google Scholar] [CrossRef]
- Shaharuddin, B.; Ahmad, S.; Meeson, A.; Ali, S. Concise Review: Immunological Properties of Ocular Surface and Importance of Limbal Stem Cells for Transplantation. Stem Cells Transl. Med. 2013, 2, 614–624. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Available online: https://clinicaltrials.gov/ct2/results?term=ophthalmology&cond=stem+cells&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt= (accessed on 6 May 2023).
- Dua, H.S.; Said, D.G. Clinical evidence of the pre-Descemets layer (Dua’s layer) in corneal pathology. Eye 2016, 30, 1144–1145. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Vision. Available online: https://www.who.int/docs/default-source/documents/publications/world-vision-report-accessible.pdf?sfvrsn=223f9bf7_2 (accessed on 6 December 2022).
- Davanger, M.; Evensen, A. Role of the Pericorneal Papillary Structure in Renewal of Corneal Epithelium. Nature 1971, 229, 560–561. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Bron, A.J. Limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. Available online: https://pubmed.ncbi.nlm.nih.gov/7182957/ (accessed on 6 December 2022). [PubMed]
- Bobba, S.; Di Girolamo, N.; Mills, R.; Daniell, M.; Chan, E.; Harkin, D.; Cronin, B.G.; Crawford, G.; McGhee, C.N.; Watson, S. Nature and incidence of severe limbal stem cell deficiency in Australia and New Zealand. Clin. Exp. Ophthalmol. 2017, 45, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Baylis, O.; Figueiredo, F.; Henein, C.; Lako, M.; Ahmad, S. 13 years of cultured limbal epithelial cell therapy: A review of the outcomes. J. Cell. Biochem. 2011, 112, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S. Concise Review: Limbal Stem Cell Deficiency, Dysfunction, and Distress. Stem Cells Transl. Med. 2012, 1, 110–115. [Google Scholar] [CrossRef]
- Ramírez, B.E.; Sánchez, A.; Herreras, J.M.; Fernández, I.; García-Sancho, J.; Nieto-Miguel, T.; Calonge, M. Stem Cell Therapy for Corneal Epithelium Regeneration following Good Manufacturing and Clinical Procedures. BioMed Res. Int. 2015, 2015, 408495. [Google Scholar] [CrossRef]
- Oie, Y.; Nishida, K. Regenerative Medicine for the Cornea. BioMed Res. Int. 2013, 2013, 428247. [Google Scholar] [CrossRef]
- Daya, S.M.; Chan, C.C.; Holland, E.J.; Members of The Cornea Society Ocular Surface Procedures Nomenclature Committee. Cornea Society nomenclature for ocular surface rehabilitative procedures. Cornea 2011, 30, 1115–1119. [Google Scholar] [CrossRef]
- Daya, S.M. Conjunctival-limbal autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef]
- Thoft, R.A. Conjunctival transplantation. Arch Ophthalmol. 1977, 95, 1425–1427. [Google Scholar] [CrossRef]
- Kenyon, K.R.; Tseng, S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Buenaga, R.; Aiello, F.; Zaher, S.S.; Grixti, A.; Ahmad, S. Twenty years of limbal epithelial therapy: An update on managing limbal stem cell deficiency. BMJ Open Ophthalmol. 2018, 3, e000164. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Tseng, S.C. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Investig. Opthalmol. Vis. Sci. 1991, 32, 2219–2233. [Google Scholar]
- Shanbhag, S.S.; Tarini, S.; Kunapuli, A.; Basu, S. Simultaneous surgical management of unilateral limbal stem cell deficiency and symblepharon post chemical burn. BMJ Case Rep. 2020, 13, e237234. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Schwab, I.R. Cultured corneal epithelia for ocular surface disease. Trans. Am. Ophthalmol. Soc. 1999, 97, 891–986. [Google Scholar]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Sotozono, C.; Kinoshita, S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 2001, 108, 1569–1574. [Google Scholar] [CrossRef]
