Simple Summary
This study evaluated two Bacillus halotolerans-beneficial strains (Cal.l.30 and Cal.f.4) for the levels of their compatibility and their potential to be effective biofertilizers and in parallel biocontrol agents. We investigated their plant growth-promoting effect and ability to induce the expression of defense-related genes in the leaves of young tomato seedling plants, applying them individually or in combination under in vitro and greenhouse conditions using seed biopriming and soil drenching as inoculum delivery systems. The results showed that B. halotolerans strains (singly or in combination) are promising bioproducts for sustainable horticulture as they combine both direct antifungal activity against plant pathogens and the ability to prime plant immunity and enhance plant growth.
Abstract
Evaluating microbial-based alternatives to conventional fungicides and biofertilizers enables us to gain a deeper understanding of the biocontrol and plant growth-promoting activities. Two genetically distinct Bacillus halotolerans strains (Cal.l.30, Cal.f.4) were evaluated for the levels of their compatibility. They were applied individually or in combination under in vitro and greenhouse conditions, using seed bio-priming and soil drenching as inoculum delivery systems, for their plant growth-promoting effect. Our data indicate that application of Cal.l.30 and Cal.f.4 as single strains and as a mixture significantly enhanced growth parameters of Arabidopsis and tomato plants. We investigated whether seed and an additional soil treatment with these strains could induce the expression of defense-related genes in leaves of young tomato seedling plants. These treatments mediated a long lasting, bacterial-mediated, systemic-induced resistance as evidenced by the high levels of expression of RP3, ACO1 and ERF1 genes in the leaves of young tomato seedlings. Furthermore, we presented data showing that seed and soil treatment with B. halotolerans strains resulted in an effective inhibition of Botrytis cinerea attack and development on tomato leaves. Our findings highlighted the potential of B. halotolerans strains as they combine both direct antifungal activity against plant pathogens and the ability to prime plant innate immunity and enhance plant growth.
1. Introduction
To meet the growing demand for humans’ food and animals’ feed, increasing agronomic inputs such as chemical fertilizers and pesticides raise public concerns on the environment and human health. Hence, new alternatives have been sought that would lead to an increase in agricultural production via eco-friendly and sustainable strategies [1]. Thus, the use of natural compounds and plant-beneficial microorganisms have been proposed [1]. These beneficial microorganisms may be bacteria associated with below- or above-ground plant areas, or bacteria inhabiting the plant endosphere (endophytic bacteria) and/or the rhizosphere (rhizobacteria), collectively known as plant growth-promoting bacteria (PGPB) [2]. These PGPB are equipped with a number of traits through which they improve plant growth in diverse agricultural settings. The mechanisms of plant growth promotion include the facilitation of nutrient acquisition and the synthesis of plant hormones, restriction of pathogens’ growth or even the elimination of pathogens through antibiotic production and/or enzyme production, competition for nutrients and appropriate colonization of niches at the root surface [1]. Furthermore, PGPB protect plants against pathogens by activating physiological, biochemical and molecular defense responses in the plant [3].
Inoculation of plants with beneficial microorganisms can be performed with a single isolate or with more than one, called co-inoculation. In co-inoculation, beneficial microorganisms may interact synergistically, increasing the efficiency of exerted plant growth-promoting activities [4,5]. For the inoculation of plants with beneficial microorganisms, different methods are being employed including seed treatment, root dipping, soil drenching and foliar spraying inoculation, with seed inoculation or soil drenching being the most widely used methods in various agricultural settings [6]. The colonization of host plant tissues (e.g., roots) by PGPB can prime, sensitize or activate plants’ innate immune defense system and, thus, can render the plant capable of responding quickly and strongly as soon as the pathogen is perceived [7,8]. In general, the priming process sensitizes or activates the expression of the genes of different hormonal pathways [9,10]. Two types of induced defenses were proposed, ISR (induced systemic resistance) and SAR (systemic acquired resistance), depending on the hormone implicated and the type of elicitor [10]. Accordingly, ISR is initiated by beneficial rhizobacteria or other non-pathogenic microorganisms, while SAR is induced by pathogens or chemical compounds [10]. The signal transduction pathway of ISR is regulated by the Jasmonic acid/Ethylane (JA/ET) pathway and is associated with the expression of JA/ET marker genes such as ACC oxidase (ACO), which is involved in the final step for ethylene biosynthesis (ACO1) and genes coding for ethylene response factor 1 (ERF1), a transcription factor involved in the regulation of gene expression responding to biotic and abiotic stresses [10]. SAR, on the other hand, is controlled by the SA-dependent signaling pathway and characterized by the expression of SA marker genes encoding pathogenesis-related (PR) proteins [10]. ISR is commonly regarded as SA-independent and develops without the accumulation of PR proteins [7]; however, there are a few exceptions where there is a positive SA-JA interplay, resulting in the simultaneous induction of SA and JA signaling pathways. For example, the colonization of Arabidopsis thaliana by Enterobacter radicincitans DSM 16,656 enhanced plant growth and induced the priming of plant defense responses, as was evidenced by the accumulation of ISR or SAR marker genes, PR1, PR2, PR5 and PDF1.2 as compared to untreated plants [11]. The presence of Bacillus amyloliquefaciens MBI600 in the in-tomato rhizosphere or phyllosphere activated the induction and interplay of ISR and SAR, as was evidenced by the up-regulation of salicylic acid, jasmonic acid or ethylene-responsive marker genes [12].
The most promising PGPB that are able to function as priming agents and plant growth-promoting agents belong to Bacillus and Pseudomonas species [5,13,14]. In most cases, the inoculation of plants with beneficial microorganisms has been performed with a single isolate. However, recent studies have demonstrated that inoculation with mixtures of more than one isolate may interact synergistically, resulting in improved plant development and enhanced priming effect compared to single strain inoculations [5,15].
The aim of this study was to combine the novel endophytic B. halotolerans strains Cal.l.30 and Cal.f.4, two promising biological control agents, and evaluate their plant growth-promoting and plant protection abilities as mono- and co-cultures. Cal.l.30 and Cal.f.4 were selected ahead of other isolates due to their ability to combine direct plant growth effect and strong antimicrobial activity against several plant pathogens with the secretion of novel secondary metabolites [16,17]. Strains were tested for their compatibility under in vitro conditions and their co-colonization capacity on A. thaliana roots. We used seed bio-priming and soil-drenching methods as inoculum delivery systems to apply both strains individually or in combination on tomato plants and evaluate their plant growth promotion. We further studied the activation of tomato plant defense mechanisms after the application of inoculants through seed priming and soil drenching and evaluated their ability to protect plants against gray mold disease. Finally, we evaluated the direct suppression of B. cinerea under ex vivo application on detached grape berries.
2. Materials and Methods
2.1. Bacterial Preparation
Both B. halotolerans Cal.l.30 and B. halotolerans Cal.f.4 are natural endophytes isolated from Calendula officinalis, as recently described by Tsalgatidou et al., 2023 [16]. Routinely, both strains were inoculated in 5 mL liquid nutrient broth (NB) medium and incubated overnight with shaking on an orbital platform (200 rpm) at 30 °C. Bacteria were maintained on solid nutrient agar (NA) plates (1.5% w/v agar) until further use.
2.2. Compatibility Assays In Vitro
The in vitro compatibility of Cal.l.30 and Cal.f.4 was studied by the modified mixed colony spot [18] and pairwise interaction methods [19]. Cal.l.30 and Cal.f.4 have different colonial morphology and could be differentiated based on this. To further support the results, the rifampicin-resistant strain of Cal.l.30 (Cal.l.30-rif) was used to evaluate coexistence of Cal.l.30 and Cal.f.4. A mixed colony spot was performed between bacterial strains Cal.l.30-rif and Cal.f.4 onto an NA medium in 5 cm diameter Petri dishes. Both bacterial strains, each containing ~108 CFU/mL (OD600 = 0.5)—based on standard curves of each bacterial strain—were equally combined, creating a 1:1 mixture. After mixing thoroughly, an aliquot of 10 μL of the mixture (5 μL of each bacterial strain) was spot inoculated on the agar surface and incubated at 30 °C for five days. Colony growth was captured and measured on a daily basis until the fifth day of observation. The bacterial cell density of mono- and co-culture colonies was calculated daily after pouring 1 mL of sterilized ddH2O onto petri plates, excluding the cells from the NA medium with a Pasteur pipette and, finally, serially diluting and plating 100 μL of the resuspended cells on NA plates supplemented with or without rifampicin. Plates were incubated overnight at 30 °C. Experiments were conducted in triplicate and repeated three times.
The pairwise interaction method was performed between B. halotolerans Cal.l.30 and Cal.f.4 strains onto the NA medium in 5 cm Petri dishes. An aliquot of 5 μL of each bacterial strain of cell suspension of 108 CFU/mL (OD600 = 0.5) was spot inoculated at a 1 cm distance between them. Each strain was singly inoculated as a control treatment. Plates were incubated at 30 °C for 7 days and images were taken on a daily basis. For each treatment, four biological replicates were run in three technical replicates.
2.3. Bacterial Interactions on A. thaliana Plantlets
Bacterial strains Cal.l.30 and Cal.f.4 were studied for their in planta compatibility by the root colonization of A. thaliana seedlings and the plant growth effect, as mono- and co-cultures. A. thaliana seeds were surface sterilized and grown on solid half-strength Murashige and Skoog (½ MS) medium (Duchefa Biochemie, Haarlem, The Netherlands) supplemented with 0.5% (w/v) sucrose and 0.8% (w/v) bacteriological agar (Sigma, Burlington, MA, USA) as previously described by Tsalgatidou et al., 2023 [16], until further use.
To evaluate the simultaneous A. thaliana root colonization ability of Cal.l.30 and Cal.f.4 as a mixture, a modified protocol of Beauregard et al., 2013 [20] was conducted. Eight-day-old seedlings grown on solid ½ MS medium were transferred to Eppendorf’s containing 300 mL ½ MS medium, inoculated with bacterial cells of ~108 CFU/root (OD600 = 0.5) of mono- (Cal.l.30, Cal.f.4) and co-cultures (Cal.l.30 + Cal.f.4) and put on an orbital shaker at 100 rpm for 18 h. For this experiment, the rifampicin resistant strain Cal.l.30-rif was used. To determine colonization ability, plants were removed from each tube, washed two times in 1 mL of ½ MS after shaking thoroughly for 30 sec and finally washed in 1 mL of ½ MS with vortex agitation for 1 min to remove all cells slightly attached to the roots. Cell-containing solution was serially diluted with ½ MS before plating on NA medium supplemented with rifampicin, only for Cal.l.30-rif determination. The CFU/root was determined 24 h after incubation at 30 °C. For each treatment, six biological replicates were run in three technical replicates. Single strain bacterial cells’ colonization was visualized under an Olympus BX40 microscope.
The growth effect on A. thaliana morphological characteristics was determined after bacterial mono- (Cal.l.30 and Cal.f.4) and co-culture (Cal.l.30 + Cal.f.4) inoculation at 3 cm distance from root tips, as previously described by Tsalgatidou et al., 2023 [16]. Both bacterial strains, each containing ~108 CFU/mL (OD600 = 0.5), were equally combined, creating a 1:1 mixture, and an aliquot of 10 μL cell suspension was inoculated. For each treatment six biological replicates were run in three technical replicates. Seedlings’ fresh weight, primary root length, lateral roots and root hairs were determined as previously described by Tsalgatidou et al., 2023 [16].
