The Effects of Obesity on the Inflammatory, Cardiovascular, and Neurobiological Responses to Exercise in Older Adults
Abstract
Simple Summary
Abstract
1. Introduction
2. The Effect of Exercise on Inflammation in Obese Older Adults
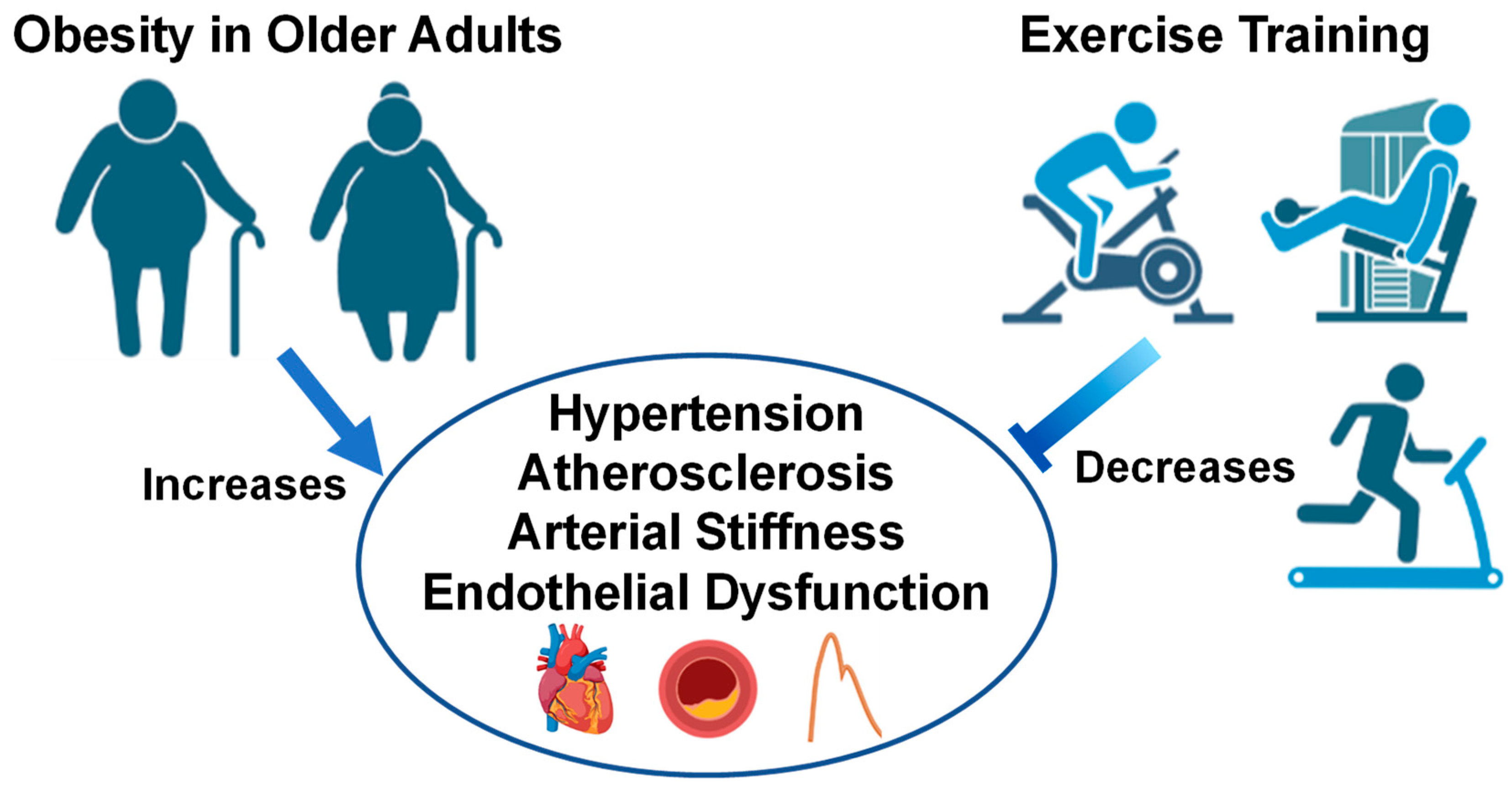
3. Aging: Cardiovascular Responses to Exercise in Obesity
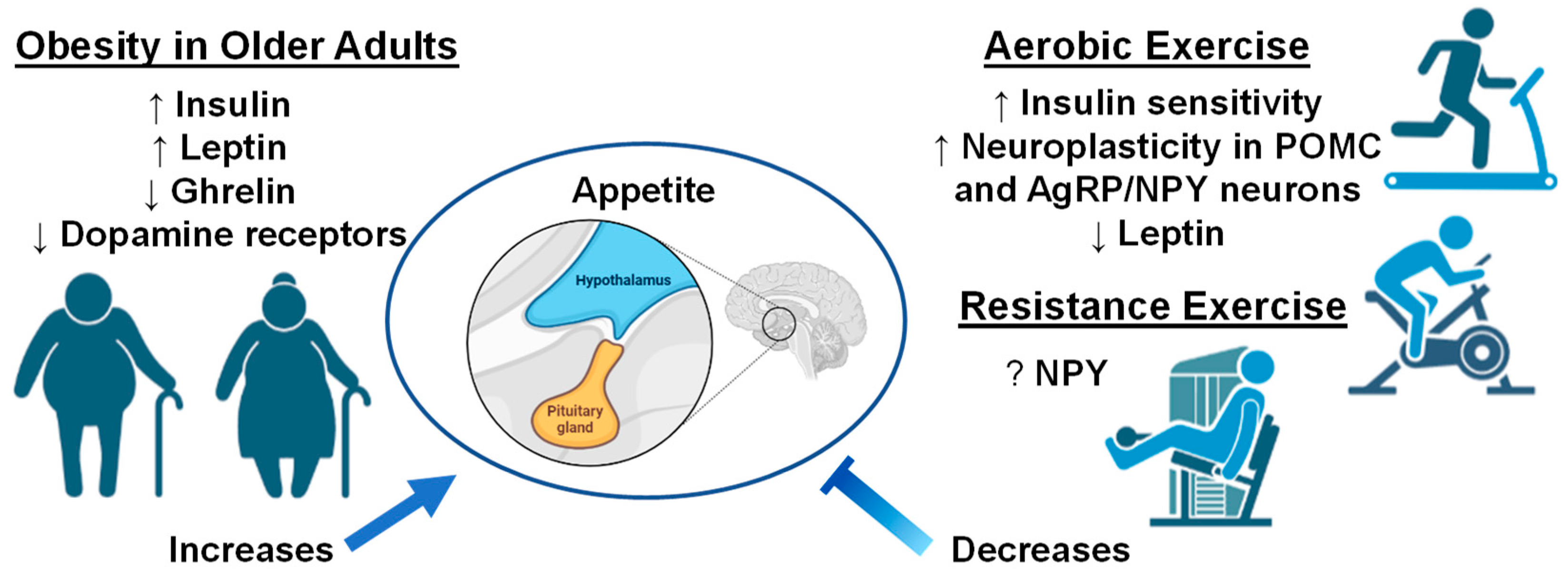
4. Neurobiological Responses in Older Adults with Obesity and Effects of Exercise
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.; Alter, D. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011, 12, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D. Several areas of overlap between obesity and aging indicate obesity as a biomarker of accelerated aging of human B cell function and antibody responses. Immun. Ageing 2022, 19, 48. [Google Scholar] [CrossRef]
- Xu, H.; Cupples, L.A.; Stokes, A.; Liu, C.-T. Association of Obesity with Mortality Over 24 Years of Weight History. JAMA Netw. Open 2018, 1, e184587. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obesity as a Neuroendocrine Reprogramming. Medicina 2021, 57, 66. [Google Scholar] [CrossRef]
- Bischof, G.N.; Park, D.C. Obesity and Aging: Consequences for Cognition, Brain Structure, and Brain Function. Psychosom. Med. 2015, 77, 697–709. [Google Scholar] [CrossRef]
- Hassing, L.B.; Dahl, A.K.; Pedersen, N.L.; Johansson, B. Overweight in Midlife Is Related to Lower Cognitive Function 30 Years Later: A Prospective Study with Longitudinal Assessments. Dement. Geriatr. Cogn. Disord. 2010, 29, 543–552. [Google Scholar] [CrossRef]
- Marti, A.; Marcos, A.; Martinez, J.A. Obesity and immune function relationships. Obes. Rev. 2001, 2, 131–140. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Bouloumie, A.; Casteilla, L.; Lafontan, M. Adipose tissue lymphocytes and macrophages in obesity and insulin resistance. Arter. Thromb. Vasc. Biol. 2008, 28, 1211–12132008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, R.; Gu, H.; Zhang, E.; Qu, J.; Cao, W.; Huang, X.; Yan, H.; He, J.; Cai, Z. Metabolic reprogramming in macrophage responses. Biomark. Res. 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.-Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Aljada, A.; Hofmeyer, D.; Syed, T.; Mohanty, P.; Dandona, P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004, 110, 1564–1571. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Thomas, A.L.; Alarcon, P.C.; Divanovic, S.; Chougnet, C.A.; Hildeman, D.A.; Moreno-Fernandez, M.E. Implications of inflammatory states on dysfunctional immune responses in aging and obesity. Front. Aging 2021, 2, 732414. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef]
- Park, H.-K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metabolism 2015, 64, 24–34. [Google Scholar] [CrossRef] [PubMed]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Koerner, A.; Kratzsch, J.; Kiess, W. Adipocytokines: Leptin—The classical, resistin—The controversical, adiponectin—The promising, and more to come. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 525–546. [Google Scholar] [CrossRef]
- Park, Y.; Myers, M.; Vieira-Potter, V. Adipose tissue inflammation and metabolic dysfunction: Role of exercise. Mo. Med. 2014, 111, 65–72. [Google Scholar]
- Ferrandi, P.J.; Fico, B.G.; Whitehurst, M.; Zourdos, M.C.; Bao, F.; Dodge, K.M.; Rodriguez, A.L.; Pena, G.; Huang, C.-J. Acute high-intensity interval exercise induces comparable levels of circulating cell-free DNA and Interleukin-6 in obese and normal-weight individuals. Life Sci. 2018, 202, 161–166. [Google Scholar] [CrossRef]
- Huang, C.-J.; Rodriguez, A.L.; Visavadiya, N.P.; Fico, B.G.; Slusher, A.L.; Ferrandi, P.J.; Whitehurst, M. An exploratory investigation of apoptotic and autophagic responses in peripheral blood mononuclear cells following maximal aerobic exercise in obese individuals. Arch. Physiol. Biochem. 2022, 128, 209–216. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Fico, B.G.; Garten, R.S.; Zourdos, M.C.; Whitehurst, M.; Ferrandi, P.J.; Dodge, K.M.; Pena, G.S.; Rodriguez, A.A.; Huang, C.-J. The Impact of Obesity on C1q/TNF-Related Protein-9 Expression and Endothelial Function following Acute High-Intensity Interval Exercise vs. Continuous Moderate-Intensity Exercise. Biology 2022, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Slusher, A.L.; Fico, B.G.; Dodge, K.M.; Garten, R.S.; Ferrandi, P.J.; Rodriguez, A.A.; Pena, G.; Huang, C.-J. Impact of acute high-intensity interval exercise on plasma pentraxin 3 and endothelial function in obese individuals—A pilot study. Eur. J. Appl. Physiol. 2021, 121, 1567–1577. [Google Scholar] [CrossRef]
- Rejeski, W.J.; Marsh, A.P.; Fanning, J.; Ambrosius, W.T.; Walkup, M.P.; Nicklas, B.J. Dietary Weight Loss, Exercise, and Inflammation in Older Adults with Overweight or Obesity and Cardiometabolic Disease. Obesity 2019, 27, 1805–1811. [Google Scholar] [CrossRef]
- Fico, B.G.; Alkatan, M.; Tanaka, H. No Changes in Appetite-Related Hormones Following Swimming and Cycling Exercise Interventions in Adults with Obesity. Int. J. Exerc. Sci. 2020, 13, 1819–1825. [Google Scholar] [PubMed]
- Zhuang, M.; Jin, M.; Lu, T.; Lu, L.; Ainsworth, B.E.; Liu, Y.; Chen, N. Effects of three modes of physical activity on physical fitness and hematological parameters in older people with sarcopenic obesity: A systematic review and meta-analysis. Front. Physiol. 2022, 13, 917525. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-like growth factor-1 and neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef]
- Ringseis, R.; Eder, K.; Mooren, F.C.; Krüger, K. Metabolic signals and innate immune activation in obesity and exercise. Exerc. Immunol. Rev. 2015, 21, 793575724. [Google Scholar]
- McMurray, R.G.; Hackney, A.C. Interactions of metabolic hormones, adipose tissue and exercise. Sport. Med.-ADIS Int. 2005, 35, 393–412. [Google Scholar] [CrossRef]