- Behaegel, J.; Dhubhghaill, S.N.; Koppen, C.; Zakaria, N. Safety of Cultivated Limbal Epithelial Stem Cell Transplantation for Human Corneal Regeneration. Stem Cells Int. 2017, 2017, 6978253. [Google Scholar] [CrossRef]
- Schwab, I.R.; Reyes, M.; Isseroff, R.R. Successful Transplantation of Bioengineered Tissue Replacements in Patients with Ocular Surface Disease. Cornea 2000, 19, 421–426. [Google Scholar] [CrossRef]
- Bobba, S.; Chow, S.; Watson, S.; Di Girolamo, N. Clinical outcomes of xeno-free expansion and transplantation of autologous ocular surface epithelial stem cells via contact lens delivery: A prospective case series. Stem Cell Res. Ther. 2015, 6, 23. [Google Scholar] [CrossRef]
- Chotikavanich, S.; Prabhasawat, P.; Ekpo, P.; Uiprasertkul, M.; Tesavibul, N. Efficacy of cultivated corneal epithelial stem cells for ocular surface reconstruction. Clin. Ophthalmol. 2012, 6, 1483–1492. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef]
- Basu, S.; Shanbhag, S.; Patel, C.; Goyal, R.; Donthineni, P.; Singh, V. Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J. Ophthalmol. 2019, 67, 1265–1277. [Google Scholar] [CrossRef]
- Holland, E.J. Epithelial transplantation for the management of severe ocular surface disease. Trans. Am. Ophthalmol. Soc. 1996, 94, 677–743. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Holland, E.J. Keratolimbal allograft. Curr. Opin. Ophthalmol. 2017, 28, 377–381. [Google Scholar] [CrossRef]
- Sepsakos, L.; Cheung, A.Y.; Nerad, J.A.; Mogilishetty, G.; Holland, E.J. Donor-Derived Conjunctival-Limbal Melanoma After a Keratolimbal Allograft. Cornea 2017, 36, 1415–1418. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Sotozono, G.; Yamada, J.; Nakamura, T.; Inatomi, T. Concept and Clinical Application of Cultivated Epithelial Transplantation for Ocular Surface Disorders. Ocul. Surf. 2004, 2, 21–33. [Google Scholar] [CrossRef]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: A review. Stem Cell Res. Ther. 2020, 11, 301. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef]
- Nakamura, T.; Endo, K.-I.; Cooper, L.J.; Fullwood, N.J.; Tanifuji, N.; Tsuzuki, M.; Koizumi, N.; Inatomi, T.; Sano, Y.; Kinoshita, S. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2003, 44, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, D.; Orzechowska-Wylegala, B.; Wowra, B.; Wroblewska-Czajka, E.; Grolik, M.; Szczubialka, K.; Nowakowska, M.; Puzzolo, D.; Wylęgała, E.; Micali, A.; et al. Cultivated Oral Mucosa Epithelium in Ocular Surface Reconstruction in Aniridia Patients. BioMed Res. Int. 2015, 2015, 281870. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Higa, K.; Kato, N.; Satake, Y. Barrier Function of Cultivated Limbal and Oral Mucosal Epithelial Cell Sheets. Investig. Opthalmol. Vis. Sci. 2009, 50, 5672–5680. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Yamato, M.; Nishida, K.; Okano, T. Evidence of the Survival of Ectopically Transplanted Oral Mucosal Epithelial Stem Cells After Repeated Wounding of Cornea. Mol. Ther. 2014, 22, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.-Y.; Xie, H.-T.; Zhao, X.-Y.; Xu, W.-H.; Zhang, M.-C. Limbal niche cells can reduce the angiogenic potential of cultivated oral mucosal epithelial cells. Cell. Mol. Biol. Lett. 2019, 24, 3. [Google Scholar] [CrossRef]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef]
- Jiang, T.-S.; Cai, L.; Ji, W.-Y.; Hui, Y.-N.; Wang, Y.-S.; Hu, D.; Zhu, J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol. Vis. 2010, 16, 1304–1316. Available online: https://pubmed.ncbi.nlm.nih.gov/20664793/ (accessed on 6 December 2022).