2.4. Pot Experiment Design
2.4.1. Tomato Seed Bio-Priming and Soil Drenching with Cal.l.30 and Cal.f.4 as Single- and Dual-Strain Inoculant
Tomato seeds (Solanum lycopersicum var. Chondrokatsari Messinias) were bacterized with B. halotolerans strains Cal.l.30 and Cal.f.4 as a single-strain formulation and as a mixture under greenhouse conditions with a completely randomized block design with three replicates. Tomato seeds were kindly provided by Dr Costas Delis (Department of Agriculture at the University of the Peloponnese). Sterilization and bio-priming of tomato seeds were conducted, as previously described, by Thomloudi et al., 2021 [21]. Briefly, tomato seeds were surface sterilized by immersion in a 3% (v/v) aqueous solution of commercial bleach (5% sodium hypochlorite solution) for 6 min, rinsed six times in double distilled water and air-dried in a UV-sterilized laminar flow cabinet. Dried seeds were immersed in a solution containing a single strain and a 1:1 mixture of bacterial suspension adjusted to ~108 CFU/mL (OD600 = 0.5), supplemented with 1% (w/v) Carboxyl Methyl Cellulose (CMC) (Sigma-Aldrich®, Merk KGaA, Burlington, MA, USA). Control seeds were immersed in a solution containing only CMC. Seeds were incubated for 1.5 h at 25 °C under agitation and then air-dried in a UV-sterilized laminar flow cabinet, until sowing in 7 × 7 cm2 pots (one plant/pot) using a substrate of peat and perlite (2:1 v/v).
Additional bacterial application was done by the soil drenching (10 mL/pot) of Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4 (1:1 mixture) suspension adjusted to ~108 CFU/mL, at 15 d 30 d after sowing. The pot experiment was conducted in a greenhouse (average air temperature 25 °C, relative air humidity 60% and an average 10½ h photoperiod) from October to December of 2019. Plant growth parameters (shoot fresh and dry weight, root fresh and dry weight and shoot length) were recorded 45 days after sowing (das). Plants were captured with a digital camera and shoot length was measured using ImageJ software v.1.8.0 [22].
2.4.2. Rhizosphere Colonization Assay
Bacterial rhizosphere colonization and soil survival were estimated at two time points: at 15 das, to measure the number of cells of each treatment in the rhizosphere soil with bio-priming being the only inoculation system, and at 45 days after two extra bacterial inoculations were conducted through soil drenching. For this experiment, the rifampicin resistant strain Cal.l.30-rif was used. Plants were carefully uprooted and, after removing most of the soil from the roots, one gram of the remaining soil tightly adhered to the roots was taken. The soil was transferred to a falcon tube containing 10 mL of sterilized ddH2O and vortexed for 1 min. The bacterial population of each treatment was enumerated at each sample day by a standard dilution plate count method on NA medium. The colonies of Cal.l.30-rif were counted by supplementing the NA medium with rifampicin. The CFU/g of soil were determined 24 h after incubation at 30 °C. For each treatment, four biological replicates were run in three technical replicates.
2.4.3. B. cinerea Infection Assay
After 45 days of bacterial inoculation through seed bio-priming and additional soil drenching applications at 15 and 30 das, B. cinerea was inoculated on tomato leaves in vivo. A droplet of 10 μL of spore suspension (5 × 105 CFU/mL) was inoculated on one side of the midvein of the leaves (one leaf/tomato plant). Inoculated plants were covered with transparent plastic to maintain high humidity and plants were left in the greenhouse for five more days. After 5 days post infection (dpi), the lesion area created by B. cinerea on tomato leaves was measured and disease severity index (DSI %) was calculated as described by Lee et al., 2006 [23]. The lesion area was measured using the ImageJ software v.1.8.0 analysis tool [22]. Disease severity (DS %) was calculated as the percentage of infected leaf area on which a 0-to-4 rating scale was created, with 0 = healthy leaves, 1 = 0.1–5%, 2 = 5.1–20%, 3 = 20.1–40% and 4 = 40.1–100% lesion area [23]. The disease severity index (DSI %) was estimated using the above severity scale and based on the following formula: % Disease Severity Index (DSI) = [∑(number of infected leaves in a specific value of disease rating scale × corresponding value of disease scale)/(maximum rating scale value × total number of leaves rated)] × 100.
2.5. Quantitative Analysis of Defense-Related Gene Expression
The induction of defense-related genes after bacterial formulation (Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4) through seed bio-priming and both seed bio-priming and an additional root drenching (15 d), was evaluated by quantitative real-time PCR. Tomato plantlets’ leaf samples were collected for RNA extraction 15 das of bio-primed seeds and 24 h after an additional bacterial soil drenching application.
Total RNA was extracted from tomato leaves (100 μg/sample) of four different treatments (non-inoculated plants (control), inoculation with B. halotolerans Cal.l.30, inoculation with B. halotolerans Cal.f.4 and inoculation with the mixture of B. halotolerans strains Cal.l.30 + Cal.f.4) at two different time points (15 das of bacterial bio-primed seeds and 24 h after additional soil drenching) following the protocol from the manufacturer, Macherey-Nagel (NucleoSpin® RNA Plus, Macherey-Nagel, Loughborough, Leicestershire, UK). DNA was DNAase eliminated and cDNA was synthesized with 3 μg of purified RNA using the PrimeScript 1st strand cDNA, Synthesis Kit (Takara Bio, Kusatsu, Shiga, Japan), according to the manufacturer’s instructions. The conditions of RT-qPCR and the relative quantification of specific mRNA levels were performed using PowerUp SYBR Green Master Mix Assay (Applied Biosystems, Waltham, MA, USA)) containing 1 μL cDNA template, 10 μL SYBR GREEN PowerUp mix and 2 μL each of 10 μM tomato gene-specific primer pair (Supplementary Table S1). The cycling conditions were as follows: pre-incubation at 95 °C for 2 min, followed by 40 cycles at 95 °C for 15 sec, at 60 °C for 15 sec and at 72 °C for 1 min. Ubi3 gene coding for ubiquitin [24] was used as a housekeeping gene and for the normalization of gene expression. Primers used against genes coding for pathogenesis-related proteins PR2; PR2 [25] and PR3; PR3 (Beacon Designer v.7.9), for proteins related to ethylene synthesis and response such as ERF1; ERF1 [26] and ACO1; ACO1 (Beacon Designer v.7.9), and the lipoxygenase A encoding gene TomloxA [27] that catalyzes the first step in the octadecanoid pathway as a response to biotic and abiotic stress, were selected.
The efficiency of the reaction for each target gene derived from LinRegPCR software [28] through linear analysis regression on the logarithm of the fluorescence intensity values in each cycle reaction. Relative levels of target gene transcripts for each sample were calculated based on the formula derived from the Pfaffl equation: ErefCref/Ectargetcttarget [29].
2.6. Detached Fruit Assay
The direct biological control ability of B. halotolerans Cal.l.30 and B. halotolerans Cal.f.4 as individual strains and as a consortium was evaluated on detached grape berries (Vitis vinifera L. cv. Sultanina) against gray mold disease caused by B. cinerea as previously described by Tsalgatidou et al., 2022 [17]. Briefly, an aliquot of 10 μL of single strain (Cal.l.30 and Cal.f.4) and a 1:1 mixture (Cal.l.30 + Cal.f.4) of bacterial suspension adjusted to ~108 CFU/mL (OD600 = 0.5) were inoculated on a 3 mm wound on the surface-sterilized grape berries (20 fruit/replicate). The grapes were incubated for 1 h at room temperature until infection with 10 μL of B. cinerea spore suspension (~1 × 105 CFU/mL). The grapes were placed into plastic boxes and transferred to a dark growth chamber at 25 °C for 5 d. After 5 dpi, the lesion area created by B. cinerea was measured and the disease severity index and disease incidence were calculated as previously described by Tsalgatidou et al., 2022 [17]. The lesion area was measured using ImageJ software v.1.8.0. Finally, the colonization ability on grapes of both single strains (Cal.f.4 and Cal.l.30) and their mixture was evaluated at 24 h post inoculation as previously described by Tsalgatidou et al., 2022 [17]. For this experiment, the rifampicin-resistant strain Cal.l.30-rif was used to determine colonization on grapes.
2.7. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA). Plots were created with Sigma Plot, version 12.0 (Systat Software, San Jose, CA, USA). In bacterial compatibility assays, A. thaliana and tomato pot experiments were performed and in detached grapes assays, statistical analysis was performed with ANOVA followed by Tukey’s honestly significant difference (HSD) test (p < 0.05) to allow for comparisons among all means. To evaluate the bacterial cell number, data were transformed to the logarithmic scale followed by Tukey analysis. Plots indicate mean values with standard deviation as error bars. Different letters represent statistically significant differences among treatments. Data expressed as percentages were arcsine transformed prior to Tukey analysis (p < 0.05). For gene expression analysis, statistical analysis was performed on the logarithmic values of the relative expression (on ΔCt) to ensure the assumptions of parametric statistical analysis where data follow a normal distribution and homogeneity of variances. Graphs indicate the mean of gene expression for each treatment and a 95% confidence interval (Mean ± CI).
3. Results
3.1. Co-Existence of B. halotolerans Cal.l.30 and Cal.f.4 in In Vitro Compatibility Assays
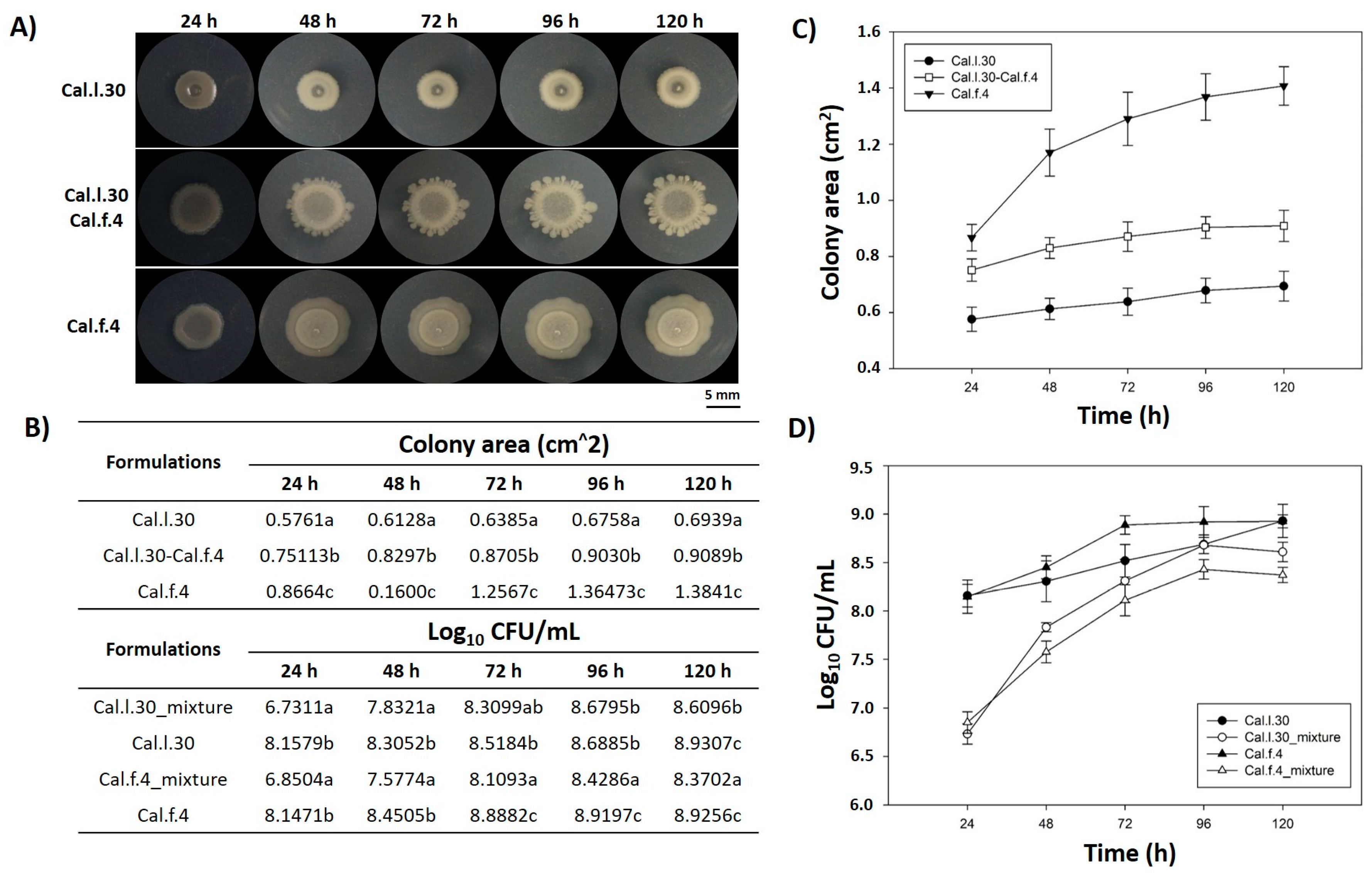
Members of a bacterial consortium are considered as compatible when they do not inhibit each other’s growth during their in vitro co-culture and/or in rhizosphere colonization competition assays [15]. In this context, the in vitro compatibility of the strain mixture involving Cal.l.30 and Cal.f.4 was evaluated by determining the level of co-existence during growth in colony and biofilm modes in a series of co-culture experiments. First of all, we dropped, inoculated, each strain either singly or as bacterial mixture (1:1) on an NA agar plate and measured the colony morphology (Figure 1A) as well as the number of bacterial cells (Figure 1D) in each of the evolving colonies. The results shown in Figure 1B,D indicate that the number of Cal.l.30 and Cal.f.4 cells in the spot-inoculated two-strain mixture-developing colony remained almost identical when compared to the number of cells found in each monoculture, suggesting that Cal.l.30 and Cal.f.4 strains grown in close proximity do not inhibit the growth of each other and, thus, can co-exist, occupying the same niche under the given environmental conditions.