- Arikawa, A.Y.; Thomas, W.; Schmitz, K.H.; Kurzer, M.S. Sixteen weeks of exercise reduces C-reactive protein levels in young women. Med. Sci. Sport. Exerc. 2011, 43, 1002–1009. [Google Scholar] [CrossRef]
- Kohut, M.; McCann, D.; Russell, D.; Konopka, D.; Cunnick, J.; Franke, W.; Castillo, M.; Reighard, A.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of β-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006, 20, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Iliadis, F.; Angelopoulou, N.; Perrea, D.; Ampatzidis, G.; Liapis, C.D.; Alevizos, M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 837–843. [Google Scholar] [CrossRef]
- Xing, H.; Lu, J.; Yoong, S.Q.; Tan, Y.Q.; Kusuyama, J.; Wu, X.V. Effect of Aerobic and Resistant Exercise Intervention on Inflammaging of Type 2 Diabetes Mellitus in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2022, 23, 823–830.e13. [Google Scholar] [CrossRef]
- Bianchi, A.; Marchetti, L.; Hall, Z.; Lemos, H.; Vacca, M.; Paish, H.; Green, K.; Elliott, B.; Tiniakos, D.; Passos, J.F. Moderate exercise inhibits age-related inflammation, liver steatosis, senescence, and tumorigenesis. J. Immunol. 2021, 206, 904–916. [Google Scholar] [CrossRef]
- Olson, T.P.; Dengel, D.; Leon, A.; Schmitz, K. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int. J. Obes. 2007, 31, 996–1003. [Google Scholar] [CrossRef]
- Donges, C.E.; Duffield, R.; Drinkwater, E.J. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med. Sci. Sport. Exerc. 2010, 42, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Sardeli, A.V.; Tomeleri, C.M.; Cyrino, E.S.; Fernhall, B.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Effect of resistance training on inflammatory markers of older adults: A meta-analysis. Exp. Gerontol. 2018, 111, 188–196. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Hsu, F.C.; Brinkley, T.J.; Church, T.; Goodpaster, B.H.; Kritchevsky, S.B.; Pahor, M. Exercise training and Plasma C-reactive Protein and Interleukin-6 in elderly people. J. Am. Geriatr. Soc. 2008, 56, 2045–2052. [Google Scholar] [CrossRef]
- Colleluori, G.; Napoli, N.; Phadnis, U.; Villareal, R.-A.; Villareal, D.T. Effect of Weight Loss, Exercise, or Both on Undercarboxylated Osteocalcin and Insulin Secretion in Frail, Obese Older Adults. Oxidative Med. Cell. Longev. 2017, 2017, 4807046. [Google Scholar] [CrossRef] [PubMed]
- Bouchonville, M.; Armamento-Villareal, R.; Shah, K.; Napoli, N.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: Results of a randomized controlled trial. Int. J. Obes. 2014, 38, 423–431. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.-S.; Lang Lehrskov, L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019, 29, 844–855.e3. [Google Scholar] [CrossRef] [PubMed]
- Čížková, T.; Štěpán, M.; Daďová, K.; Ondrůjová, B.; Sontáková, L.; Krauzová, E.; Matouš, M.; Koc, M.; Gojda, J.; Kračmerová, J. Exercise training reduces inflammation of adipose tissue in the elderly: Cross-sectional and randomized interventional trial. J. Clin. Endocrinol. Metab. 2020, 105, e4510–e4526. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Zanuso, S.; Cardelli, P.; Salerno, G.; Fallucca, S.; Nicolucci, A.; Pugliese, G. Supervised exercise training counterbalances the adverse effects of insulin therapy in overweight/obese subjects with type 2 diabetes. Diabetes Care 2012, 35, 39–41. [Google Scholar] [CrossRef]
- Bruun, J.M.; Helge, J.W.; Richelsen, B.; Stallknecht, B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E961–E967. [Google Scholar] [CrossRef]
- Gonzalo-Encabo, P.; Maldonado, G.; Valadés, D.; Ferragut, C.; Pérez-López, A. The Role of Exercise Training on Low-Grade Systemic Inflammation in Adults with Overweight and Obesity: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 13258. [Google Scholar] [CrossRef]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. Effects of chronic exercise training on inflammatory markers in Australian overweight and obese individuals in a randomized controlled trial. Inflammation 2013, 36, 625–632. [Google Scholar] [CrossRef]
- Brady, T.M. The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr. Hypertens. Rep. 2016, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Rescaldani, M.; Sala, C.; Grassi, G. Left-ventricular hypertrophy and obesity: A systematic review and meta-analysis of echocardiographic studies. J. Hypertens. 2014, 32, 16–25. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac remodeling in obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef] [PubMed]
- Haass, M.; Kitzman, D.W.; Anand, I.S.; Miller, A.; Zile, M.R.; Massie, B.M.; Carson, P.E. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ. Heart Fail. 2011, 4, 324–331. [Google Scholar] [CrossRef]
- Strasser, B.; Arvandi, M.; Pasha, E.; Haley, A.; Stanforth, P.; Tanaka, H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Association, A.H. Heart Disease and Stroke Statistics 2018–At-a-Glance; American Heart Association: Dallas, TX, USA, 2018. [Google Scholar]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, J.R. Exploring vascular benefits of endothelium-derived nitric oxide. Am. J. Hypertens. 2005, 18, 177S–183S. [Google Scholar] [CrossRef]
- Gewaltig, M.T.; Kojda, G. Vasoprotection by nitric oxide: Mechanisms and therapeutic potential. Cardiovasc. Res. 2002, 55, 250–260. [Google Scholar] [CrossRef]
- Tziros, C.; Freedman, J.E. The many antithrombotic actions of nitric oxide. Curr. Drug Targets 2006, 7, 1243–1251. [Google Scholar] [CrossRef]
- Engin, A. Endothelial dysfunction in obesity. Obes. Lipotoxicity 2017, 960, 345–379. [Google Scholar]