- Nieto-Miguel, T.; Galindo, S.; Reinoso, R.; Corell, A.; Martino, M.; Pérez-Simón, J.A.; Calonge, M. In Vitro Simulation of Corneal Epithelium Microenvironment Induces a Corneal Epithelial-like Cell Phenotype from Human Adipose Tissue Mesenchymal Stem Cells. Curr. Eye Res. 2013, 38, 933–944. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Kao, W.W.-Y. Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System. Ocul. Surf. 2016, 14, 121–134. [Google Scholar] [CrossRef]
- Nieto-Nicolau, N.; Martínez-Conesa, E.M.; Fuentes-Julián, S.; Arnalich-Montiel, F.; García-Tuñón, I.; De Miguel, M.P.; Casaroli-Marano, R.P. Priming human adipose-derived mesenchymal stem cells for corneal surface regeneration. J. Cell. Mol. Med. 2021, 25, 5124–5137. [Google Scholar] [CrossRef]
- Monteiro, B.G.; Serafim, R.C.; Melo, G.B.; Silva, M.C.P.; Lizier, N.F.; Maranduba, C.M.C.; Smith, R.L.; Kerkis, A.; Cerruti, H.; Gomes, J.A.P.; et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009, 42, 587–594. [Google Scholar] [CrossRef]
- Li, Q.; Shen, Y.; Wu, S.; Wei, H.; Zou, J.; Xu, S.; Ling, Q.; Kang, M.; Huang, H.; Chen, X.; et al. MLN4924 Promotes Self-Renewal of Limbal Stem Cells and Ocular Surface Restoration. J. Pers. Med. 2023, 13, 379. [Google Scholar] [CrossRef]
- Jang, E.; Jin, S.; Cho, K.J.; Kim, D.; Rho, C.R.; Lyu, J. Wnt/β-catenin signaling stimulates the self-renewal of conjunctival stem cells and promotes corneal conjunctivalization. Exp. Mol. Med. 2022, 54, 1156–1164. [Google Scholar] [CrossRef]
- Uyama, H.; Mandai, M.; Takahashi, M. Stem-cell-based therapies for retinal degenerative diseases: Current challenges in the establishment of new treatment strategies. Dev. Growth Differ. 2021, 63, 59–71. [Google Scholar] [CrossRef]
- Wang, X.; Gericke, A.; Ackermann, M.; Wang, S.; Neufurth, M.; Schröder, H.C.; Pfeiffer, N.; Müller, W.E.G. Polyphosphate, the physiological metabolic fuel for corneal cells: A potential biomaterial for ocular surface repair. Biomater. Sci. 2019, 7, 5506–5515. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Chen, W.; Jiang, Z.; Luo, Y.; Wang, D.; Zheng, Y.; Liu, Y. Acellularized Uvea Hydrogel as Novel Injectable Platform for Cell-Based Delivering Treatment of Retinal Degeneration and Optimizing Retinal Organoids Inducible System. Adv. Healthc. Mater. 2022, 11, e2202114. [Google Scholar] [CrossRef]
- Fernandes, R.A.B.; Lojudice, F.H.; Ribeiro, L.Z.; da Cruz, N.F.S.; Polizelli, M.U.; Cristovam, P.C.; Innocenti, F.; Morimoto, L.; Magalhães, O.J.; Sallum, J.M.F.; et al. Transplantation of subretinal stem cell–derived retinal pigment epithelium for stargardt disease: A Phase I Clinical Trial. Retina 2022, 43, 263–274. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Wang, L.; Wang, F.; Zhao, T.; Li, Q.; Xu, H.; Meng, X.; Hao, J.; Zhou, Q.; et al. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years’ follow-up. Cell Prolif. 2021, 54, e13100. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Nature. Reversing Blindness with Stem Cells. Available online: https://www.nature.com/articles/d41586-021-02629-w (accessed on 6 December 2022).