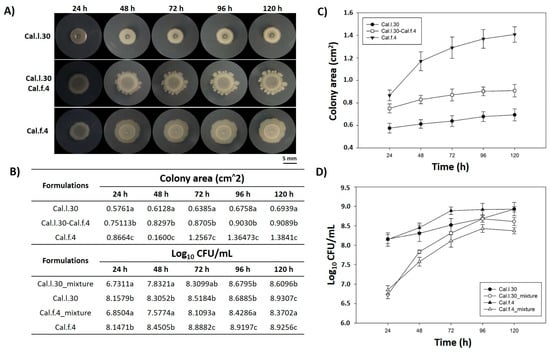
Figure 1.
Mixed colony spot of B. halotolerans Cal.l.30 and B. halotolerans Cal.f.4 as a two-strain mixture on NA agar plates. (A) Colony expansion patterns derived from single- and two-strain communities were recorded at the indicated time intervals (24, 48, 72, 96 and 120 h). (B–D) Plots and tables of the average colony-covered area and number of bacterial cells versus time for the spot-inoculated two-strain and single-strain mixture. Graphs represent the mean ± SD from three independent experiments (n = 4). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test (p < 0.05). Different letters indicate statistically significant differences among formulations at each time point.
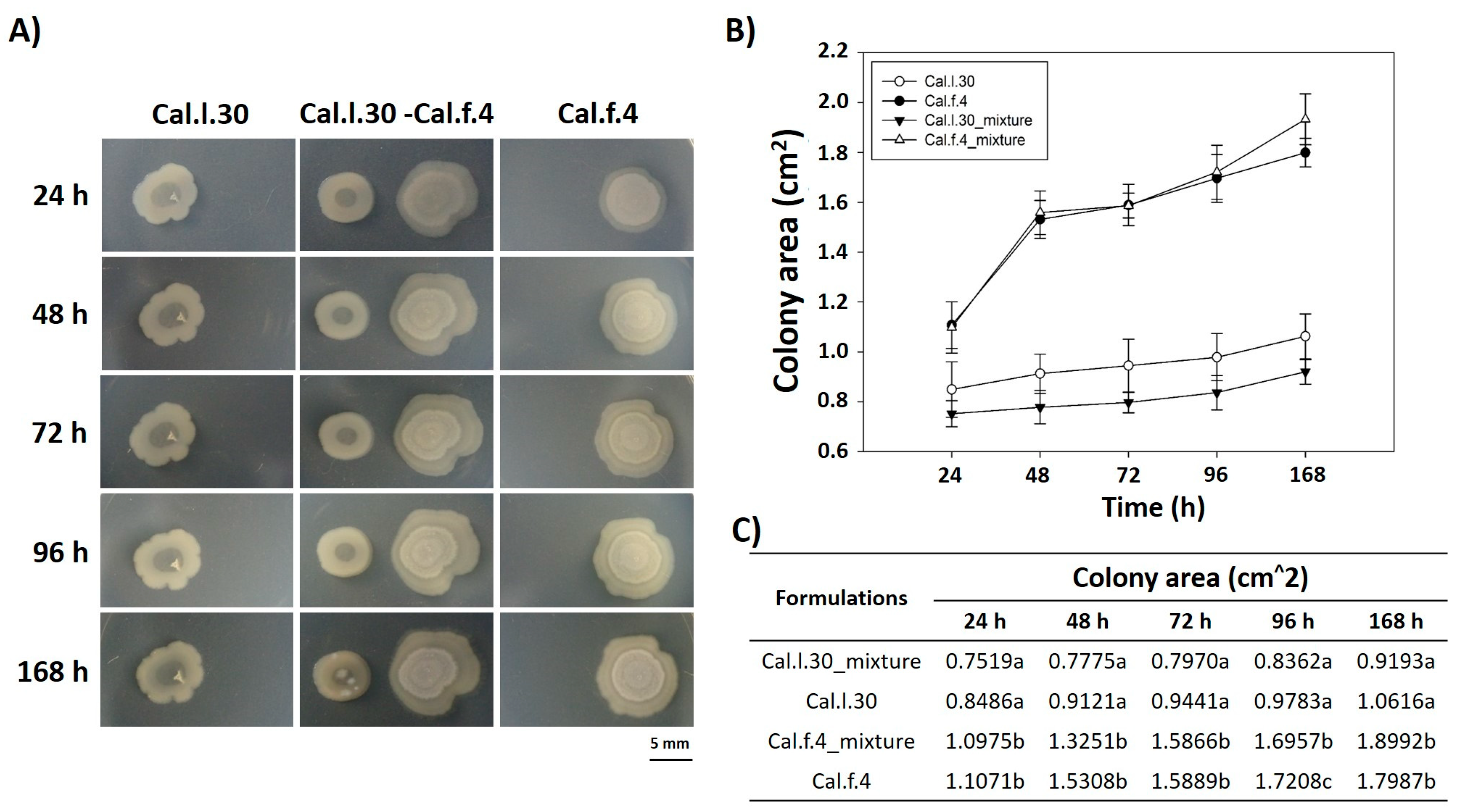
We also carried out experiments in which two different colonies (Cal.l.30 and Cal.f.4) were inoculated on NA agar plates with a separation of 10 mm, and their external (in the direction opposite to the other colony) and internal (in the direction of the other colony) radii were periodically depicted and measured for 7 days. As shown in Figure 2A, when Cal.l.30 and Cal.f.4 strains are inoculated in close proximity to each other, although there is a slight decrease of the Cal.f.4 internal radius as compared to the external one, the area covered by the interacting macrocolonies was largely unaffected as compared to singly grown macrocolonies (Figure 2B,C). These data suggested that both strains interacted, and no antagonistic relationship was found, suggesting that Cal.f.4 coexisted well with Cal.30.
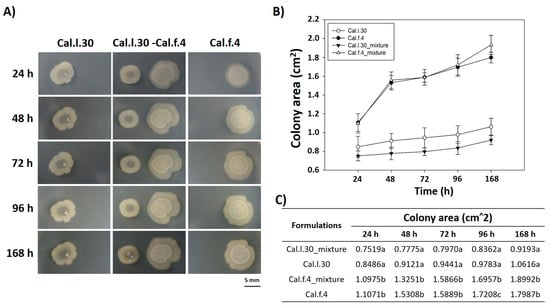
Figure 2.
Time course of pairwise interactions between Cal.l.30 and Cal.f.4 bacterial colonies inoculated with a separation of 10 mm. (A) Colony expansion patterns derived from single- and two-strain communities were recorded at the indicated time intervals, 24 h, 48 h, 72 h, 96 h and 168 h. (B,C) Plot and table of the average colony-covered area versus time for the single strain and the interacting strains. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test (p < 0.05). Different letters suggest statistical differences among treatments at each time point.
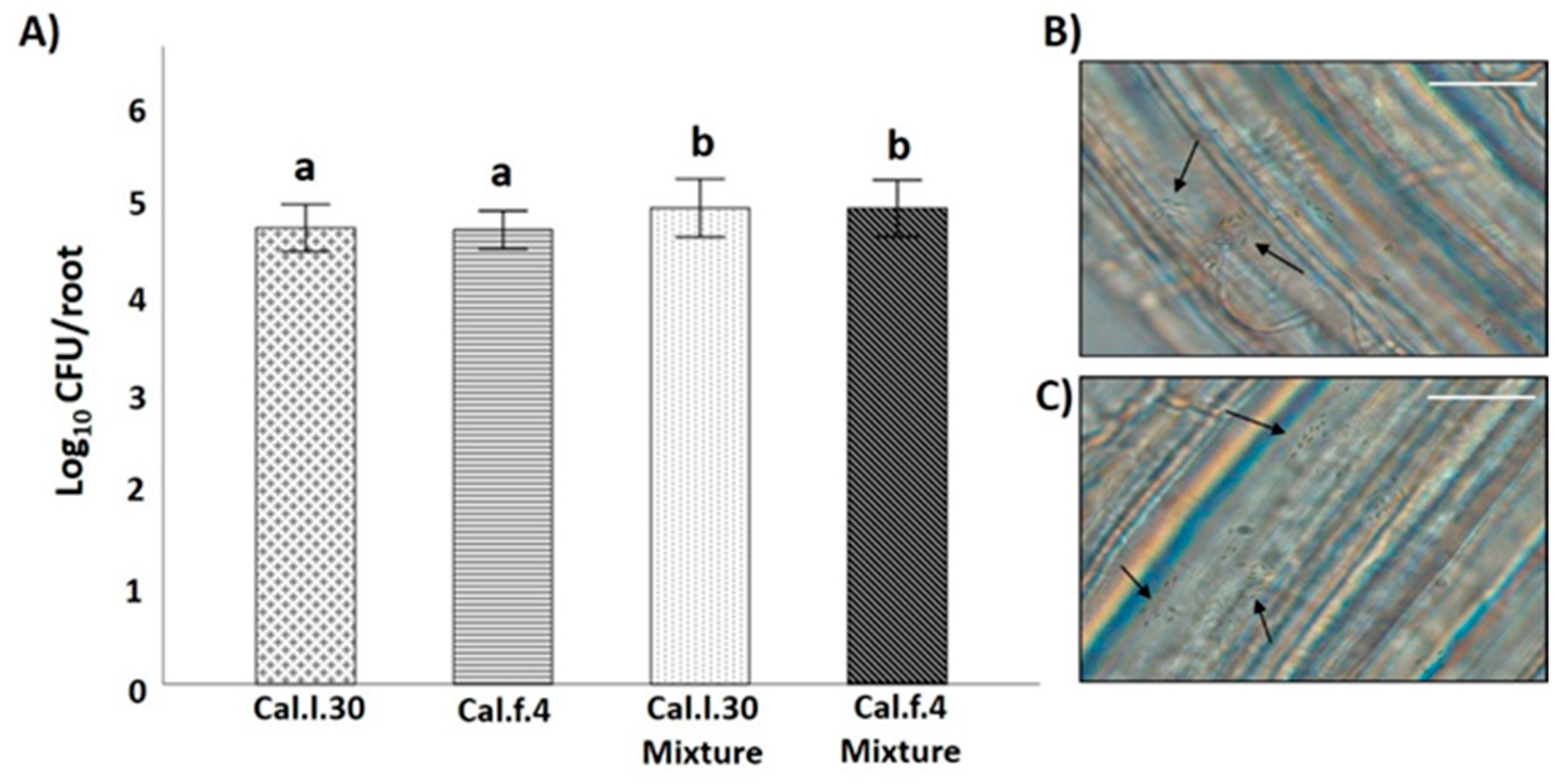
Compatibility of the two B. halotolerans strains was also evaluated by investigating whether antagonism between two B. halotolerans strains occurring on the root may affect population density of each strain, using an in vitro root colonization assay. As shown in Figure 3B,C, both bacterial strains can successfully colonize the root surface of A. thaliana plantlets either as single cells or as aggregates (black arrows). Inoculation of Cal.l.30 and Cal.f.4 as single cultures on hydroponic grown A. thaliana Col-0 resulted in similar levels (3–4 × 104 CFU/root) of colonization by each species, whereas inoculation with a mixture (1:1) of both strains resulted in increased and cumulative colonization of roots by both strains (6–7 × 104 CFU/root from each strain) (Figure 3A). These data suggested that both bacterial strains could co-exist on roots in a synergistic manner.