- Perticone, F.; Ceravolo, R.; Pujia, A.; Ventura, G.; Iacopino, S.; Scozzafava, A.; Ferraro, A.; Chello, M.; Mastroroberto, P.; Verdecchia, P. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001, 104, 191–196. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Prior, S.J.; Ryan, A.S.; Blumenthal, J.B.; Watson, J.M.; Katzel, L.I.; Goldberg, A.P. Sarcopenia is associated with lower skeletal muscle capillarization and exercise capacity in older adults. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2016, 71, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Fang, F.; Li, F.; Ren, Y.; Hu, J.; Cao, J. Sarcopenia is associated with hypertension in older adults: A systematic review and meta-analysis. BMC Geriatr. 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Chung, G.E.; Lee, H.; Kim, M.J.; Choi, S.Y.; Lee, W.; Yoon, J.W. Significance of Low Muscle Mass on Arterial Stiffness as Measured by Cardio-Ankle Vascular Index. Front. Cardiovasc. Med. 2022, 9, 857871. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, K.; Klich-Rączka, A.; Skalska, A.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. Pulse Wave Velocity and Sarcopenia in Older Persons-A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6477. [Google Scholar] [CrossRef]
- Amarasekera, A.T.; Chang, D.; Schwarz, P.; Tan, T.C. Does vascular endothelial dysfunction play a role in physical frailty and sarcopenia? A systematic review. Age Ageing 2021, 50, 725–732. [Google Scholar] [CrossRef]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship between sarcopenia and cardiovascular diseases in the elderly: An overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef]
- Sasaki, K.I.; Fukumoto, Y. Sarcopenia as a comorbidity of cardiovascular disease. J. Cardiol. 2022, 79, 596–604. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Atkins, J.L. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef]
- Stephen, W.C.; Janssen, I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J. Nutr. Health Aging 2009, 13, 460–466. [Google Scholar] [CrossRef]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Endocrinol. Metab. 2013, 28, 86–89. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, D.R.; Janssen, I. Dynapenic-obesity and physical function in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 71–77. [Google Scholar] [CrossRef]
- Batsis, J.A.; Zbehlik, A.J.; Pidgeon, D.; Bartels, S.J. Dynapenic obesity and the effect on long-term physical function and quality of life: Data from the osteoarthritis initiative. BMC Geriatr 2015, 15, 118. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Sanders, K.M.; Aitken, D.; Hayes, A.; Ebeling, P.R.; Jones, G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity 2014, 22, 1568–1574. [Google Scholar] [CrossRef]
- Lv, D.; Shen, S.; Chen, X. Association Between Dynapenic Abdominal Obesity and Fall Risk in Older Adults. Clin. Interv. Aging 2022, 17, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Nakamura, T.; Yamashita, M.; Maekawa, E.; Reed, J.L.; Yamaoka-Tojo, M.; et al. Prognostic utility of dynapenia in patients with cardiovascular disease. Clin. Nutr. 2021, 40, 2210–2218. [Google Scholar] [CrossRef]
- Yoo, J.I.; Kim, M.J.; Na, J.B.; Chun, Y.H.; Park, Y.J.; Park, Y.; Hah, Y.S.; Ha, Y.C.; Park, K.S. Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J. Cachexia Sarcopenia Muscle 2018, 9, 1034–1041. [Google Scholar] [CrossRef]
- Kivimäki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Brunner, E.J.; Fransson, E.; Goldberg, M. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef] [PubMed]
- Dubbert, P.M.; Carithers, T.; Hall, J.E.; Barbour, K.A.; Clark, B.L.; Sumner, A.E.; Crook, E.D. Obesity, physical inactivity, and risk for cardiovascular disease. Am. J. Med. Sci. 2002, 324, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Tataranni, P.A. Dietary fat and human obesity. J. Am. Diet. Assoc. 1997, 97, S42–S46. [Google Scholar] [CrossRef]
- Al Suwaidi, J.; Higano, S.T.; Holmes, D.R.; Lennon, R.; Lerman, A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J. Am. Coll. Cardiol. 2001, 37, 1523–1528. [Google Scholar] [CrossRef]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef]
- Zebekakis, P.E.; Nawrot, T.; Thijs, L.; Balkestein, E.J.; Van Der Heijden-Spek, J.; Van Bortel, L.M.; Struijker-Boudier, H.A.; Safar, M.E.; Staessen, J.A. Obesity is associated with increased arterial stiffness from adolescence until old age. J. Hypertens. 2005, 23, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Dangardt, F.; Chen, Y.; Berggren, K.; Osika, W.; Friberg, P. Increased Rate of Arterial Stiffening with Obesity in Adolescents: A Five-Year Follow-Up Study. PLoS ONE 2013, 8, e57454. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Hulens, M.; Vansant, G.; Lysens, R.; Claessens, A.; Muls, E. Exercise capacity in lean versus obese women. Scand. J. Med. Sci. Sport. 2001, 11, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.S.; Francis, G.S.; Okin, P.M.; Pashkow, F.J.; Snader, C.E.; Marwick, T.H. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999, 281, 524–529. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.-P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef]
- Arbit, B.; Azarbal, B.; Hayes, S.W.; Gransar, H.; Germano, G.; Friedman, J.D.; Thomson, L.; Berman, D.S. Prognostic contribution of exercise capacity, heart rate recovery, chronotropic incompetence, and myocardial perfusion single-photon emission computerized tomography in the prediction of cardiac death and all-cause mortality. Am. J. Cardiol. 2015, 116, 1678–1684. [Google Scholar] [CrossRef]
- Dipla, K.; Nassis, G.P.; Vrabas, I.S. Blood pressure control at rest and during exercise in obese children and adults. J. Obes. 2012, 2012, 298953. [Google Scholar] [CrossRef]
- Hargens, T.A.; Guill, S.G.; Zedalis, D.; Gregg, J.M.; Nickols-Richardson, S.M.; Herbert, W.G. Attenuated heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. Sleep 2008, 31, 104–110. [Google Scholar] [CrossRef]