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kang, J.S.; Lee, M.; Baek, A.; Kim, S.; Chung, S.-K.; Lee, M.-O.; Kim, J. Simplified Brain Organoids for Rapid and Robust Modeling of Brain Disease. Front. Cell Dev. Biol. 2020, 8, 594090. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Yi, S.A.; Jang, H.J.; Han, J.-W.; Lee, J. In vitro modeling for inherited neurological diseases using induced pluripotent stem cells: From 2D to organoid. Arch. Pharmacal Res. 2020, 43, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Vaccarino, F.M. Breakthrough Moments: Yoshiki Sasai’s Discoveries in the Third Dimension. Cell Stem Cell 2019, 24, 837–838. [Google Scholar] [CrossRef]
- Eiraku, M.; Sasai, Y. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nat. Protoc. 2011, 7, 69–79. [Google Scholar] [CrossRef]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef]
- Eiraku, M.; Sasai, Y. Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr. Opin. Neurobiol. 2012, 22, 768–777. [Google Scholar] [CrossRef]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Canto-Soler, Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef]
- Jin, Z.-B.; Gao, M.-L.; Deng, W.-L.; Wu, K.-C.; Sugita, S.; Mandai, M.; Takahashi, M. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 2018, 69, 38–56. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Aguzzi, E.A.; Johnson, T.V. Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration. Cells 2021, 10, 1426. [Google Scholar] [CrossRef]
- Benowitz, L.I.; He, Z.; Goldberg, J.L. Reaching the brain: Advances in optic nerve regeneration. Exp. Neurol. 2017, 287 Pt 3, 365–373. [Google Scholar] [CrossRef]
- Trakhtenberg, E.F.; Li, Y.; Feng, Q.; Tso, J.; Rosenberg, P.; Goldberg, J.L.; Benowitz, L.I. Zinc chelation and Klf9 knockdown cooperatively promote axon regeneration after optic nerve injury. Exp. Neurol. 2018, 300, 22–29. [Google Scholar] [CrossRef]
- Moore, D.L.; Blackmore, M.G.; Hu, Y.; Kaestner, K.H.; Bixby, J.L.; Lemmon, V.P.; Goldberg, J.L. KLF Family Members Regulate Intrinsic Axon Regeneration Ability. Science 2009, 326, 298–301. [Google Scholar] [CrossRef]
- de Lima, S.; Koriyama, Y.; Kurimoto, T.; Oliveira, J.T.; Yin, Y.; Li, Y.; Gilbert, H.-Y.; Fagiolini, M.; Martinez, A.M.B.; Benowitz, L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 9149–9154, Erratum in Proc. Natl. Acad. Sci. USA 2012, 109, 13465. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; Meng, H.; Li, Y.; Dmitriev, P.; Tian, F.; Page, J.C.; Lu, Q.R.; He, Z. Robust Myelination of Regenerated Axons Induced by Combined Manipulations of GPR17 and Microglia. Neuron 2020, 108, 876–886.e4. [Google Scholar] [CrossRef]
- Pearson, R.A.; Barber, A.C.; Rizzi, M.; Hippert, C.; Xue, T.; West, E.L.; Duran, Y.; Smith, A.J.; Chuang, J.Z.; Azam, S.A.; et al. Restoration of vision after transplantation of photoreceptors. Nature 2012, 485, 99–103. [Google Scholar] [CrossRef]
- Pearson, R.A.; Gonzalez-Cordero, A.; West, E.L.; Ribeiro, J.R.; Aghaizu, N.; Goh, D.; Sampson, R.D.; Georgiadis, A.; Waldron, P.V.; Duran, Y.; et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 2016, 7, 13029. [Google Scholar] [CrossRef]
- Singh, M.S.; Balmer, J.; Barnard, A.R.; Aslam, S.A.; Moralli, D.; Green, C.M.; Barnea-Cramer, A.; Duncan, I.; MacLaren, R.E. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 2016, 7, 13537. [Google Scholar] [CrossRef]
- Ripolles-Garcia, A.; Dolgova, N.; Phillips, M.J.; Savina, S.; Ludwig, A.L.; Stuedemann, S.A.; Nlebedum, U.; Wolfe, J.H.; Garden, O.A.; Maminishkis, A.; et al. Systemic immunosuppression promotes survival and integration of subretinally implanted human ESC-derived photoreceptor precursors in dogs. Stem Cell Rep. 2022, 17, 1824–1841. [Google Scholar] [CrossRef]
- Tay, H.G.; Andre, H.; Chrysostomou, V.; Adusumalli, S.; Guo, J.; Ren, X.; Tan, W.S.; Tor, J.E.; Moreno-Moral, A.; Plastino, F.; et al. Photoreceptor laminin drives differentiation of human pluripotent stem cells to photoreceptor progenitors that partially restore retina function. Mol. Ther. 2023, 31, 825–846. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT05187104?view=record (accessed on 6 May 2023).