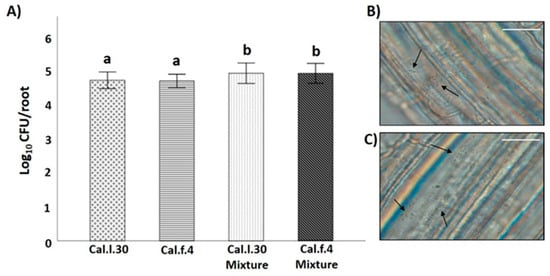
Figure 3.
B. halotolerans Cal.l.30 and Cal.f.4 co-colonizing A. thaliana roots. (A) Colonization ability of the bacteria was measured as CFUs per root (n = 6). The samples were collected at 18 h. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test (p < 0.05). Different letters suggest statistical differences among treatments. A. thaliana root colonization (black arrows) by (B) Cal.l.30 and (C) Cal.f.4 using an Olympus BX40 optical microscope (Scale bar: 20 nm).
3.2. Application of B. halotolerans Strains in Combination and Individually Enhanced the Growth Parameters of Arabidopsis Plants Grown under In Vitro Conditions
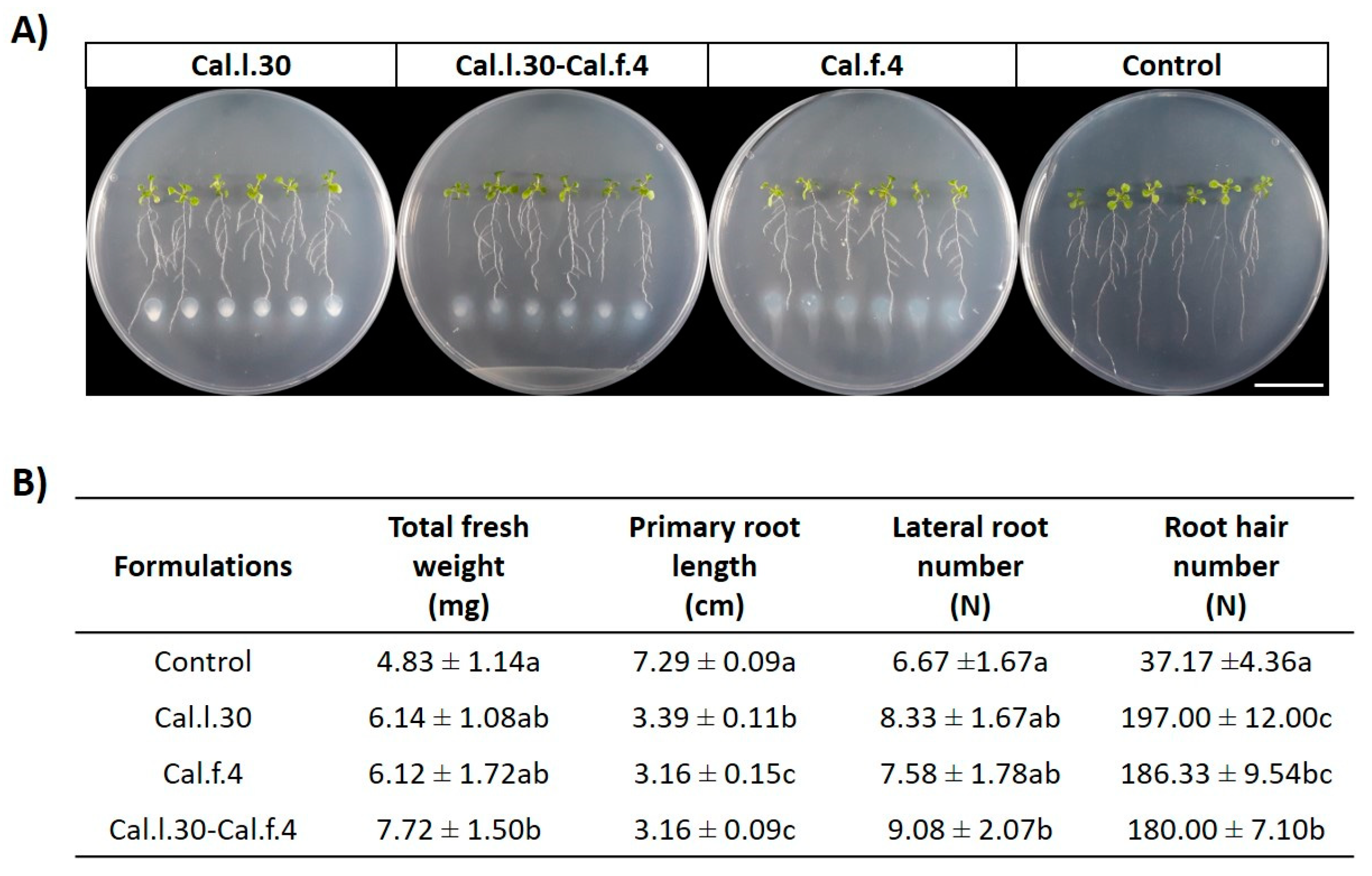
As a first step towards evaluating the plant growth effect of B. halotolerans strains, we evaluated the capacity of Cal.l.30 + Cal.f.4 consortium to promote plant growth in comparison with single-strain applications, using an in vitro A. thaliana co-cultivation approach (Figure 4A). Treatment with Cal.l.30, Cal.f.4 and a Cal.l.30 + Cal.f.4 mixture significantly increased the growth of A. thaliana as evidenced by the enhanced values of all growth parameters measured (fresh weight, number of lateral roots and root hairs) (Figure 4B). Treatment of Arabidopsis by the two-strain mixture resulted in plants with higher total fresh weight and lateral roots compared to the single-strain formulations and the control plantlets (Figure 4B). The single-strain formulation of Cal.l.30 resulted in the highest root hair number compared to the other two formulations.
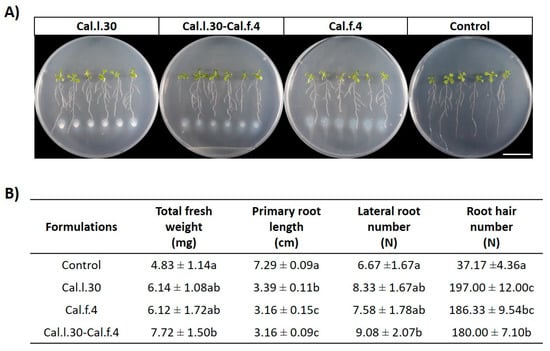
Figure 4.
(A) Representative phenotypic responses of co-cultivation of A. thaliana seedlings with Cal.l.30, Cal.f.4 or Cal.l.30 + Cal.f.4 bacterial mixture inoculated at 3 cm distance from root tips. Physical changes were observed at 6 days after bacterial application (Scale bar: 20 mm). (B) Effect of endophytic bacterial strains Cal.l.30 and Cal.f.4 as mono- and co-cultures on different growth parameters (total fresh weight, primary root length, lateral root number and root hair number) of A. thaliana seedlings under in vitro conditions. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test (p < 0.05). Different letters suggest statistical differences among treatments.
3.3. Application of B. halotolerans Strains as a Mixture and Individually Enhanced Growth Parameters of Tomato Plants under Greenhouse Conditions
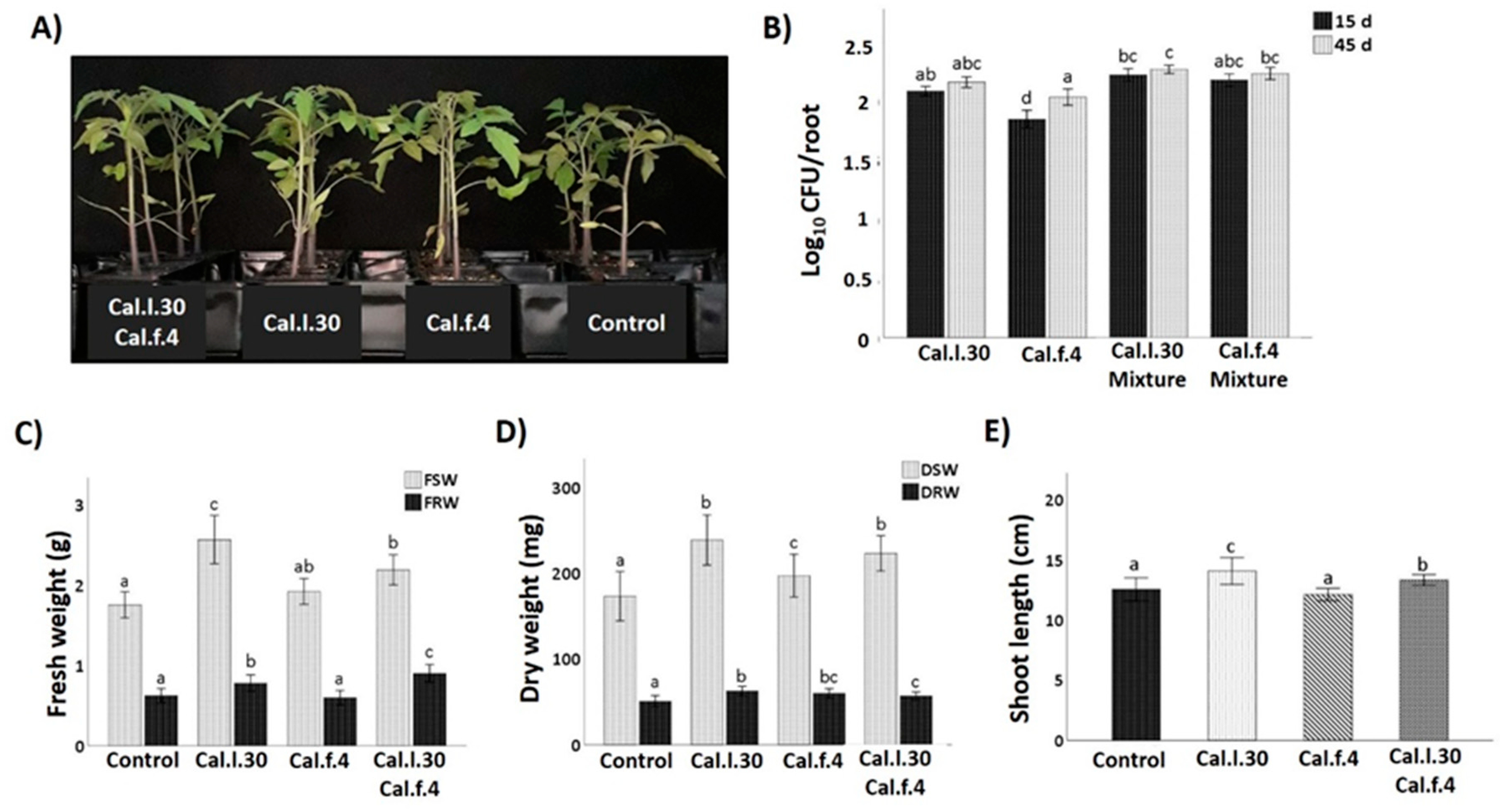
The plant growth promotion capacity of Cal.l.30 and Cal.f.4 was investigated when single strains and bacterial mixtures were applied as tomato seed treatment followed by two additional soil drenching treatments at 15 and 30 das under greenhouse conditions. Plants were harvested at 45 das and growth parameters were measured (total shoot and root, fresh and dry weight and shoot length). As illustrated in Figure 5, seed and soil treatment with Cal.l.30 and Cal.f.4, individually or in combination, resulted in a pronounced growth of tomato plants’ growth parameters compared to untreated plants (control). The results presented in Figure 5 revealed that compared to the control, maximum shoot and root biomass was recorded in plants treated with Cal.l.30, followed by plants treated with Cal.l.30 + Cal.f.4 (Figure 5C,D). Examination of plants’ rhizospheric bacterial populations revealed that Cal.l.30 and Cal.f.4 remained viable in interactions with roots at 15 and 45 das, supporting their long lasting effects to plants.