- Deniz, F.; Katircibasi, M.T.; Pamukcu, B.; Binici, S.; Sanisoglu, S.Y. Association of metabolic syndrome with impaired heart rate recovery and low exercise capacity in young male adults. Clin. Endocrinol. 2007, 66, 218–223. [Google Scholar] [CrossRef]
- Itagi, A.B.H.; Jayalakshmi, M.; Yunus, G. Effect of obesity on cardiovascular responses to submaximal treadmill exercise in adult males. J. Fam. Med. Prim. Care 2020, 9, 4673. [Google Scholar] [CrossRef]
- Franklin, N.C.; Ali, M.; Goslawski, M.; Wang, E.; Phillips, S.A. Reduced vasodilator function following acute resistance exercise in obese women. Front. Physiol. 2014, 5, 253. [Google Scholar] [CrossRef]
- Marcal, I.R.; Goessler, K.F.; Buys, R.; Casonatto, J.; Ciolac, E.G.; Cornelissen, V.A. Post-exercise hypotension following a single bout of high intensity interval exercise vs. a single bout of moderate intensity continuous exercise in adults with or without hypertension: A systematic review and meta-analysis of randomized clinical trials. Front. Physiol. 2021, 12, 675289. [Google Scholar] [CrossRef]
- Tibana, R.; Pereira, G.; Navalta, J.; Bottaro, M.; Prestes, J. Acute effects of resistance exercise on 24-h blood pressure in middle aged overweight and obese women. Int. J. Sport. Med. 2013, 34, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Baillot, A.; Chenail, S.; Barros Polita, N.; Simoneau, M.; Libourel, M.; Nazon, E.; Riesco, E.; Bond, D.S.; Romain, A.J. Physical activity motives, barriers, and preferences in people with obesity: A systematic review. PLoS ONE 2021, 16, e0253114. [Google Scholar] [CrossRef] [PubMed]
- O’Neil-Pirozzi, T.M.; Cattaneo, G.; Solana-Sánchez, J.; Gomes-Osman, J.; Pascual-Leone, A. The importance of motivation to older adult physical and cognitive exercise program development, initiation, and adherence. Front. Aging 2022, 3, 1. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Yang, Y.-J.; Ge, R.-K.; Zhou, M.; Hu, H.; Liu, H.; Cui, J.; Li, L.-L.; Dong, Y.-F. Aerobic exercise improves endothelial function and serum adropin levels in obese adolescents independent of body weight loss. Sci. Rep. 2017, 7, 17717. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.P.; Dengel, D.R.; Leon, A.S.; Schmitz, K.H. Moderate resistance training and vascular health in overweight women. Med. Sci. Sport. Exerc. 2006, 38, 1558–1564. [Google Scholar] [CrossRef]
- D’Agostino, E.M.; Day, S.E.; Konty, K.J.; Armstrong, S.C.; Skinner, A.C.; Neshteruk, C.D. Longitudinal Association between Weight Status, Aerobic Capacity, Muscular Strength, and Endurance among New York City Youth, 2010–2017. Child. Obes. 2022, 19, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Alberga, A.S.; Farnesi, B.-C.; Lafleche, A.; Legault, L.; Komorowski, J. The effects of resistance exercise training on body composition and strength in obese prepubertal children. Physician Sportsmed. 2013, 41, 103–109. [Google Scholar]
- Farah, B.; Ritti-Dias, R.; Balagopal, P.; Hill, J.; Prado, W. Does exercise intensity affect blood pressure and heart rate in obese adolescents? A 6-month multidisciplinary randomized intervention study. Pediatr. Obes. 2014, 9, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Manolio, T.A.; Burke, G.L.; Savage, P.J.; Sidney, S.; Gardin, J.M.; Oberman, A. Exercise blood pressure response and 5-year risk of elevated blood pressure in a cohort of young adults: The CARDIA study. Am. J. Hypertens. 1994, 7, 234–241. [Google Scholar]
- Janz, K.; Dawson, J.; Mahoney, L. Increases in physical fitness during childhood improve cardiovascular health during adolescence: The Muscatine Study. Int. J. Sport. Med. 2002, 23, 15–21. [Google Scholar] [CrossRef]
- Farinatti, P.; Neto, S.R.M.; Dias, I.; Cunha, F.A.; Bouskela, E.; Kraemer-Aguiar, L.G. Short-term resistance training attenuates cardiac autonomic dysfunction in obese adolescents. Pediatr. Exerc. Sci. 2016, 28, 374–380. [Google Scholar] [CrossRef]
- Amano, M.; KANDA, T.; UE, H.; MORITANI, T. Exercise training and autonomic nervous system activity in obese individuals. Med. Sci. Sport. Exerc. 2001, 33, 1287–1291. [Google Scholar] [CrossRef]
- Swift, D.L.; Earnest, C.P.; Katzmarzyk, P.T.; Rankinen, T.; Blair, S.N.; Church, T.S. The effect of different doses of aerobic exercise training on exercise blood pressure in overweight and obese postmenopausal women. Menopause 2012, 19, 503–509. [Google Scholar] [CrossRef]
- Swift, D.L.; Earnest, C.P.; Blair, S.N.; Church, T.S. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: Results from the DREW study. Br. J. Sport. Med. 2012, 46, 753–758. [Google Scholar] [CrossRef]
- Figueroa, A.; Kalfon, R.; Wong, A. Whole-body vibration training decreases ankle systolic blood pressure and leg arterial stiffness in obese postmenopausal women with high blood pressure. Menopause 2015, 22, 423–427. [Google Scholar] [CrossRef]
- Figueroa, A.; Vicil, F.; Sanchez-Gonzalez, M.A.; Wong, A.; Ormsbee, M.J.; Hooshmand, S.; Daggy, B. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am. J. Hypertens. 2013, 26, 416–423. [Google Scholar] [CrossRef]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef]
- National Center for Health Statistics (US). Health, United States: 2010, with Special Feature on Death and Dying; February Report No.: 2011-1232; National Center for Health Statistics: Washington, DC, USA, 2011. [Google Scholar]
- Lima, R.; Wofford, M.; Reckelhoff, J.F. Hypertension in postmenopausal women. Curr. Hypertens. Rep. 2012, 14, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Yanes, L.L.; Reckelhoff, J.F. Postmenopausal hypertension. Am. J. Hypertens. 2011, 24, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Prelevic, G.M.; Kwong, P.; Byrne, D.J.; Jagroop, I.A.; Ginsburg, J.; Mikhailidis, D.P. A cross-sectional study of the effects of hormon replacement therapy on the cardiovascular disease risk profile in healthy postmenopausal women. Fertil. Steril. 2002, 77, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Kalantaridou, S.N.; Naka, K.K.; Papanikolaou, E.; Kazakos, N.; Kravariti, M.; Calis, K.A.; Paraskevaidis, E.A.; Sideris, D.A.; Tsatsoulis, A.; Chrousos, G.P. Impaired endothelial function in young women with premature ovarian failure: Normalization with hormone therapy. J. Clin. Endocrinol. Metab. 2004, 89, 3907–3913. [Google Scholar] [CrossRef]