- Karl, M.O.; Hayes, S.; Nelson, B.R.; Tan, K.; Buckingham, B.; Reh, T.A. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 2008, 105, 19508–19513. [Google Scholar] [CrossRef] [PubMed]
- Lahne, M.; Nagashima, M.; Hyde, D.R.; Hitchcock, P.F. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 2020, 6, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Do, H.; Mazo, K.W.; Chopra, M.; Wahlin, K.J. Restoring vision and rebuilding the retina by Müller glial cell reprogramming. Stem Cell Res. 2023, 66, 103006. [Google Scholar] [CrossRef]
- Goldman, D. Müller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Thummel, R.; Kassen, S.C.; Enright, J.M.; Nelson, C.M.; Montgomery, J.E.; Hyde, D.R. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 2008, 87, 433–444. [Google Scholar] [CrossRef]
- Langhe, R.; Chesneau, A.; Colozza, G.; Hidalgo, M.; Ail, D.; Locker, M.; Perron, M. Müller glial cell reactivation in Xenopus models of retinal degeneration. Glia 2017, 65, 1333–1349. [Google Scholar] [CrossRef]
- Fischer, A.J.; McGuire, C.R.; Dierks, B.D.; Reh, T.A. Insulin and Fibroblast Growth Factor 2 Activate a Neurogenic Program in Müller Glia of the Chicken Retina. J. Neurosci. 2002, 22, 9387–9398. [Google Scholar] [CrossRef]
- Mandai, M. Pluripotent stem cell-derived retinal organoid/cells for retinal regeneration therapies: A review. Regen. Ther. 2023, 22, 59–67. [Google Scholar] [CrossRef]
- Stewart, R.M.K.; Sheridan, C.M.; Hiscott, P.S.; Czanner, G.; Kaye, S.B. Human Conjunctival Stem Cells are Predominantly Located in the Medial Canthal and Inferior Forniceal Areas. Investig. Opthalmol. Vis. Sci. 2015, 56, 2021–2030. [Google Scholar] [CrossRef]
- Pellegrini, G.; Golisano, O.; Paterna, P.; Lambiase, A.; Bonini, S.; Rama, P.; De Luca, M. Location and Clonal Analysis of Stem Cells and Their Differentiated Progeny in the Human Ocular Surface. J. Cell Biol. 1999, 145, 769–782. [Google Scholar] [CrossRef]
- Walkden, A. Amniotic Membrane Transplantation in Ophthalmology: An Updated Perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Koivusalo, L.; Karvinen, J.; Sorsa, E.; Jönkkäri, I.E.M.; Väliaho, J.; Kallio, P.; Ilmarinen, T.; Miettinen, S.; Skottman, H.; Kellomäki, M. Hydrazone crosslinked hyaluronan-based hydrogels for therapeutic delivery of adipose stem cells to treat corneal defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 85, 68–78. [Google Scholar] [CrossRef]
- Ke, Y.; Wu, Y.; Cui, X.; Liu, X.; Yu, M.; Yang, C.; Li, X. Polysaccharide Hydrogel Combined with Mesenchymal Stem Cells Promotes the Healing of Corneal Alkali Burn in Rats. PLoS ONE 2015, 10, e0119725. [Google Scholar] [CrossRef]
- Zhong, Z.; Deng, X.; Wang, P.; Yu, C.; Kiratitanaporn, W.; Wu, X.; Schimelman, J.; Tang, M.; Balayan, A.; Yao, E.; et al. Rapid bioprinting of conjunctival stem cell micro-constructs for subconjunctival ocular injection. Biomaterials 2021, 267, 120462. [Google Scholar] [CrossRef]
- Arnhold, S.; Semkova, I.; Andressen, C.; Lenartz, D.; Meissner, G.; Sturm, V.; Kochanek, S.; Addicks, K.; Schraermeyer, U. Iris pigment epithelial cells: A possible cell source for the future treatment of neurodegenerative diseases. Exp. Neurol. 2004, 187, 410–417. [Google Scholar] [CrossRef]
- Tay, C.Y.; Sathiyanathan, P.; Chu, S.W.; Stanton, L.W.; Wong, T.T. Identification and Characterization of Mesenchymal Stem Cells Derived from the Trabecular Meshwork of the Human Eye. Stem Cells Dev. 2012, 21, 1381–1390. [Google Scholar] [CrossRef]