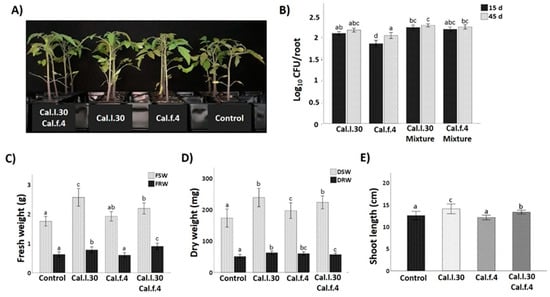
Figure 5.
Effect of seed bio-priming with B. halotolerans strains, single or as mixture, on plant biomass accumulation in tomato at 45 days. (A) Representative image of the tomato plants inoculated with mono- and co-cultures of B. halotolerans strains at 45 days. (B) B. halotolerans populations of mono- and co-cultures colonizing tomato roots at 15 and 45 das. (C–E) The effect of different treatments on tomato plant height (shoot length), fresh shoot weight (FSW), fresh root weight (FRW), dry shoot weight (DSW) and dry root weight (DRW). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test (p < 0.05). Different letters suggest statistical differences among treatments.
3.4. Seed Priming and Additional Soil Drenching with Cal.l.30 and Cal.f.4 Strains Singly or in Combination Protect Tomato Plants against Gray Mold Disease
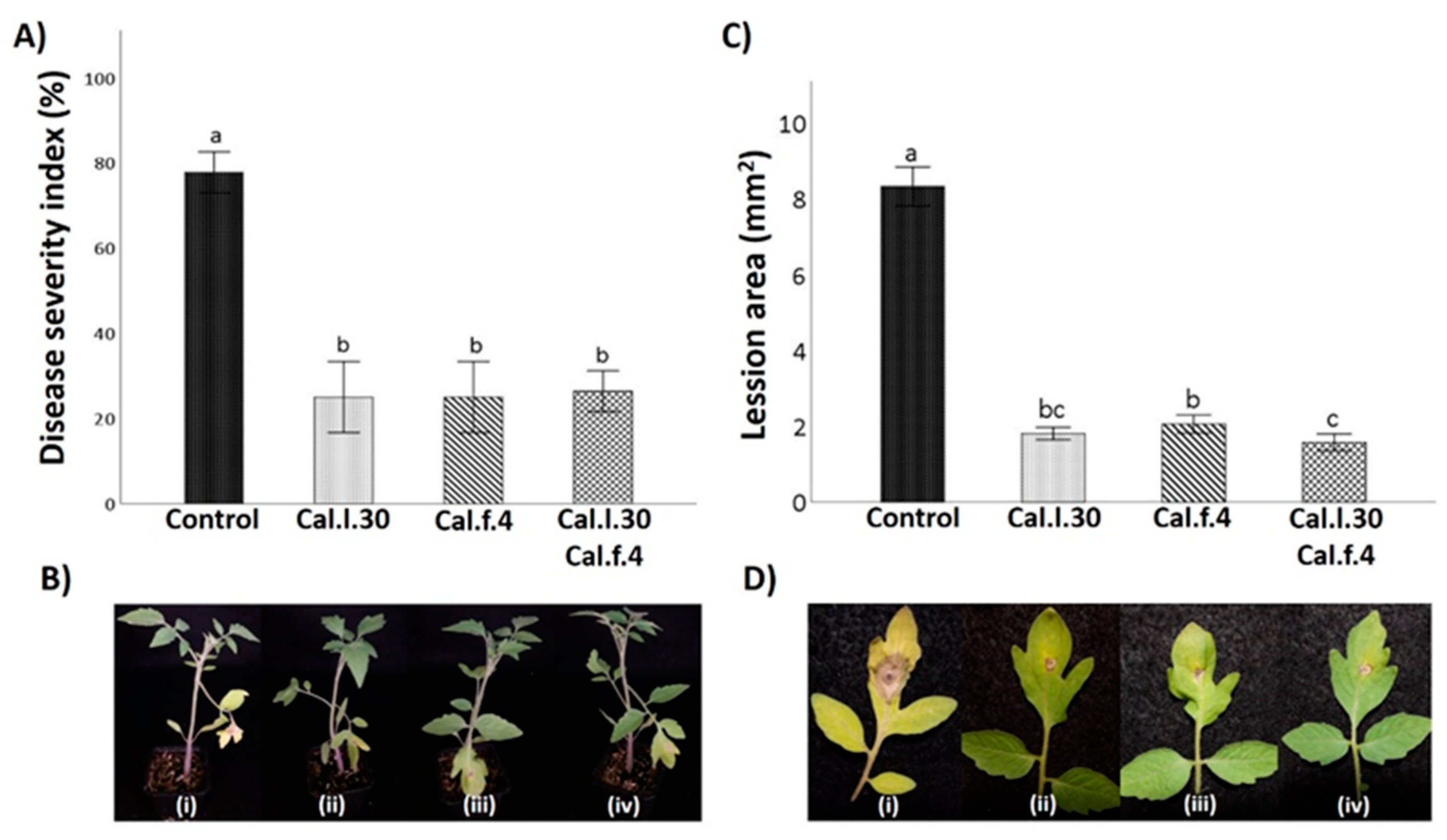
To evaluate whether seed and soil treatment with Cal.l.30, Cal.f.4 and their combination (Cal.l.30 + Cal.f.4) induce systemic resistance of tomato against gray mold disease, disease symptoms on leaves caused by B. cinerea were examined at 5 days after infection with the pathogen (Figure 6B). Five days after pathogenic fungus inoculation, leaves belonging to Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4-treated plants presented minor disease symptoms (brown spots and necrotic lesion) around inoculating loci, compared to untreated plants (Figure 6D). The lesion area was 1.8 cm2, 2.1 cm2 and 1.6 cm2 in Cal.l.30, Cal.f.4 or Cal.l.30 + Cal.f.4-treated plants, respectively, compared to 8.2 cm2 in untreated plants, suggesting that Cal.l.30 + Cal.f.4 treated plants exhibited the best inhibition disease development (Figure 6C).
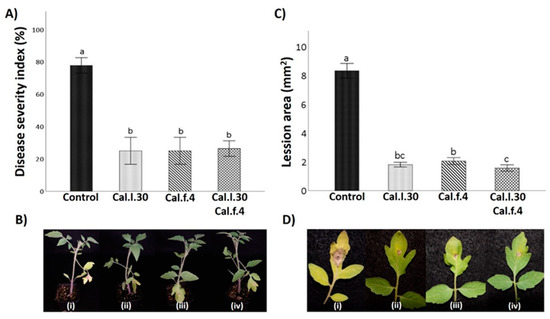
Figure 6.
Effect of B. halotolerans strains Cal.l.30 and Cal.f.4 (singly or as mixture) on tomato leaf area covered with B. cinerea infection. (B–D) Representative images of B. cinerea symptoms development in leaves treated with Cal.l.30 (ii), Cal.f.4 (iii), Cal.l.30 + Cal.f.4 (iv) and control (i) tomato plants. (A) Disease severity index (%) and (C) Lesion area of infected tomato leaves was calculated. Values with a same letter are not significantly (p ≤ 0.05) different according to Turkey’s test. Numerical values were mean ± SD of ten replicates.
To further identify the biocontrol efficacy of Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4-treated plants against B. cinerea, the disease severity index was calculated. As presented in Figure 6A, the disease severity index (%) of control plants is 78.0%, compared to the low values of Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4-treated plants ranging between 25–26%, suggesting that the bacterial mixture and the single strains were equally effective in controlling gray mold disease.
3.5. Activation of Long-Lasting Defense-Related Genes of Tomato Plantlets
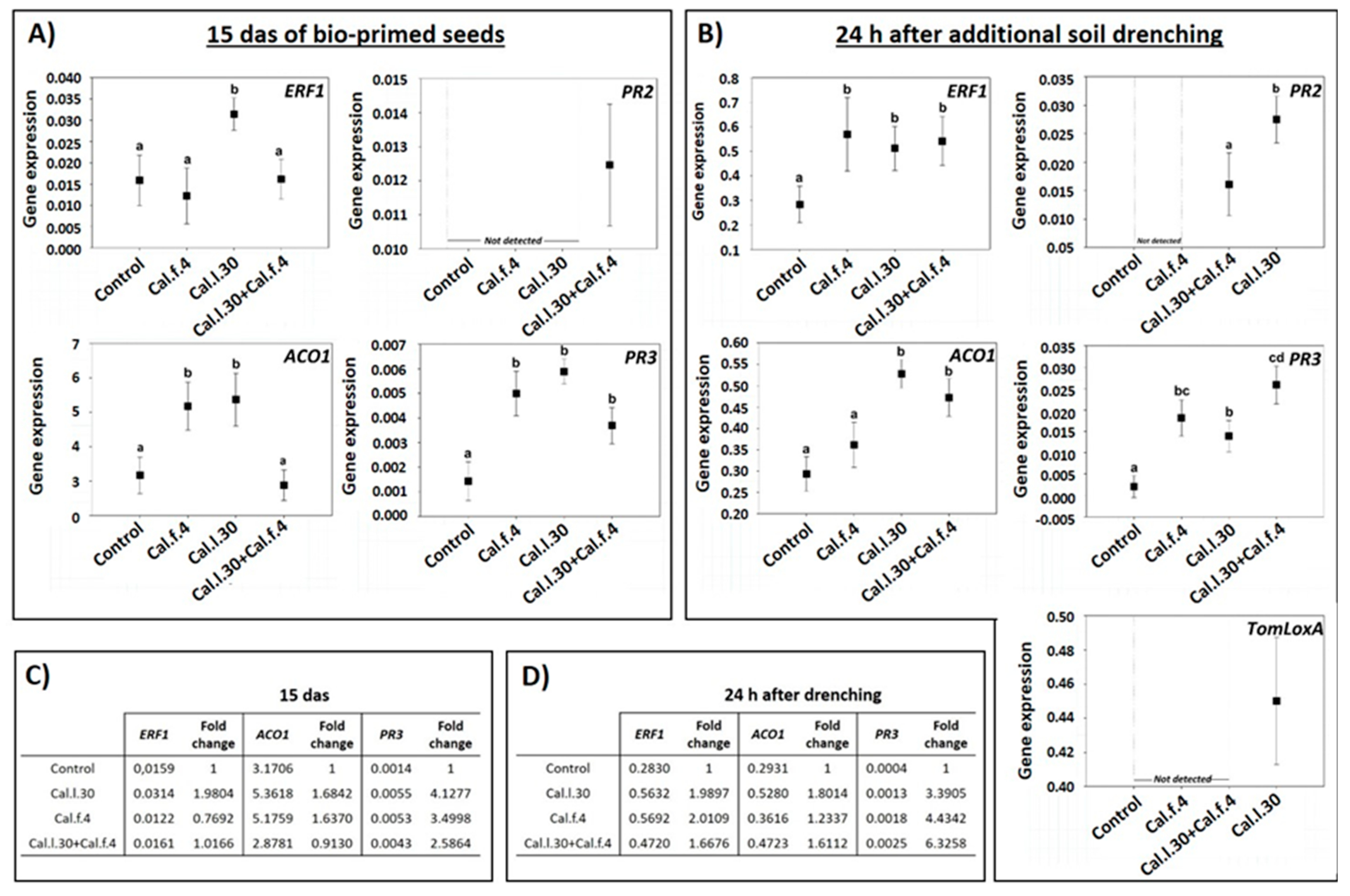
Besides the impact of B. halotolerans strains in control efficacy against B. cinerea, to understand whether seed bio-priming and soil drenching with Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4 could induce the expression of the long-lasting defense-related genes, RT-qPCR analysis was conducted. Therefore, we analyzed the expression of the SA-responsive genes PR3 (encoding the PR protein 2 β-1,3-glucanase) and PR2 (encoding the PR protein 2 β-1,3-glucanase), the JA synthesis-related gene TomLoxA (encoding for lipoxygenase A), the ET-responsive gene ERF1 and the ethylene biosynthesis-related gene ACO1 in the leaves of young plantlets after being seed bio-primed with Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4 (15 das) and after additional application through root drenching with Cal.l.30, Cal.f.4 or Cal.l.30 + Cal.f.4 (24 h post additional soil drenching).