- Writing Group for The Women, S.H.I.I. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results from the Women’s Health Initiative Randomized Controlled Trial. JAMA J. Am. Med. Assoc. 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Herrington, D.M. The HERS trial results: Paradigms lost? Heart and Estrogen/progestin Replacement Study. Ann. Intern. Med. 1999, 131, 463–466. [Google Scholar] [CrossRef]
- Grady, D.; Herrington, D.; Bittner, V.; Blumenthal, R.; Davidson, M.; Hlatky, M.; Hsia, J.; Hulley, S.; Herd, A.; Khan, S. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002, 288, 49–57. [Google Scholar] [CrossRef]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health 2012, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Jung, W.-S.; Hong, K.; Kim, Y.-Y.; Kim, S.-W.; Park, H.-Y. Effects of moderate combined resistance-and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: A pilot study. Int. J. Environ. Res. Public Health 2020, 17, 7233. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen-Hinderling, V.B.; Hesselink, M.K.; Meex, R.; Van Der Made, S.; Schär, M.; Lamb, H.; Wildberger, J.E.; Glatz, J.; Snoep, G.; Kooi, M.E. Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. J. Clin. Endocrinol. Metab. 2010, 95, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Konieczna, J.; Zulet, M.A.; Galmés-Panades, A.M.; Ibero-Baraibar, I.; Babio, N.; Estruch, R.; Vidal, J.; Toledo, E.; Razquin, C.; et al. Association of lifestyle factors and inflammation with sarcopenic obesity: Data from the PREDIMED-Plus trial. J. Cachexia Sarcopenia Muscle 2019, 10, 974–984. [Google Scholar] [CrossRef]
- Hershberger, D.; Bollinger, L. Sarcopenic Obesity: Background and Exercise Training Strategies. Strength Cond. J. 2015, 37, 78–83. [Google Scholar] [CrossRef]
- França, G.O.; Frantz, E.D.C.; Magliano, D.C.; Bargut, T.C.L.; Sepúlveda-Fragoso, V.; Silvares, R.R.; Daliry, A.; Nascimento, A.R.D.; Borges, J.P. Effects of short-term high-intensity interval and continuous exercise training on body composition and cardiac function in obese sarcopenic rats. Life Sci. 2020, 256, 117920. [Google Scholar] [CrossRef]
- Sénéchal, M.; Bouchard, D.R.; Dionne, I.J.; Brochu, M. The effects of lifestyle interventions in dynapenic-obese postmenopausal women. Menopause 2012, 19, 1015–1021. [Google Scholar] [CrossRef]
- Upadhya, B.; Haykowsky, M.J.; Eggebeen, J.; Kitzman, D.W. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2015, 12, 205–214. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Del Buono, M.G.; Canada, J.M.; Kim, Y.; Damonte, J.I.; Trankle, C.R.; Halasz, G.; Mihalick, V.; Vecchié, A.; Markley, R.R.; et al. Sarcopenic Obesity Is Associated with Reduced Cardiorespiratory Fitness Compared with Nonsarcopenic Obesity in Patients with Heart Failure with Reduced Ejection Fraction. Circ. Heart Fail. 2022, 15, e009518. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Simon, F.; Achiardi, O.; Vilos, C.; Cabrera, D.; Cabello-Verrugio, C. The Critical Role of Oxidative Stress in Sarcopenic Obesity. Oxidative Med. Cell. Longev. 2021, 2021, 4493817. [Google Scholar] [CrossRef]
- Bellanti, F.; Romano, A.D.; Lo Buglio, A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Baumgartner, R.N.; Atkinson, H.H.; Penninx, B.W.; Lenchik, L.; Palla, S.L.; Ambrosius, W.T.; Tracy, R.P.; Pahor, M. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am. J. Clin. Nutr. 2005, 82, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Parise, G.; Brose, A.N.; Tarnopolsky, M.A. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp. Gerontol. 2005, 40, 173–180. [Google Scholar] [CrossRef]
- Takahashi, M.; Miyashita, M.; Kawanishi, N.; Park, J.H.; Hayashida, H.; Kim, H.S.; Nakamura, Y.; Sakamoto, S.; Suzuki, K. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur. J. Appl. Physiol. 2013, 113, 1117–1126. [Google Scholar] [CrossRef]
- Kang, Y.; Dillon, K.N.; Martinez, M.A.; Maharaj, A.; Fischer, S.M.; Figueroa, A. Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women. Nutrients 2022, 15, 74. [Google Scholar] [CrossRef]
- Coles, K.E. Investigation into the Antioxidant Capacity of L-Arginine and L-Citrulline in Relation to Their Vascular Protective Properties. Ph.D. Thersis, Cardiff University (United Kingdom), Wales, UK, 2007. [Google Scholar]
- Figueroa, A.; Jaime, S.J.; Morita, M.; Gonzales, J.U.; Moinard, C. L-Citrulline Supports Vascular and Muscular Benefits of Exercise Training in Older Adults. Exerc. Sport Sci. Rev. 2020, 48, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Caballero-García, A.; Pascual-Fernández, J.; Noriega-González, D.C.; Bello, H.J.; Pons-Biescas, A.; Roche, E.; Córdova-Martínez, A. L-Citrulline Supplementation and Exercise in the Management of Sarcopenia. Nutrients 2021, 13, 3133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cash, R.E.; Bower, J.K.; Focht, B.C.; Paskett, E.D. Physical activity and risk of cardiovascular disease by weight status among US adults. PLoS ONE 2020, 15, e0232893. [Google Scholar]
- Piercy, K.L.; Troiano, R.P. Physical activity guidelines for Americans from the US department of health and human services: Cardiovascular benefits and recommendations. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e005263. [Google Scholar] [CrossRef]