- Du, Y.; Roh, D.S.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L.; Schuman, J.S. Multipotent Stem Cells from Trabecular Meshwork Become Phagocytic TM Cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 1566–1575. [Google Scholar] [CrossRef]
- Wang, E.; Jiang, X. Stem cells from trabecular meshwork cells can secrete extracellular matrix. Biochem. Biophys. Res. Commun. 2020, 523, 522–526. [Google Scholar] [CrossRef]
- Cicero, S.A.; Johnson, D.; Reyntjens, S.; Frase, S.; Connell, S.; Chow, L.M.L.; Baker, S.J.; Sorrentino, B.P.; Dyer, M.A. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 6685–6690. [Google Scholar] [CrossRef]
- Ni, A.; Wu, M.J.; Nakanishi, Y.; Chavala, S.H. Facile and Efficient Reprogramming of Ciliary Body Epithelial Cells into Induced Pluripotent Stem Cells. Stem Cells Dev. 2013, 22, 2543–2550. [Google Scholar] [CrossRef]
- Gualdoni, S.; Baron, M.; Lakowski, J.; Decembrini, S.; Smith, A.J.; Pearson, R.A.; Ali, R.R.; Sowden, J.C. Adult Ciliary Epithelial Cells, Previously Identified as Retinal Stem Cells with Potential for Retinal Repair, Fail to Differentiate into New Rod Photoreceptors. Stem Cells 2010, 28, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Inoue, Y.; Kawase, Y.; Uchida, S.; Tamaki, Y.; Araie, M.; Okochi, H. Properties of growth and molecular profiles of rat progenitor cells from ciliary epithelium. Exp. Eye Res. 2006, 82, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Mamalis, N.; Davis, B.; Nilson, C.D.; Hickman, S.M.; Leboyer, R.M. Complications of foldable intraocular lenses requiring explantation or secondary intervention—2003 survey update. J. Cataract. Refract. Surg. 2004, 30, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ouyang, H.; Zhu, J.; Huang, S.; Liu, Z.; Chen, S.; Cao, G.; Li, G.; Signer, R.A.J.; Xu, Y.; et al. Lens regeneration using endogenous stem cells with gain of visual function. Nature 2016, 531, 323–328, Erratum in Nature 2017, 541, 558. [Google Scholar] [CrossRef]
- Sasai, Y.; Eiraku, M.; Suga, H. In vitro organogenesis in three dimensions: Self-organising stem cells. Development 2012, 139, 4111–4121. [Google Scholar] [CrossRef]
- Cvekl, A.; Camerino, M.J. Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology. Cells 2022, 11, 3516. [Google Scholar] [CrossRef]
- Janowski, M.; Bulte, J.W.; Handa, J.T.; Rini, D.; Walczak, P. Concise Review: Using Stem Cells to Prevent the Progression of Myopia—A Concept. Stem Cells 2015, 33, 2104–2113. [Google Scholar] [CrossRef]
- Lin, K.-J.; Loi, M.-X.; Lien, G.-S.; Cheng, C.-F.; Pao, H.-Y.; Chang, Y.-C.; Ji, A.T.-Q.; Ho, J.H.-C. Topical administration of orbital fat-derived stem cells promotes corneal tissue regeneration. Stem Cell Res. Ther. 2013, 4, 72. [Google Scholar] [CrossRef]
| Limbal Autografts | Limbal Allografts | Non-LESCs Transplantation |
|---|---|---|
|
|
|
|
| |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Winkler-Lach, W.; Tomczyk-Socha, M.; Misiuk-Hojło, M. Advancements in Ocular Regenerative Therapies. Biology 2023, 12, 737. https://doi.org/10.3390/biology12050737
Tomczak W, Winkler-Lach W, Tomczyk-Socha M, Misiuk-Hojło M. Advancements in Ocular Regenerative Therapies. Biology. 2023; 12(5):737. https://doi.org/10.3390/biology12050737
Chicago/Turabian StyleTomczak, Wojciech, Weronika Winkler-Lach, Martyna Tomczyk-Socha, and Marta Misiuk-Hojło. 2023. "Advancements in Ocular Regenerative Therapies" Biology 12, no. 5: 737. https://doi.org/10.3390/biology12050737
APA StyleTomczak, W., Winkler-Lach, W., Tomczyk-Socha, M., & Misiuk-Hojło, M. (2023). Advancements in Ocular Regenerative Therapies. Biology, 12(5), 737. https://doi.org/10.3390/biology12050737