As illustrated in Figure 7A, the expression level of ERF1, ACO1 and PR3 at 15 das were strong induced in plants inoculated with Cal.l.30 through seed bio-priming in comparison to the control (unprimed seeds). Specifically, gene transcript levels of ERF1, ACO1 and PR3 were up-regulated 1.98-, 1.68- and 4.12-fold, respectively, compared to the control (Figure 7C). Tomato plants raised from Cal.f.4 bio-primed seeds presented up-regulated gene transcript levels of ACO1 and PR3 genes (1.63- and 3.49-fold, respectively), while ERF1 expression was down-regulated (Figure 7C). Bio-primed plants with a Cal.l.30 + Cal.f.4 consortium presented a significant up-regulation (2.58-fold) of PR3 transcripts compared to control plants, while ERF1 and ACO1 gene expressions presented a similar level in gene expression in both non- and bio-primed plants (Figure 7C). Expression of PR2 was only observed in plants inoculated with Cal.l.30 + Cal.f.4 consortium (Figure 7A). These results indicated that defense mechanisms were less activated in plants with combined bacterial priming treatment compared to the individual treatments.
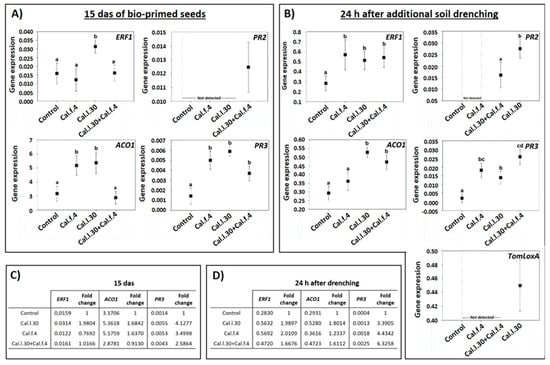
Figure 7.
Expression of defense-related genes in leaves of young tomato plantlets after Cal.f.4, Cal.l.30 and Cal.l.30 + Cal.f.4 bacterial inoculation. (A) Gene expression of ERF1, ACO1, PR2 and PR3 15 das of bio-primed tomato seeds. (B) Gene expression levels of ERF1, ACO1, PR2, PR3 and TomLoxA 24 h after additional bacterial soil drenching. Graphs indicate the mean of gene expression for each treatment and a 95% confidence interval (Mean ± CI). Different letters suggest statistical differences among treatments after one-way ANOVA followed by Tukey’s multiple comparison post hoc test (p < 0.05). (C) Fold-change in gene expression of ERF1, ACO1 and PR3 15 das of bio-primed tomato seeds. (D) Fold-change in gene expression of ERF1, ACO1 and PR3 24 h after additional bacterial soil drenching.
The gene expression levels of ERF1, ACO1, PR2, PR3 and TomLoxA were significantly differentiated after both seed bio-priming and additional bacterial formulation via soil drenching at 15 das (Figure 7B). As reported in Figure 7D, transcript levels of ERF1, ACO1 and PR3 genes of all bacterial inoculated treatments were up regulated compared to untreated plants. Specifically, in Cal.l.30-treated plants, transcript levels of the ERF1, ACO1 and PR3 genes were up-regulated 2-, 1.8- and 3-fold when compared to control plants, while in Cal.f.4-inoculated plants transcript levels were up-regulated 2-, 1.2- and 4.4-fold, respectively. Finally, the expression levels of the ERF1, ACO1 and PR3 genes after consortium’s treatment were up-regulated 1.7-, 1.6- and 6-fold (Figure 7D). Expression of the TomLoxA gene was only recorded in plants inoculated with Cal.l.30, while PR2 gene expression was only triggered after inoculated plants with Cal.l.30 and the consortium (Cal.l.30 + Cal.f.4) (Figure 7B). These results indicated that soil drenching with B. halotolerans strains singly or as a two-strain mixture efficiently activated the expression of JA/ET- and SA-responsive genes in the leaves of tomato plants under greenhouse conditions.
3.6. B. halotolerans Strains Cal.l.30 and Cal.f.4 (Singly or as a Mixture) Suppress Grape’s Postharvest Gray Mold Disease
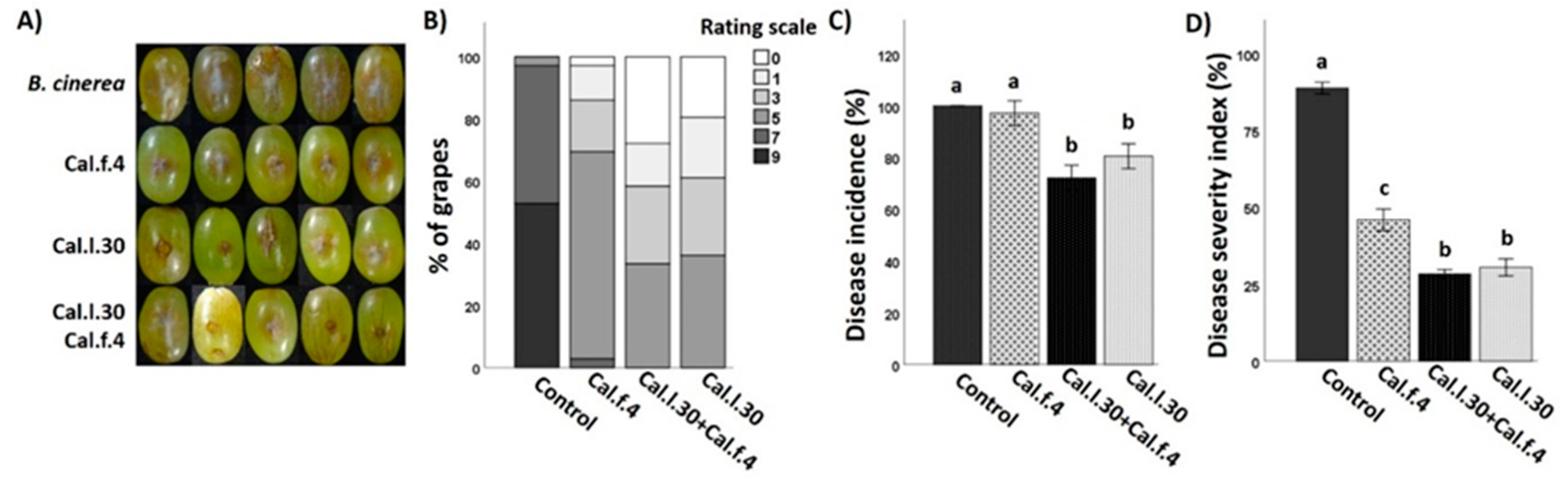
Although the efficacy of B. halotolerans Cal.l.30 and B. halotolerans Cal.f.4 as single-strain biological control agents against B. cinerea in ex vivo applications has already been tested in previous studies [16,17], the application of these strains as a consortium is studied here for the first time. All three applications were tested on detached grape berries in a small-scale storage experiment against B. cinerea. Both single bacterial applications, as well as their mixture, successfully colonized grapes at the point of application, showing a similar cell number (1–3 × 107 CFU/wound).
As presented in Figure 8A,B, Cal.l.30 and Cal.f.4 single-strain applications, as well as their 1:1 mixture, presented a strong inhibition against B. cinerea by reducing both the number of infected grapes and the brown lesion area. Grapes infected only with the pathogenic fungus developed extended brown lesion and visible mycelia growth around the point of formulation and reached 100% infection rate for B. cinerea (Figure 8A). Negative control grape berries that were wounded artificially but not infected with the fungal pathogen appeared to be unaffected after 5 days of storage. Both single-strain formulations significantly reduced the disease severity index, with strain Cal.l.30 being the most efficient with 31% of DSI and 81% of DI, in comparison to the 46% DSI and 97% DI of Cal.f.4 (Figure 8C,D). However, treatment with Cal.l.30 + Cal.f.4 synergistically suppressed disease severity, reaching the lowest DI value of 72% and DSI value of 28% at 5 day of incubation, presenting, along with the Cal.l.30 single-strain formulation, the most impressive results.
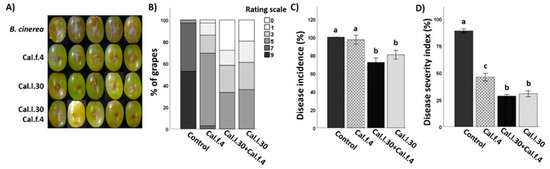
Figure 8.
Inhibition of B. cinerea on wounded grape berries after Cal.l.30 and Cal.f.4 single strain and co-culture formulation. (A) Inhibition of gray mold disease after mono- and co-culture of Cal.l.30 and Cal.f.4 compared to control (B. cinerea) treatment. (B) Infected grapes (%) as assessed by color in control (B. cinerea) and inoculated grapes (n = 20). Colored blocks within each column represent the percentage of grapes corresponding to the scale value of the disease severity of B. cinerea. Visual disease rating scale of symptoms caused by B. cinerea is represented as 0 = healthy fruits, 1 = 1–10%, 3 = 11–25%, 5 = 26–50%, 7 = 51–75% and 9 ≥ 75% infected area. (C) Disease severity index (%) and (D) disease incidence (%) of wounded grapes, respectively. Bars represent the mean ± SD of 3 independent biological replicates. Letters indicate statistically significant differences among treatments after Tukey analysis (p < 0.05).