- Dibben, G.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2021, 11, Cd001800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, H.; Jiang, P.; Tang, H. Cardiac rehabilitation in acute myocardial infarction patients after percutaneous coronary intervention: A community-based study. Medicine 2018, 97, e9785. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ratchford, S.M.; Lee, J.F.; Bunsawat, K.; Alpenglow, J.K.; Zhao, J.; Ma, C.L.; Ryan, J.J.; Khor, L.L.; Wray, D.W. The impact of obesity on the regulation of muscle blood flow during exercise in patients with heart failure with a preserved ejection fraction. J. Appl. Physiol. 2022, 132, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.J.; O’Driscoll, J.M. Exercise Training in Heart failure with Preserved and Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. Sport. Med.-Open 2022, 8, 76. [Google Scholar] [CrossRef]
- Lee, D.J.; Elias, G.J.; Lozano, A.M. Neuromodulation for the treatment of eating disorders and obesity. Ther. Adv. Psychopharmacol. 2018, 8, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Belfort-DeAguiar, R.; Seo, D. Food cues and obesity: Overpowering hormones and energy balance regulation. Curr. Obes. Rep. 2018, 7, 122–129. [Google Scholar] [CrossRef]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s reward system in health and disease. Circadian Clock Brain Health Dis. 2021, 1344, 57–69. [Google Scholar]
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef]
- Biebermann, H.; Castañeda, T.R.; van Landeghem, F.; von Deimling, A.; Escher, F.; Brabant, G.; Hebebrand, J.; Hinney, A.; Tschöp, M.H.; Grüters, A. A role for β-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 2006, 3, 141–146. [Google Scholar] [CrossRef]
- Kastin, A. Handbook of Biologically Active Peptides; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef] [PubMed]
- Yaswen, L.; Diehl, N.; Brennan, M.B.; Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 1999, 5, 1066–1070. [Google Scholar] [CrossRef]
- Greenman, Y.; Kuperman, Y.; Drori, Y.; Asa, S.L.; Navon, I.; Forkosh, O.; Gil, S.; Stern, N.; Chen, A. Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Mol. Endocrinol. 2013, 27, 1091–1102. [Google Scholar] [CrossRef]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef]
- Yang, S.B.; Tien, A.C.; Boddupalli, G.; Xu, A.W.; Jan, Y.N.; Jan, L.Y. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 2012, 75, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Wilsey, J.; Scarpace, P. Hypothalamic pro-opiomelanocortin gene delivery ameliorates obesity and glucose intolerance in aged rats. Diabetologia 2005, 48, 2376–2385. [Google Scholar] [CrossRef]
- Kowalski, C.; Micheau, J.; Corder, R.; Gaillard, R.; Conte-Devolx, B. Age-related changes in cortico-releasing factor, somatostatin, neuropeptide Y, methionine enkephalin and β-endorphin in specific rat brain areas. Brain Res. 1992, 582, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wolden-Hanson, T.; Marck, B.T.; Matsumoto, A.M. Blunted hypothalamic neuropeptide gene expression in response to fasting, but preservation of feeding responses to AgRP in aging male Brown Norway rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R138–R146. [Google Scholar] [CrossRef]
- Carter, S.; Caron, A.; Richard, D.; Picard, F. Role of leptin resistance in the development of obesity in older patients. Clin. Interv. Aging 2013, 8, 829–844. [Google Scholar]
- Ono, H. Molecular mechanisms of hypothalamic insulin resistance. Int. J. Mol. Sci. 2019, 20, 1317. [Google Scholar] [CrossRef]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e183. [Google Scholar] [CrossRef]
- Henn, R.E.; Noureldein, M.H.; Elzinga, S.E.; Kim, B.; Savelieff, M.G.; Feldman, E.L. Glial-neuron crosstalk in health and disease: A focus on metabolism, obesity, and cognitive impairment. Neurobiol. Dis. 2022, 170, 105766. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yamashita, T. The Effects of Leptin on Glial Cells in Neurological Diseases. Front. Neurosci. 2019, 13, 828. [Google Scholar] [CrossRef]
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC neuronal heterogeneity in energy balance and beyond: An integrated view. Nat. Metab. 2021, 3, 299–308. [Google Scholar] [CrossRef]
- Veyrat-Durebex, C.; Quirion, R.; Ferland, G.; Dumont, Y.; Gaudreau, P. Aging and long-term caloric restriction regulate neuropeptide Y receptor subtype densities in the rat brain. Neuropeptides 2013, 47, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Freire-Regatillo, A.; Diaz-Pacheco, S.; Frago, L.M.; Arévalo, M.; Argente, J.; Garcia-Segura, L.M.; de Ceballos, M.L.; Chowen, J.A. Sex Differences in Hypothalamic Changes and the Metabolic Response of TgAPP Mice to a High Fat Diet. Front. Neuroanat. 2022, 16, 910477. [Google Scholar] [CrossRef]
- MacNicol, B. The biology of addiction. Can. J. Anesth. J. Can. D’anesthésie 2017, 64, 141–148. [Google Scholar] [CrossRef]
- Cooper, S.; Robison, A.; Mazei-Robison, M.S. Reward circuitry in addiction. Neurotherapeutics 2017, 14, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.-J.; Morris, M.J. The role of reward circuitry and food addiction in the obesity epidemic: An update. Biol. Psychol. 2018, 131, 31–42. [Google Scholar] [CrossRef]
- Lerma-Cabrera, J.M.; Carvajal, F.; Lopez-Legarrea, P. Food addiction as a new piece of the obesity framework. Nutr. J. 2015, 15, 5. [Google Scholar] [CrossRef]
- Adams, R.C.; Sedgmond, J.; Maizey, L.; Chambers, C.D.; Lawrence, N.S. Food addiction: Implications for the diagnosis and treatment of overeating. Nutrients 2019, 11, 2086. [Google Scholar] [CrossRef]
- Lee, P.C.; Dixon, J.B. Food for thought: Reward mechanisms and hedonic overeating in obesity. Curr. Obes. Rep. 2017, 6, 353–361. [Google Scholar] [CrossRef]
- Reynolds, S.M.; Berridge, K.C. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J. Neurosci. 2002, 22, 7308–7320. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, A. It wasn’t me; it was my brain–Obesity-associated characteristics of brain circuits governing decision-making. Physiol. Behav. 2017, 176, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Temple, J.L.; Roemmich, J.N.; Bouton, M.E. Habituation as a determinant of human food intake. Psychol. Rev. 2009, 116, 384. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Jayne, M.; Franceschi, D.; Wong, C.; Gatley, S.J.; Gifford, A.N.; Ding, Y.S. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 2002, 44, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. ‘Liking’and ‘wanting’food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 2009, 97, 537–550. [Google Scholar] [CrossRef]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Wang, G.-J.; Volkow, N.D.; Felder, C.; Fowler, J.S.; Levy, A.V.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusil, N. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport 2002, 13, 1151–1155. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef]
- Raefsky, S.M.; Mattson, M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic. Biol. Med. 2017, 102, 203–216. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Villareal, D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 2021, 155, 111561. [Google Scholar] [CrossRef]
- Beavers, K.M.; Ambrosius, W.T.; Rejeski, W.J.; Burdette, J.H.; Walkup, M.P.; Sheedy, J.L.; Nesbit, B.A.; Gaukstern, J.E.; Nicklas, B.J.; Marsh, A.P. Effect of exercise type during intentional weight loss on body composition in older adults with obesity. Obesity 2017, 25, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Ibeas, K.; Herrero, L.; Mera, P.; Serra, D. Hypothalamus-skeletal muscle crosstalk during exercise and its role in metabolism modulation. Biochem. Pharmacol. 2021, 190, 114640. [Google Scholar] [CrossRef] [PubMed]
- Jiaxu, C.; Weiyi, Y. Influence of acute and chronic treadmill exercise on rat brain POMC gene expression. Med. Sci. Sport. Exerc. 2000, 32, 954–957. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Gao, Y.; Alhadeff, A.L.; Castorena, C.M.; Huang, Y.; Lieu, L.; Afrin, S.; Sun, J.; Betley, J.N.; Guo, H. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol. Metab. 2018, 18, 107–119. [Google Scholar] [CrossRef]
- Mueller, K.; Möller, H.E.; Horstmann, A.; Busse, F.; Lepsien, J.; Blüher, M.; Stumvoll, M.; Villringer, A.; Pleger, B. Physical exercise in overweight to obese individuals induces metabolic-and neurotrophic-related structural brain plasticity. Front. Hum. Neurosci. 2015, 9, 372. [Google Scholar] [CrossRef]
- Yi, C.X.; Al-Massadi, O.; Donelan, E.; Lehti, M.; Weber, J.; Ress, C.; Trivedi, C.; Müller, T.D.; Woods, S.C.; Hofmann, S.M. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol. Behav. 2012, 106, 485–490. [Google Scholar] [CrossRef]
- Gaspar, R.C.; Muñoz, V.R.; Formigari, G.P.; Kuga, G.K.; Nakandakari, S.; Botezelli, J.D.; da Silva, A.S.R.; Cintra, D.E.; de Moura, L.P.; Ropelle, E.R.; et al. Acute physical exercise increases the adaptor protein APPL1 in the hypothalamus of obese mice. Cytokine 2018, 110, 87–93. [Google Scholar] [CrossRef]
- Rochete Ropelle, E.; Ramos da Silva, A.S.; Esper Cintra, D.; Pereira de Moura, L.; Teixeira, A.M.; Rodrigo Pauli, J. Physical Exercise: A Versatile Anti-Inflammatory Tool Involved in the Control of Hypothalamic Satiety Signaling. Exerc. Immunol. Rev. 2021, 27, 7–23. [Google Scholar]
- Wilson, R.A.; Stathis, C.G.; Hayes, A.; Cooke, M.B. Intermittent Fasting and High-Intensity Exercise Elicit Sexual-Dimorphic and Tissue-Specific Adaptations in Diet-Induced Obese Mice. Nutrients 2020, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Onambélé-Pearson, G.L.; Breen, L.; Stewart, C.E. Influence of exercise intensity in older persons with unchanged habitual nutritional intake: Skeletal muscle and endocrine adaptations. Age 2010, 32, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. A cross-sectional study evaluating the effects of resistance exercise on inflammation and neurotrophic factors in elderly women with obesity. J. Clin. Med. 2020, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Chen, H.T.; Chung, Y.C.; Chen, Y.J.; Ho, S.Y.; Wu, H.J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Assari, S.; Bazargan, M. Baseline Obesity Increases 25-Year Risk of Mortality due to Cerebrovascular Disease: Role of Race. Int. J. Environ. Res. Public Health 2019, 16, 3705. [Google Scholar] [CrossRef]
- Marcell, T.J.; McAuley, K.A.; Traustadóttir, T.; Reaven, P.D. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism 2005, 54, 533–541. [Google Scholar] [CrossRef]

| Inflammatory Responses | |||
|---|---|---|---|
| Reference | Population | Type/Duration of Study | Conclusions |
| Balducci et al., 2012 [52] | Overweight and obese diabetic middle-aged and older adults (N = 36) | Aerobic and resistance exercise (75 min of aerobic exercise and four resistance exercises) for 12 months |
|
| Bruun et al., 2006 [53] | Severely obese adults (N = 27) | Aerobic exercise (2–3 h, 5×/week) for 15 weeks |
|
| Čížková et al., 2020 [51] | Overweight older females (N = 51) | Aerobic and resistive training (3×/week) for 4 months |
|
| Donges et al., 2010 [45] | Overweight and obese adults (N = 76) | Aerobic training (N = 41) or resistance training (N = 35) for 10 weeks |
|
| Ho et al., 2013 [55] | Overweight and obese middle-aged and older adults (N = 48) | Aerobic (N = 15), resistance (N = 16), or combination exercise (N = 17) for 12 weeks |
|
| Kadoglou et al., 2007 [41] | Overweight and obese diabetic older adults (N = 30) | Aerobic exercise (45–60 min, 4×/week) for 6 months |
|
| Kohut et al., 2006 [40] | Obese older adults (N = 48) | Aerobic exercise (45 min, 3×/week) for 10 months |
|
| Marcell et al., 2005 [213] | Overweight and obese insulin resistant middle-aged adults (N = 37) | Aerobic exercise (30 min, 5×/week) for 16 weeks |