4. Discussion
Plant growth-promoting bacteria (PGPB) can provide multiple benefits in agriculture by mediating enhancement in crop productivity and nutrient content and suppressing the growth of pathogens. Although members of the B. subtilis species complex have been extensively studied for their ability to promote vegetative plant growth, reduce disease incidence and promote plant tolerance against fungal and bacterial pathogens by inducing the systemic resistance of host plants, the quest for new Bacillus-based biological control agents (BCAs) along with effective methods for their delivery is an ongoing process [30,31].
B. halotolerans strains Cal.l.30 and Cal.f.4 have a wealth of plant growth-promoting traits and exhibit a strong antimicrobial activity against fungal pathogens through the production of multiple antifungal metabolites [16,17]. The present study provides evidence that Cal.l.30 and Cal.f.4 could form a compatible consortium under in vitro, ex vivo and greenhouse conditions. The efficient coexistence of Cal.l.30 and Cal.f.4 was observed when both strains successfully colonized A. thaliana roots in in vitro application, presenting a greater adhesion of their cells on the roots when applied as a mixture than as single strains. Cal.l.30 + Cal.f.4 mixture synergistically promoted A. thaliana plant growth parameters under in vitro conditions, compared to single treatments.
Recent studies have demonstrated that seed bio-priming and soil drenching are the most promising methods for delivering the beneficial microbes to the host plant when PGPB are evaluated for their plant growth-promoting activity under greenhouse or field conditions [32,33]. Furthermore, it has been demonstrated that tomato, cow pea and mung bean seed bio-priming followed soil drenching induced significant plant growth parameters as compared to single treatment [34,35]. In this regard, in our studies, we evaluated the plant growth effect of B. halotolerans Cal.l.30 and B. halotolerans Cal.f.4 when applied to tomato, singly or in combination, through seed bio-priming and additional soil drenching. Our data demonstrated that tomato seed bio-priming followed by soil drenching with all inoculum formulations (Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4) gave better results in plant growth-promotion parameters compared to untreated plants. These findings are in line with previous studies showing that tomato plants inoculated through seed treatment and soil drenching methods with Pseudomonas sp. strain S3 led to more improved morphological characteristics (root/shoot length, fresh weight and dry weight) compared to a single-method treatment [36]. It should be pointed out that, in contrast to Arabidopsis studies where the bacterial mixture showed a better performance compared to the individual strains, when applied on tomato plants the effect of the Cal.l.30 + Cal.f.4 mixture was more pronounced when compared to the single-strain Cal.f.4 treatment, and somewhat inferior compared to treatment with Cal.l.30, suggesting that consortia may provide better plant growth-promoting effects than some individual strains. This is in line with another study, where it was observed that the effect of seed bio-priming and soil drenching in biomass-promoting effects on Withania somnifera is more pronounced when B. amyloliquefaciens and Pseudomonas fluorescens were applied as a mixture compared to individual treatments [5].
These findings highlights that the plant growth-promoting effect observed in tomato plants treated with Cal.l.30 and Cal.f.4 could partially be due to the known plant growth-promoting traits of Cal.l.30 and Cal.f.4, such as production of IAA and siderophores, phosphate solubilization [16] and/or due to the PGPB-mediated long-lasting induction of the expression of genes related to plant growth and development [37,38,39].
Our data indicated that tomato plants treated with Cal.l.30 and Cal.f.4 through seed bio-priming and soil drenching showed a defense-priming effect; plants raised from Cal.l.30 and Cal.f.4-treated seeds showed elevated levels of transcripts of SA and JA/ET defense-related genes, while plants raised from the Cal.l.30 + Cal.f.4 mixture-treated seeds showed enhanced levels of SA-responsive genes. However, plants raised from Cal.l.30, Cal.f.4 or Cal.l.30 + Cal.f.4 bio-primed seeds that also received a soil drenching application displayed a relatively high level of expression of SA and JA/ET defense-related marker genes, indicating that Cal.l.30 and Cal.f.4 strains, singly or in combination, do not only sensitize the plants’ SA and JA/ET immune responsive systems but also enable the plant to enter the defense-priming state. In this regard, plant’s priming is able to maintain a long-lasting state of readiness that does not confer resistance per se, as the accumulation of defense-related transcripts is below the threshold for effective resistance, but rather allows accelerated activation of inducible defense as soon as a pathogen or pest invasion occurs [40,41].
These findings are consistent with previous studies where it was reported that seed treatment followed by soil drenching with certain beneficial ISR-inducing microorganisms, including Bacillus sp. and Trichoderma spp., reprograms their transcriptional apparatus and up-regulates inducible defenses (enhanced expression of SA- and/or ET-defense genes and/or genes coding antioxidative enzymes and enzymes involved in callose deposition and lignification) [9,37,38,42,43,44,45,46].
The duration of the priming state in tomato plants raised from bio-primed seeds or root-drenched plants is not known. Priming-mediated defenses can be durable and maintain throughout the plant’s life cycle and can even be transmitted to the subsequent generation [9]. However, recent studies demonstrated that the inoculum application mode, and most importantly the number of applications, is important for the duration and efficacy of PGPM (plant growth-promoting microorganism) priming-mediated defense [47]. Seed dressing and soil drenching with species of B. amyloliquefaciens FS6 [48], Bacillus subtilis JW-1 [49], Bacillus thuringiensis SE and Pseudomonas aeruginosa KA19 [50] and Bacillus velezensis SZAD2 [51], were found to be effective methods for the control of fungal pathogens. However, when seed treatment was followed by soil drenching, the biocontrol efficacy appeared to be more effective than either treatment alone [48,49]. Suppression of Rhizoctonia solani was found more effective after two soil drenching applications of B. amyloliquefaciens FZB42 at short time intervals, compared to single treatment of tomato plants through soil drenching [52]. Similarly, suppression of potato virus Y was found more in tomato plants subjected to B. amyloliquefaciens MBI600 triple soil drench application compared to a single application through soil drenching prior to germination [53]. In addition, suppression of Fusarium was found more when tomato plants were treated with MBI600 dual drenching (soil drenching prior to germination followed by additional soil drenching), compared to single soil drenching just after sowing [33,46]. Taken together, these data suggested that the back-to-back application of ISR-inducing PGPB (e.g., seed bio-priming followed by soil drenching, seedling treatment), as compared to a single ISR-inducing PGPB application, may prolong the priming defense state of the host plant and, thus, keep the elevated levels of gene expression of defense-related genes. This offers better prospects for an accelerated activation of inducible defenses as soon as an invasion occurs [39,40].
In our studies, the biocontrol efficacy of Cal.l.30, Cal.f.4 and Cal.l.30 + Cal.f.4 was evaluated on tomato plants raised from bio-primed seeds that received two additional doses of bacteria by soil drenching and then challenged with B. cinerea. Under this scheme of inoculum application and seed bio-priming followed by two soil drenchings at short time intervals, tomato plants may sustain the primed defense state (elevated levels of gene expression of SA and ET/JA defense-related genes), thus possessing the means to develop a fast and effective defense response upon facing the ingressive B. cinerea. Based on their disease-suppression abilities, when plants were treated with Cal.l.30 + Cal.f.4, a significant reduction in the plant disease severity was achieved with respect to the control, but this result was not significantly different than those observed in the individual-strain treatments. However, plants treated with Cal.l.30 + Cal.f.4 exhibited better disease inhibition development around the infection zone compared to Cal.l.30 or Cal.f.4 treated plants, suggesting a strong synergistic effect against B. cinerea ingression and development in tomato leaves.
The ability of PGPM in sensitizing and priming the plant immune defense mainly relies on extracellular compounds synthesized and secreted by the beneficial microbes [54]. Recent studies have demonstrated that cell-free culture filtrates (CFCF) of ISR-inducing beneficial B. amyloliquefaciens MBI600, when inoculated by leaf spraying or root drenching, activated the expression of SA and/or JA defense-related genes [12]. The ISR-eliciting activity of CFCF was attributed to Bacillus sp.-secreted metabolites with antibiotic functions, such as iturinic-type lipopeptides, iturin A and bacilomycin D, as well as to the lipopeptides fengycin and surfactin [55]. In particular, it has been demonstrated that the spraying of A. thaliana leaves with iturin A or mucosubtilin activated the expression of SA and JA defense-related genes, PR1 and PDF1.2 [56], while A. thaliana seedling treatment with mycosubtilin from B. subtilis J-15 activated the expression of ET and JA defense-related genes [57]. Similarly, root treatment of tomato plants with fengycin stimulated the induction of SA-responsive genes [58]. In addition, treatment of mandarin fruit with each of the lipopeptides Iturin A, fengycin and surfactin secreted from B. subtillis ABS-S14 activated the expression of SA and JA defense-related genes [59], while treatment of tomato fruit with iturin A or bacilomycin D activated the expression of SA defense-related genes [60,61]. Previous integrated genomic and metabolomic analysis demonstrated that B. halotolerans strains Cal.l.30, Cal.f.4 and Hil4 have the ability to constitutively and simultaneously synthesize and secrete the lipopeptides fengycin, surfactin and the iturinic-type lipopeptide mojavensin [17,21], suggesting that colonization of plant tissues with these B. halotolerans strains may activate and sustained the defense-priming state of the host plant.
5. Conclusions
Beneficial microorganisms have been thought to trigger mild plant responses that do not affect the plant host defense-related transcriptome overall and contribute to sensitization rather than the actual induction of defense-related genes [7]. In the present study, B. halotolerans strains Cal.l.30 and Cal.f.4 belong to a special group of beneficial microorganisms that are able not only to sensitize the induction of defense-related genes upon pathogen challenge but also enable the host plant to maintain elevated levels of defense components around the threshold for effective resistance. Cal.l.30 and Cal.f.4 created a compatible consortium with both plant growth-promoting effects and direct biocontrol activity against B. cinerea. Furthermore, our findings highlighted that seed treatment followed by the soil drenching of tomato plants with the novel ISR-inducing B. halotolerans strains Cal.l.30 and Cal.f.4, as both individual strains and as a compatible consortium, could be an effective and practical approach for controlling gray mold disease under greenhouse crop production conditions. In this regard, the ability of B. halotolerans strains to promote growth and activate plants’ innate immune responses, along with their potential to synthesize numerous ISR-inducing and antifungal metabolites, make these beneficial Bacillus strains important for the development of effective biological control agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology12060779/s1, Table S1: Primers used for RT-qPCR.