|
| Rejeski et al., 2019 [33] | Overweight and obese older adults (N = 154) | Aerobic training (N = 79) or resistance training (N = 75) for 18 months |
|
| Cardiovascular Responses | |||
| Reference | Population | Type/Duration of Study | Conclusions |
| Amano et al., 2001 [119] | Obese middle-aged males and females (N = 18) | Aerobic exercise (30 min/session, 3×/week) for 12 weeks |
|
| Figueroa et al., 2015 [122] | Obese postmenopausal women with high blood pressure (N = 36) | Whole-body vibration training stratified by ankle systolic blood pressure into 3 groups (high blood pressure, normal blood pressure, non-exercising control) for 12 weeks |
|
| Figueroa et al., 2013 [123] | Obese postmenopausal women (N = 41) | Low-intensity resistance exercise training, hypocaloric diet, or combined exercise + diet for 12 weeks |
|
| Ho et al., 2013 [55] | Overweight/obese middle-aged males and females (N = 64, all participants) | Aerobic exercise for 30 min (group 1), resistance exercise for 30 min (group 2), combined aerobic and resistance (15 min each modality, group 3), and non-exercising controls for 12 weeks |
|
| Swift et al., 2012 [121] | Obese postmenopausal women with elevated blood pressure (N = 155) | Aerobic exercise at different energy expenditure doses (4, 8, and 12 kcal/kg/week vs controls) for 6 months |
|
| Park et al., 2020 [134] | Obese older males (N = 20) | Combined elastic band resistance and aerobic exercise training 3 days/week vs non-exercising controls for 12 weeks |
|
| Schrauwen-Hinderling et al., 2010 [135] | Overweight/obese middle-aged males (N = 14) | Combined aerobic and resistance exercise training for 12 weeks |
|
| Neurobiological Responses | |||
| Reference | Population | Type/Duration of Study | Conclusions |
| Chen et al., 2017 [211] | Sarcopenic obese older adults (N = 60) randomized into a control, aerobic, resistance, or concurrent training group. | 8-week intervention study. Aerobic training group: 60 min of moderately intense aerobic exercise 2×/week. Resistance training group: series of 10 movements targeting multiple muscle groups at 60–70% 1-repetition maximum, 2×/week. |
|
| Gaspar et al., 2018 [204] | 6-week-old Swiss mice split into control and obese groups (high fat diet). | Acute treadmill exercise consisting of three 45 min bouts with 15 min. rest between bouts. |
|
| He et al., 2018 [201] | Transgenic POMC/NPY mice split into either a sedentary or exercise group | Exercise group intervention: combination of high intensity interval training and continuous exercise. Control group: mice placed on static treadmill for the same amount of time as exercise mice. |
|
| Jiaxu et al., 2000 [200] | Male Sprague-Dawley rats separated into a sedentary control, and exercise group. | Exercise group: 35 min of treadmill running for 2 weeks after 5-week exercise week acclimation protocol. Control group: no exercise or treadmill placement during entirety of study. |
|
| Mueller et al., 2015 [202] | Young overweight/obese women (N = 9) | 60 min of concurrent exercise training 2/week for 12 weeks. |
|
| Onambélé-Pearson et al., 2010 [207] | Sedentary older adults (N = 30) | Participants split into either a low or high resistance exercise intervention 3×/week for 12 weeks. |
|
| Roh et al., 2020 [208] | Obese older women (N = 26) | Resistance training 3×/week for 12 weeks, or no resistance exercise control. |
|
| Villareal et al., 2017 [210] | Obese older adults (N = 141) randomized into a control, aerobic, resistance, or concurrent training group, the latter three with added weight management programs. | 26-week intervention study. Control group: educational sessions about diet. Aerobic group: weight management program + 60 min of aerobic exercise 3×/week. Resistance group: weight management program + 40 min of resistance exercise 3×/week. Concurrent training group: weight management program + aerobic and resistance exercise training 3×/week. |
|
| Wilson et al., 2020 [206] | Male (N = 39) and Female (N = 49) C57BL/6 mice with high fat diet assigned to 12–24 weeks of Intermittent fasting, high intensity interval training, intermittent fasting + high intensity interval training, or control group. | Intermittent fasting group: 2 alternative days fasting/8-day cycle. High intensity interval training group: 3 days of 6–8 repetitions 20 s. sprints, 40 s. walking/ 8-day cycle. Intermittent fasting + high intensity interval training group: 2 non-consecutive days fasting/ 8-day cycle + high intensity interval protocol 3 non-fast days/ 8-day cycle. |
|
| Yi et al., 2012 [203] | Low-density lipoprotein deficient mice split into exercise (N = 10) or sedentary (N = 6) groups. | Exercise group intervention: 30 min of treadmill running at 10% grade for 26 weeks. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fico, B.G.; Maharaj, A.; Pena, G.S.; Huang, C.-J. The Effects of Obesity on the Inflammatory, Cardiovascular, and Neurobiological Responses to Exercise in Older Adults. Biology 2023, 12, 865. https://doi.org/10.3390/biology12060865
Fico BG, Maharaj A, Pena GS, Huang C-J. The Effects of Obesity on the Inflammatory, Cardiovascular, and Neurobiological Responses to Exercise in Older Adults. Biology. 2023; 12(6):865. https://doi.org/10.3390/biology12060865
Chicago/Turabian StyleFico, Brandon G., Arun Maharaj, Gabriel S. Pena, and Chun-Jung Huang. 2023. "The Effects of Obesity on the Inflammatory, Cardiovascular, and Neurobiological Responses to Exercise in Older Adults" Biology 12, no. 6: 865. https://doi.org/10.3390/biology12060865
APA StyleFico, B. G., Maharaj, A., Pena, G. S., & Huang, C.-J. (2023). The Effects of Obesity on the Inflammatory, Cardiovascular, and Neurobiological Responses to Exercise in Older Adults. Biology, 12(6), 865. https://doi.org/10.3390/biology12060865