Author Contributions
Conceptualization, P.C.T., A.V. and P.K.; methodology, P.C.T., E.-E.T. and A.V.; investigation and formal analysis, P.C.T. and E.-E.T.; validation: P.C.T.; writing—original draft, P.C.T. and P.K.; editing, A.Z.; elaborating the research questions, analyzing the data, formal analysis, software, writing and reviewing the article: P.C.T., E.-E.T., C.D., K.N., A.V. and P.K.; supervision and funding acquisition, P.K. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The research of Polina C. Tsalgatidou was co-financed by Greece and the European Union (European Social Fund, ESF) through the Operational Programme “Human Resources Development, Education and Lifelong Learning” in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research, 2nd cycle” (MIS-5000432), implemented by the State Scholarships Foundation (IKΥ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The Bacillus halotolerans strains Cal.l.30 and Cal.f.4 whole genome projects are available in the NCBI database under the accession numbers JAEACK000000000, JAEACI000000000 (GenBank), respectively, and SAMN16949411, SAMN16949417, (BioSample) and PRJNA681331, PRJNA681333, (BioProject), respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Orozco-Mosqueda, M.D.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Sharma, C.K.; Vishnoi, V.K.; Dubey, R.C.; Maheshwari, D.K.A. Twin rhizospheric bacterial consortium induces systemic resistance to a phytopathogen Macrophomina phaseolina in mung bean. Rhizosphere 2018, 5, 71–75. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, S. Microbe-based inoculants: Role in next green revolution. In Environmental Concerns and Sustainable Development; Volume 2: Biodiversity, Soil and Waste Management; Vertika, S., Narendra, K., Eds.; Springer: Singapore, 2019; pp. 191–246. [Google Scholar]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Ann. Rev. Pphytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Ann. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Yang, Z.; Zhi, P.; Chang, C. Priming seeds for the future: Plant immune memory and application in crop protection. Front. Plant Sci. 2022, 13, 961840. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Brock, A.K.; Berger, B.; Mewis, I.; Ruppel, S. Impact of the PGPB Enterobacter radicincitans DSM 16656 on growth, glucosinolate profile, and immune responses of Arabidopsis thaliana. Microb. Ecol. 2013, 65, 661–670. [Google Scholar] [CrossRef]
- Dimopoulou, A.; Theologidis, I.; Liebmann, B.; Kalantidis, K.; Vassilakos, N.; Skandalis, N. Bacillus amyloliquefaciens MBI600 differentially induces tomato defense signaling pathways depending on plant part and dose of application. Sci. Rep. 2019, 9, 19120. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, W.; Hu, Y.; Tian, R.; Wang, Z. Bacillus velezensis YYC promotes tomato growth and induces resistance against bacterial wilt. Biol. Control. 2022, 172, 104977. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Ma, Q.; Bian, L.; Liu, X.; Xu, Y.; Zhang, H.; Shao, J.; Liu, Y. Bacillus velezensis CLA178-induced systemic resistance of Rosa multiflora against crown gall disease. Front. Microbiol. 2020, 11, 587667. [Google Scholar] [CrossRef] [PubMed]
- Thomloudi, E.E.; Tsalgatidou, P.C.; Douka, D.; Spantidos, T.N.; Dimou, M.; Venieraki, A.; Katinakis, P. Multistrain versus single-strain plant growth promoting microbial inoculants-The compatibility issue. Hell. Plant Prot. J. 2019, 12, 61–77. [Google Scholar] [CrossRef]
- Tsalgatidou, P.C.; Thomloudi, E.E.; Nifakos, K.; Delis, C.; Venieraki, A.; Katinakis, P. Calendula officinalis—A Great Source of Plant Growth Promoting Endophytic Bacteria (PGPEB) and Biological Control Agents (BCA). Microorganisms 2023, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Tsalgatidou, P.C.; Thomloudi, E.E.; Baira, E.; Papadimitriou, K.; Skagia, A.; Venieraki, A.; Katinakis, P. Integrated genomic and metabolomic analysis illuminates key secreted metabolites produced by the novel endophyte Bacillus halotolerans Cal. l. 30 involved in diverse biological control activities. Microorganisms 2022, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- McCully, L.M.; Bitzer, A.S.; Seaton, S.C.; Smith, L.M.; Silby, M.W. Interspecies social spreading: Interaction between two sessile soil bacteria leads to emergence of surface motility. MSphere 2019, 4, e00696-18. [Google Scholar] [CrossRef]
- Molina-Santiago, C.; Pearson, J.R.; Navarro, Y.; Berlanga-Clavero, M.V.; Caraballo-Rodriguez, A.M.; Petras, D.; Romero, D. The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization. Nature Commun. 2019, 10, 1919. [Google Scholar] [CrossRef]
- Beauregard, P.B.; Chai, Y.; Vlamakis, H.; Losick, R.; Kolter, R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc. Natl. Acad. Sci. USA 2013, 110, E1621–E1630. [Google Scholar] [CrossRef]
- Thomloudi, E.E.; Tsalgatidou, P.C.; Baira, E.; Papadimitriou, K.; Venieraki, A.; Katinakis, P. Genomic and metabolomic insights into secondary metabolites of the novel Bacillus halotolerans Hil4, an endophyte with promising antagonistic activity against gray mold and plant growth promoting potential. Microorganisms 2021, 9, 2508. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.P.; Lee, S.W.; Kim, C.S.; Son, J.H.; Song, J.H.; Lee, K.Y.; Moon, B.J. Evaluation of formulations of Bacillus licheniformis for the biological control of tomato gray mold caused by Botrytis cinerea. Biolog. Control 2006, 37, 329–337. [Google Scholar] [CrossRef]
- Rotenberg, D.; Thompson, T.S.; German, T.L.; Willis, D.K. Methods for effective real-time RT-PCR analysis of virus-induced gene silencing. J. Virol. Meth. 2006, 138, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, C.; Zhao, R.; Wang, L.; Chen, L.; Yu, W.; Shen, L. CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Boil. 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, L.; Liu, L.; Wang, J.; Li, C.; Wang, Q. Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternata f. sp. lycopersici. J. Experiment. Bot. 2013, 64, 637–650. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT—PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocont. Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; del Carmen Orozco-Mosqueda, M.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de Los Santos-Villalobos, S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre-and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosatka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Roberts, D.P.; Xie, L.; Qin, L.; Li, Y.; Liao, X.; Han, P.; Yu, C.; Liao, X. Seed treatment containing Bacillus subtilis BY-2 in combination with other Bacillus isolates for control of Sclerotinia sclerotiorum on oilseed rape. Biol. Control 2019, 133, 50–57. [Google Scholar] [CrossRef]
- Dawar, S.; Wahab, S.; Tariq, M.; Zaki, M.J. Application of Bacillus species in the control of root rot diseases of crop plants. Arch. Phytopatol. Plant Prot. 2010, 43, 412–418. [Google Scholar] [CrossRef]
- Samaras, A.; Efthimiou, K.; Roumeliotis, E.; Karaoglanidis, G.S. Biocontrol potential and plant-growth-promoting effects of Bacillus amyloliquefaciens MBI 600 against Fusarium oxysporum f. sp. radicis-lycopersici on tomato. V Int. Symp. Tomato Dis. Perspect. Future Dir. Tomato Prot. 2016, 1207, 139–146. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S. Evaluation of Pseudomonas sp. for its multifarious plant growth promoting potential and its ability to alleviate biotic and abiotic stress in tomato (Solanum lycopersicum) plants. Sci. Rep. 2020, 10, 20951. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, S.P.; Mahfooz, S.; Singh, S.P.; Bhattacharya, A.; Mishra, N.; Nautiyal, C.S. Endophyte-mediated modulation of defense-related genes and systemic resistance in Withania somnifera (L.) Dunal under Alternaria alternata stress. Appl. Environ. Microbiol. 2018, 84, e02845-17. [Google Scholar] [CrossRef]
- Samaras, A.; Kamou, N.; Tzelepis, G.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Root transcriptional and metabolic dynamics induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on cucumber plants. Plants 2022, 11, 1218. [Google Scholar] [CrossRef]
- Wu, X.; Fan, Y.; Wang, R.; Zhao, Q.; Ali, Q.; Wu, H.; Gu, Q.; Borriss, R.; Xie, Y.; Gao, X. Bacillus halotolerans KKD1 induces physiological, metabolic and molecular reprogramming in wheat under saline condition. Front. Plant Sci. 2022, 13, 978066. [Google Scholar] [CrossRef]
- Wilkinson, S.W.; Magerøy, M.H.; López Sánchez, A.; Smith, L.M.; Furci, L.; Cotton, T.A.; Ton, J. Surviving in a hostile world: Plant strategies to resist pests and diseases. Ann. Rev. Phytopathol. 2019, 57, 505–529. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Patel, R.R.; Patel, D.D.; Bhatt, J.; Thakor, P.; Triplett, L.R.; Thakkar, V.R. Induction of pre-chorismate, jasmonate and salicylate pathways by Burkholderia sp. RR18 in peanut seedlings. J. Appl. Microbiol. 2021, 131, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.S.P.; Ito, S.I. Different mechanisms of Trichoderma virens-mediated resistance in tomato against Fusarium wilt involve the jasmonic and salicylic acid pathways. Mol. Plant Pathol. 2018, 19, 870–882. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chun, S.C. Induction of defence-related enzymes in tomato (Solanum lycopersicum) plants treated with Bacillus subtilis CBR05 against Xanthomonas campestris pv. vesicatoria. Biocontrol Sci. Technol. 2016, 26, 1366–1378. [Google Scholar] [CrossRef]
- Takishita, Y.; Charron, J.B.; Smith, D.L. Biocontrol rhizobacterium Pseudomonas sp. 23S induces systemic resistance in tomato (Solanum lycopersicum L.) against bacterial canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 2119. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Swain, B.B.; Mohapatra, P.K.; Naik, S.K.; Mukherjee, A.K. Biopriming for induction of disease resistance against pathogens in rice. Planta 2022, 255, 113. [Google Scholar]
- Wang, R.; Wang, C.; Zuo, B.; Liang, X.; Zhang, D.; Liu, R.; Yang, L.; Lu, B.; Wang, X.; Gao, J. A novel biocontrol strain Bacillus amyloliquefaciens FS6 for excellent control of gray mold and seedling diseases of ginseng. Plant Dis. 2021, 105, 1926–1935. [Google Scholar] [CrossRef]
- Kwon, J.W.; Kim, S.D. Characterization of an antibiotic produced by Bacillus subtilis JW-1 that suppresses Ralstonia solanacearum. J. Microbiol. Biotechnol. 2014, 24, 13–18. [Google Scholar] [CrossRef]
- Mishra, S.; Arora, N.K. Evaluation of rhizospheric Pseudomonas and Bacillus as biocontrol tool for Xanthomonas campestris pv campestris. World J. Microbiol. Biotechnol. 2012, 28, 693–702. [Google Scholar] [CrossRef]
- Sherzad, Z.; Canming, T. A new strain of Bacillus velezensis as a bioagent against Verticillium dahliae in cotton: Isolation and molecular identification. Egypt. J. Biol. Pest Control 2020, 30, 118. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef] [PubMed]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 10320. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Pršić, J.; Ongena, M. Elicitors of plant immunity triggered by beneficial bacteria. Front. Plant Sci. 2020, 11, 594530. [Google Scholar] [CrossRef]
- Kawagoe, Y.; Shiraishi, S.; Kondo, H.; Yamamoto, S.; Aoki, Y.; Suzuki, S. Cyclic lipopeptide iturin A structure-dependently induces defense response in Arabidopsis plants by activating SA and JA signaling pathways. Bioch. Bioph. Res. Commun. 2015, 460, 1015–1020. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, H.; You, J.; Yang, J.; Zhang, Q.; Zhao, J.; Aimaier, R.; Zhang, J.; Han, S.; Zhao, H.; et al. Transcriptome and metabolome analyses reveal that Bacillus subtilis BS-Z15 lipopeptides mycosubtilin homologue mediates plant defense responses. Front. Plant Sci. 2022, 13, 1088220. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Gao, X. Suppression of Sclerotinia sclerotiorum by the induction of systemic resistance and regulation of antioxidant pathways in tomato using fengycin produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef]
- Tunsagool, P.; Leelasuphakul, W.; Jaresitthikunchai, J.; Phaonakrop, N.; Roytrakul, S.; Jutidamrongphan, W. Targeted transcriptional and proteomic studies explicate specific roles of Bacillus subtilis iturin A, fengycin, and surfactin on elicitation of defensive systems in mandarin fruit during stress. PLoS ONE 2019, 14, e0217202. [Google Scholar] [CrossRef]
- Lin, F.; Xue, Y.; Huang, Z.; Jiang, M.; Lu, F.; Bie, X.; Miao, S.; Lu, Z. Bacillomycin D inhibits growth of Rhizopus stolonifer and induces defense-related mechanism in cherry tomato. Appl. Microbiol. Biotechnol. 2019, 103, 7663–7674. [Google Scholar] [CrossRef]
- Jiang, M.; Pang, X.; Liu, H.; Lin, F.; Lu, F.; Bie, X.; Lu, Z.; Lu, Y. Iturin A induces resistance and improves the quality and safety of harvested cherry tomato. Molecules 2021, 26, 6905. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).